INTRODUCTION
Laparoscopic surgery has gained popularity in the last few decades, replacing open standard technique in several procedures from general surgery, gastrointestinal surgery, gynecology and urology. In fact, it has been considered standard of care in many cases such as cholecystectomy, appendectomy, colectomy, hysterectomy, pyeloplasty, nephrectomy, and others[1-5]. Laparoscopic approach has been associated with decreased postoperative pain, shorter hospitalization, faster recovery, and better cosmetics[1-5]. Although surgeons are interested in adopting laparoscopic techniques in their practices, most are lacking formal training in laparoscopy. Barriers such as new technology, inadequate training availability, concerns about complications, and willingness to negotiate learning curves make the transition to minimally invasive procedures challenging.
Currently, more realistic training opportunities involving weekend courses, video libraries, hands-on conferences, and traveling proctors are helping in laparoscopy dissemination. In addition, new generation of surgeons has been trained in laparoscopy during medical residence or fellowship programs. Inanimate models, virtual-reality simulators, and animal and cadaver laboratory have been incorporated to surgical education and are providing a positive impact on minimally invasive surgeon’s performance.
Herein, we aim to describe the activities performed in a dry and animal-model training laboratory and to evaluate the impact of different kinds of laparoscopic surgery training courses on surgeon’s performance.
DRY LABORATORY
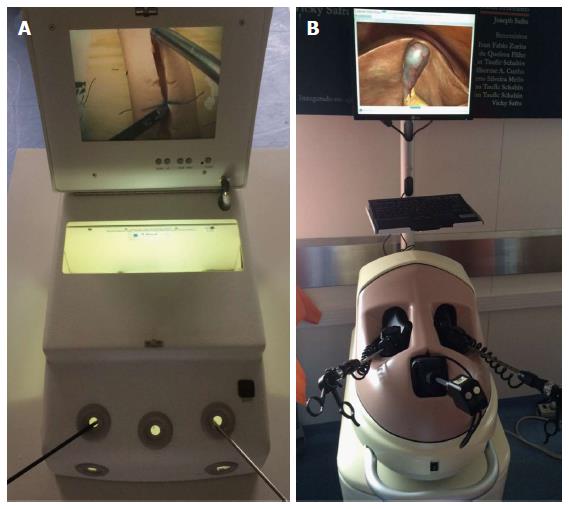
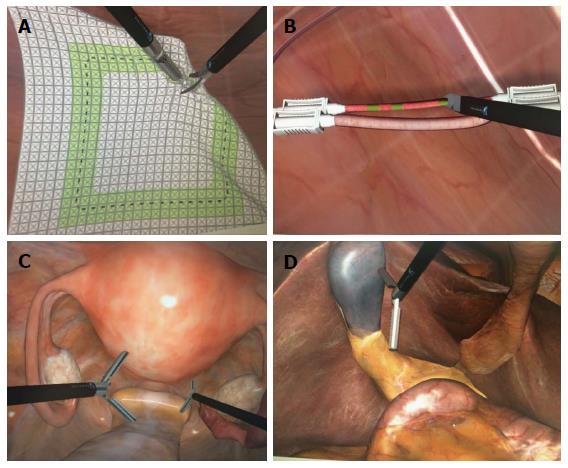
Dry laboratory training comprises box models (consisting of physical inanimate materials) and virtual reality simulators (Figure 1). Similarly, there are physical and virtual reality training models available for robot-assisted laparoscopic surgery. As the fundamentals of laparoscopic surgery (e.g., camera navigation, cutting, suturing, grasping) require different skills from surgeons familiarized with conventional surgery, training models begin with basic principles and can offer more sophisticated exercises, including physical or virtual simulation of complete procedures and surgeries (Figure 2). Each model has particularities regarding cost, availability and performance measures.
Figure 1 Laboratory tools for surgical training.
A: Box training; B: Virtual reality simulator.
Figure 2 Virtual reality simulator.
A: Cutting task; B: Clipping task; C: Hysterectomy; D: Cholecystectomy.
Evaluation of a model’s validity for training includes face, content and construct validity[6]. Face validity refers to the subjective perception of a test being able to measure what it is set out to measure, which means, in the case of training models, the impression of realism. Content validity is the extent to which a test measures and represents all relevant aspects of a given construct (i.e., whether a model can thoroughly evaluate all aspects of surgical skills). Construct validity refers to the ability of a test to effectively measure what it claims to measure. A manifestation of construct validity in surgical simulators is the ability of the system to differentiate novices from experts. Evaluation of a trainee performing tasks may take into account time for completion, accuracy of movements, number of movements, and distance needed to complete a given task[7,8]. Camera skills evaluation also takes into account percentage of time with optimal framing. For complex procedures, ability to finish a surgical step and complications within steps are also considered. A composite score is usually generated to evaluate the whole of the performance.
Box model training
Surgical box models consist of real instruments used for laparoscopy inserted into a box with a camera to simulate the human abdomen. The surgeon will manipulate targets inside the box that simulate tissues (e.g., silicon models to mimic bowels or a bladder). Advantages of these models include low cost and high availability; trainees may even purchase models and practice at home. Another strength is the use of real instruments. Face validity is a shortcoming of this method, since rubber or silicon models used are limited in realism regarding aspects such as consistency and ability to simulate bleeding. Another drawback of the method is the limited repertoire of surgeries and the complexity of tasks that a single model can provide. Yet, to date, these models appear to be effective in improving basic technical skills in subjects with no previous experience in laparoscopic surgery. Studies with medical students have shown improvement in quality and speed of sutures[9] as well as improved camera skills after training in box models[10]. Similarly, studies have shown greater accuracy, precision and speed for cutting among novice students trained with box models[11]. Subjects appear to develop greater speed, travel lesser distances and perform lesser movements to complete tasks after training, although these results have not been replicated in all studies[12,13]. Trainees also seem to present lower error rates after training, although it is unclear whether box models or virtual reality simulators offer better results[9,12,14]. Overall, despite existence of conflicting results and the difficulty in accurately assessing improvement, box model training seems to improve performance of basic skills in laparoscopy for trainees with no previous experience[15].
Virtual reality simulators
Virtual reality simulators (VRS) of numerous manufacturers have been released in the market. These models consist of sophisticated softwares that generate representations of laparoscopic exercises, from simple tasks to whole surgeries (e.g., nephrectomy, colectomy). The trainee manipulates instruments that mimic those used in real laparoscopy. VRS have been tested and validated for face, content and construct validity[16,17]. Strengths of VRS include greater realism and the possibility of a wide range of procedures of different complexity[18]. Furthermore, performance of an individual can be recorded, measured against objective standards and compared to other trainees. However, low availability and high prices, beginning at EUR 60000 are a limitation for the widespread use of these instruments[19]. Studies have suggested that VRS provide comparable skill acquisition in relation to box model training, and it has also been suggested that these 2 methods may have complementary roles in laparoscopic training[11,20]. The individual role of VRS alone in final surgical performance is still unclear[21].
Robotic surgery simulators
Similar to virtual simulators of conventional laparoscopy, robotic surgery simulators have been developed and validated, offering representation of surgical tasks and incorporating the technical differences between the two surgical techniques[6,22,23]. These models share the same strengths of conventional laparoscopy VRS, especially realism and standardized evaluation. Similarly, robotic surgery simulators are of limited dispersion due to their high prices. To date, skill transfer properties of these models are still unclear[24].
ANIMAL AND CADAVER MODEL LABORATORY
Teaching minimally invasive techniques in the operating room has become increasingly difficult due to economic and patient safety concerns. Laparoscopic surgical training includes live animal training (Figure 3), animal cadaver training, training using the box-trainer and virtual reality training. Virtual reality training has been used primarily to develop component skills, i.e., diathermy, clipping, suturing. It usually does not allow the student to perform the entire procedure and does not take into consideration possible anatomic variations that might be encountered. In addition, real laparoscopic instruments are not used, current technology has limitations, and high costs limit widespread applicability of virtual reality simulators. Yet, the combination of virtual and box-trainer with the animal model training might shorten the learning curve. La Torre et al[25] showed that the ability and time to knot-tying might be reduced if the surgeon underwent training in the virtual simulator prior to the animal model. More important, if the surgeon is exposed to repetitive animal model training, surgical time and intraoperative complications are reduced and the level of confidence and expertise measured by the global operative assessment of laparoscopic skills (GOALS) are significantly improved. Animal model training and surgeon evaluation through GOALS might be used to identify all areas of skill deficiency that require improvement. Supplementary training and mentoring can be offered to address skill deficiencies. In addition, surgeons’ performances might be evaluated and compared in relation to the mean of the performances of other surgeons with the same training or those with high proficiency.
Figure 3 Pig model for laparoscopic training.
Residents usually prefer animal models for training rather than a virtual simulator model because the first are more realistic to the real scenario of operating on a patient. Tissue handling and haptic feedback are advantages compared to virtual simulators and box models. Also, intraoperative complications such as bleeding and organ lesions are only realistic in the animal model[26,27]. Zimmerman et al[28] evaluated 36 surgical residents of a multimodality intensive laparoscopic training course who underwent a 5-d intensive training on the porcine model and found that the post-course performance scores improved by 100% to 200% with respect to the pre-course scores. The main areas with significant interest on laparoscopic training during residency are general surgery, urology, gastrointestinal surgery, and gynecology. Since Rassweiler et al[29] highlighted the importance of preclinical training on pelvic trainer and animal studies before advancing to real-time laparoscopic nephrectomy, there has been an increase in number of training models being utilized and reported in literature in regards to urological procedures. The most common models for training are the porcine or chicken models[30]. Initially, authors studied the learning curve for ablative procedures such as total nephrectomy. Later, with the advancement in minimally invasive surgeries, the learning of complex surgical skills with multiple models were developed for partial nephrectomy, pyeloplasty, single port surgery, natural orifice transluminal endoscopic surgery, orthotopic renal transplants, and finally radical prostatectomy. More recently, 2-dimensional (2D) was compared to 3-dimensional (3D) laparoscopy during residence laboratory training[31]. The authors found that the 3D technology facilitated the surgical performance of inexperienced surgeons during complex laparoscopic kidney procedures on a porcine model.
Although most general surgery program directors consider skills labs effective for improving operating room performance, only half of those programs have in fact an implemented skill lab training program in the residency curriculum[32]. Torricelli et al[18] have demonstrated that with a 10-wk dedicated laparoscopic training program, first-year urology residents were able to perform more than one hundred procedures with low and high complexity in the porcine model under supervision of a more experienced proctor[18]. The improvements on laparoscopic skills lead to a high degree of familiarization with the actual operative field. Also, it shortens operative time, decreases operative complications and ultimately increases patient safety. In the same study, the authors emphasize that residents from more than one surgical specialty might train in the same laboratory. However, a cross-specialty training program is also feasible and has proved validity[33-35]. Benefits of this arrangement for a training program comprise more frequent disposal of courses and a more effective use of training resources.
IMPACT OF LAPAROSCOPIC TRAINING COURSES
Several different laparoscopic courses are available for surgeons who aim to improve their skills in minimally invasive surgery. There are short length courses that range from 2 to 5 d well as full year fellowship programs, which are designated for senior residents interested in laparoscopic and robotic procedures. Each course has its particularities and has proved to be able of achieving specific goals.
Asano et al[36] in a 2-d laparoscopic intestinal workshop including interactive discussions during live laparoscopic resection, didactic teaching, video clips and supervised hands-on practice of laparoscopic colon resection on cadaveric models reported 62.5% of participants who were not performing laparoscopic colectomies prior to the course had performed at least one 6 mo after the training. Okrainee et al[37] in a 3-d course described the impact of the “fundaments of laparoscopic surgery” (FLS) program in small group of 20 surgeons and trainees (general surgery, urology, and gynecology). FLS is an educational program developed by the Society of American Gastrointestinal and Endoscopic Surgeons for teaching the basic cognitive knowledge and technical skills required for laparoscopic surgery[37]. It includes a didactic component presented in a standardized fashion CD-ROM, a simulation-based technical skills component (peg transfer, pattern cutting, ligating loop, extracorporeal suture, and intracorporeal suture), and an assessment component that measures both cognitive and technical skills. In this course, although the mean posttest scores were significantly higher than pretests for each FLS task and for the total normalized FLS simulator score, only two surgeons achieved a passing score on both cognitive and skills assessment required to obtain FLS certification. This study indicates that FLS course can positively impact on surgeons’ performance, however a longer period of training is probably required for surgeons obtain FLS certification[38].
“Mini-residency” is another modality of laparoscopic training, usually performed in a 5-d period. Chou et al[39] described their experience with 16 participants who had individual didactic sessions with expert faculty and skills-training sessions with inanimate models, pelvic trainers, virtual reality simulators, and the animal and cadaver laboratory. Overall, the participants did not show a statistically significant improvement in their overall laparoscopic skills scores. When subcategories (ring transfer, thread suture, cutting line, suturing) of laparoscopic skills were examined, only the task of threading suture through loops showed a statistically significant improvement after mini-residency. On the follow-up survey, two laparoscopically naive participants had performed laparoscopic nephrectomy, and of the eight participants who had prior renal-ablative laparoscopic experience, four had performed advanced reconstructive laparoscopic cases[39]. In a similar study with 32 participants, Corica et al[40] reported their experience with a 5-d mini-residency program that included inanimate model skills training, animal laboratory, and operating room observation. Eight months after mini-residency program, 26 (81%) participants were performing laparoscopic surgery. Compared with before the mini-residency program, laparoscopic radical nephrectomy (P = 0.008), nephroureterectomy (P < 0.0005), and pyeloplasty (P = 0.008) were performed considerably more often by participants after training. Concomitantly, participants performed hand-assisted laparoscopic surgery considerably less often (P = 0.008)[40]. In a large sample including 106 urologists, Kolla et al[41] reported similar findings to those described before. In a study evaluating the impact of 5-d mini fellowship program that included tutorial sessions, hands-on inanimate and animate skills training, and clinical case observations, there was also a significant increase in the laparoscopic procedures performed by the participants after the program. Of the surgeons with prior experience with laparoscopy, there was an increase in the practice of laparoscopic radical nephrectomy (88% vs 72%), nephroureterectomy (56% vs 13%), pyeloplasty (40% vs 6%) and partial nephrectomy (32% vs 6%). Of the laparoscopic naive surgeons, the take rate was 76%, 52%, 34%, and 32% for laparoscopic radical nephrectomy, nephroureterectomy, pyeloplasty and partial nephrectomy[41]. From all these studies, it is noted that short period training can improve laparoscopic surgical skills, although most of times it is not enough to confer laparoscopic expertise for participants. But one point is clear, short period training is able to increase the laparoscopic practice of surgeons in their communities.
When evaluating the learning process in robot-assisted laparoscopic procedures, the findings are similar to those described above. One or 2-d courses, as well as mini-fellowship training program, have proved their efficiency of improving participant’s robotic skills. Moreover, these courses also are increasing the number of robot-assisted cases performed by the participants in their institutions[42,43].
Full year laparoscopic fellowship programs are another way of improving laparoscopic skills. In a retrospective analysis including more than 4000 surgical cases, the percentage of total cases performed laparoscopically increased from 12.1% to 48.3% after integrating a fellowship-trained surgeon into an established practice. The integration of a fellowship-trained colleague into a general surgery practice resulted in a 300% increase in the proportion of appendectomies, ventral hernias, inguinal hernias, and colectomies performed laparoscopically by the other members of the practice. In this study, when surveyed, the surgeons felt that mentoring by a colleague with laparoscopic training was the most effective method for adopting minimally invasive surgery into their practice[44].
LAPAROSCOPIC TRAINING AND LEARNING CURVE
Sandy et al[45] evaluated if laparoscopic skills could be objectively quantified by measuring specific skill parameters during training in a virtual reality surgical simulator. The authors compared the performance of ten medical students with no laparoscopic experience at all with the performance of ten urology residents with some degree of expertise in regards to basic laparoscopic skills, e.g., camera handling, cutting, peg transfer and clipping skills (Immersion Lap VR, San Jose, CA, United States). They found that most individuals in both groups exhibited a significant improvement in their task completion time and error rate, proving that there was a learning curve effect on training. Moreover, the mean time taken to complete tasks was significantly shorter for the urology residents. In addition, this more experienced group of surgeons could complete the tasks with fewer errors. The authors concluded that laparoscopic skills might be objectively measured in a virtual reality surgical simulator based on quantified skill parameters, including the time spent to complete skill tasks and the associated error rate. In a subsequent study from the same group, Duarte et al[46] aimed to determine the minimal number of simulator sessions of basic laparoscopic tasks required to elaborate an ideal virtual reality training curriculum. Eleven medical students with no previous laparoscopic experience were enrolled in the study and underwent simulator training sessions starting at level 1, including sequentially camera handling, peg and transfer, clipping and cutting. Each student trained twice a week until a total of ten sessions were completed. By a non-linear regression method analysis, the authors found after 4.26 sessions all students reached the plateau of 80% of the estimated acquired knowledge. From the fifth session till the last, some students could reach 96% of the expected improvement, though the gain of knowledge was not significant.
Training is certainly crucially important for laparoscopic skills learning. However, there are other factors, which should be considered in this equation, and surgeon aptitude is one of this. Buckley et al[47] recruited twenty medical students and divided them in two groups according to their aptitude in regards to visual-spatial ability, depth perception, and psychomotor ability. All individuals were tested consecutively using the ProMIS III simulator until they reached proficiency performing laparoscopic suturing. Students with high aptitude achieved proficiency after a mean of 7 attempts, ranging from 4 to 10 trials. In converse, only 30% of subjects with low aptitude achieved proficiency after a mean of 14 attempts, ranging from 10 to 16 tries. In addition, in the group with low aptitude, 40% showed improvement but did not reach proficiency, and 30% failed to progress. The authors concluded that the fundamental ability of distinguish individuals lead to distinct learning curves for laparoscopic suturing, where high aptitude is directly related to earlier completion of the learning curve.
Another factor that has been proved to influence on the learning curve for laparoscopic training is coaching[48,49]. Cole et al[48] compared the effects of structured coaching with an autodidactic training in simulated laparoscopic surgery. Seventeen surgically inexperienced medical students were randomized into two groups, eight being placed into an intervention group which received structured coaching, and nine being placed into a control group who received no training at all. All subjects performed ten laparoscopic cholecystectomies on a virtual reality simulator and the surgical quality of the first, fifth, and tenth operations was evaluated by two independent blinded assessors using the competency assessment tool (CAT) for cholecystectomy. They found that the coached group scored significantly higher on the CAT assessment and knowledge test of procedures one, five, and ten, with increasing disparity. The learning curve for error frequency of the coached group reached competency after operation seven, while the control group did not plateau by the last procedure. The authors concluded that structured coaching might represent a key element in the acquisition of laparoscopic surgical skills. In the same sense, Ahlberg et al[49] evaluated individual learning curves for a cohort of surgeons performing laparoscopic fundoplication and analyzed if the Procedicus MIST-simulator (Mentice Inc., Göteborg, Sweden) could predict surgical performance. For that, twelve centers participated and each contributed with a “master” and a “pupil” surgeon. Pupils were tested in the simulator and then performed their first twenty supervised operations. All procedures were recorded and thereafter appraised by three independent reviewers. The authors found the master to significantly affect the pupil’s score and concluded that Individual learning curves varied, and the teacher was shown to be the most important factor influencing the pupil’s performance score.
More recently, a technological advancement allowed for a shorter learning curve during laparoscopic training. Romero-Loera et al[50] tested the potential benefit of in-depth perception of 3D images in laparoscopic surgery. They recruited 40 individuals with no experience in laparoscopic surgery and divided them in two groups: 20 began the skills in the 2D modality and then performed them in 3D, and the other 20 began in 3D and then shifted to 2D. Each subject was used as his own control. Of all skills evaluated, there was a significantly difference in time improvement between groups, being 72% in the 3D group compared to 37% in the 2D modality. In addition, the accomplishment percentage using the 3D laparoscopy was greater for both groups. Finally, subjects’ preference was also evaluated and 52.5% of participants preferred 3D laparoscopy, only 15% preferred 2D, and 32.5% had no preferences. The authors concluded that 3D laparoscopic surgical training is feasible and superior to 2D, with a shorter learning curve.
Finally, the learning curve has a potential benefit in terms of cost-savings. Stefanidis et al[51] compared a group of ten medical students who trained until proficiency was achieved on five basic laparoscopic tasks with a group of ten students who received no training. After this initial step, both groups underwent a supervised training on the Fundamentals of Laparoscopic Surgery suturing model until previously reported proficiency levels were achieved and then two weeks later they were retested to evaluate their retention scores, training parameters, instruction requirements, and cost between groups. The initial performance on the simulator was better for individual with basic skills training, their suturing learning curve was shorter, and they required less active instruction. Although the overall time required to finish the curriculum was similar for both cohorts, the subjects who underwent a previous training strategy cost less, with mean savings of USD148 per student. Therefore, they determined that teaching novices basic laparoscopic skills before a more complex laparoscopic task allows substantial cost savings.
CONCLUSION
Laparoscopic surgery has been replacing the open standard technique in several procedures. However, the learning curve required to obtain laparoscopic expertise has been an issue in medical community. Laparoscopic surgery training laboratory was developed to overcome this barrier. Although a short period of training can improve laparoscopic surgical skills, full laparoscopic training in medical residence or fellowship program is the best way of stimulating laparoscopic dissemination.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chatzimavroudis G, Milone M, Zhang WJ S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL