Published online May 27, 2022. doi: 10.4240/wjgs.v14.i5.482
Peer-review started: December 16, 2021
First decision: March 13, 2022
Revised: March 16, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 27, 2022
The life-threatening complications following pancreatoduodenectomy (PD), intra-abdominal hemorrhage, and postoperative infection, are associated with leaks from the anastomosis of pancreaticoduodenectomy. Although several methods have attempted to reduce the postoperative pancreatic fistula (POPF) rate after PD, few have been considered effective. The safety and short-term clinical benefits of omental interposition remain controversial.
To investigate the safety and feasibility of omental interposition to reduce the POPF rate and related complications in pancreaticoduodenectomy.
In total, 196 consecutive patients underwent PD performed by the same surgical team. The patients were divided into two groups: An omental interposition group (127, 64.8%) and a non-omental interposition group (69, 35.2%). Propensity score-matched (PSM) analyses were performed to compare the severe complication rates and mortality between the two groups.
Following PSM, the clinically relevant POPF (CR-POPF, 10.1% vs 24.6%; P = 0.025) and delayed postpancreatectomy hemorrhage (1.4% vs 11.6%; P = 0.016) rates were significantly lower in the omental interposition group. The omental inter
The application of omental interposition is an effective and safe approach to reduce the CR-POPF rate and related complications after PD.
Core Tip: Postoperative pancreatic fistula (POPF) is a life-threatening complication after pancreaticoduodenectomy. Multiple methods have been described in the literature to prevent POPF; however, few trials have demonstrated that a certain method can achieve good clinical outcomes. In this study, we proved that the application of omental interposition can reduce the incidence of clinically relevant POPF, which is associated with a trend towards accelerated recovery.
- Citation: Li Y, Liang Y, Deng Y, Cai ZW, Ma MJ, Wang LX, Liu M, Wang HW, Jiang CY. Application of omental interposition to reduce pancreatic fistula and related complications in pancreaticoduodenectomy: A propensity score-matched study. World J Gastrointest Surg 2022; 14(5): 482-493
- URL: https://www.wjgnet.com/1948-9366/full/v14/i5/482.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i5.482
Pancreaticoduodenectomy is the gold standard for benign or malignant tumors in the periampullary region. Despite advances in surgical techniques and perioperative care, the postoperative morbidity rate remains high (20-50%), even in high-volume comprehensive hospitals[1-3]. Postoperative pancreatic fistula (POPF) is a life-threatening complication because of its interrelationship with delayed postpancreatectomy hemorrhage (PPH) and postoperative intraabdominal infection[4]. POPF is responsible for erosion of the gastroduodenal artery stump (GDAS), skeletonized hepatic artery (HA), or other adjacent abdominal vessels due to activated pancreatic enzymes.
During the last quarter of the 20th century, multiple methods have been described in the literature to prevent POPF and subsequent complications, including the usage of somatostatin or octreotide, introduction of pancreatic duct stenting, creation of various anastomosis techniques (e.g., duct-to-mucosa, pancreatogastrostomy, invagination), use of polyethylene glycolic acid mesh to reinforce around the pancreatojejunostomy (PJ) site, and use of fibrin glue over the PJ site[5-9]. However, few trials have demonstrated that a certain method will reinforce the PJ site in PD with favorable clinical outcomes.
Currently, the greater omentum has been widely used to reinforce anastomoses and compensate for tissue defects in the fields of thoracic, urinary, and general surgery[10-12]. Recently, some centers have shown that fixing the omental interposition behind the anastomotic site of the PJ to protect the GDAS and nearby HA from erosive pancreatic juices is the most promising approach to reduce the incidence of severe complications[13,14], but they did not have control group data.
Our study investigated whether the application of the omental interposition could effectively reduce the incidence of POPF and its related complications after pancreaticoduodenectomy.
Between January 2015 and December 2019, 196 consecutive patients underwent pancreaticoduodenectomy performed by the same surgical team at our institution. The first 69 consecutive patients did not use omental interpositions, and the remaining 127 used omental interpositions. According to whether the omental interposition was applied, the patients were divided into two groups: the omental group (79 males, 48 females; mean age: 64.8 years) and the non-omental interposition group (44 males, 25 females; mean age: 62.1 years). Propensity score matching (PSM) was used to minimize bias from the nonrandomized treatment assignments. We summarized the data on the general clinical characteristics, short-term surgical outcomes, and recovery. Moreover, the laboratory data on the drain fluid amylase obtained on the first postoperative day (DFA1) were pooled. All data were prospectively collected in our electronic media database. This study was approved by the ethics review committee of Huadong Hospital Affiliated to Fudan University (2019K087; Shanghai, China).
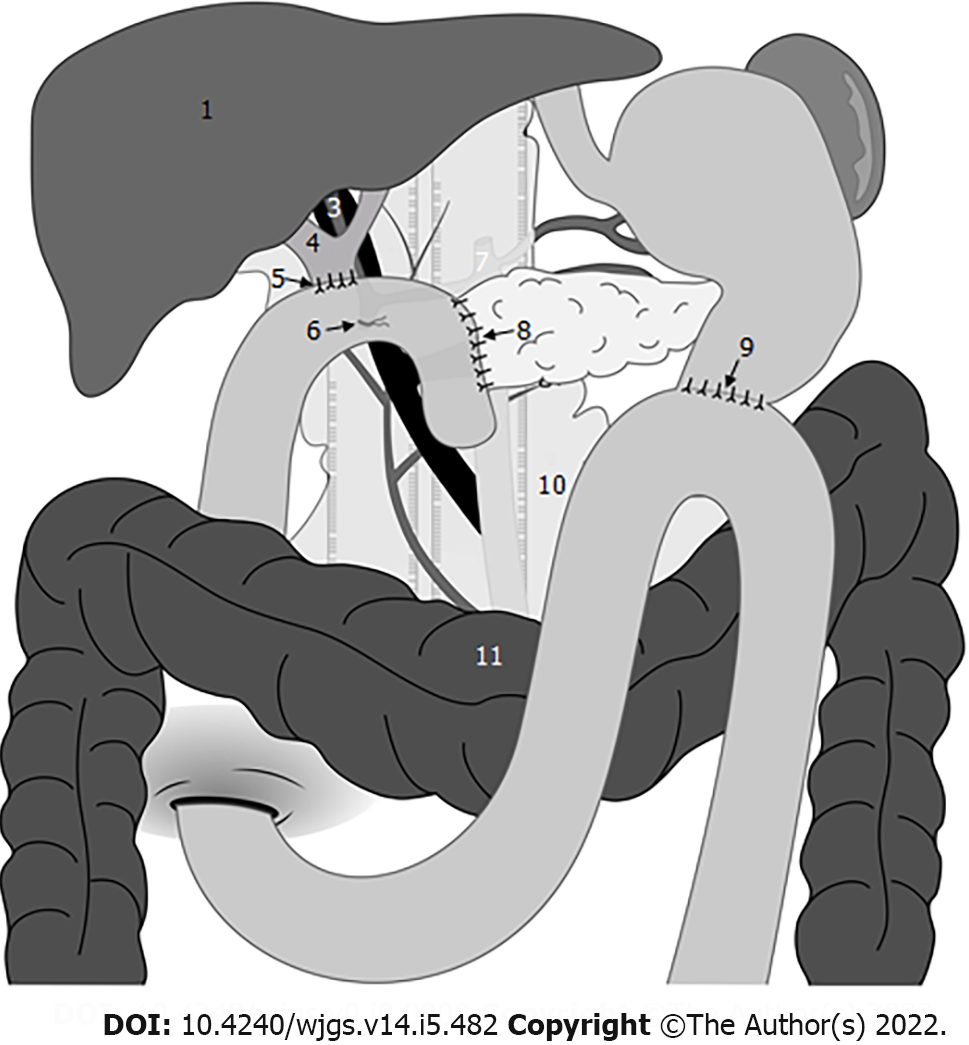
At our institution, PD was accomplished with a standard approach. After the head of the pancreas had been removed, intestinal reconstruction was achieved with a modified version of the method described by Child. A reconstruction PJ was performed (by duct-to-mucosa, end-to-side reconstruction) and a pancreatic drainage tube was placed. (1) Insert the pancreatic juice drainage tube into 3-5 cm and use 4-0 polydioxanone suture to insert the needle from the ventral side of the pancreatic duct, penetrate the anterior and posterior walls of the pancreatic juice drainage tube, and suture from the back of the pancreatic duct to fix the drainage tube; (2) Place the pancreatic juice drainage tube into the distal end of the jejunal loop, and purse suture of the jejunal incision; and (3) Use 3-0 prolene to suture of seromuscular layer of pancreas and jejunum. Hepaticojejunostomy (HJ) was performed with continuous barbed sutures or interrupted sutures. Gastrojejunostomy (GJ) was performed with interrupted 3-0 polypropylene monofilament sutures.
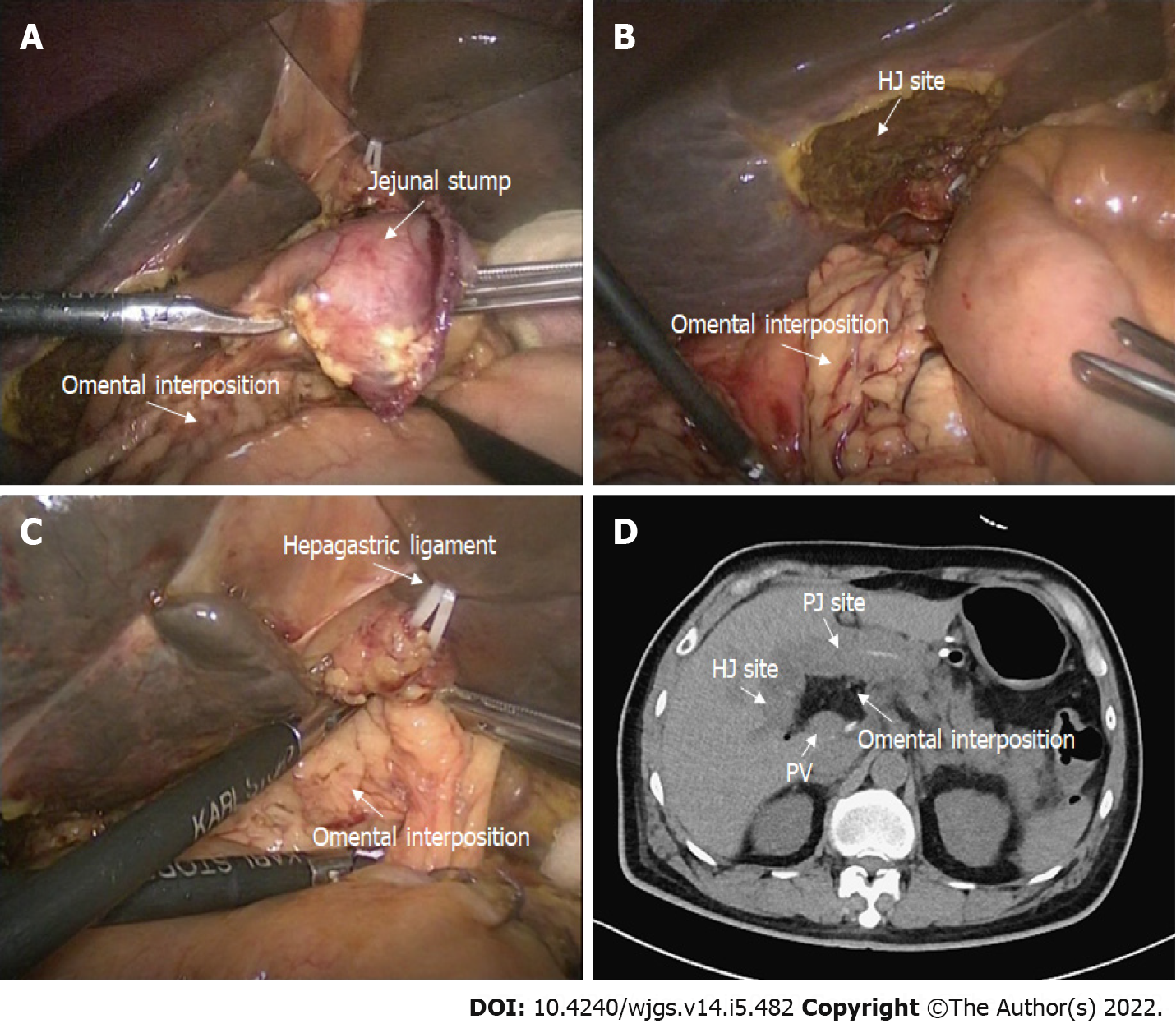
In the omental interposition group, following complete anastomosis, we routinely placed a pedicled omental interposition in front of the adjacent vessels (HA, PV, and GDAS) and behind the anastomosis where the pancreas stump was fixed to the jejunum[15]. The omental interposition was fixed to the hepatic portal and hepatogastric ligament with several sutures to prevent postoperative mobilization (Figure 1). Generally, the upper boundary of the omental interposition was the level of the hepatogastric ligament, the left boundary was the level of the pancreatic body, and the right boundary was the right margin of the inferior vena cava, so that the omental interposition could separate skeletonized vessels from a possible anastomotic leakage. Then two double catheterization cannulas (PJ tube and HJ tube) were placed at the left anterior of the PJ anastomosis site and right posterior of the HJ anastomosis site, respectively. The blood flow of the omental interposition was reconfirmed before the abdominal cavity was closed. The application of the omental interposition in PD is shown in Figure 2.
In the non-omental interposition group, we simply placed the two drainage tubes at the aforementioned positions after completing the anastomosis. After the operation, the amylase concentration from the drainage fluid was measured daily. If the drain fluid amylase obtained on DFA1 exceeded 2000 U/L, abdominal irrigation was used to dilute the concentration of pancreatic juice around the anastomosis as soon as possible. Approximately 3000 mL normal saline was irrigated every day, with a flow rate of 200 mL/h. The flow of irrigation was modulated frequently according to the character of the secretion. The suction pressure was set with low-pressure suction between 20 and 30 cm water. Once the amylase level of the dilution fluid was lower than 30 U/L, the use of abdominal irrigation was stopped. The drainage tubes were removed until the amylase concentration was less than three times the upper limit of the normal serum level. All patients underwent routine postoperative computed tomography (CT) examinations before the drain tubes were removed to assess the presence of potential complications and peritoneal effusion.
POPF was defined and graded according to the modified definition by the International Study Group of Pancreatic Fistula (ISGPF)[16]. Clinically relevant POPF (CR-POPF) was considered grades B and C. Delayed gastric emptying (DGE) and PPH were defined and classified by the International Study Group for Pancreatic Surgery[17,18]. Intra-abdominal infections were diagnosed according to the definition proposed by the Surgical Infection Society and the Infectious Diseases Society of America[19].
All statistical analyses were conducted using SPSS 23.0. The χ2 test or Fisher’s exact test was used for categorical variables, whereas the Student’s t-test or Wilcoxon rank-sum test (whether the variables were normally distributed) were used for continuous variables. P < 0.05 was considered statistically significant. After matching, each patient who received an omental interposition was matched to a patient in the non-omental interposition group by using nearest-neighbor matching in a 1:1 ratio. A PSM analysis was used to reduce the impact of the treatment selection bias when estimating the omental interposition values using original observational indicators. Multivariable logistic regression was performed with adjustments for the propensity scores using the associated covariates.
The demographic and clinically related variables of all patients including age, sex, body mass index (BMI), American Society of Anesthesiologists score, serum albumin content, main pancreatic duct size and pathology were similar between the two groups (P > 0.05). However, patients in the omental interposition group had a higher median serum bilirubin than those in the non-omental interposition group (96.5 [17.9-107.0] vs 20.5 [9.6-148.5]; P = 0.015). Laparoscopic pancreaticoduodenectomy (LPD) was more frequently performed in the omental interposition group than in the non-omental interposition group (69, 54.3% vs 19, 27.5%; P < 0.001). The details are shown in Table 1.
| Before PSM | After PSM | |||||
| Omental interposition group (127) | Non-omental interposition group (69) | P value | Omental interposition group (69) | Non-omental interposition group (69) | P value | |
| Male/female | 79/48 | 44/25 | 0.919 | 46/23 | 44/25 | 0.721 |
| Age (yr) | 64.8 ± 10.5 | 62.1 ± 9.9 | 0.083 | 64.2 ± 9.5 | 62.1 ± 9.9 | 0.210 |
| BMI (mean ± SD, kg/m2) | 21.9 ± 3.0 | 22.0 ± 2.8 | 0.844 | 21.9 ± 3.2 | 22.0 ± 2.9 | 0.933 |
| ASA score, n (%) | 0.126 | 0.168 | ||||
| I | 65 (51.2) | 42 (60.9) | 34 (49.3) | 42 (60.9) | ||
| II | 60 (47.2) | 24 (34.8) | 34 (49.3) | 24 (34.8) | ||
| III | 2 (1.6) | 3 (4.3) | 1 (1.4) | 3 (4.3) | ||
| Serum ALB [n (%), g/L] | 0.152 | 1.00 | ||||
| < 35 | 13 (10.2) | 12 (17.4) | 12 (17.4) | 12 (17.4) | ||
| ≥ 35 | 114 (89.8) | 57 (82.6) | 57 (82.6) | 57 (82.6) | ||
| Serum bilirubin (μmol/L) | 96.5 (17.9-107.0) | 20.5 (9.6-148.5) | 0.015 | 29.8 (12.4-153.7) | 20.5 (9.6-148.5) | 0.753 |
| Main pancreatic duct size [n (%), mm] | 0.080 | 0.173 | ||||
| < 3 | 57 (44.9) | 40 (58.0) | 32 (46.4) | 40 (58.0) | ||
| ≥ 3 | 70 (55.1) | 29 (42.0) | 37 (53.6) | 29 (42.0) | ||
| Operation method, n (%) | 0.005 | 0.708 | ||||
| LPD | 69 (54.3) | 19 (27.5) | 21 (30.4) | 19 (27.5) | ||
| OPD | 58 (45.7) | 50 (72.5) | 48 (69.6) | 50 (72.5) | ||
| Pathology, n (%) | 0.009 | 0.151 | ||||
| PDAC | 53 (41.7) | 25 (36.2) | 36 (52.2) | 25 (36.2) | ||
| Bile duct cancer | 10 (7.9) | 13 (18.8) | 4 (5.8) | 13 (18.8) | ||
| Ampulla of Vater cancer | 18 (14.2) | 15 (21.7) | 10 (14.5) | 15 (21.7) | ||
| Duodenal cancer | 11 (8.7) | 2 (2.9) | 2 (2.9) | 2 (2.9) | ||
| Other carcinoma | 19 (15.0) | 2 (2.9) | 3 (4.3) | 2 (2.9) | ||
| Benign tumor | 16 (12.6) | 12 (17.4) | 14 (20.3) | 12 (17.4) | ||
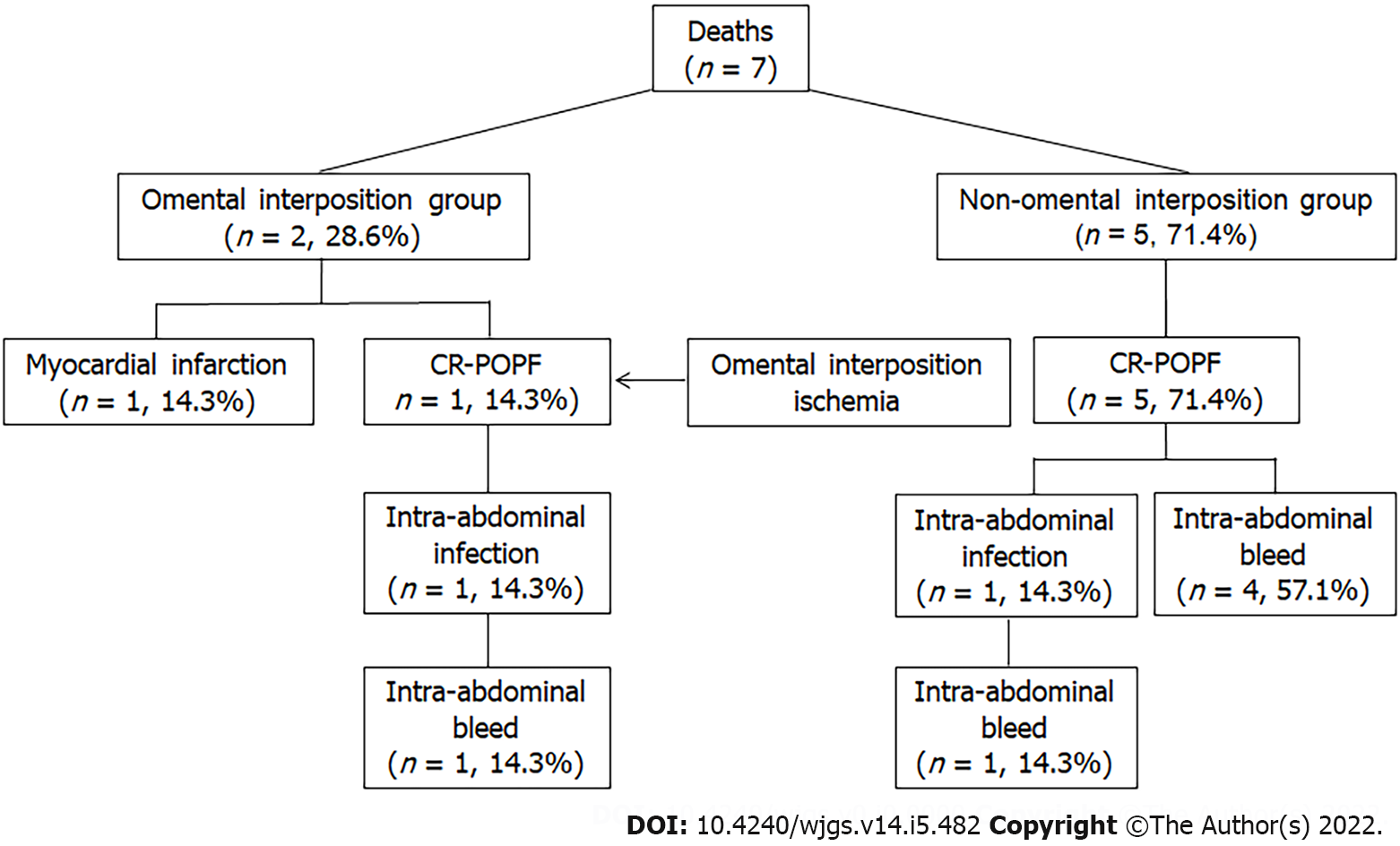
Regarding postoperative complications, a comparison revealed that the rates of CR-POPF (13, 10.2% vs 17, 24.6%; P = 0.028), biliary fistula (BF, 2,1.6% vs 5, 7.2%; P = 0.041), delayed PPH associated with POPF (1, 0.8% vs 8, 11.6%; P = 0.002), and postoperative transfusion (18,14.2% vs 20, 29.0%; P = 0.012) were significantly lower in the omental interposition group than in the non-omental interposition group. The rates of other surgery-related complications, including DGE, intra-abdominal abscess, and reoperation, did not significantly differ between the two groups. Regarding mortality, there was no significant difference between the two groups (2, 1.6% vs 5, 7.2%; P = 0.101). However, the CR-POPF-related mortality in the omental interposition group was significantly lower than the mortality in the non-omental interposition group (1, 0.8% vs 5, 7.2%; P = 0.021). The details on the deaths that occurred are shown in Table 2 and Figure 3. Fewer complications in the omental group may be related to passing the laparoscopic learning curve. However, among the 108 cases of OPD, 58 cases applied the omental interposition technique, and the omental interposition group had lower incidence of complications (6, 10.3% vs 9, 18%; P = 0.008) and lower mortality rate (0, 0% vs 4, 8%; P = 0.007).
| Before PSM | After PSM | |||||
| Omental interposition group (127) | Non-omental interposition group (69) | P value | Omental interposition group (69) | Non-omental interposition group (69) | P value | |
| CR-POPF | 13 (10.2%) | 17 (24.6%) | 0.028 | 7 (10.1%) | 17 (24.6%) | 0.025 |
| Operation time (mean ± SD, min) | 388.3 ± 68.8 | 365.2 ± 75.0 | 0.031 | 392.6 ± 74.1 | 365.2 ± 75.0 | 0.033 |
| BF, n (%) | 2 (1.6) | 5 (7.2) | 0.041 | 1 (1.4) | 5 (7.2) | 0.208 |
| DGE, n (%) | 4 (3.1) | 6 (8.7) | 0.178 | 1 (1.4) | 6 (8.7) | 0.115 |
| PPH, n (%) | 1 (0.8) | 8 (11.6) | 0.002 | 1 (1.4) | 8 (11.6) | 0.016 |
| Intra-abdominal abscess, n (%) | 15 (11.8) | 12 (17.4) | 0.286 | 8 (11.6) | 12 (17.4) | 0.333 |
| Reoperation, n (%) | 3 (2.4) | 6 (8.7) | 0.096 | 2 (2.9) | 6 (8.7) | 0.274 |
| Mortality in 30 d, n (%) | 2 (1.6) | 5 (7.2) | 0.101 | 2 (2.9) | 5 (7.2) | 0.438 |
| Mortality related to POPF, n (%) | 1 (0.8) | 5 (7.2) | 0.038 | 1 (1.4) | 5 (7.2) | 0.210 |
| DFA1 around the HJ site (U/L) | 300.0 (74.3-893.0) | 599.8 (171.1-2064.7) | 0.002 | 200.0 (57.5-659.8) | 599.8 (171.1-2064.7) | 0.003 |
| DFA1 around the PJ site (U/L) | 546.8 (76.4-3094.0) | 350.0 (50.0-2577.4) | 0.255 | 325.0 (69.5-2972.5) | 350.0 (50.0-2577.4) | 0.951 |
| Duration until removal of the tube around the HJ site (d) | 7 (5-9) | 9 (7-14) | 0.000 | 8 (6-11) | 9 (7-14) | 0.115 |
| Duration until removing the tube around the PJ site (d) | 7 (6-11) | 10 (7-15) | 0.004 | 8 (6-12) | 10 (7-15) | 0.100 |
| Required blood transfusions, n (%) | 18 (14.2) | 20 (29.0) | 0.012 | 9 (13.0) | 20 (29.0) | 0.022 |
| Length of hospital stay (d) | 15 (11-22) | 21 (13-32) | 0.004 | 16 (12-24) | 21 (13-32) | 0.031 |
| Duration until restarting diet (d) | 6 (5-8) | 8 (6-15) | 0.001 | 7 (5-8) | 8 (6-15) | 0.048 |
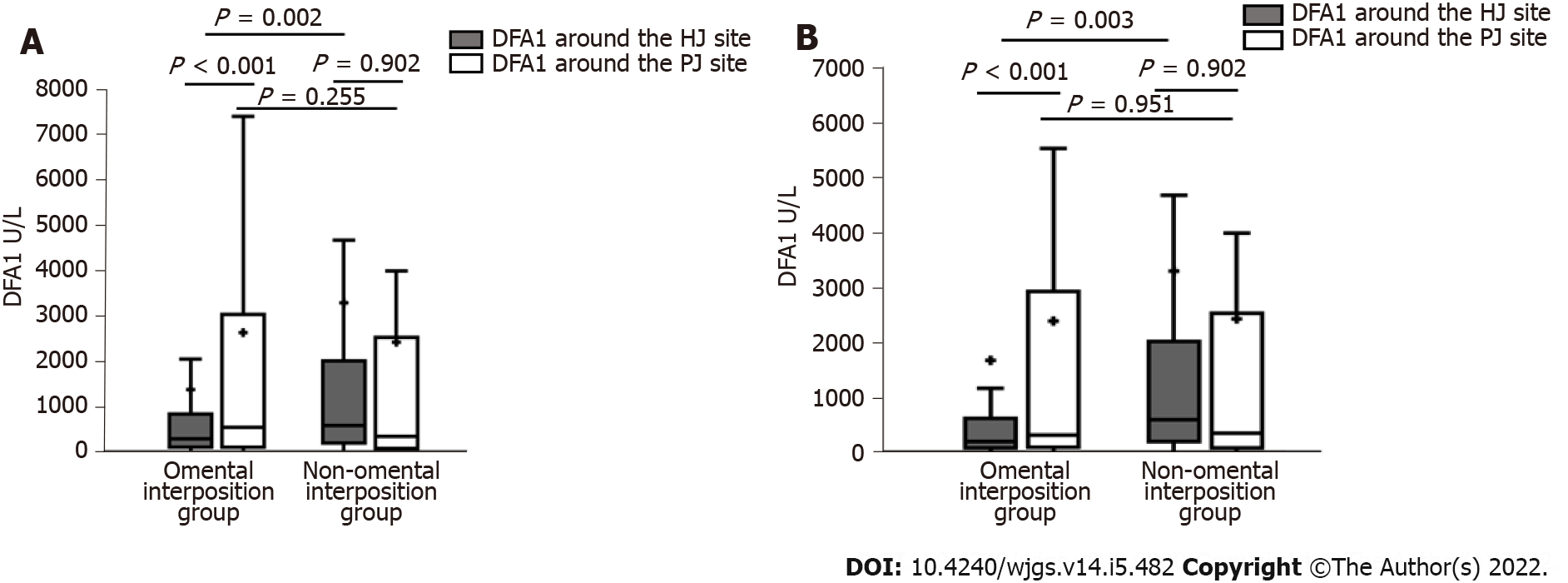
When comparing relevant data on the enhanced recovery after surgery between the two groups, the HJ and PJ drainage tubes were removed earlier in the omental interposition group than in the non-omental interposition group (both P < 0.05). The omental interposition group of patients had significantly shorter postoperative durations of restarting their diet and shorter length of hospital stay than the non-omental interposition group patients (both P < 0.01). Based on the laboratory test results, the DFA1 around the HJ in the omental interposition group was dramatically lower than that in the non-omental interposition group (300.0 [74.3-893.0] vs 599.8 [171.1-2064.7]; P = 0.002). In the omental interposition group, the drain amylase values from the tube around the HJ were lower than those around the PJ (300.0 [74.3-893.0] vs 546.8 [76.4-3094.0]; P < 0.001). However, the difference disappeared in the non-omental interposition group. The details are shown in Table 2 and Figure 4A.
To reduce the impact of selection bias and the role of the procedure (LPD and OPD), PSM was performed using nine selected baseline characteristics. After PSM, the patient demographic and clinically related characteristics, including preoperative serum bilirubin and operation methods, were similar between the two groups. The rates of CR-POPF (7, 10.1% vs 17, 24.6%; P = 0.025), delayed PPH associated with POPF (1, 1.4% vs 8, 11.6%; P = 0.016) and postoperative transfusion (9, 13.0% vs 20, 29.0%; P = 0.022) remained significantly lower in the omental interposition group than in the non-omental interposition group after PSM. The operation time in the omental interposition group was slightly longer both before (388.3 ± 68.8 vs 365.2 ± 75.0) and after (392.6 ± 74.1 vs 365.2 ± 75.0) the match, which may be related to the selection, cutting, and fixing of the omental interposition. Moreover, the omental interposition group of patients had a significantly shorter postoperative duration to restart their diet (7 [5-8] vs 8 [6-15]; P = 0.048) and shorter hospital stays (16 [12-24] vs 21 [13-32]; P = 0.031] than the non-omental interposition group of patients. The non-omental interposition group had greater mortality related to POPF than the omental interposition group (5 [7.2%] vs 1 [1.4%]), but there was no significant difference, which may be related to the small number of cases. The details are shown in Table 2.
Following PSM, the omental interposition group had dramatically lower DFA1 around the HJ than the non-omental interposition group (200.0 [58-610.6] vs 599.8 [171.1-2064.7] P = 0.003). In the omental interposition group, the DFA1 around the HJ was lower than the DFA1 around the PJ (200.0 [58-610.6] vs 325.0 [75.3-2869], P < 0.001). The details on DFA1 are shown in Table 2 and Figure 4B.
Table 3 shows the univariate and multivariate analyses of the PSM data to evaluate the risk factors associated with CR-POPF after PD. Male sex, BMI ≥ 23 kg/m2, nonapplication of omental interposition, DFA1 around HJ ≥ 1000 U/L, and main pancreatic duct size < 3 mm were significantly associated with the development of CR-POPF after PD. Multivariate logistic regression analyses showed that a high BMI (odds ratio [OR] = 6.094, 95% confidence interval [CI]: 2.021-18.374; P = 0.001), nonapplication of omental interposition (OR = 3.145, 95%CI: 1.040-9.509; P = 0.042), and main pancreatic duct diameter < 3 mm (OR = 5.663, 95%CI: 1.456-22.033; P = 0.012) were independent factors that were significantly associated with the development of CR-POPF after PD.
| CR-POPF (24) | No CR-POPF (114) | P value | Multivariate analysis | |||
| OR | 95%CI | P value | ||||
| Age (mean ± SD, yr) | 63.5 ± 7.9 | 63.1 ± 10.1 | 0.829 | |||
| Sex, n (%) | 0.040 | |||||
| Male | 20 (83.3%) | 70 (61.4%) | 2.436 | 0.692-8.574 | 0.165 | |
| Female | 4 (16.7%) | 44 (38.6%) | Reference | |||
| Operation method, n (%) | 0.143 | |||||
| LPD | 4 (16.7%) | 36 (31.6%) | ||||
| OPD | 20 (83.3%) | 78 (68.4%) | ||||
| BMI (kg/m2) | 0.000 | |||||
| ≥ 23 | 18 (75.0%) | 33 (28.9%) | 6.094 | 2.021-18.374 | 0.001 | |
| < 23 | 6 (25.0%) | 81 (71.1%) | Reference | |||
| Serum bilirubin (μmol/L) | 96.6 (16.1-180.4) | 67 (13.8-111.2) | 0.185 | |||
| Serum ALB (g/L) | 0.843 | |||||
| ≥ 35 | 21 (87.5%) | 98 (86.0%) | ||||
| < 35 | 3 (12.5%) | 16 (14.0%) | ||||
| ASA score, n (%) | 0.122 | |||||
| Grade I | 11 (45.8%) | 66 (57.9%) | ||||
| Grade II | 11 (45.8%) | 47 (41.2%) | ||||
| Grade III | 2 (8.3%) | 1 (0.9%) | ||||
| Pathology, n (%) | 0.196 | |||||
| Malignancy | 23 (95.8%) | 96 (84.2%) | ||||
| Benign | 1 (4.2%) | 18 (15.8%) | ||||
| Omental interposition, n (%) | 0.025 | |||||
| Yes | 7 (29.2%) | 62 (54.4%) | Reference | |||
| No | 17 (70.8%) | 52 (45.6%) | 3.145 | 1.040-9.509 | 0.042 | |
| Operating time (mean ± SD, min) | 387.1±82.5 | 377.7±71.2 | 0.609 | |||
| HJ DFA1 (U/L) | 0.010 | |||||
| ≥ 1000 | 13 (54.2%) | 31 (27.2%) | 1.000 | 1.000-1.000 | 0.834 | |
| < 1000 | 11 (45.8%) | 83 (72.8%) | Reference | |||
| PJ DFA1 (U/L) | 0.115 | |||||
| ≥ 1000 | 13 (54.2%) | 42 (36.8%) | ||||
| < 1000 | 11 (45.8%) | 72 (63.2%) | ||||
| Main pancreatic duct size [n (%), mm] | 0.000 | |||||
| ≥ 3 | 3 (12.5%) | 64 (56.1%) | Reference | |||
| < 3 | 21 (87.5%) | 50 (43.9%) | 5.663 | 1.456-22.033 | 0.012 | |
To date, POPF remains the most fatal complication after PD. Pancreatic fistula, especially clinically related postoperative fistula, is the most common cause of delayed PPH and intra-abdominal infections after PD[1-4]. Leaked activated pancreatic juice is highly corrosive. Once the drainage tubes fail to effectively work, pancreatic juice accumulates in the potential cavity gap around the anastomosis. This condition may erode the vulnerable anastomosis and adjacent vascular wall. Various efforts[5-8] have been tested for their ability to reduce the incidence of CR-POPF after PD, such as improved anastomosis and the use of somatostatin. However, few randomized control trials have significantly prevented CR-POPF.
Since pancreatic fistulas are almost inevitable after PD, it is necessary to improve the surgical techniques and accelerate the healing process of fistulas to strive for “harmless” pancreatic fistulas. Experimental results have shown that the greater omentum can resist corrosion, provide anti-infection properties, absorb the peritoneal effusion, regenerate blood vessels and repair tissue defects. Thus, we hypothesized that the omental interposition could seal the posterior wall of the PJ anastomosis, fill the potential cavity to avoid effusion at the surgical site, cover the skeletonized vessels to avoid erosion and accelerate the regeneration of blood vessels to improve the blood supply of the anastomosis. The study shows that the incidence of CR-POPF and delayed PPH were lower in the omental interposition group than in the non-omental interposition group. As a result of the reduced complications, the average duration to restart diet and the length of hospital stay were shorter in the omental interposition group. Previous studies on OPD have reached similar conclusions. Maeda[14] covered the major splanchnic arteries and the PV with an omental flap in 100 patients. Although the author concluded that the incidence of POPF (20%) was not significantly different from that in other articles, he did not rule out biochemical fistulas based on the modified definition by the ISGPF. Matsuda et al[20] emphasized the preventive effect of omental flaps in PD against postoperative pseudoaneurysm formation. Shah et al[21] wrapped the omental flap around the PJ site in 101 patients and showed that it could reduce the incidence of POPF (4.0% vs 17.4%), PPH (0% vs 6.5%), BF (1.0% vs 13.0%), and DGE (4.0% vs 17.4%) compared to those in the non-omental interposition group.
In addition to the physiological function of the omental interposition, our method could elevate the height of the anastomosis and fill the potential cavity due to the physical characteristics. Because the omental interposition can elevate the position of the HJ anastomosis (Figure 2D), the erosive pancreatic fluid will flow to the left instead of remaining around the skeletonized vessels in the right upper quadrant of the abdomen. The difference in DFA1 between HJ and PJ sites confirm these physical characteristics in the omental interposition group. This finding also confirms that the application of the omental interposition, by preventing leakage from the anastomosis, reduces the incidence of delayed PPH. Because of the effective control of serious complications, the omental interposition group had their drainage tubes removed earlier, required fewer postoperative transfusions, restarted their diet earlier and had a shorter hospital stay than the non-omental interposition group. These findings are highly consistent with the aforementioned studies showing the efficacy of the omental interposition in PD.
PSM of nine baseline characteristics was performed to reduce selection bias and potential confounding factors between the two groups. After matching, the incidences of CR-POPF and delayed PPH remain significantly lower in the omental interposition group. Similarly, the difference in median DFA1 values between HJ and PJ sites in the omental interposition group remained observable. However, in the non-omental interposition group, the DFA1 around the PJ site was significantly higher than the DFA1 around the HJ site. Due to the physical characteristics of the omental interposition, the corrosive pancreatic juice would flow to the left upper quadrant of the abdomen because of gravity. Obviously, these details matter tremendously.
Previous studies[22-24] have reported that the risk factors for POPF include a high BMI, soft pancreatic texture, and small pancreatic duct size. In our study, univariate and multivariate analyses revealed that a high BMI, nonapplication of omental interposition, and main pancreatic duct diameter < 3 mm were independent factors significantly associated with the development of CR-POPF after PD. The developed statistical model had a c-index of 0.848. These findings were partially consistent with previous POPF risk scores.
Only one patient in the omental group died of delayed PPH caused by ischemic infection due to poor blood supply of the omental interposition, which resulted in delayed hemorrhage. This was the eighth case in which we applied the omental interposition with insufficient emphasis on ensuring good blood supply to the omental interposition. Since then, we detached the gastrocolic ligament along the gastric wall to ensure good blood supply to the omental interposition.
This study had several limitations, including its design as a single-center, retrospective observational study. However, all clinically related data were prospectively collected, and all operations were performed by the same surgical group with the same surgical technology. Thus, the majority of the potential confounding factors were controlled.
In conclusion, we believe that the application of the omental interposition is technically simple and may help prevent CR-POPF and the associated complications following PD.
Postoperative pancreatic fistula (POPF) is a life-threatening complication after pancreaticoduodenectomy (PD).
Several methods have attempted to reduce the POPF after PD, few have been considered effective. The safety and short-term clinical benefits of omental interposition remain controversial.
To investigate the safety and feasibility of omental interposition to reduce the POPF rate and related complications in PD.
In total, 196 consecutive patients underwent PD performed by the same surgical team, the patients were divided into two groups: an omental interposition group (127, 64.8%) and a non-omental interposition group (69, 35.2%). Propensity score-matched analyses were performed to compare the severe complication rates and mortality between the two groups.
The clinically relevant POPF (CR-POPF; 10.1% vs 24.6%; P = 0.025) and delayed postpancreatectomy hemorrhage (1.4% vs 11.6%; P = 0.016) rates were significantly lower in the omental interposition group. The omental interposition technique was associated with a shorter time to resume food intake (7 vs 8 d; P = 0.048) and a shorter hospitalization period (16 vs 21 d; P = 0.031).
The application of the omental interposition is an effective and safe approach to reduce the CR-POPF rate and related complications after PD.
Prospective studies are needed on the role of omental interposition in reducing CR-POPF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kessel B, Israel; Suc B, France; Vasavada B, India S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Ellis RJ, Brock Hewitt D, Liu JB, Cohen ME, Merkow RP, Bentrem DJ, Bilimoria KY, Yang AD. Preoperative risk evaluation for pancreatic fistula after pancreaticoduodenectomy. J Surg Oncol. 2019;119:1128-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Yamao J, Kim S, Matsui Y, Takai S, Mergental H, Kamiyama Y; Department of Surgery, Kansai Medical University, Osaka, Japan. A new guideline to reduce postoperative morbidity after pancreaticoduodenectomy. Pancreas. 2008;37:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Roberts KJ, Sutcliffe RP, Marudanayagam R, Hodson J, Isaac J, Muiesan P, Navarro A, Patel K, Jah A, Napetti S, Adair A, Lazaridis S, Prachalias A, Shingler G, Al-Sarireh B, Storey R, Smith AM, Shah N, Fusai G, Ahmed J, Abu Hilal M, Mirza DF. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg. 2015;261:1191-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 107] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 4. | Chen JF, Xu SF, Zhao W, Tian YH, Gong L, Yuan WS, Dong JH. Diagnostic and therapeutic strategies to manage post-pancreaticoduodenectomy hemorrhage. World J Surg. 2015;39:509-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kang JS, Han Y, Kim H, Kwon W, Kim SW, Jang JY. Prevention of pancreatic fistula using polyethylene glycolic acid mesh reinforcement around pancreatojejunostomy: the propensity score-matched analysis. J Hepatobiliary Pancreat Sci. 2017;24:169-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Hong S, Wang H, Yang S, Yang K. External stent versus no stent for pancreaticojejunostomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg. 2013;17:1516-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Martin I, Au K. Does fibrin glue sealant decrease the rate of anastomotic leak after a pancreaticoduodenectomy? HPB (Oxford). 2013;15:561-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Kurumboor P, Palaniswami KN, Pramil K, George D, Ponnambathayil S, Varma D, Aikot S. Octreotide Does Not Prevent Pancreatic Fistula Following Pancreatoduodenectomy in Patients with Soft Pancreas and Non-dilated Duct: A Prospective Randomized Controlled Trial. J Gastrointest Surg. 2015;19:2038-2044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Montorsi M, Zerbi A, Bassi C, Capussotti L, Coppola R, Sacchi M; Italian Tachosil Study Group. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: a multicenter, randomized, controlled trial. Ann Surg. 2012;256:853-9; discussion 859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Ye P, Cao JL, Li QY, Wang ZT, Yang YH, Lv W, Hu J. Mediastinal transposition of the omentum reduces infection severity and pharmacy cost for patients undergoing esophagectomy. J Thorac Dis. 2016;8:1653-1660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Ye J, Li Q, Liu R, Zhang K, Nie Z, Chen J, Jin F, Huo W. Pedicled greater omentum graft: a new technique to repair recurrent urinary fistulae after kidney transplantation. Cell Biochem Biophys. 2012;62:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Karabulut B, Sönmez K, Türkyilmaz Z, Demiroğullari B, Karabulut R, Sezer C, Sultan N, Başaklar AC, Kale N. Omentum prevents intestinal adhesions to mesh graft in abdominal infections and serosal defects. Surg Endosc. 2006;20:978-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Seyama Y, Kubota K, Kobayashi T, Hirata Y, Itoh A, Makuuchi M. Two-staged pancreatoduodenectomy with external drainage of pancreatic juice and omental graft technique. J Am Coll Surg. 1998;187:103-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Maeda A, Ebata T, Kanemoto H, Matsunaga K, Bando E, Yamaguchi S, Uesaka K. Omental flap in pancreaticoduodenectomy for protection of splanchnic vessels. World J Surg. 2005;29:1122-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Jiang CY, Liang Y, Wang HW, Hu PF, Cai ZW, Wang W. Management of the uncinate process via the artery first approach in laparoscopic pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2019;26:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2435] [Cited by in F6Publishing: 2372] [Article Influence: 338.9] [Reference Citation Analysis (1)] |
| 17. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1771] [Cited by in F6Publishing: 2043] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 18. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1411] [Cited by in F6Publishing: 1660] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 19. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt). 2010;11:79-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Matsuda H, Sadamori H, Umeda Y, Shinoura S, Yoshida R, Satoh D, Utsumi M, Yagi T, Fujiwara T. Preventive effect of omental flap in pancreaticoduodenectomy against postoperative pseudoaneurysm formation. Hepatogastroenterology. 2012;59:578-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Shah OJ, Bangri SA, Singh M, Lattoo RA, Bhat MY. Omental flaps reduces complications after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int. 2015;14:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 22. | Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, Konishi M. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:1744-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, Hamdy E, Atef E, El Hanafy E, El-Geidie A, Abdel Wahab M, Abdallah T. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013;37:1405-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Polanco PM, Zenati MS, Hogg ME, Shakir M, Boone BA, Bartlett DL, Zeh HJ, Zureikat AH. An analysis of risk factors for pancreatic fistula after robotic pancreaticoduodenectomy: outcomes from a consecutive series of standardized pancreatic reconstructions. Surg Endosc. 2016;30:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |