Published online Apr 27, 2017. doi: 10.4240/wjgs.v9.i4.109
Peer-review started: September 2, 2016
First decision: October 20, 2016
Revised: December 11, 2016
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: April 27, 2017
To prospectively evaluate the postoperative morbi-mortality and weight loss evolution of patients who underwent a laparoscopic sleeve gastrectomy (LSG) as a primary bariatric procedure during 5 years of follow-up.
Since 2006, data from patients undergoing a highly restrictive primary LSG have been prospectively registered in a database and analysed. Preoperative co-morbid conditions, operating time, hospital stay, early and late complications rate and evolution of weight loss after 5 years of follow-up were analysed.
A total of 156 patients were included, 74.3% of whom were women. The mean age was 43.2 ± 13.1 years and the mean body mass index (BMI) was 41.5 ± 7.9 kg/m2. Seventy patients (44.8%) presented a BMI under 40 kg/m2. The mortality rate was 0%. The leakage rate was 1.2%, and the total 30-d morbidity rate was 5.1% (8/156). With a mean follow-up of 32.7 ± 28.5 (range 6-112) mo, the mean percent of excess of weight loss (%EWL) was 82.0 ± 18.8 at 1 year, 76.7 ± 21.3 at 3 years and 60.3 ± 28.9 at 5 years. The mean percent of excess of BMI loss (%EBMIL) was 94.9 ± 22.4 at 1 year, 89.4 ± 27.4 at 3 years and 74.8 ± 29.4 at 5 years. Patients with preoperative BMI less than 40 kg/m2 achieved greater weight loss than did the overall study population. Diabetes remitted in 75% of the patients and HTA improved in 71.7%. CPAP masks were withdrawn in all patients with obstructive sleep apnoea.
LSG built with a narrow 34 F bougie and starting 3 cm from the pylorus proved to be safe and highly effective in terms of weight loss as a stand-alone procedure, particularly in patients with a preoperative BMI lower than 40 kg/m2.
Core tip: The number of laparoscopic sleeve gastrectomies (LSGs) performed worldwide as a primary bariatric procedure has grown exponentially in recent years, given the simplicity of the technique, the low complication rate and the good short- and mid-term results regarding weight loss and the resolution of co-morbidities. However, there are a limited data from long-term studies. In this study, a standardized LSG proved to be safe (no mortality and a leakage rate of 1.2%) and highly effective in terms of weight loss after 5-year of follow-up, particularly in patients with a low preoperative body mass index. This manuscript provides additional evidence supporting the role of laparoscopic sleeve gastrectomy as a stand-alone procedure for selected morbidly obese patients.
- Citation: Hoyuela C. Five-year outcomes of laparoscopic sleeve gastrectomy as a primary procedure for morbid obesity: A prospective study. World J Gastrointest Surg 2017; 9(4): 109-117
- URL: https://www.wjgnet.com/1948-9366/full/v9/i4/109.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i4.109
The laparoscopic bariatric procedure commonly referred to as “sleeve gastrectomy” (LSG) is a left partial gastrectomy of the fundus and body to create a long tubular gastric conduit constructed along the lesser curve of the stomach[1].
LSG was initially proposed as a first-stage procedure to reduce the mortality and postoperative morbidity of more complex bariatric procedures in higher-risk patients[2], such as the duodenal switch, to complete the biliopancreatic diversion or the Roux-en-Y gastric bypass (RYGB) in a second stage. Soon, it was noted that many patients frequently lost sufficient weight such that a second-stage operation became unnecessary[3]. LSG is not merely a restrictive procedure. LSG provokes a rapid gastric emptying of solid food, accelerates intestinal transit and induces a favourable change in the gut hormones, thereby facilitating weight loss through restriction and appetite suppression, given the reduction in the ghrelin levels after resection of the gastric fundus[3-7]. Since then, LSG has been performed as a primary and definitive bariatric procedure in patients whose weight and medical condition are not sufficiently severe to require a complex bariatric operation, moving to a second stage only in those selected patients in which weight loss was inadequate[8]. Eventually, LSG was performed in some patients with special conditions in which the usual bariatric operations might be too aggressive[9].
The number of LSGs performed worldwide has grown exponentially over the last decade, because it appears to be an easier and safer technique[10-13]. Many surgeons now perform LSG as their standard bariatric operation[3]. The advantages of the LSG include its technical simplicity, shorter operative time, maintenance of bowel integrity and preservation of the pylorus[3,10]. The long-term problems associated with other complex bariatric procedures, including internal hernias and small bowel obstruction are avoided with LSG. In addition, patients who underwent LSG had fewer nutritional deficiencies than that did patients who underwent RYGB or biliopancreatic diversion[14]. The LSG can later be modified by a laparoscopic approach if required, to a more complex procedure (such as RYGB or duodenal switch) in patients who develop severe gastroesophageal reflux symptoms or those who regain weight.
LSG has proven highly effective at achieving durable weight loss and co-morbidity reduction over the short and intermediate terms and is comparable in some aspects to RYGB, the current gold standard in bariatric surgery[7,15-18]. However, some questions must be answered regarding the long-term results of LSG because there are a limited data from long-term studies and because of the variability in both the reported follow-up among series and the rate of patients lost to follow-up.
The aim of this study was to assess the safety and outcomes of patients who underwent a LSG as a primary bariatric procedure in analysing mortality, postoperative morbidity rate, late complications and evolution of weight loss after 5 years of follow-up.
From 2006 to January 2016, data from patients who underwent a LSG as a single procedure treating morbid obesity were collected in an electronic database (Microsoft Access 2003 Microsoft Corporation, Redmond, QA, United States) for analysis. All study participants, or their legal guardian, provided informed written consent prior to study enrolment. The study was officially registered under the identification number researchregistry 1580 on researchregistry.com.
The indications for LSG included patients with body mass index (BMI) less than 45 kg/m2, primary procedure in super-obese patients as the initial stage of a two-staged approach for weight loss (RYGB or BPD in 2 stages), adolescents (under 18 years old of age) with morbid obesity and obese patients with impaired medical conditions or other important co-morbidities such as liver cirrhosis.
The first endpoint of this study was to assess the safety of the procedure by analysing the 30-d mortality and early postoperative complications: Suture leak rate, haemorrhages, wound infection rate, deep venous thrombosis, pulmonary embolism and cardiac and pulmonary complications.
The second endpoint was to evaluate the outcome of LSG in terms of weight loss 5 years after the procedure. Weight loss was measured using BMI evolution and the percentage of excess weight loss (%EWL). Given the variability of %EWL depending on the definition of ideal body weight, we also used the percentage of excess body mass index loss (%EBMIL)[19]. Excessive BMI itself was defined as initial BMI minus 25. Values are reported as the mean ± standard deviation.
The following variables were also evaluated: Resolution of preoperative co-morbid conditions [diabetes, hypertension, obstructive sleep apnoea syndrome (OSA)], length of hospital stay and late complications (stricture, functional obstruction, gastroesophageal reflux, trocar-site hernia rate).
Under general anaesthesia the patients were placed in the reverse Trendelenburg position with the surgeon standing between the legs. All patients received intravenous antibiotic prophylaxis with 2 g of cefazoline. Compression stockings were used during the operation to prevent deep vein thrombosis and thromboembolism.
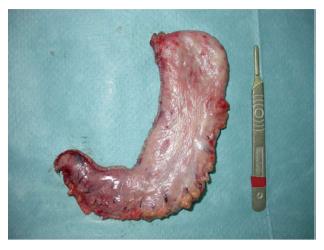
The procedure was performed using 4 or 5 ports (two or three 12-mm trocars and two 5-mm trocars). The greater curvature of the stomach was completely freed starting from the antrum (3 cm proximal to pylorus) until the left pillar of the diaphragm and the gastroesophageal junction were completely exposed. If a hiatal hernia is identified, dissection should be carried posteriorly to achieve appropriate closure of the crus. If a hernia is found, it should be repaired[10]. A harmonic scalpel (Ultracision®, Ethicon Endo-Surgery Inc., Johnson and Johnson, Cincinnati, OH, United States) was used to divide the gastroepiploic and the short gastric vessels. Then, the adhesions of the posterior side of the stomach were dissected to achieve an appropriate sleeved stomach. The LSG was performed by sequentially firing an articulating linear stapler (Echelon Flex™ Endopath, Ethicon Endo-Surgery Inc., Johnson and Johnson, Cincinnati, OH, United States). The gastric division started at 3 cm proximal to the pylorus. Two 60-mm green staple cartridges (open height = 4.1 mm) were usually used to transect the antrum, and gold (3.8 mm) and blue loads (3.6 mm) were later applied at the gastric corpus and fundus. The whole fundus had to be removed. Special attention was required at that point to avoid rotation and functional obstruction of the sleeve by ensuring equal (and not excessive) traction on both walls of the stomach. It is of utmost importance to align the stapler firings properly to avoid excessive narrowing, especially at the level of the incisura angularis (Figure 1).
The calibration of the LSG was obtained using a 34 F oral gastric tube (1.13 cm). The gastric stapled line was always oversewn with a 2/0 absorbable running suture (Monoplus®, B. Braun, Melsungen, Germany) in the 125 initial cases. A bovine pericardial strip (BPS-Peristrip) was used in 5 patients. Since 2014, bioabsorbable membranes (Gore Seamguard® from WL Gore and Associates, Newark, DE, United States) were used instead of the reinforcement suture to achieve better hemostasis and reduce the suture leakage rate[15]. Intraoperative leak testing using methylene blue dye was routinely performed. A suction Blake or Jackson-Pratt drain was placed along the suture line. Finally, the gastric specimen was withdrawn through the right 12-mm port. All 12-mm wounds were closed with Monoplus® or Monomax® 2/0 sutures (B. Braun, Melsungen, Germany) using an Endoclose™ trocar-site closure device (Covidien Products, Medtronic, Minneapolis, MN, United States).
Patients started to walk 8 to 12 h after the procedure. A liquid diet was initiated on the first postoperative day and was implemented for two weeks. The patients were usually discharged on the second or third postoperative day. The treatment included oral analgesia, proton-pump inhibitors (PPI) and low molecular weight heparin against deep vein thrombosis for 30 d.
The first follow-up control was scheduled at the medical office eight days after the procedure. Follow-up data were obtained at the medical office after 15 d, 1, 3, 6 mo, 1 year and semi-annually thereafter by the surgeon who performed the procedure and by a nutritionist. All data were prospectively collected.
Data from 156 patients who underwent LSG until January 2016 were analysed. Of the patients, 116 (74.4%) were women, and 40 (25.6%) were men; overall, the mean age was 43.2 ± 13.1 (range 16-71) years, and the mean BMI was 41.5 ± 7.9 (range 34-76) kg/m2. Seventy patients (44.9%) presented BMI under 40 kg/m2, and only 15 patients (9.6%) were super-obese (BMI greater than 50 kg/m2). All the procedures were performed laparoscopically by the same surgeon. The mean hospital stay was 3.5 ± 0.7 d (range: 1-18). All patients completed the 6-mo outpatient follow-up at the medical office. The mean follow-up was 32.7 ± 28.5 mo (Table 1).
The mean operating time was 95 ± 14.1 min. Conversion to laparotomy was necessary in 2 patients (1.2%) due to intraoperative haemorrhage. One patient was a woman suffering from a cavernous transformation of the portal vein and the other required a lateral segmentectomy to remove a bleeding 8-cm liver haemangioma.
| Number of patients | 156 |
| Age1 (yr) | 43.2 ± 13.2 (16-71) |
| Gender (Female/male) | 116/40 |
| BMI1 (kg/m2) | 41.5 ± 7.9 |
| BMI < 40 kg/m2 | 70 (44.9) |
| BMI 40-50 kg/m2 | 71 (45.5) |
| BMI > 50 kg/m2 | 15 (9.6) |
| Comorbidity | |
| HTA | 39 (25) |
| Diabetes | 12 (7.6) |
| Obstructive sleep apnea (with CPAP) | 21 (13.4) |
| Other | 67 (42.9) |
| Operating time1 (min) | 95 ± 14.1 (65-155) |
| Hospital stay1 (d) | 3.5 ± 0.7 (1-18) |
| Follow-up1 (mo) | 32.7 ± 28.5 (6-112) |
No mortality was observed in this series. The total 30-d postoperative complication rate was 5.1% (8/156 patients). The type and severity of complications are listed in Table 2. A leakage in the staple-line was detected in 2 women (1.2%). The first woman (after oversewing the staple line) healed successfully with medical management 14 d after. The second (Peristrips® reinforcement) required a laparoscopic reoperation to drain a subphrenic abscess secondary to a leak at the angle of His. No endoprosthesis or self-expanded wall-stent was needed. There was no relationship between leakage and patients’ BMI, age or technical difficulties during the sleeve gastrectomy procedure. Intraoperative leak testing was not predictive of the later development of staple line leaks. No patients presented with deep vein thrombosis or pulmonary embolism.
| Mortality | 0 |
| Total 30-d complications | 8 (5.1) |
| Staple line leakage | 2 (1.2) |
| Staple line haemorrhage | 1 (0.6) |
| Wound infection | 2 (1.2) |
| Pneumonia | 1 (0.6) |
| Cutaneous rash | 1 (0.6) |
| Urethral bleeding | 1 (0.6) |
| Late complications | |
| Symptomatic gastroesophageal reflux | 24 (15.3) |
| Hiatal hernia needing laparoscopic repair | 1 (0.6) |
| Gastric stricture – conversion to gastric by-pass | 1 (0.6) |
| Symptomatic cholelithiasis | 7 (4.4) |
Regarding late complications, one patient (without symptoms of previous staple-line leak) developed a gastric stricture 10 mo after the LSG and submitted to a laparoscopic gastric bypass (0.6%). Twenty-four patients (15.3%) referred to new-onset symptoms suggesting gastroesophageal reflux requiring daily low-dose of PPI. One of these patients developed a hiatal hernia and underwent laparoscopic hiatoplasty and a Hill gastropexy with good outcomes. To date, three patients (1.9%) have developed a trocar-site hernia. Cholecystectomy due to symptomatic gallstones was performed during the follow-up in 7 patients (4.4%); 2 of them presented with acute pancreatitis. There were no data on the cholelithiasis rate in asymptomatic patients.
The mean follow-up was 32.7 ± 28.5 mo (range 6-112). There were 140 patients with at least 1 year of follow-up. Fifty-one patients reached more than 5 years of follow-up.
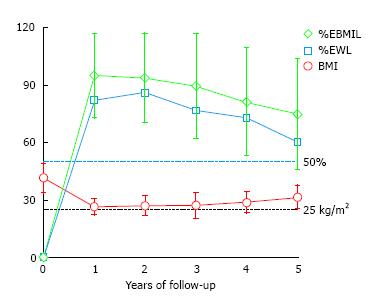
The mean initial BMI was 41.5 ± 7.9 kg/m2 (range 34.2-76.0), and the mean initial percentage of excess of weight (%EW) was 83.1% ± 18.1%. The preoperative BMI of 72 patients (44.9%) was less than 40 kg/m2. Marked weight loss was observed during the first year in all patients, achieving a mean BMI of 26.4 kg/m2, with a mean %EWL of 82.0 ± 18.8 and a mean %EBMIL of 94.9 ± 22.4 after the 1-year follow-up. However, weight loss dropped progressively during the follow-up with remarkable differences among the patients (Figure 2). The mean %EBMIL was 89.4 ± 27.4 at 3 years and 74.8 ± 29.4 (range: 27.2-119.0) at 5 years. The evolution of mean BMI, %EWL and %EBMIL at different follow-up points is shown in Figure 2 and Table 3.
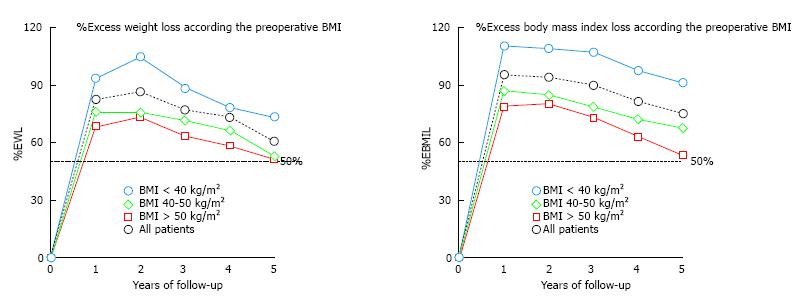
The overall success rate, defined when %EWL is > 50%, was 96.1% of the patients after 1 year, 95.1% after 2 years, 89.5% after 3 years, 82.1% after 4 years and 73.0% after 5 years. It must be highlighted that the patients with a lower initial BMI, especially those with initial BMI under 40 kg/m2, achieve excellent results in terms of %EWL and %EBMIL (Figure 3).
During postoperative follow-up, re-operation because of weight regain from %EWL > 50% to %EWL < 30% was necessary in 4 patients (2.5%), all of them beyond the fourth year of follow-up. A 70-year-old woman received a laparoscopic re-sleeve, one patient underwent a SADI’s and two received a laparoscopic RYGB.
After the first postoperative year, the rate of remission or improvement of hypertension was 71.7% (total remission in 25 patients and improvement in 3). CPAP was withdrawn in all patients with obstructive sleep apnoea (OSA). Complete remission of type 2 diabetes (T2DM) was observed in 75% (9/12) of preoperative diabetic patients (remission was considered when anti-diabetic medication was discontinued and blood glucose level was under 120 mg/mL). One patient receiving preoperative insulin improved and now receives per-oral anti-diabetic medication.
The first endpoint of this study was to assess the safety of LSG as a primary bariatric procedure. LSG has gained popularity in recent years given its theoretical technical simplicity and low rate of complications[10,11,15]. However, LSG can be a very difficult procedure even for laparoscopic surgeons with advanced skills. The surgeon’s experience and some technical aspects, such as the bougie size (less than 40 F) and the distance to the pylorus being less than 4 cm from the first stapling, have been previously reported as risk factors for the development of complications after a LSG[13].
The mortality rate in this series was nil and the rate of 30-d severe complications related to the procedure was 1.9% (Table 1). The rate of staple-line leak and fistula, which is the most feared postoperative complication after LSG, was low in this series (1.2%), even when using a thin bougie to calibrate the stomach and sectioning the stomach at a short distance from the pylorus. According to the International Sleeve Gastrectomy Expert Panel[10], the average leak rate is 1.06% ± 1.13%. There is currently no consensus on the most effective measures to prevent the leakage and fistula, but we share the concept that reinforcing the staple line (with sutures or buttressing material) during LSG can significantly reduce the leakage rate[7,15,20]. The method for doing so is still a matter of debate[21]. Some reports showed no differences between oversewing of the staple line and the use of buttresses[22-24]. However, a systematic review of 88 included studies representing 8920 patients[15] found that the leak rate in LSG was significantly lower using absorbable membrane (Seamguard®) staple-line reinforcement (1.1%) than was oversewing (2.0%), bovine pericardial strip (BPS-Peristrips®) reinforcement (3.3%), or no reinforcement (2.6%). We observed one leak after oversewing of the staple line and another after the use of Peristrips®. No leaks were observed in the Seamguard® subgroup but the small number of patients in this series does not allow further analysis. It must be noted that the significantly highest incidence of leaks was reported when using both sutures and buttressing material (3.6%); consequently, this approach should always be avoided[24].
The second endpoint was to evaluate the evolution of weight loss after LSG as a primary bariatric procedure. The overall results of this study reinforce the evidence that LSG was effective at achieving a significant weight loss over short- and mid-term follow-up. Comparable outcomes in terms of weight loss over a 5-year period were reported at the 3rd International Summit of Sleeve Gastrectomy[3], with a mean percentage of excess weight loss of 62.7%, 64.7%, 64.0%, 57.3%, and 60.0% after 1, 2, 3, 4, and 5 years, respectively. These data are all consistent with other studies published to date[16,25-38] (Table 4). LSG outcomes are comparable to the gold standard procedure in bariatric surgery, the RYGB[6], thus supporting the role of LSG as a stand-alone bariatric operation for morbid obesity.
| Author | Year | Patients with 5-yr follow-up | Mean initial BMI (kg/m2) | %EWL 1 yr | %EWL 5 yr | %EBMIL 1 yr | %EBMIL 5 yr |
| Bohdjalian[26] | 2010 | 26 | 48.2 ± 1.3 | 57.5 ± 4.5 | 55.0 ± 6.8 | ||
| Himpens[27] | 2010 | 30 | 39 | 53.3 | |||
| D’Hondt[28] | 2011 | 83 | 39.3 | 78.5 | 54.4 | ||
| Braghetto[29] | 2012 | 60 | 38.4 ± 5.1 | 57.3 | 57.3 | ||
| Sarela[30] | 2012 | 13 | 45.9 | 76 | 69 (8 yr) | ||
| Rawlins[31] | 2013 | 49 | 65 | 56 | 85.8 | 91 | |
| Sieber[32] | 2014 | 62 | 43.0 ± 8.0 | 61.5 ± 23.4 | 57.4 ± 24.7 | ||
| Boza[33] | 2014 | 112 | 34.9 | 88 | 62.9 | ||
| Liu[34] | 2015 | 44 | 41.0 ± 7.0 | 70.5 | 57.2 | ||
| Lemanu[35] | 2015 | 55 | 50.7 | 56 | 40 | ||
| Pok[36] | 2015 | 61 | 37.3 ± 8.1 | 76.5 | 72.6 | ||
| Alexandrou[37] | 2015 | 30 | 55.5 ± 1.7 | 65.2 ± 6.1 | 56.4 ± 5.8 | ||
| Perrone[38] | 2016 | 162 | 47.4 ± 4.2 | 75.1 ± 18.9 | 78.8 ± 23.5 | ||
| Hoyuela | 2016 | 51 | 41.5 ± 7.9 | 82.0 ± 18.8 | 60.3 ± 28.9 | 94.9 ± 22.4 | 74.8 ± 29.4 |
However, a significant amount of patients may regain weight over time after LSG. Long-term results of LSG still are an ongoing concern, and 10-year follow-up data are actually scarce. Furthermore, a high rate of patients lost to long-term follow-up is not uncommon in previously reported series. Although weight regain was evident with time, data from our series and some long-term observational studies indicate that a significant number of patients maintained good weight loss beyond 5 years of follow-up (Table 4). A recent systematic review of 16 long-term studies including 492 patients revealed the %EWL to be 62.3%, 53.8%, 43% and 54.8% at 5, 6, 7 and 8 or more years of follow-up, respectively[25]. Arman et al[39] reported a mean %EBMIL of 62.5% in patients who kept the simple sleeve construction (74.6% overall-study series) after a mean follow-up of 11.7 years.
It is still unclear why LSG ceases to be effective over time in terms of weight loss in some patients, but several reasons could be involved, including dilation of the gastric tube, insufficient gastric fundus resection (where ghrelin is produced) or hyperactivity of previously silent ghrelin-producing cells and other hormonal changes[6,26,39,40]. Inadequate adherence to aftercare changes in eating behaviour and lack of physical activity could play a role of paramount importance in patients with poorer maintenance of weight loss. A recent systematic review by Karmali et al[41] concluded that the underlying causes leading to weight regain are multi-factorial and related to patient- and procedure-specific factors.
Our data showed better results regarding weight loss when the initial BMI was lower. Patients with an initial BMI less than 40 kg/m2 registered excellent results (73% of EWL and 90.8% of EBMIL at 5 years) compared with the overall study population (Figure 3). Age > 60 years, pre-existing co-morbidities and BMI superior to 50 kg/m2 were identified as prognostic factors of poorer outcome after LSG. Super-obese patients also had poorer weight loss results in this series. These results allow us to suggest that LSG could be routinely used as a sole bariatric technique for patients whose BMI was less than 40 kg/m2.
However, we observed high variability among patients regarding weight loss maintenance over time, even in patients with similar characteristics. No other significant differences were found between subgroups of patients probably due to the small sample of patients with 5 years of follow-up. Identifying preoperative predictive factors of success might be useful for developing strategies to improve bariatric surgery outcomes and patient selection. Further long-term follow-up randomized studies that include a larger number of patients are needed to identify which patients would benefit the most from LSG.
The last endpoint was to analyse the resolution of preoperative co-morbidities in the patients who underwent a LSG. LSG allowed CPAP to be withdrawn in all patients in the series with preoperative OSA and achieved the resolution of hypertension and T2DM in more than 70%. The improvement of T2DM occurred soon after surgery, even without significant weight loss yet being achieved, and this fact could be attributed to hormonal changes, such as increased GLP-1 secretion or decreased ghrelin[6]. The long-term effects of LSG on T2DM evolution are under continuous evaluation, and Aminian et al[42] recently reported a 44% of long-term relapse of T2DM after initial remission and continuous complete remission for ≥ 5 years (“cure”) was achieved in only 3% of the patients. LSG and RYGB showed comparable remission rates of T2DM in a long-term observational study[18], but a meta-analysis including 6526 patients confirmed that RYGB achieved a higher diabetes remission rate (HR = 1.49, 95%CI: 1.04-2.12)[16]. Current data suggesting the long-term superiority of RYGB over LSG in the metabolic control of T2DM could be accounted for by the greater weight loss and by a larger contribution of weight-loss-independent mechanisms[43-45].
In our opinion, the main limitations of this study are the sample size of the series and the heterogeneity of the patients included in the series, precluding to discover significant differences between subgroups of patients (for example, only 15 super-obese patients are included in this series). In addition, only 32% (51/156) of patients reached 5-years of follow-up. The lack of adherence to follow-up was reported previously, and it can be related to several issues, including the distance to the medical office and a lack of trust or rapport with the surgeon or the medical team[46]. However, the most relevant strength of this study is that all patients underwent a standardized LSG operative technique, first, because surgeon expertise is a key issue to lower the complications rate[13,24] and second, because there were no technical differences that may influence the weight loss results. We always tried to perform a more restrictive LSG by using a thinner bougie and beginning the dissection 3 cm from the pylorus to achieve greater weight loss, as suggested by Baltasar et al[8,31]. In addition, the long-term follow-up of the patients was always carried out by the same surgeon who performed the procedure.
In conclusion, a LSG built with a narrow 34 F bougie and starting 3 cm from the pylorus, proved to be safe and highly effective in terms of weight loss as a stand-alone procedure, especially in patients with preoperative BMI lower than 40 kg/m2. In our opinion, LSG could be accepted as the first stand-alone procedure for morbidly obese patients with low BMI. Prospective randomized trials analysing long-term results (beyond ten years of follow-up) will help elucidate whether LSG is comparable to more aggressive techniques.
The unselfish support of Eric Herrero, MD and Fernando Carvajal, MD is highly acknowledged.
The number of laparoscopic sleeve gastrectomies (LSGs) performed worldwide as a primary bariatric procedure has grown exponentially in recent years, given the simplicity of the technique, the low complication rate and the good short- and mid-term results regarding weight loss and the resolution of co-morbidities. However, the long-term results of LSG still are an ongoing concern because a significant amount of patients may regain weight over time after LSG.
Bariatric surgery is safe and efficient and allows not only to lose weight but treat conditions such diabetes, hypertension and sleep apnoea in morbidly obese people. Probably, the indications of bariatric and metabolic surgery will increase in the future treating such comorbidities, given its good results and low morbi-mortality.
The current prospective study suggests that LSG could be the procedure of choice for those morbid patients with a low preoperative body mass index (BMI) and without severe comorbidities. However, strict nutritional and behavioural monitoring and follow-up by the surgical team seem to be of paramount importance.
This study provides additional evidence supporting the role of LSG as a stand-alone procedure for morbidly obese patients, particularly in patients with a low preoperative BMI.
Sleeve gastrectomy: Is a left partial gastrectomy of the fundus and body to create a long tubular gastric conduit constructed along the lesser curve of the stomach. The body mass index (BMI) is the main parameter to assess morbid obesity and is defined as the body mass (weight in kilograms) divided by the square of the body height and is universally expressed in units of kg/m2. The changes in weight and BMI expressed by means of percentage of excess weight loss and percentage of excess of BMI loss help to evaluate the success of bariatric surgery.
The article addresses an important entity and many newly qualified surgeons may find this article interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fogli L, Maleki AR, Mann O S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | ASMBS Clinical Issues Committee. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2012;8:e21-e26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13:861-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 531] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 3. | Deitel M, Gagner M, Erickson AL, Crosby RD. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749-759. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19:1515-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Kandeel AA, Sarhan MD, Hegazy T, Mahmoud MM, Ali MH. Comparative assessment of gastric emptying in obese patients before and after laparoscopic sleeve gastrectomy using radionuclide scintigraphy. Nucl Med Commun. 2015;36:854-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, Flores-Le Roux JA. Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21:11804-11814. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Sánchez-Santos R, Masdevall C, Baltasar A, Martínez-Blázquez C, García Ruiz de Gordejuela A, Ponsi E, Sánchez-Pernaute A, Vesperinas G, Del Castillo D, Bombuy E. Short- and mid-term outcomes of sleeve gastrectomy for morbid obesity: the experience of the Spanish National Registry. Obes Surg. 2009;19:1203-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 325] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Baltasar A, Serra C, Bou R, Bengochea M, Andreo L. Sleeve gastrectomy in a 10-year-old child. Obes Surg. 2008;18:733-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of & gt; 12,000 cases. Surg Obes Relat Dis. 2012;8:8-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 713] [Cited by in F6Publishing: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 11. | Gagner M, Deitel M, Erickson AL, Crosby RD. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obes Surg. 2013;23:2013-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Spaniolas K, Kasten KR, Brinkley J, Sippey ME, Mozer A, Chapman WH, Pories WJ. The Changing Bariatric Surgery Landscape in the USA. Obes Surg. 2015;25:1544-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Sánchez-Santos R, Corcelles Codina R, Vilallonga Puy R, Delgado Rivilla S, Ferrer Valls JV, Foncillas Corvinos J, Masdevall Noguera C, Socas Macias M, Gomes P, Balague Ponz C. Prognostic Factors for Morbimortality in Sleeve Gastrectomy. The Importance of the Learning Curve. A Spanish-Portuguese Multicenter Study. Obes Surg. 2016;26:2829-2836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20:447-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10:713-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Li JF, Lai DD, Lin ZH, Jiang TY, Zhang AM, Dai JF. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, Schultes B, Laederach K, Bueter M, Schiesser M. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690-694; discussion 695. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Montero PN, Stefanidis D, Norton HJ, Gersin K, Kuwada T. Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: a plea for standardization. Surg Obes Relat Dis. 2011;7:531-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Shikora SA, Mahoney CB. Clinical Benefit of Gastric Staple Line Reinforcement (SLR) in Gastrointestinal Surgery: a Meta-analysis. Obes Surg. 2015;25:1133-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:750-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 22. | Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 23. | Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 24. | Stroh C, Köckerling F, Volker L, Frank B, Stefanie W, Christian K, Christiane B, Thomas M; Obesity Surgery Working Group, Competence Network Obesity. Results of More Than 11,800 Sleeve Gastrectomies: Data Analysis of the German Bariatric Surgery Registry. Ann Surg. 2016;263:949-955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Diamantis T, Apostolou KG, Alexandrou A, Griniatsos J, Felekouras E, Tsigris C. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, Prager G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 393] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 27. | Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 28. | D'Hondt M, Vanneste S, Pottel H, Devriendt D, Van Rooy F, Vansteenkiste F. Laparoscopic sleeve gastrectomy as a single-stage procedure for the treatment of morbid obesity and the resulting quality of life, resolution of comorbidities, food tolerance, and 6-year weight loss. Surg Endosc. 2011;25:2498-2504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012;22:479-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Sarela AI, Dexter SP, O’Kane M, Menon A, McMahon MJ. Long-term follow-up after laparoscopic sleeve gastrectomy: 8-9-year results. Surg Obes Relat Dis. 2012;8:679-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Rawlins L, Rawlins MP, Brown CC, Schumacher DL. Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis. 2013;9:21-25. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Sieber P, Gass M, Kern B, Peters T, Slawik M, Peterli R. Five-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Boza C, Daroch D, Barros D, León F, Funke R, Crovari F. Long-term outcomes of laparoscopic sleeve gastrectomy as a primary bariatric procedure. Surg Obes Relat Dis. 2014;10:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Liu SY, Wong SK, Lam CC, Yung MY, Kong AP, Ng EK. Long-term Results on Weight Loss and Diabetes Remission after Laparoscopic Sleeve Gastrectomy for A Morbidly Obese Chinese Population. Obes Surg. 2015;25:1901-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Lemanu DP, Singh PP, Rahman H, Hill AG, Babor R, MacCormick AD. Five-year results after laparoscopic sleeve gastrectomy: a prospective study. Surg Obes Relat Dis. 2015;11:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Pok EH, Lee WJ, Ser KH, Chen JC, Chen SC, Tsou JJ, Chin KF. Laparoscopic sleeve gastrectomy in Asia: Long term outcome and revisional surgery. Asian J Surg. 2016;39:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Alexandrou A, Athanasiou A, Michalinos A, Felekouras E, Tsigris C, Diamantis T. Laparoscopic sleeve gastrectomy for morbid obesity: 5-year results. Am J Surg. 2015;209:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Perrone F, Bianciardi E, Benavoli D, Tognoni V, Niolu C, Siracusano A, Gaspari AL, Gentileschi P. Gender Influence on Long-Term Weight Loss and Comorbidities After Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study With a 5-Year Follow-up. Obes Surg. 2016;26:276-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Arman GA, Himpens J, Dhaenens J, Ballet T, Vilallonga R, Leman G. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:1778-1786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 40. | Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 583] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 41. | Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922-1933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 42. | Aminian A, Brethauer SA, Andalib A, Punchai S, Mackey J, Rodriguez J, Rogula T, Kroh M, Schauer PR. Can Sleeve Gastrectomy “Cure” Diabetes? Long-term Metabolic Effects of Sleeve Gastrectomy in Patients With Type 2 Diabetes. Ann Surg. 2016;264:674-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Vidal J, Jiménez A, de Hollanda A, Flores L, Lacy A. Metabolic Surgery in Type 2 Diabetes: Roux-en-Y Gastric Bypass or Sleeve Gastrectomy as Procedure of Choice? Curr Atheroscler Rep. 2015;17:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1639] [Cited by in F6Publishing: 1509] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 45. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1125] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 46. | Moroshko I, Brennan L, O’Brien P. Predictors of attrition in bariatric aftercare: a systematic review of the literature. Obes Surg. 2012;22:1640-1647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |