Published online Apr 27, 2021. doi: 10.4240/wjgs.v13.i4.366
Peer-review started: November 8, 2020
First decision: December 20, 2020
Revised: December 28, 2020
Accepted: January 21, 2021
Article in press: January 21, 2021
Published online: April 27, 2021
Remnant gastric cancer (RGC) is defined as a tumor that develops in the stomach after a previous gastrectomy and is generally associated with a worse prognosis. However, there little information available regarding RGCs and their prognostic factors and survival.
To evaluate the clinicopathological characteristics and prognosis of RGC after previous gastrectomy for benign disease.
Patients who underwent curative resection for primary gastric cancer (GC) at our institute between 2009 and 2019 were retrospectively evaluated. All RGC resections with histological diagnosis of gastric adenocarcinoma were enrolled in this study. Primary proximal GC (PGC) who underwent total gastrectomy was selected as the comparison group. Clinical and pathological data were collected from a prospective medical database.
A total of 41 patients with RGC and 120 PGC were included. Older age (P = 0.001), lower body mass index (P = 0.006), hemoglobin level (P < 0.001), and number of resected lymph nodes resected (LN) (P < 0.001) were associated with the RGC group. Lauren type, pathological tumor-node-metastasis, and perioperative morbimortality were similar between RGC and PGC. There was no difference in disease-free survival (P = 0.592) and overall survival (P = 0.930) between groups. LN status was the only independent factor related to survival.
RGC had similar clinicopathological characteristics to PGC. Despite the lower number of resected LN, RGC had a similar prognosis.
Core Tip: This is a retrospective study to evaluate the clinicopathological character-istics, surgical outcomes, and survival of remnant gastric cancer (RGC) after previous gastrectomy for benign disease. We compared the RGC patients with primary proximal gastric cancer (PGC) who underwent total gastrectomy. The findings indicated that RGC and PGC had similar clinicopathological characteristics, including Lauren type and pathological tumor-node-metastasis stage, but RGC patients were older and had a lower number of resected lymph nodes. Although RGC is generally associated with a worse prognosis, there was no significant difference in perioperative morbimortality and survival between the groups.
- Citation: Ramos MFKP, Pereira MA, Dias AR, Dantas ACB, Szor DJ, Ribeiro Jr U, Zilberstein B, Cecconello I. Remnant gastric cancer: An ordinary primary adenocarcinoma or a tumor with its own pattern? World J Gastrointest Surg 2021; 13(4): 366-378
- URL: https://www.wjgnet.com/1948-9366/full/v13/i4/366.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i4.366
Gastric cancer (GC) is the fifth most common cancer in the world, and it is estimated that more than one million new cases of GC occur annually[1]. Among the types of gastric tumors, remnant gastric cancer (RGC) is defined as a tumor that develops in the gastric remnant more than 5 years after a previous gastrectomy.
GC carcinogenesis is a multistep process that involves the interaction of several genetic, epigenetic, and environmental factors[2]. Risk factors commonly associated with the development of GC include chronic infection with Heliobacter pylori (H. pylori), low fruit and vegetable intake, high salt intake, smoking, and alcohol consumption[3]. After gastric resection, environmental changes induce chronic damage to the previous normal gastric mucosa of the remnant, initiating a de novo carcinogenic pathway with a longer period for the development of RGC[4,5]. Another factor contributing to the remnant carcinogenesis is the vagotomy performed during the previous procedure, which causes denervation of the gastric mucosa leading to hypochlorhydria[6]. On the other hand, the frequency of H. pylori infection decreases in the mucosa remnant, leading to a protective effect[6-8].
The incidence of RGC was reported in the range of 2%-6% of all GC[6,7,9,10]. It can occur in the remnant stomach after a previous resection for either benign or malignant lesions. Nevertheless, these tumors seem to have different behaviors and etiologies according to this origin.
Over the past few decades, the introduction of histamine 2-receptor antagonists and proton pump inhibitors drastically reduced the number of gastric resections due to peptic disease. However, since the time for the development of the disease is long, the occurrence of RGC is still part of the current reality due to gastric resection for treatment of peptic ulcer in the past.
Within the group of patients who had previous distal gastrectomy, a long-term follow-up is recommended for early detection of RGC[5,9]. But even with these recommendations, there is a common sense that RGC is generally related to more advanced clinical stage and worse prognosis[6,10,11]. The long period of carcinogenic effect after resection, as well as the previous diagnosis of benign disease, makes patients less likely to perform follow-up assessments for early detection of the gastric remnant tumor.
Completion total gastrectomy with radical lymphadenectomy is usually the treatment of choice for RGC. It is a technically challenging procedure, which may be associated with higher morbidity and mortality rates[12,13]. The change in lymphatic drainage after the first resection may impact the alteration in the pattern of lymph node (LN) spread. Furthermore, which LN stations must be removed and how to stage the disease in these patients persist as unanswered questions[14]. Therefore, it is essential to understand the characteristics of RGC to determine its prognosis and decide on the appropriate treatment strategies.
Thus, this study aimed to evaluate the clinicopathological characteristics and prognosis of RGC after the previous gastrectomy for the benign disease compared to patients with primary proximal gastric cancer (PGC) undergoing total gastrectomy (TG) for primary cancer.
Patients who underwent curative resection for GC at our institute between 2009 and 2019 were retrospectively evaluated from a prospectively collected medical database. All RGC resections with histological diagnosis of gastric adenocarcinoma were enrolled in this study. As a comparison group, patients with primary PGC of corpus/fundus/cardia who underwent total gastrectomy with curative intent were selected. Non-adenocarcinoma histology, palliative resections, and patients with the previous resection due to gastric adenocarcinoma were excluded from the study.
Clinical data were collected on the following variables: Age, sex, preoperative body mass index, albumin level, hemoglobin level, neutrophil-lymphocyte ratio, and physical status based on the American Society of Anesthesiologists classification[15]. Comorbidities were recorded for all patients following Charlson-Deyo comorbidity index[16], without the inclusion of age and GC as comorbidity. Time from previous gastrectomy and type of reconstruction were also analyzed in RGC patients.
The preoperative staging was performed through abdominal and pelvis computed tomography, endoscopy, and laboratory tests[17]. Tumor location and size was defined by endoscopy. Cardia tumors were classified according to Siewert classification, and those invading the previous anastomosis were considered as tumors located at the anastomotic site.
The number of LN retrieved was also evaluated according to the lymph node ratio (LR), as proposed by Deng et al[18]. Patients were classified into four categories based on the following cutoff points: LR0 < 10%, LR1 = 10%-20%, LR2 = 20%-40%, and LR3 > 40%. Tumor-node-metastasis (TNM) staging was determined according to the 8th edition of the American Joint Committee on Cancer manual[19].
All cases were operated in a high-volume center by specialist surgeons. The extent of LN dissection, as well as resection of adjacent organs during the surgery, was established by the attending surgeon to achieve a complete R0 resection. The extension of LN dissection and LN stations in total gastrectomy followed the recommendations of the Japanese Gastric Cancer Association guidelines[20]. The surgical approach (open or laparoscopic) was decided based on the surgeon´s decision during a multidiscipli
Postoperative complications (POC) were graded according to Clavien-Dindo's classification[21], and Clavien III-V were determined as a major complication. Mortality at 30 d and 90 d after the surgical procedure was also an outcome assessed. Adjuvant or perioperative platin-based chemotherapy was administered according to clinical indication (T3/T4 and/or N+).
Postoperative follow-up medical appointments were performed quarterly in the first year and every 6 mo in the following years. Follow-up image tests for recurrence detection were performed based on the presence of symptoms. Lost to follow-up was defined as an absence for more than 12 mo in follow-up visits. The study was approved by the hospital ethics committee (CAAE: 25516719.3.0000.0065).
The chi-square tests were used for categorical variables and t-tests for continuous variables. Survival was defined as the interval in months between the date of surgery and the date of recurrence, death for any cause, or the date of the last appointment. Overall survival (OS) and disease-free survival (DFS) were estimated using the method of Kaplan–Meier. The log-rank test was used to identify differences between the survival curves. To determine factors associated with DFS and OS, univariate and multivariate survival analysis was performed by Cox proportional hazards model. All tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc, Armonk, NY, United States).
Among the 623 patients who underwent gastrectomy with lymphadenectomy with curative intent at our institute, RGC resection was performed in 60 patients. Of these, 41 patients who had the previous resection due to benign disease were enrolled. For the comparison group, 120 patients with primary proximal gastric adenocarcinoma submitted to TG in the same period met the inclusion criteria and were included in the study.
Concerning RGC, all patients were previously operated on by an open approach and underwent gastrojejunostomy (Billroth II) reconstruction. The mean age of patients at the time of first surgery was 31.7 years (range 19-53 years), and the median time between the first and the second surgery was 37 years (mean 36.5 ± 8.4 years). The tumor was located at the gastric corpus along with the previous anastomosis in 29 cases (70.7%).
Clinical and surgical characteristics of the RGC and PGC groups are summarized in Table 1. Older age (P = 0.001), lower body mass index (P = 0.006), and lower hemoglobin level (P < 0.001) were associated with patients in the RGC group. No differences were observed regarding sex, comorbidities, American Society of Anesthesiologists classification, and surgical access between the groups. D2 lymphadenectomy was performed in 85% of PGC cases. Preoperative or adjuvant CMT was more commonly administered for PGC (P < 0.001). Three and 34 patients received neoadjuvant treatment in the RGC group and PGC group, respectively. Considering the postoperative outcomes, POC and mortality rates at 30 d and 90 d were similar in both groups.
| Variable | PGC, n = 120 (%) | RGC, n = 41 (%) | P value |
| Sex | 0.216 | ||
| Female | 32 (26.7) | 7 (17.1) | |
| Male | 88 (73.3) | 34 (82.9) | |
| Age in yr, mean ± SD | 62.6 ± 12.7 | 68.1 ± 7.6 | 0.001 |
| Body mass index in kg/cm², mean ± SD | 24.9 ± 4.9 | 22.5 ± 4.2 | 0.006 |
| Hemoglobin in g/dL, mean ± SD | 12.5 ± 2.1 | 10.6 ± 2.2 | < 0.001 |
| Albumin in g/dL, mean ± SD | 4.3 ± 3.2 | 3.8 ± 0.5 | 0.402 |
| Neutrophil lymphocyte ratio, mean ± SD | 3.02 (2.94) | 3.01 (2.37) | 0.994 |
| Charlson–Deyo comorbidity index | 0.391 | ||
| 0 | 76 (63.3) | 29 (70.7) | |
| > 1 | 44 (36.7) | 12 (29.3) | |
| American Society of Anesthesiologists | 0.561 | ||
| I/II | 82 (68.3) | 30 (73.2) | |
| III/IV | 38 (31.7) | 11 (26.8) | |
| Tumor site | 0.002 | ||
| Cardia Siewert II | 16 (13.3) | 0 (0) | |
| Cardia Siewert III | 32 (26.7) | 5 (12.2) | |
| Corpus/fundus | 72 (60) | 36 (87.8) | |
| Surgical acess | 0.693 | ||
| Open | 108 (90) | 36 (87.9) | |
| Laparoscopic | 12(10) | 5 (12.1) | |
| Combined resection of other organs | 0.789 | ||
| No | 96 (80) | 32 (78) | |
| Yes | 24 (20) | 9 (22) | |
| Grade of Postoperative complication | 0.867 | ||
| 0-I-II | 98 (81.7) | 33 (80.5) | |
| III-IV | 22 (18.3) | 8 (19.5) | |
| Hospital length of stay, mean ± SD | 16.1 (9.1) | 20.7 (17.9) | 0.120 |
| Neoadjuvant / adjuvant chemotherapy | < 0.001 | ||
| No | 43 (38.5) | 29 (70.7) | |
| Yes | 77 (64.5) | 12 (29.3) | |
| Mortality | |||
| 30 d | 7 (5.8) | 2 (4.9) | 1.0 |
| 90 d | 10 (8.3) | 5 (12.2) | 0.535 |
Pathological characteristics are shown in Table 2. There was no difference related to Lauren type, the grade of differentiation, and lymphatic/vascular/perineural invasion. Category pathological (p)T, pN, and final pTNM stage were also similar between the groups. RGC had a significantly lower number of retrieved LN than the PGC group (43.2 vs 26; P < 0.001), but there was no difference regarding the LN ratio categories between RGC and PGC. Among the eight RGC patients with small bowel mesenteric LNs dissected, the presence of metastasis was observed in two cases: One case with one LN+ and the other with five LN+.
| Variables | PGC, n = 120 (%) | RGC, n = 41 (%) | P value |
| Tumor size, mean ± SD | 5.1 (3.4) | 5.3 (3.4) | 0.785 |
| Lauren type | 0.302 | ||
| Intestinal | 68 (56.7) | 27 (65.9) | |
| Diffuse/mixed | 52 (43.3) | 14 (34.1) | |
| Grade of histological differentiation | 0.256 | ||
| Well/moderately differentiated | 55 (45.8) | 23 (56.1) | |
| Poorly differentiated | 65 (54.2) | 18 (43.9) | |
| Lymphatic invasion | 0.484 | ||
| No | 51 (42.5) | 20 (48.8) | |
| Yes | 69 (57.5) | 21 (51.2) | |
| Venous invasion | 0.069 | ||
| No | 75 (62.5) | 32 (78) | |
| Yes | 45 (37.5) | 9 (22) | |
| Perineural invasion | 0.602 | ||
| No | 50 (41.7) | 19 (46.3) | |
| Yes | 70 (58.3) | 22 (53.7) | |
| pT | 0.842 | ||
| pT1/T2 | 46 (38.3) | 15 (36.6) | |
| pT3/T4 | 74 (61.7) | 26 (63.4) | |
| Number of resected LNs, mean ± SD | 43.2 (20.8) | 26 (14) | < 0.001 |
| LN metastasis | 0.204 | ||
| pN0 | 45 (37.5) | 20 (48.8) | |
| pN+ | 75 (62.5) | 21 (51.2) | |
| Lymph node ratio | 0.915 | ||
| LR0 | 76 (63.3) | 25 (61) | |
| LR1 | 21 (17.5) | 9 (22) | |
| LR2 | 11 (9.2) | 4 (9.8) | |
| LR3 | 12 (10) | 3 (7.3) | |
| pTNM | 0.773 | ||
| I | 36 (30) | 13 (31.7) | |
| II | 27 (22.5) | 11 (28.8) | |
| III | 57 (47.5) | 17 (41.5) | |
| Adjuvant chemotherapy | < 0.001 | ||
| No | 45 (37.5) | 29 (70.7) | |
| Yes | 71 (62.5) | 12 (29.3) | |
| Recurrence | 0.607 | ||
| No | 87 (72.5) | 28 (68.3) | |
| Yes | 33 (27.5) | 13 (31.7) | |
| Pattern of recurrence1 | 0.322 | ||
| Locoregional | 11 (33.3) | 5 (38.5) | |
| Distant | 12 (36.4) | 5 (38.5) | |
| Peritoneal | 14 (42.4) | 8 (61.5) | |
After a mean follow-up of 32.1 mo, there were 33 recurrences in the PGC groups and 13 in the RGC group. At the time of this study, 70 patients died (18 in RGC and 52 in the PGC group). The DFS and OS rate for PGC was 64.3% and 48.4%, respectively. In RGC patients, DFS and OS rate was 61.3% and 49.3%, respectively. The median OS for the entire cohort was 58.5 mo.
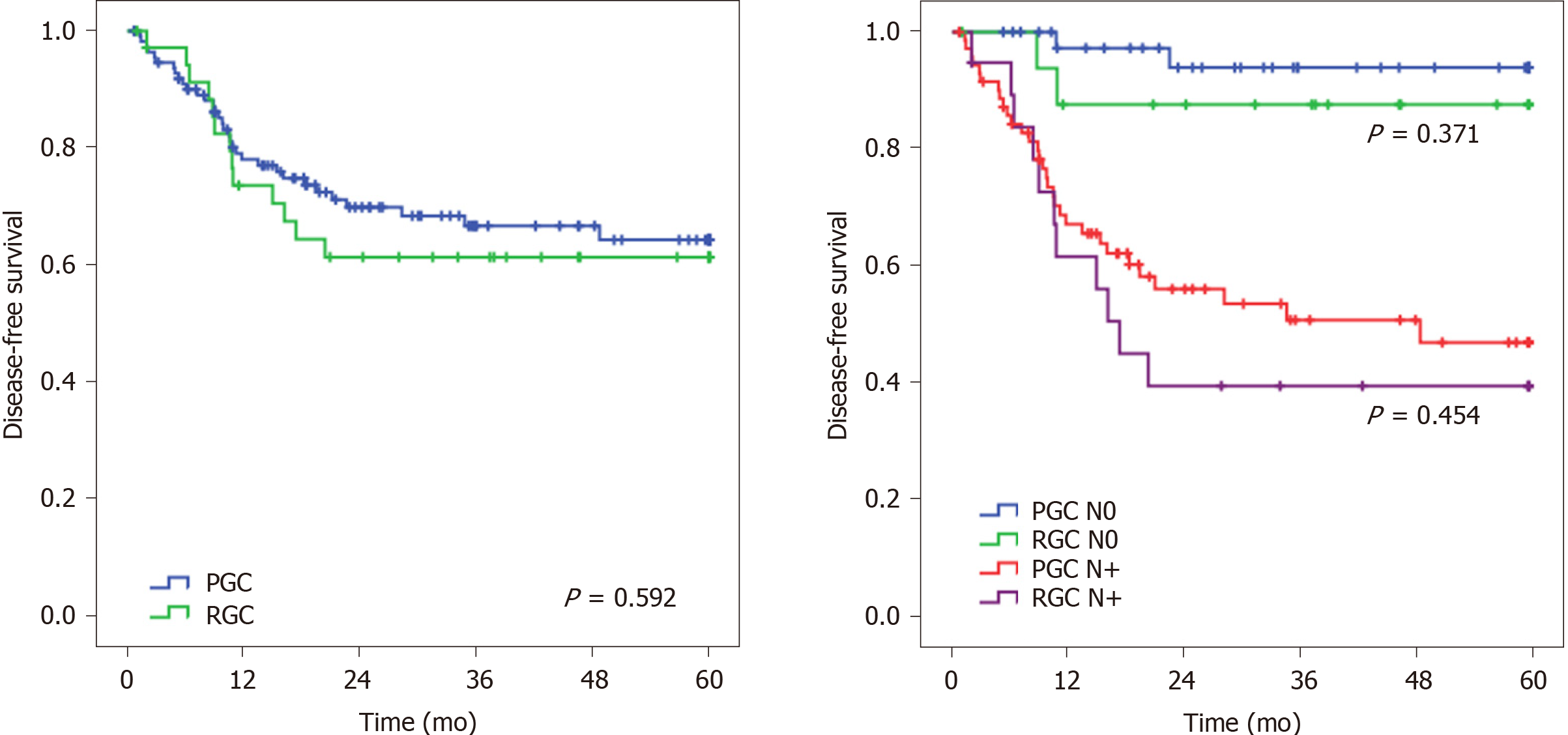
The DFS rate was similar between RGC and PGC patients (P = 0.592) (Figure 1). When adjusted for the pN stage, there was no statistical difference in DFS between pN0 RGC and PGC (P = 0.371) and between pN+ RGC and pN+ PGC groups (P = 0.454). Regarding the recurrence site, the most common site was peritoneal, particularly for RGC (61.5% vs 42.4%). Locoregional recurrence was similar between RGC and PGC groups (33.3% vs 38.5%, respectively; P = 0.322).
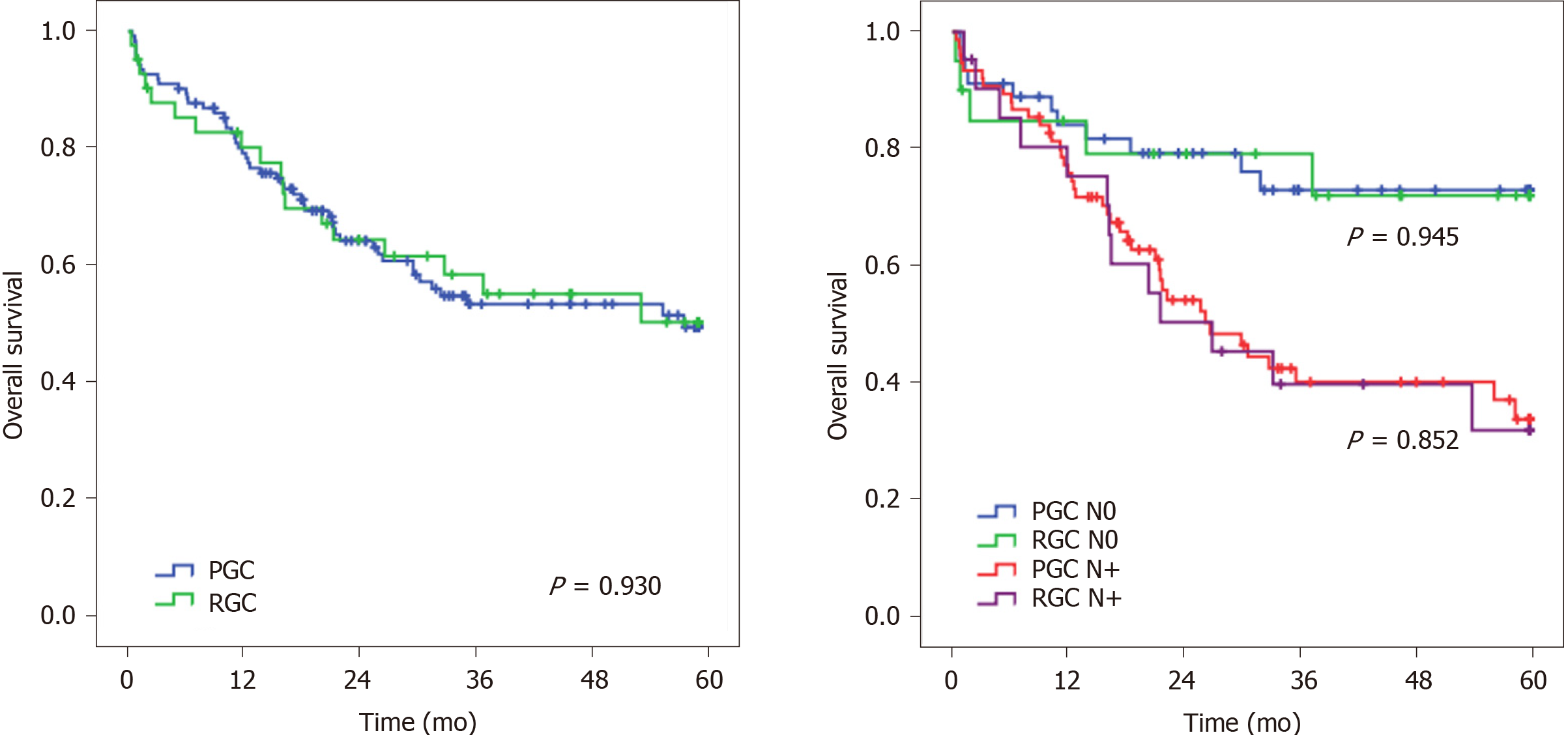
Considering the OS, no significant difference was observed between RGC and PGC patients (P = 0.930), even when adjusted for pN status (P = 0.945 and P = 0.852 for pN0 and pN+ status, respectively) (Figure 2).
After adjusting for significant predictors of survival; tumor size, depth of tumor invasion, LN status, and CMT were associated with DFS in univariate analysis. In the multivariate analysis, only LN status remained a factor related to survival. Tumor size, pT, and pN status were associated with OS in univariate analyses. In the multivariate model, LN status was the only independent factor associated with OS. RGC was not a prognostic factor for both DFS and OS compared to PGC (Table 3).
| Disease-free survival | Univariate | Multivariate | Overall survival | Univariate | Multivariate | ||||||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value | Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Female (vs male) | 0.78 | 0.41-1.48 | 0.445 | - | - | - | Female (vs male) | 1.13 | 0.66-1.93 | 0.658 | - | - | - |
| Age ≥ 65 (vs < 65 yr) | 0.85 | 0.47-1.51 | 0.570 | - | - | - | Age ≥ 65 (vs < 65 yr) | 1.19 | 0.74-1.90 | 0.467 | - | - | - |
| ASA III/IV (vs I/II) | 0.97 | 0.50-1.88 | 0.972 | - | - | - | ASA III/IV (vs I/II) | 1.51 | 0.92-2.48 | 0.101 | - | - | - |
| Diffuse/mixed type (vs others) | 1.43 | 0.80-2.54 | 0.228 | - | - | - | Diffuse/mixed type (vs others) | 1.18 | 0.73-1.89 | 0.495 | - | - | - |
| Tumor size ≥ 4.0 cm (vs < 4.0 cm) | 4.41 | 1.97-9.87 | < 0.001 | 2.00 | 0.80-4.98 | 0.138 | Tumor size ≥ 4.0 cm (vs < 4.0 cm) | 2.12 | 1.24-3.63 | 0.006 | 1.35 | 0.72-2.53 | 0.351 |
| pT3/pT4 status (vs pT1/pT2) | 4.97 | 2.11-11.73 | < 0.001 | 1.89 | 0.69-5.14 | 0.215 | pT3/pT4 status (vs pT1/pT2) | 2.29 | 1.31-4.01 | 0.004 | 1.38 | 0.70-2.72 | 0.347 |
| pN+ (vs pN0) | 9.21 | 3.30-25.73 | < 0.001 | 5.79 | 1.92-17.45 | 0.002 | pN+ (vs pN0) | 2.75 | 1.57-4.81 | < 0.001 | 2.10 | 1.12-3.96 | 0.021 |
| non-CMT vs (CMT) | 0.48 | 0.25-0.92 | 0.028 | 1.25 | 0.62-2.53 | 0.530 | non-CMT vs (CMT) | 0.96 | 0.60-1.55 | 0.867 | - | - | - |
| RGC (vs PGC) | 1.91 | 0.63-2.26 | 0.593 | - | - | - | RGC (vs PGC) | 0.98 | 0.57-1.67 | 0.930 | - | - | - |
Gastric resection for benign disease, commonly performed until the late 1980s, contributed to the emergence of patients at risk of developing tumors in the gastric remnant. Due to its rarity and diversity, the characteristics of RGC, as well as the prognostic and survival factors related to this neoplasm, remain uncertain. Therefore, in the present study, we report a cohort of 41 RGC patients treated at a single institution to provide more information about this type of disease.
Among patients treated with curative intent, the frequency of RGC due to benign disease in our series was 6.6%. As tumor behavior and characteristics may be different according to the tumor location, we only selected primary PGC as the comparison group to avoid this bias. In general, the RGC group presented characteristics that resemble PGC patients[22]. Also, despite the expected lower number of retrieved LN, there was no difference in survival between the RGC and PGC groups, which suggest that both GC groups have a similar prognosis.
After previous gastric resection for malignant disease, the cumulative carcinogenic effect on the gastric mucosa is maintained[2]. For this reason patients with the previous gastrectomy for cancer develop RGC in a significantly shorter period than patients with previous benign lesions[7,10,11,23]. Conversely, after gastric resection for benign disease, the time required for the changes in the remnant gastric mucosa lead to the development of RGC is more than 20 years[10,23-28]. Following this evidence, we found a mean time from previous resection of 36.4 years. Besides, the older age-related to the RGC group seems to reflect the long period of inflammatory gastritis needed to induce carcinogenesis in the gastric mucosa.
Indeed, whether the changes that occur in the gastric mucosa after previous gastrectomy actually lead to a higher incidence of GC in the remaining stomach, or whether they only reflect the normal risk of GC in the general population, is still under discussion. This discrepancy in reports could result from difference in incidence rates of GC in the general population from different countries. Regions with a low incidence of GC tend to have a higher proportion of RGC than PGC compared to regions with a high incidence of GC. Hanyu et al[7] reported an incidence of 5.4% of RGC with Billroth I reconstruction after 20 years, similar to the incidence of primary GC in the Japanese population. In contrast, Lagergren et al[9] found an increased risk of RGC after 30 years in the low-risk Swedish population.
The relation between the type of reconstruction and risk of RGC remains uncertain. Billroth I reconstruction keeps the flow of ingested food from the remnant stomach into the duodenum, but due to the resection of the pylorus, the duodenal-gastric biliary reflux is increased[4]. Billroth II (BII) reconstruction drives the inflow of bile from the afferent jejunal limb into the remnant stomach. The constant flow makes alkaline gastritis more common and severe after BII. This leads to mucosal inflammation and regeneration, which may be associated with a higher risk of RGC. Despite some reports in the literature, this association is still not a consensus[7,10,11,27,28]. Conversely, Roux-en-Y reconstruction avoids biliary reflux to the remnant stomach, but it is seldom performed for benign resections. In our series, all cases had previous BII reconstruction. It must be emphasized that it is the common practice in our country to use BII for reconstruction after benign disease and Roux-en-Y for malignant disease. Therefore, the association of BII with RGC probably reflects a habit of reconstruction rather than a relationship of cause and effect in our cohort.
We found that 87% of RGC cases had the tumor at the anastomotic site. Again, this predisposition may be attributed to the closer contact of the previous anastomosis with the biliary reflux[6,10]. The PGC group had a higher proportion of cardia tumors. It has been suggested that RGC from the non-anastomotic sites may have different behavior than the ones on the anastomotic line; but due to the low number of cases, we could not verify this hypothesis[8,11,29].
Intestinal adenocarcinoma is more commonly associated with multistep carcinogenesis. Meanwhile, the diffuse type is considered to have a direct carcinogenesis pathway sometimes related to inherited germline mutations[2]. Considering the continuous aggression of the biliary reflux causing chronic inflammation, we expected a higher proportion of the intestinal type in RGC patients. However, there was no difference between the groups, which represents an interesting finding that has already been described in other studies[25,28].
As in PGC, surgery resection with regional D2 lymphadenectomy is the cornerstone of RGC treatment. Adhesion to adjacent organs and displacement of anatomical structures are common challenges during the surgery, making it longer and more likely to combine other organs during repair or resection[10,23,27,30]. However, it remains uncertain whether RGC resection has higher postoperative morbidity and mortality rates. Usually, surgical procedure is performed by the conventional open approach, but recently minimally invasive laparoscopic and robotic approaches have been increasing[13,31].
It has been suggested that the characteristics of LN metastasis in RGC are different due to the interruption of the lymphatic pathway in the first procedure. The type of reconstruction and the previous indication of the first gastrectomy do not seem to influence the incidence of LN metastasis but its station location[23]. This may lead to more involvement of the splenic artery, splenic hilum, lower mediastinum, and jejunal mesentery LN[6,23,32]. However, the standard extension of lymphadenectomy is not yet defined. Similar to PGC, splenic hilum lymphadenectomy is indicated only if the tumor invades the greater curvature[33]. LN metastasis in the jejunal mesentery has a poor prognosis[6,10]. It is known that extended lymphadenectomy in the area can severely affect the postoperative quality of life. Therefore, the extent of mesentery lymphadenectomy should be determined based on the extent of LN involvement, considering a balance between risk and benefit[6,14]. As expected, in our series the number of retrieved LN was smaller in RGC than in PGC. This is a common finding due to the absence of the distal stomach altogether with its LN stations. Furthermore, in RGC cases, the higher frequency of older patients with more comorbidities and the non-definition of the standard lymphadenectomy may lead to a higher proportion of D1 lymphadenectomy[6,10].
Even though the TNM system is applied to all GC, the staging system for RGC has not been established. For proper pTNM staging, it is recommended to retrieve at least 15 LN to avoid under-staging. In an attempt to overcome this limitation, the proposal of LN staging bases on LN ratio has been suggested[34,35]. LN ratio is determined by dividing the number of metastatic LN by the number of retrieved LN. However, differences in cut-off values for the ratio and inconclusive results in studies have limited its use[18,34]. As the number of retrieved LNs is expected to be insufficient to determine the pN stage in some RGC patients, we also analyzed the groups using the LN ratio, but no differences were found.
Commonly, some studies associate RGC with a diagnosis in more advanced stages[13,28]. As these patients are used to previous symptoms of the peptic disease and post-gastrectomy alkaline gastritis, it has been suggested that they may delay seeking medical care. Another factor that may lead to late diagnosis is the limited access to surveillance endoscopy in developing countries[11,13]. However, we did not find a higher proportion of advanced pathological stages or tumor with higher dimensions. The fact that the main site of RGC was at the anastomosis line may have impaired the ingestion of food, worsening their usual complaints.
DFS and OS did not differ between groups, even considering that patients in PGC had greater adherence to CMT treatment compared to RGC. This probably occurred due to the advanced age of patients in the RGC group, since the staging and frequency of POC were similar compared with PGC[36]. To elucidate further the impact on survival, we also evaluated survival concerning the presence of LN metastasis. This was a planned pre-analysis decision due to the previously explained concern regarding the change of the lymphatic flow after the prior surgery. Therefore, patients with positive LNs could have a higher risk of aberrant spread outside the field of usual lymphadenectomy, including the jejunal mesentery. However, DFS did not differ between groups, and the pattern of site recurrence was similar between the groups. There is relatively sparse data on survival, and many studies include previous gastric resection for neoplasia. Our results are consistent with other reports that demonstrated a similar survival outcome of RCG after the previous gastrectomy for a benign disease compared to PGC[11,13,23,28].
A retrospective study has inherent drawbacks related to its design. The date of the first procedure sometimes is not accurate. We only included patients submitted to surgical resection. Therefore, RGC patients referred exclusively to palliative treatment were not included, and we do not know if survival would be different in this setting. Finally, some analyses were not possible due to the insufficient number of patients. Therefore, multicentric studies or studies with a larger number of patients and long-term follow-up will be needed to confirm our findings and ensure external validation of results.
As for strengths, to our knowledge, this is the largest series of RGC from a single Western institution for the past 10 years, which included only previous gastric resection due to benign disease. Other Western reports have more cases; however, they are multicenter studies and had an inclusion period of more than 20 years. This may affect the outcome analyses, since recent improvements in adjuvant treatments as well as perioperative care were not yet incorporated[10,27,28,30,37]. To ensure the homogeneity of the study population, we excluded patients with previous resection for malignant disease. Even if we consider it as a de novo tumor after 5 years of the previous resection, we believe that the inclusion of these patients would bias the comparison with PGC; especially since in these cases, cancer probably originates from the same precancerous condition that had already existed before the initial operation. Moreover, the previous lymphadenectomy would change the pattern of dissemination[7,10].
RGC had similar clinicopathological characteristics to primary PGC. Despite the lower number of resected LN, RGC had also a similar prognosis than PGC patients. Considering the number of gastric resections performed to treat benign peptic disease in the past, and the long period of carcinogenesis required for tumor development, it is expected that surgeons will still encounter RGC patients for the foreseeable future.
Remnant gastric cancer (RGC), defined as a tumor that develops in the stomach after a previous gastrectomy, is often reported as a tumor with a poor prognosis. However, due to its rarity and diversity, factors related to its prognosis and survival remain unclear.
The occurrence of RGC continues to be part of the reality of gastric cancer treatment, due to peptide ulcer resections performed in the past. Most studies that have investigated the surgical treatment of RGC enrolled only a few patients and provided only a brief descriptive analysis of their complications.
This study aimed to evaluate the clinicopathological characteristics and prognosis of RGC after previous gastrectomy for benign disease.
All patients who underwent gastrectomy between 2009 and 2019 were retrospectively evaluated from a prospective medical database. RGC resections with histological diagnosis of gastric adenocarcinoma were enrolled in this study. Primary proximal GC (PGC) who underwent total gastrectomy was selected as the comparison group.
A total of 41 RGC patients were included, and 120 PGC served as a comparison group. Despite presenting differences about some clinical characteristics, there was no significant difference between RGC and RGC patients concerning pathological tumor-node-metastasis and the occurrence of postoperative complications. Also, the survival rates of RGC were similar to the PGC group.
RGC had clinicopathological characteristics and prognosis similar to PGC, including short-term outcomes.
Our findings provide additional data about the characteristics and outcomes of the RGC that may assist to clarify factors related to the survival of these patients. As we suggest that RGC does not adversely affect patient prognosis and postoperative course, the inclusion of these patients in future trials could contribute to data that improve the survival of patients with RGC.
Manuscript source: Invited manuscript
Corresponding author's membership(s) in professional societies: American College of Surgery; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract; International Gastric Cancer Association; Brazilian Society of Oncological Surgery; and Brazilian Association of Gastric Cancer.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ammendola M, Taira K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50811] [Article Influence: 8468.5] [Reference Citation Analysis (44)] |
| 2. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 3. | Ramos MFKP, Ribeiro Júnior U, Viscondi JKY, Zilberstein B, Cecconello I, Eluf-Neto J. Risk factors associated with the development of gastric cancer - case-control study. Rev Assoc Med Bras (1992). 2018;64:611-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kondo K. Duodenogastric reflux and gastric stump carcinoma. Gastric Cancer. 2002;5:16-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Safatle-Ribeiro AV, Ribeiro Júnior U, Sakai P, Iriya K, Ishioka S, Gama-Rodrigues J. Gastric stump mucosa: is there a risk for carcinoma? Arq Gastroenterol. 2001;38:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 55] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Hanyu T, Wakai A, Ishikawa T, Ichikawa H, Kameyama H, Wakai T. Carcinoma in the Remnant Stomach During Long-Term Follow-up After Distal Gastrectomy for Gastric Cancer: Analysis of Cumulative Incidence and Associated Risk Factors. World J Surg. 2018;42:782-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Chowdappa R, Tiwari AR, Ranganath N, Kumar RV. Is there difference between anastomotic site and remnant stump carcinoma in gastric stump cancers? J Gastrointest Oncol. 2019;10:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lagergren J, Lindam A, Mason RM. Gastric stump cancer after distal gastrectomy for benign gastric ulcer in a population-based study. Int J Cancer. 2012;131:E1048-E1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Di Leo A, Pedrazzani C, Bencivenga M, Coniglio A, Rosa F, Morgani P, Marrelli D, Marchet A, Cozzaglio L, Giacopuzzi S, Tiberio GA, Doglietto GB, Vittimberga G, Roviello F, Ricci F. Gastric stump cancer after distal gastrectomy for benign disease: clinicopathological features and surgical outcomes. Ann Surg Oncol. 2014;21:2594-2600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Huang H, Wang W, Chen Z, Jin JJ, Long ZW, Cai H, Liu XW, Zhou Y, Wang YN. Prognostic factors and survival in patients with gastric stump cancer. World J Gastroenterol. 2015;21:1865-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Kwon IG, Cho I, Choi YY, Hyung WJ, Kim CB, Noh SH. Risk factors for complications during surgical treatment of remnant gastric cancer. Gastric Cancer. 2015;18:390-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kwon IG, Cho I, Guner A, Choi YY, Shin HB, Kim HI, An JY, Cheong JH, Noh SH, Hyung WJ. Minimally invasive surgery for remnant gastric cancer: a comparison with open surgery. Surg Endosc. 2014;28:2452-2458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Irino T, Hiki N, Ohashi M, Nunobe S, Tokunaga M, Sano T, Yamaguchi T. Characteristics of gastric stump cancer: A single hospital retrospective analysis of 262 patients. Surgery. 2016;159:1539-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Doyle DJ, Goyal A, Bansal P, Garmon EH. American Society of Anesthesiologists Classification. 2020 Jul 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. [PubMed] [Cited in This Article: ] |
| 16. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32099] [Cited by in F6Publishing: 34912] [Article Influence: 943.6] [Reference Citation Analysis (0)] |
| 17. | Zilberstein B, Malheiros C, Lourenço LG, Kassab P, Jacob CE, Weston AC, Bresciani CJ, Castro O, Gama-Rodrigues J; Grupo do Consenso; Borin AA; Buchpiegel C; Montagnini A; Leite CV; Deutsch CR; Kruel CD; Mucrino D; Wohnrath D; Ilias E; Mrué F; Maluf-Filho F; Rocha F; de Souza F; Tomasich FS; Ishak G; Laporte G; de Souza HP; Cecconello I; Eisig J; Ohana J; Sabagga J; del Grande JC; de Jesus JP; Soares J; Dias LA; Moreira LF; Correa M; Carvalho M; Andreollo NA; Áquila ND; Czeczko NG; Kruel N; Forones NM; da Motta OM; Malafaia O; Assumpção P; Leonardi P; Sakai P; Rocha PR; Colleoni R; Gurgel R; Coral RP; Chalub S; Ribeiro- Junior U; Alves VA; Vasquez Vde L; Nadalin V; Brazilian Association of Gastric Cancer. Brazilian consensus in gastric cancer: guidelines for gastric cancer in Brazil. Arq Bras Cir Dig. 2013;26:2-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Deng J, Liang H, Wang D, Sun D, Ding X, Pan Y, Liu X. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol. 2010;17:1043-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 702] [Cited by in F6Publishing: 801] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1793] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 21. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18532] [Cited by in F6Publishing: 21888] [Article Influence: 1094.4] [Reference Citation Analysis (0)] |
| 22. | Ramos MFKP, Pereira MA, Yagi OK, Dias AR, Charruf AZ, Oliveira RJ, Zaidan EP, Zilberstein B, Ribeiro-Júnior U, Cecconello I. Surgical treatment of gastric cancer: a 10-year experience in a high-volume university hospital. Clinics (Sao Paulo). 2018;73:e543s. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Shimada H, Fukagawa T, Haga Y, Oba K. Does remnant gastric cancer really differ from primary gastric cancer? Gastric Cancer. 2016;19:339-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Tokunaga M, Sano T, Ohyama S, Hiki N, Fukunaga T, Yamada K, Yamaguchi T. Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancer. J Gastrointest Surg. 2013;17:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Kitajima M; Society for the Study of Postoperative Morbidity after Gastrectomy. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Song XH, Liu K, Sun LF, Chen XL, Zhao LY, Zhang WH, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Clinicopathological characteristics and prognostic factors of remnant gastric cancer: A single-center retrospective analysis of 90 patients. Int J Surg. 2018;51:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Mezhir JJ, Gonen M, Ammori JB, Strong VE, Brennan MF, Coit DG. Treatment and outcome of patients with gastric remnant cancer after resection for peptic ulcer disease. Ann Surg Oncol. 2011;18:670-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Costa-Pinho A, Pinto-de-Sousa J, Barbosa J, Costa-Maia J. Gastric stump cancer: more than just another proximal gastric cancer and demanding a more suitable TNM staging system. Biomed Res Int. 2013;2013:781896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Sasaki K, Fujiwara Y, Kishi K, Motoori M, Yano M, Ohigashi H, Ohue M, Noura S, Maruhashi S, Takahashi H, Gotoh K, Shingai T, Yamamoto T, Tomita Y, Ishikawa O. Pathological findings of gastric mucosa in patients with gastric remnant cancer. Hepatogastroenterology. 2014;61:251-254. [PubMed] [Cited in This Article: ] |
| 30. | St-Louis E, Gowing SD, Mossallanejad P, Leimanis ML, Mueller C, Ferri LE. Outcomes after completion total gastrectomy for gastric remnant cancer: experience from a Canadian tertiary centre. Can J Surg. 2018;61:270-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Booka E, Kaihara M, Mihara K, Nishiya S, Handa K, Ito Y, Shibutani S, Egawa T, Nagashima A. Laparoscopic total gastrectomy for remnant gastric cancer: A single-institution experience. Asian J Endosc Surg. 2019;12:58-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Iguchi K, Kunisaki C, Sato S, Tanaka Y, Miyamoto H, Kosaka T, Akiyama H, Endo I, Rino Y, Masuda M. Evaluation of Optimal Lymph Node Dissection in Remnant Gastric Cancer Based on Initial Distal Gastrectomy. Anticancer Res. 2018;38:1677-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Watanabe M, Kinoshita T, Morita S, Yura M, Tokunaga M, Otsuki S, Yamagata Y, Kaito A, Yoshikawa T, Katai H. Clinical impact of splenic hilar dissection with splenectomy for gastric stump cancer. Eur J Surg Oncol. 2019;45:1505-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Son SY, Kong SH, Ahn HS, Park YS, Ahn SH, Suh YS, Park DJ, Lee HJ, Kim HH, Yang HK. The value of N staging with the positive lymph node ratio, and splenectomy, for remnant gastric cancer: A multicenter retrospective study. J Surg Oncol. 2017;116:884-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Nakagawa M, Choi YY, An JY, Hong JH, Kim JW, Kim HI, Cheong JH, Hyung WJ, Choi SH, Noh SH. Staging for Remnant Gastric Cancer: The Metastatic Lymph Node Ratio vs. the UICC 7th Edition System. Ann Surg Oncol. 2016;23:4322-4331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Ramos MFKP, de Castria TB, Pereira MA, Dias AR, Antonacio FF, Zilberstein B, Hoff PMG, Ribeiro U Jr, Cecconello I. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J Gastrointest Surg. 2020;24:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Tran TB, Hatzaras I, Worhunsky DJ, Vitiello GA, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber S, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Norton JA, Poultsides GA. Gastric remnant cancer: A distinct entity or simply another proximal gastric cancer? J Surg Oncol. 2015;112:877-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |