Published online May 15, 2017. doi: 10.4239/wjd.v8.i5.187
Peer-review started: October 31, 2016
First decision: January 14, 2017
Revised: February 13, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Diabetes mellitus (DM) is the third most common non-infectious disease leading to early disability and high mortality. Moreover, the number of patients is growing every year. The main symptom of DM is hyperglycemia. Increased levels of blood glucose activate polyol, hexosamine, and protein kinase metabolic pathways cause the intensification of non-enzymatic glycosylation and nitration of macromolecules. This, in turn, leads to the development of oxidative and nitrative stresses and secondary complications, such as different kinds of micro- and macroangiopathies. Metabolic disorders caused by insulin deficiency in diabetes significantly impede the functioning of a homeostasis system, which change the physical, biochemical, morphological, and functional properties of blood cells. As a result, the oxygen-transport function of red blood cells (RBCs), rheological properties of the blood, and functions of immunocompetent cells as well as the process of apoptosis are primarily affected. Modern pharmacotherapy focuses on the search for new preparations that aim to decrease blood glucose levels. Undesirable side effects and adverse reactions caused by synthetic medicines led to the search and investigation of new preparations of natural origin. Medicinal mushrooms play an important role among such new preparations. They are a source of a large number of high- and low-molecular compounds with pronounced biological effects. Our investigations show pronounced hypoglycemic and anti-anemic action of submerged cultivated mycelium powder of medicinal mushrooms Agaricus brasiliensis (A. brasiliensis) and Ganoderma lucidum (G. lucidum) on streptozotocin-induced DM in rats. Also, we showed that mycelium powders have membrane protective properties as evidenced by the redistribution of RBC populations towards the growth of full functional cell numbers. Normalization of parameters of leukocyte formula and suppression of apoptosis of white blood cells in diabetic rats treated with A. brasiliensis and G. lucidum mycelia indicates pronounced positive effects of these strains of mushrooms. Thus, the use of medicinal mushrooms for treatment of DM and in prevention development of its secondary complications might be a new effective approach of this disease’s cure. This article is aimed at summarizing and analyzing the literature data and basic achievements concerning DM type 1 treatment using medicinal mushrooms and showing the results obtained in our research.
Core tip: Diabetes mellitus (DM) is the third most common non-infectious disease leading to early disability and high mortality. The main symptom of DM is hyperglycemia. Metabolic disorders caused by insulin deficiency in diabetes significantly impede the functioning of a homeostasis system. Medicinal mushrooms play an important role among new preparations that aim to decrease blood glucose levels. This investigations show pronounced hypoglycemic, anti-anemic, membrane protective, apoptosis suppressive effects of Agaricus brasiliensis and Ganoderma lucidum on streptozotocin-induced DM in rats. Thus, medicinal mushrooms might be new effective approach for treatment and prevention of DM.
- Citation: Vitak T, Yurkiv B, Wasser S, Nevo E, Sybirna N. Effect of medicinal mushrooms on blood cells under conditions of diabetes mellitus. World J Diabetes 2017; 8(5): 187-201
- URL: https://www.wjgnet.com/1948-9358/full/v8/i5/187.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i5.187
According to the World Health Organization, diabetes mellitus (DM) is a chronic disease caused by the diminished secretion of insulin, the hormone produced by the pancreas and regulating the level of glucose in the blood, or by increasing resistance of the body’s cells to this hormone[1].
According to International Diabetes Federation data, in 2015 there was 415 million of people suffering from diabetes worldwide. Almost half of them are men aged 40 to 59 (7% more than women). If the tendency for diabetes continues to grow, the number of people suffering from this disease will reach 642 million people in 2040. It should also be noted that about 80% of diabetics live in low- and middle-income countries[2].
There are three main types of DM: Type 1 diabetes (T1D) (previously known as insulin-dependent or juvenile-onset; accounting for 3%-10% of cases); type 2 diabetes (T2D) (previously known as non-insulin-dependent or adult-onset; accounting for 85%-90% of cases); and gestational diabetes (hyperglycemia occurring during pregnancy and usually resolving itself after delivery; accounting for 2%-5% of cases)[3]. Also, there are other specific types of diabetes with unknown etiology[4].
Although the percentage incidence of T1D is relatively small (e.g., compared with T2D), it is common in children and adolescence. This sets a particular danger associated with early disability and increases the rates of lethal cases[5]. T1D results from a selective destruction of pancreatic β-cells caused by cytotoxic lymphocytes, T-helpers and autoantibodies. The progressive destruction of β-cells results in insulin deficiency, which leads to dyscrasia and diabetes[6]. The etiology of insulin-dependent diabetes has not been fully examined. T1D is considered to be caused by an interaction between genetic and environmental factors among which include viruses and genetic mutations[7]. It is observed that some viruses can trigger autoimmune processes, promote them, or assume both roles simultaneously[4]. Some scientists consider that one or more viruses can also cause T1D among individuals with genetic susceptibility. The scientists presume that in the presence of infection a virus protein similar to β-cells protein is produced. T-cells and antibodies to foreign proteins attack the native proteins of pancreatic β-cells[4]. A range of viruses exists which share these properties, for instance, intestinal Coxsackie viruses. Accordingly, autoimmune processes contribute to the destruction of the islets of Langerhans that leads to diabetes. Yet, at this stage, genetic damages play a main role in causing autoimmune processes that result in T1D. Scientific researches reveal that insulin-dependent DM is inflicted not by a violation of any particular section of the genome, but by complex damage. Genes in Human Leucocyte Antigens-locus or genes of the major histocompatibility complex are responsible for T1D. The development of T1D is associated with genetic determination. Antigens of the major histocompatibility complex account for the susceptibility to disease with various immune responses[8]. Genetic investigations indicate that type 1 DM is strongly associated with at least 18 loci[9]. However, the precise mechanisms that lead to autoimmune destruction of β-cells in T1D remain poorly understood. According to modern concepts of the pathogenesis of the disease, the destruction of β-cells can occur as a result of various pathological processes such as necrosis and/or apoptosis[10]. Cytotoxic T lymphocytes are considered to be a main factor that induces the destruction of β-cells; their production is a crucial factor for this pathology. Most autoreactive T-cells of Langerhans islets produce cytokines that are characteristic of T × 1-lymphocytes, particularly, a tumor necrosis factor alpha, interferon gamma, IL-2. The latter is known to trigger a cellular immune response in which cytotoxic effectors T lymphocytes attack β-cells and induce antigen specific T-dependent immune lysis. The development of type 1 DM depends not only on the presence of autoreactive T-lymphocytes, but also on control mechanisms that ensure the maintenance of immune tolerance or inhibition of peripheral autoimmune processes[11].
The mechanism of β-cells destruction is complicated, slow and gradual, and the consecution of pathogenetic processes is not fully elucidated. However, when insulin deficiency becomes close to absolute (in which approximately 85% of β-cells are destroyed), it induces severe metabolic disorders and a clinical stage of disease occurs[12].
Thus, DM is still one of the main problems of the 21st century. The search of new, safe and available for most of people approaches in its treatment is relevant trend of investigation.
Red blood cells (RBCs) are the largest blood fraction, with unique cells lacking nuclei in the shape of biconcave disks[13]. The main function of the cells is to transport oxygen by means of hemoglobin from the lungs to all organs and tissues and then transport CO2 in the opposite direction. In adults, RBCs develop in the bone marrow and circulate for about 120 d in the bloodstream before they are eliminated by Kupffer’s cells of the liver and spleen. This process is slow and multistage and is controlled by hormones (e.g., erythropoietin) and cytokines. RBCs enter in the bloodstream at the stage of the reticulocyte. Reticulocytes do not have nuclei, however, they have a residual protein synthesis apparatus. They account for about 1%-2% of circulating RBCs and are transformed into mature cells within 24-48 h[14].
Metabolic disorders caused by insulin deficiency under DM considerably change physical, biochemical, morphological, and functional properties of RBCs. The membrane as a single structural element of these cells plays an important role in the maintenance of the cell’s stable form and provides its functional properties. Under DM, profound changes in the RBC membrane structure, which affect physicochemical properties of cells, are brought about. Thus, lipid exchange is disturbed, and the molecular architectonic of the red cell membrane lipid bilayer is changed[15]. It has been revealed that the cholesterol level rises due to a nonspecific exchange mechanism with plasma cholesterol, which is higher in diabetics[16]. The level of phospholipids with increased content of unsaturated fatty acids in RBC membranes also rises[17]. These changes lead to a decrease in membrane fluidity, which corresponds to enhanced lipid-lipid interactions, decreased protein-lipid interactions, and increased protein-water interactions resulting in displacement of proteins towards the membrane surface. Moreover, the cholesterol level is considered to affect the transport of substances via the membrane[16].
Under DM, the membrane’s total protein content (glycoproteins in particular) is shown to decrease, whereas the activity of sialidases increases[18]. Increased activity of these enzymes, in turn, causes a decrease in sialic acids on RBC surfaces. As a result, a superficial negative charge of the cells changes, aggregation properties increase, and their deformability decreases thus increasing blood viscosity, complicating its flow through microcirculation and acting as a precursor of diabetic complications.
Due to hyperglycemia, the processes of non-enzymatic glycosylation of red cell membranes and cytosol proteins are activated. An increase in glycosylated hemoglobin (HbA1c) affects the efficiency of the oxygen-transport function of RBCs. HbA1c has an enhanced affinity to O2, which complicates its return to cells in microcirculation[19]. That, in turn, promotes the development of tissue hypoxia. There is an enhanced level of fetal hemoglobin (HbF) in RBCs under the condition of diabetes. Such changes are compensatory, providing a better supply of oxygen to tissues under DM, as HbF is able to bind oxygen with greater affinity and return it at much less partial pressure[20].
In recent years, the role of RBCs and hemoglobin has been intensively discussed both as inhibitors and activators of nitric oxide (NO)-dependent signaling in NO metabolism[21]. Previously, it had been believed that RBCs fulfill solely the role of nitric oxide scavengers and its depot. However, functionally active isoforms of NO-synthase (an enzyme which produced nitric oxide) have been found in RBCs[22-24].
It is known that the NO produced in RBCs is involved in cell deformation processes providing their passage through microvessels and function performance[25]. Apart from that, nitrogen monoxide may diffuse from red cells to plasma inhibiting platelet activation and aggregation, as well as adhesion and migration of leukocytes[26]. In addition to NO formation, RBCs occupy a key role in nitric oxide pool support in the bloodstream. “Unused” NO diffuses from plasma to cells where it is turned into stable products by means of hemoglobin. On the one hand, hemoglobin takes part in the formation of stable NO metabolites, and on the other - it has nitrite reductase activity that underlies RBC-dependent vasodilatation[27]. This reaction plays a significant role under hypoxia conditions that develops during DM. Moreover, hemoglobin is involved in the deposition of NO in nitrosyl hemoglobin and S-nitrosohemoglobin forms. Mutual transformations of NO-hemoglobin derivatives under certain conditions lead to the release of this vasodilatator[28,29].
Under DM, several factors contribute to a decrease in NO bioavailability: Reduced production by NO-synthase[28]; reduced availability of substrate of NO-synthase (i.e., L-arginine)[30,31]; its inactivation by reactive oxygen species (ROS)[28]. Reduced NO bioavailability causes disturbances in RBC deformability[24] and, as a result, complicates their microcirculation, thus leading to the development of vessel complications and hypoxia. HbA1c (in the form of nitrosothiols) is known to bind NO rather tightly, which adversely affects NO diffusion into muscle cells and, respectively, their relaxation[32].
Changes of RBCs in patients with diabetes cannot be considered without taking into account the specificity of erythropoiesis during this disease. Erythropoiesis is increased in hyperglycemia conditions and accompanied by a growing number of reticulocytes in the bloodstream. The erythropoiesis stimulation can be caused by an increase in erythropoietin synthesis in response to tissue hypoxia (kidney tissues in particular). The lifespan of RBCs is shortened by 13% against the background of elevated erythropoiesis under DM[15,33].
Under hyperglycemia, autoxidation of glucose occurs, which is considered to be the major mechanism for free radical formation in RBCs[34]. In its enediol form, glucose with the help of transition metals is oxidized to an enediol-anion radical, which, in turn, is transformed into reactive ketoaldehydes and superoxide anion radical. O2-• is involved in the formation of other radicals, hydroxyl radical (•OH) and peroxynitrite in particular. Hyperglycemia promotes lipid peroxidation through a superoxide-dependent pathway, which also provides the formation of free radicals[35]. Interaction between glucose and protein amino acid residues leads to formation of Amadori products and advanced glycation end-products (AGEs). Interacting with corresponding receptors, AGEs inactivate enzymes changing their structure and function[36], thus promoting free-radical formation[37] and blocking the antiproliferation effect of NO[38]. In addition, NADH oxidase enzymes and NO-synthase isoforms, found recently in RBCs, contribute to the formation of endogenous oxidants and development of oxidative stress[39].
Changes effected by oxidative stress involve plasma proteins, membranes, and lipids. Hemoglobin is gradually oxidized to methemoglobin through long-lasting exposure to a high concentration of oxygen. Hemoglobin oxidation reduces the amount of oxygen supplied to tissues and leads to the formation of disulfide bridges between globin chains. Eventually, Heinz bodies are produced, and hemoglobin is precipitated followed by macrophage phagocytosis or RBC lysis[40]. Under the interaction between ROS and other RBC proteins, amino acids of protein molecule side chains are oxidized; protein-protein linkages are formed; protein chains are oxidized with further fragmentation and a lot of protein oxidation products are produced[41]. Such modifications can occur both in structural proteins of the cytoskeleton and membrane and in enzymes causing disturbances in their functionality.
Membrane lipids also undergo oxidation, especially polyunsaturated fatty acids, which are in abundance in the RBCs membrane. As a result, elasticity and fluidity are affected, as well as deformability and shape of red cells, which in turn influence the functional state of RBCs[42]. Since lipids are not synthesized in RBCs and the exchange is restricted between lipids and plasma[43], the damage is accumulated and eventually leads to either premature sequestration and RBCs elimination from the bloodstream or their lysis.
White blood cells are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. The main function of leukocytes is to provide the specific and the non-specific immune defenses of the body through the cells with effector and immunoregulatory activity. Diabetes is often accompanied by infectious and inflammatory processes that occur with relapses and are difficult to treat. Changes in the count of leukocytes and their ratio may be a cause of susceptibility to infectious and inflammatory processes in patients with DM. It has been shown that the development of diabetes is accompanied by leukocytosis[44] against a background of a reduction in the number of lymphocytes. Such changes may lead to impairment in functional properties of the immune system which is an additional risk factor for disability in patients with diabetes or even death[45]. In T1D a disturbance of cellular immune response occurs, which is accompanied by changes in the activity of different subpopulations of cells: By exhaustion of T lymphocytes, reduction of T suppressors’ activity, an increase in activated T lymphocytes and T helpers’ quantity and by violation of IL-2 production by T lymphocytes. Also, the functional activity of peripheral blood monocytes is reduced, although their number in diabetic patients may be increased. In leukocytes the inhibition of key enzymes of glucose anaerobic oxidation and decrease of intracellular ATP reserves occurs. The development of hyperglycemia in DM leads to a disruption of polymorphonuclear leukocyte functioning, resulting in reduced intensity of a “respiratory explosion” and their phagocytic activity during the response to bacterial infection[46].
Other important functional changes that cause angiopathy are: Increased permeability of the vascular wall, hemodynamic disorders, changes in blood viscosity, and a violation of adhesive, aggregation and migration ability of WBCs, which are dependent on their morphological and functional states. The development of an immune response occurs as a result of an interaction between leukocytes and antigens, soluble regulatory molecules and with other leukocytes. To initiate an inflammatory response to a bacterial infection, leukocytes have to migrate from the blood into the affected tissues, in relation to which they have positive chemotaxis. Peripheral blood leukocytes, in particular, neutrophilic granulocytes and lymphocytes, have a leading role in the pathogenesis of diabetic complications. The mechanism causing damage to the vascular endothelium by leukocytes is not completely elucidated, but it is known that the interaction between leukocytes and endothelial cells provides adhesive molecules, most of which are part of the membrane receptors of leukocytes and, according to chemical structure, are sialoglycoproteins. The increase in the number of exposed surface cells (sialoglycoconjugates) correlates with the damage of many cell types[47,48]. The potential significance of carbohydrate-specific interactions in the regulation of cellular functions is very important because an abundance of glycoligand structures expressed on the cell surface can be modified under hyperglycemic conditions in DM. Content redistribution and structural changes in carbohydrate determinants of glycoproteins on the plasma membrane of leukocytes under type 1 DM may contribute to an increased adhesion of leukocytes to the vascular endothelium and to the disruption of their phagocytic functions. These interactions could be the reasons for complications in clinical treatment of the disease.
Many pathological states, for example, diabetes, are accompanied by changes in sialic acid expression[49]. Under diabetes, leukocytes demonstrate increased adhesive ability to the vascular endothelium. This pathogenetic mechanism is mediated by an elevated level of cell adhesion molecules on the surface of endothelial cells and leukocytes and causes abnormal leukocyte-endothelial cell adhesion. Increasing membrane rigidity and reducing the ability of WBCs to deform may damage the capillaries. And next to the small diameter of the lumen of blood vessels, increased adhesion of leukocytes to the endothelial wall leads to their capture in capillaries (leykostaz) and to increased vascular occlusion, which is an important point in the development of diabetic microangiopathies[50].
The result of hyperglycemia in the cell is the excessive accumulation of products of nonenzymatic glycosylation in cells, and also violates many biochemical and physiological parameters causing cell depletion of energy resources and antioxidants. Accumulation of AGEs either inside or outside the cell perturbs the functions of molecules and the cell on the whole[51]. Some proteins that regulate the transcription of certain genes can participate in the process of glycosylation. The molecules of AGEs, when on the cell surface, can affect signaling and interaction between intercellular space and cells, thus disrupting cell functions. The disruption of the electron transport chain and excessive generating of ROS occur in the mitochondria. Overproduction of mitochondrial superoxide during hyperglycemia is a primary initiating mechanism that activates pathways of diabetes’ vascular tissue damage, leading to cellular redox imbalance and oxidative stress[52,53]. It is known that inflammatory processes are characterized by the activation of immunocompetent cells with the corresponding activation of inducible NO-synthase, and a local increase of concentration of NO hundreds of times above the norm, depending on the cell type[54]. Accordingly, under conditions of oxidative stress and high content of O2-• elevated concentration of NO leads to the reaction between them and induces increased concentration of peroxynitrite. In turn, peroxynitrite might affect different molecules causing their modification.
Protein tyrosine nitration causes various changes in protein structure and function and catalytic activity of enzymes. Protein tyrosine nitration, including a covalent modification of the phenolic ring, may also affect tyrosine phosphorylation/dephosphorylation. For example, peroxynitrite affects the insulin signal transduction through tyrosine nitration[55] and contributes to pathological complications of a whole range of diseases. Thus, development of oxidative-nitrative stress is accompanied by protein nitration with resultant changes in cell signaling, DNA single-strand breakage and base modification, and the formation of products which exhibit potent proapoptotic effects[56]. In addition, overproduction of ROS by high glucose compromises the antioxidant defense mechanisms, such as reduced levels of mitochondrial-specific manganese superoxide dismutase, and further aggravates oxidative stress[57]. Under diabetic and high-blood glucose conditions, excessive ROS might lead to damage of the structure and function of mitochondria, therefore prompting the release of apoptosis-inducing factors such as cytochrome C[58]. A high occurrence of apoptotic lymphocytes has been shown in diabetic patients, resulting in reduced numbers of circulating lymphocytes in these patients. A number of studies suggest that dysregulated apoptotic immune cell death may play a role in contributing to the immune dysfunction[59]. The key apoptotic proteins regulators in the mitochondrial pathway are the Bcl-2 superfamily proteins. DNA damage or intra/extra-cellular stress initiates signaling cascades in the cytoplasm, eventually leading to the phosphorylation of p53 which, in turn, increases Bax concentration while lowering Bcl-2[60]. Bax expression promotes apoptosis, whereas Bcl-2 expression protects cells against oxidative stress and inhibits apoptosis[61]. Oxidative stress, which develops under hyperglycemia, alters the balance between pro- and anti-apoptotic proteins (e.g., the Bcl-2 superfamily) in the cell and subsequently disrupts the mitochondrial trans-membrane potential and DNA structure[62].
Current approaches toward a potential cure for T1D have focused on three main targets: Ablation of the β-cell-specific autoimmune response; β-cell replacement therapy using islet transplantation; and potentiation of β-cell mass and function using pharmacologic agents capable of promoting β-cell proliferation, regeneration, and/or repair[63]. In the first method recombinant antigens that trigger an immune response of β-cell destruction are used. In this way, the tolerance of immune cells to auto-antigens is trying to be developed. However, this approach is quite new and not enough research has been conducted. The quickest and most promising approach that afforded patients complete independence from exogenous insulin is islet or β-cells’ transplantation. However, it should be noted that this method is accompanied with intake of immunosuppressants to reduce the possibility of transplant rejection[63]. Therefore, at this stage of the fight against DM, most attention is paid to the study and research of drugs which are able to potentiate the effect of residual β-cells and/or induce their regeneration.
Several approaches were made to reduce the hyperglycemia, the hallmark of DM, with treatments such as sulfonylureas, which stimulate pancreatic islet cells to secrete insulin; meteoric, which acts to reduce hepatic glucose production; alpha-glucosidase inhibitors, which interfere with glucose adsorption and insulin itself, which suppresses glucose production and augments glucose utilization[64]. These therapies have limited efficacy, limited tolerability, and significant mechanism-based side effects (Table 1)[65]. Of particular danger is the use of some medicines that can promote weight gain, hypoglycemia, and insulin resistance. Another problem particular to some medicines (e.g., sulphonylureas) is the development of resistance to the treatment. Therefore, the search for new preparations is extremely important. The growing public interest and awareness of natural medicines have led the pharmaceutical industry and academic researchers to pay more attention to medicinal plants and mushrooms. The apparent reversal of the trend from western to herbal medicine is partly due to the fact that synthetic drugs have always shown adverse reactions and other undesirable side effects. This has led to the belief that natural products are safer because they are more harmonious with biological systems[66,67]. In addition, the cost of administering modern antidiabetic drugs is beyond the reach of people in low-income groups and for those living in rural areas[68].
| Types | Drug | Adverse effect |
| Sulfonylureas | Glibenclamide | Hypoglycemia, weight gain |
| Thiazolidinedione | Troglitazone | Liver damage |
| Rosiglitazone | Cardiovascular disease | |
| Pioglitazone | Weight gain, pedal edema, bone loss, precipitation of congestive heart failure | |
| α-glucosidase inhibitors | Migltol | Gastrointestinal effects (flatulence, diarrhea, stomachache) |
| Acarbose | Gastrointestinal effects (flatulence, diarrhea, stomachache) | |
| Voglibose | Gastrointestinal effects (flatulence, stomachache) | |
| Biguanide | Metformin | Gastrointestinal effects (diarrhea, vomiting, nausea) |
| Phenformin | Lactic acidosis |
Mushrooms comprise an extremely abundant and diverse world. The number of mushroom species on Earth is currently estimated at 150000-160000; yet, perhaps only 10% are known to science[69]. Mushrooms are currently evaluated for their nutritional value and acceptability, as well as for their pharmacological properties[70]. They make up a vast and yet largely untapped source of new powerful pharmaceutical products. The majority of higher Basidiomycetes mushrooms contain many types of biologically active high-molecular weight and low-molecular weight compounds in fruit bodies, cultured mycelia, and cultured broth which has different properties[71,72].
Medicinal mushrooms have an established history of use in traditional oriental therapies. Contemporary research has validated and documented much of the ancient knowledge. Ancient oriental medicine has stressed the importance of several mushroom species, mostly Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Ling zhi or Reishi) and Lentinus edodes (Berk.) Singer (Shiitake). Mushrooms have also played an important role as a cure for ailments affecting the rural populations of Russia and other Slavic European countries. The most important species in these areas were Inonotus obliquus (Pers.: Fr.) Pilat (Chaga), Fomitopsis officinalis (Vill.: Fr.) Bond. et Singer, and Fomes fomentarius Fr.: Fr. These species were used in the treatment of gastrointestinal disorders, various forms of cancers, bronchial asthma, night sweats, etc[73]. There is also a long history of traditional use of mushrooms as curatives in Mesoamerica (especially species of the genus Psilocybe), Africa (Yoruba populations in Nigeria and Benin), Algeria, and Egypt. A very special role of fly agaric [Amanita muscaria (L.:Fr.) Pers.] is found in Siberia and Tibetan shamanism, Buddhism, and Celtic myths[71,74,75].
Nowadays, medicinal mushrooms are used as dietary food, dietary supplemental products, pharmaceuticals, natural bio-control agents in plant protection with insecticidal, fungicidal, bactericidal, herbicidal, nematicidal, and antiphytoviral activities, and cosmeceuticals[72].
Medicinal mushrooms are comparable to “medicinal plants” and can be defined as macroscopic fungi, mostly higher Basidiomycetes and some Ascomycetes, which are used in the form of extracts or powder for prevention, alleviation, or healing of diseases, and/or in providing a balanced healthy diet[76].
Modern clinical practice in Taiwan, Japan, China, South Korea, and other Asian countries rely on mushroom-derived preparations. These preparations have many active compounds, which identify their importance as food and as medicines. This includes mainly high-molecular weight compounds such as polysaccharides (e.g., β-D-glucans, glucuronoxylomannan), proteins, polysaccharide-protein complexes, lipopolysaccharides, glucoproteins, and lectins. Low-molecular weight metabolites include lactones, terpenoids, alkaloids, sterols, phenolic substances, and antibiotics with different active groups, metal chelating agents, etc. Also, medicinal mushrooms have enzymes - laccase, superoxide dismutase, glucose oxidase, peroxidase[72,77]. As dietary supplement products, mushrooms contain a small amount of lipids and cholesterol, and low levels of carbohydrates. At the same time, they are rich in fiber, protein, minerals, and vitamins[78]. The wide range of bioactive compounds determines antitumor, immunomodulating, antioxidant, radical scavenging, cardiovascular, antihypercholesterolemic, antiviral, antibacterial, antiparasitic, hepatoprotective, and antidiabetic properties of medicinal mushrooms[79,80].
Antidiabetic properties of Agaricus brasiliensis and Ganoderma lucidum
Research has shown that some mushrooms may have the potential to lower elevated blood sugar levels. But the explanation for this effect is limited, except for some mushrooms. Therefore, it would be necessary to carry out more research on mushrooms with a focus to identify the active compounds in specific mushrooms for the treatment of DM and its complications.
Agaricus brasiliensis (A. brasiliensis, Royal Sun Agaricus) is native to Brazil and widely grown in Japan. This mushroom is used in the treatment of atherosclerosis, hepatitis, hyperlipidemia, dermatitis, and cancer, and its polysaccharides, α-glucan and β-glucan, have been shown to have immunomodulating and antimutagenic effects both in vivo and in vitro[75,81]. The possible mechanisms of natural polysaccharides to DM might base on six directions: (1) the elevation of plasma insulin, and the decline of pancreatic glucagon; (2) the increase of insulin sensitivity, and the improvement of insulin resistance; (3) the restraint of α-glycosidase enzymes in bowel, and the reduction of carbohydrates decomposition and absorption; (4) the increase of hepatic glycogen, and the inhibition of sugar dysplasia; (5) the increased glucose use of peripheral tissue; (6) the scavenging free radicals and lipid peroxidation[65]. Also, hypoglycemic and antidiabetic properties of A. brasiliensis have been reported. Di Naso et al[82] showed that A. brasiliensis extract exhibited a significant antioxidant activity in streptozotocin (STZ)-induced diabetic rats decreasing lipoperoxidation and iNOS expression in the lungs. These results suggest that A. brasiliensis treatment effectively reduced the oxidative stress and contributes to tissue recovery in diabetes. Another study demonstrated that A. brasiliensis extracts derived from submerged-culture broth significantly reduced blood glucose levels in an oral glucose-tolerant test in STZ-induced diabetic rats[83]. There is also clinical evidence that A. brasiliensis combined with antidiabetic drugs can improve insulin resistance in T2D patients[84]. It is shown that β-glucans and oligosaccharides of A. brasiliensis have antihyperglycemic, antihypertriglyceridemic, antihypercholesterolemic, and anti-arteriosclerotic activity in diabetic rats. One group has suggested that the A. brasiliensis antidiabetic effect in diabetic rats is due to its suppression of OS and proinflammatory cytokine production, which then results in improvement of the mass of pancreatic β-cells[85]. But, nevertheless, additional pharmacological studies are needed to elucidate the mechanism of A. brasiliensis action as well as to assess the use of these species for the treatment of human DM.
Ganoderma lucidum (G. lucidum, Ling zhi, Reishi), has a leading place in present-day medicinal mushroom development. G. lucidum has been utilized for centuries in East Asia to prevent or treat various diseases and was used in traditional Chinese medicine as a tonic in promoting good health, perpetual youth, vitality, and longevity. It is widely grown on a commercial scale and is commonly purchased for its medicinal and spiritual properties. Worldwide, more than 250 Ganoderma species have been described[75]. Recent studies on G. lucidum have shown many interesting biological activities including antitumor, antiinflammatory, antioxidant, and antidiabetic effects[75,77].
Antihyperglycemic effects of G. lucidum have been extensively studied and have shown potential therapeutic activities. It has been shown that oral administration of water extracts of G. lucidum significantly reduced the increase in blood glucose and insulin levels in rats following the oral glucose tolerant test[86]. Prevention of the progression of diabetic renal complications as well as a lowering of the increased serum glucose and triglyceride levels was reported in STZ-induced diabetic rats[87]. Another study demonstrated that polysaccharides isolated from G. lucidum significantly increased nonenzymatic and enzymatic antioxidants and serum insulin levels, and reduced lipid peroxidation and blood glucose levels in STZ-diabetic rats[88,89]. In alloxan-induced diabetic rats, the aqueous extract of G. lucidum normalized blood glucose levels[68].
It was shown that G. lucidum consumption can provide beneficial effects in treating T2D by lowering the serum glucose levels through the suppression of the hepatic enzyme gene expression involved in gluconeogenesis[90] in a clinical study showed that Ganopoly (polysaccharide fractions extracted from G. lucidum by a patented technique) efficaciously lowered blood glucose concentration in patients with confirmed T2D. It was shown that G. lucidum polysaccharides attenuated myocardial collagen cross-linking in diabetic rats, which was related to the decreased level of AGEs and augmented activities of antioxidant enzymes[91]. Also, it was shown that ganoderol B (bioactive sterol from G. lucidum fruit body) has a strong inhibitory activity on α-glucosidase and can be proposed as a treatment for T2D[92]. Orally administered proteoglycan extract, Fudan-Yueyang-G. lucidum, to STZ-induced diabetic rats showed a significant decrease in plasma glucose levels[93]. It appears that there are a number of biologically active compounds to be explored in the mycelium, and future research should focus in that direction.
At present, more attention is paid to natural medicines which would have sugar lowering properties and are able to prevent angiopathy development, but, at the same time, do not have adverse effects. There are significant evidences of the role of higher mushrooms in maintaining health and in disease prevention[94,95].
Our investigations showed significant increases (3.6 times) of blood glucose concentration in streptozotocin-induced diabetic rats compared with the control group. The per oral administration of submerged cultured mycelium powder (SCMP) during 14 d (at a dose of 1 g/kg of body weight) to control animals did not cause significant changes: Only slight fluctuations around values of control group. At the same time, administration of A. brasiliensis and G. lucidum SCMP to diabetic rats decreased blood glucose in 2.4 and 2.7 times, respectively, compared with values of the control diabetic group. It should be noted that obtained values were close to control ones[96].
During the research, we also measured the weights of animals because it characterized the general physiological state of the body. It is known that development of DM is accompanied by decreases in this index in sick animals[97]. Obtained results showed significant growth of body weight of control rats to the end of the experiment (by 18.3%) while body weight of diabetic animals significantly decreased (by 4.0%)[98]. As insulin plays the role of gluconeogenesis inhibitor, so deficit of insulin under DM causes intensification of this process. Thus, an increased blood glucose level is an inductor of different molecular and biochemical changes. Besides, removal of glucose from organism occurs with needed for this process amount of water and electrolytical ions K+ and Na+[99]. As a result, dehydration occurs in the organism. Further, dehydration of organism increasing due to the formation of ketone bodies (acetoacetate and β-hydroxybutyrate) and loss of bicarbonate blood buffer system capacity with the appropriate induction of ketoacidosis[100]. The administration of SCMP of medicinal mushrooms to control animals did not cause significant changes in body weight. In diabetic rats administered with A. brasiliensis and G. lucidum mycelium powder, we observed significant increases in this index by 4.0% and 8.5%, respectively[98].
Therefore, the use of medicinal mushrooms caused the lowering of blood glucose concentration and promoted body weight gain in diabetic rats. Such results indicate an overall protective effect of mushroom mycelium. The mechanism of such effects can be mediated through affecting blood glucose levels and the correction of ketone body content.
The content of glycosylated hemoglobin is another index that has been recently considered as a biomarker in the development and course of DM. It is believed to be a more precise glycemic indicator than blood glucose concentrations because HbA1c is more resistant to stress and independent of food intake[101]. During long-term hyperglycemia, the content of glycosylated hemoglobin increases due to intensification of non-enzymatic glycosylation processes[102]. Obtained results showed significant increases of HbA1c in streptozotocin-induced diabetic rats. Administration of SCMP of medicinal mushrooms to control groups led to a slight (not significant) growth of the index. In diabetic rats, administration of A. brasiliensis and G. lucidum mycelium powder caused significant reduction of HbA1c by 16.7% and 24.7%, respectively. This indicates a normalizing effect of the studied mushrooms on glucose metabolism[96].
Inflammation and structural and functional disorders of the blood are additional risk factors involved in the pathogenesis of diabetes complications. Therefore, studying the impact of anti-diabetic drugs on morphological and functional parameters of blood cells is very important. It was shown that the development of diabetes occurs with a redistribution of segmented neutrophils and lymphocytes, and may indicate inflammatory processes in the animal under DM. Changes in the total number of white blood cells and their ratio, and violations in their functional properties are likely causes of susceptibility of patients with diabetes to infectious processes and immune status violations. For a preliminary assessment of the immune system of patients with diabetes type 1, the changes in white blood cell count were analyzed. There was no significant difference in the total number of leukocytes between the control group and the group with EDM, but the percentage of segmented neutrophils decreased by 27% and the percentage of lymphocytes increased by 8%, compared to the control[98].
Administration of mushroom mycelia in healthy animals did not lead to statistically significant changes in the ratio of different types of leukocytes. Under conditions of streptozotocin-induced DM, administration of mushroom SCMP resulted in normalization of the leukocyte formula, in particular, the increase in the number of segmented neutrophils. A decrease in the number of lymphocytes almost to the control values was also observed[98]. Thus, administration of powdered A. brasiliensis and G. lucidum to rats resulted in a normalization of the leukocyte formula.
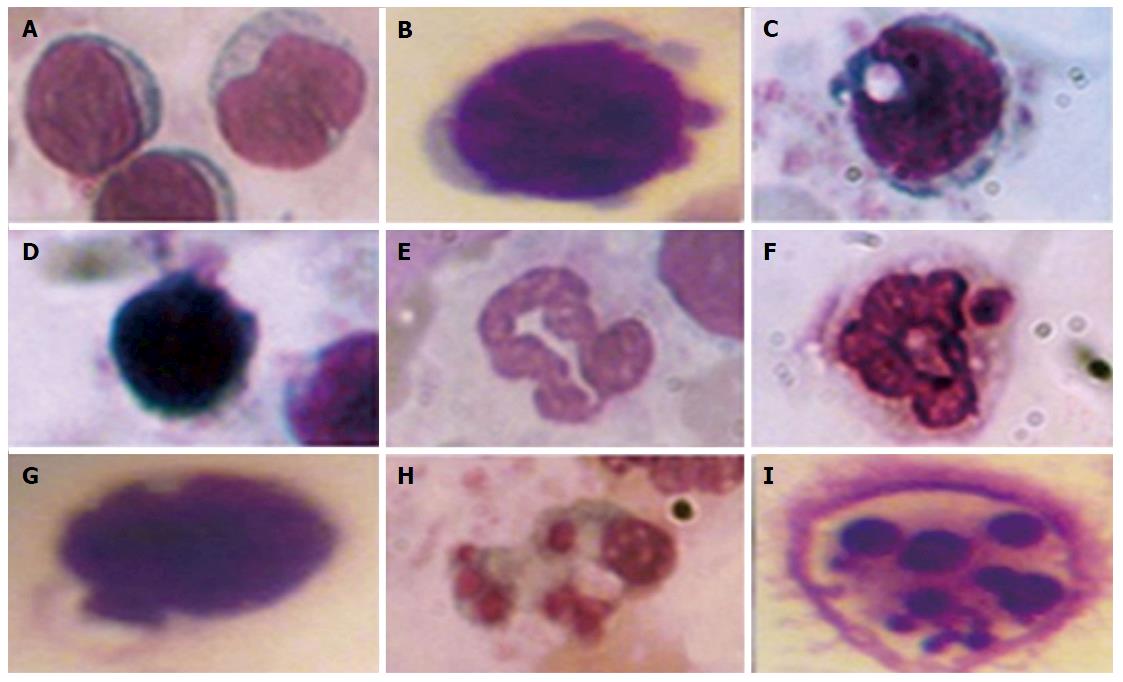
Visual assessment of processes in leukocyte apoptosis by morphological features: The decrease of leukocyte membrane glycoconjugates sialylation and violation in the genetic apparatus of cells caused by oxidative-nitrative stress under DM may lead to cell death by apoptosis. Under apoptosis, the cell reduces its size; hardens its outer cytoplasmic membrane without the cell content entering the environment; aggregates chromatin and condenses the nucleus and cytoplasm; fragments them into vesicles surrounded by plasma membrane, i.e., apoptotic bodies containing fragments of dying cells[103].
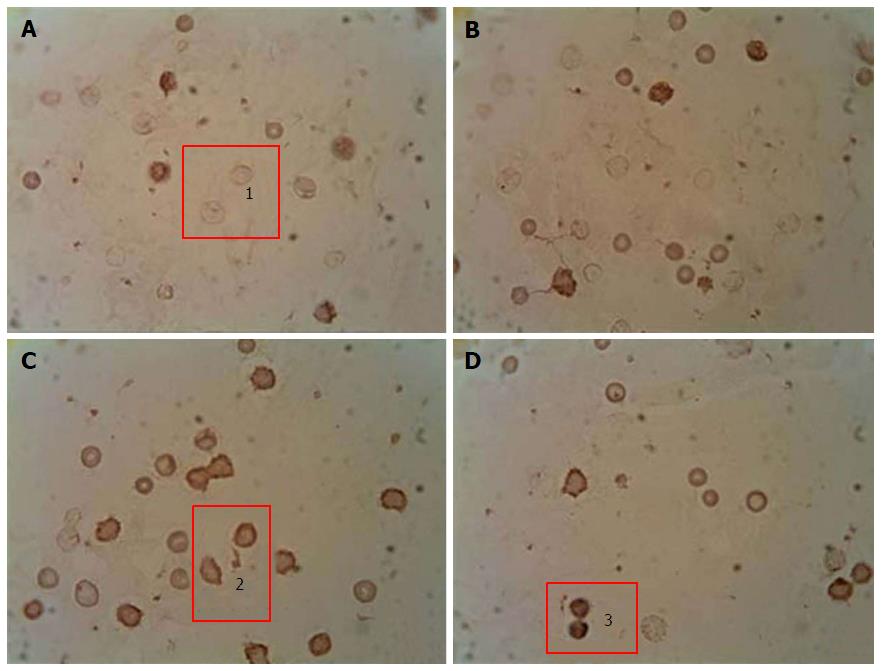
To quantify the content of apoptotic cells, the apoptotic index was calculated, i.e., the ratio between cells with morphological apoptotic features and the general quantity of cells (Figure 1). Based on the collected data it was shown that the development of diabetes was accompanied by an 8-fold increase of the apoptotic index. Whereas administration of A. brasiliensis and G. lucidum to diabetic animals led to a reduction in the apoptotic index by 3.5-fold and 3.8-fold, respectively (compared with non-treated diabetic animals)[98].
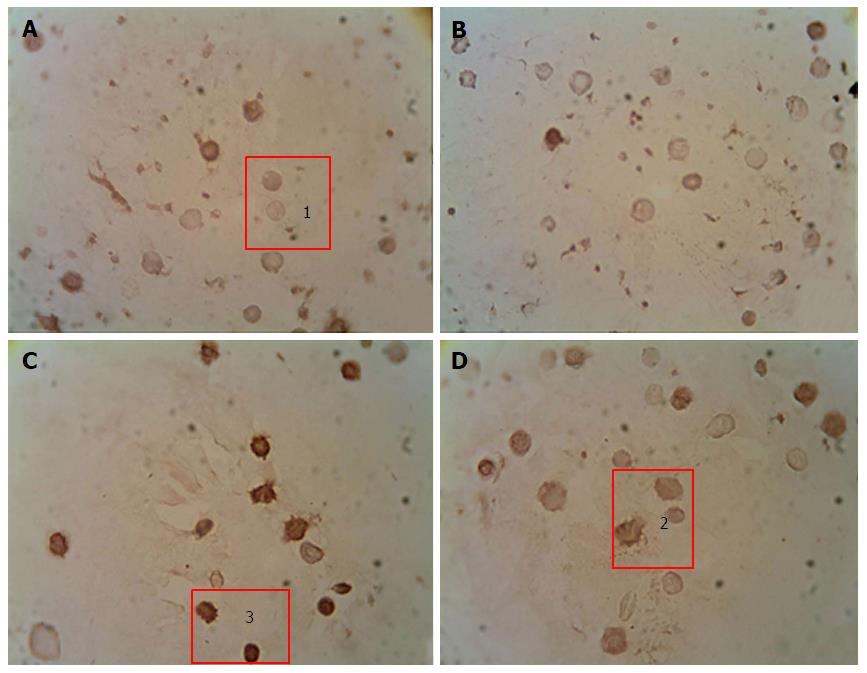
Immunocytochemical detection of the content of apoptotic protein-regulators (p53 and Bcl-2): The p-53 protein has a special place in the regulation of apoptosis. Under stress, the p-53 protein can induce apoptosis in response to numerous adverse effects leading to a variety of genetic disorders[62]. This process occurs through Bcl-2 protein inactivation by binding to Bax. As a result, a heterodimeric complex is formed, which stops the anti-apoptotic activity of the Bcl-2 protein. We showed that under streptozotocin-induced DM the number of p53++ cells (high-positive response) increased by 47%, and the number of p53+ cells (positive response) increased by 32% compared with the control, whereas the number of p53- cells (negative reaction) decreased by 25%[98] (Figure 2). Administration of selected mushroom SCMP in control animals revealed no significant changes. The administration of the mushrooms led to a decrease in the level of p53++ and p53+ cells in diabetic animals compared to untreated diabetic animals. Under A. brasiliensis administration, there was a decrease of 22% in the level of p53++ and 44.7% in the level of p53+. Also, our results have shown a significant increase in the number of cells with a p53 negative reaction by 20%. Administration of G. lucidum mycelium powder led to a decrease in the number of cells with positive response by 40.5%, cells with high-positive response by 47%, and increased the number of cells with a p53 negative reaction by 27.6% compared to untreated animals[98].
There are regulators that block or magnify destructive effects of caspases. Proteins from the Bcl-2 family belong to this group. Different members of the family can form dimers with each other, one of them enhancing or inhibiting the function of the other. In this case, the ratio between inhibitors and activators may show the disposition of cells to apoptosis[104]. Particular interest here is displayed in Bcl-2 protein. It is known that Bcl-2 inhibits p53-dependent and -independent ways of apoptosis[56].
We have shown that development of DM was accompanied by a increase in the content of Bcl-2+ and Bcl-2++ cells and a decrease in the content of Bcl-2- cells (Figure 3). Administration of studied mushroom SCMPs to control animals did not cause any significant changes. Administration of A. brasiliensis and G. lucidum to the diabetic animals caused the normalization in the percentage of cells with different levels of Bcl-2 protein expression compared to the control values[98].
Thus, these results suggest an increase of apoptotic processes in the peripheral blood leukocytes of rats with streptozotocin-induced DM. Effects of medicinal mushrooms under conditions of diabetes are aimed at normalization of the ratio of WBCs that contain proteins-regulators of apoptosis (p53 and Bcl-2) as well as reducing apoptotic index. This indicates a pronounced anti-apoptotic effect of the studied species of mushrooms.
Changes in blood erythroid link: Since hyperglycemia might cause much damage in membrane components of RBC, it can affect their number in the bloodstream. This in turn might lead to impairment of their functions. Therefore, we paid attention to the quantity of characteristics of the blood. Our research showed that the number of red cells significantly decreased in streptozotocin-induced diabetic rats[96]. Such results indicate the development of anemia that accompanies DM and is one of the causes of tissue hypoxia and secondary complications. In control animals administered with medicinal mushroom mycelium powder, we observed a tendency to decrease the RBC number, but obtained changes were not significant. In diabetic SCMP-treated rats the number of red cells significantly increased compared with non-treated animals, and the values almost reached the level of controls[96].
The impairment of RBC membrane structures in peripheral blood caused by chronic hyperglycemia and insulin deficiency leads to changes in the functional state of the cells. One of the methods used for its evaluation is RBC resistance to acid hemolysis. The method of acidic erythrograms helps to estimate the value of the hydrophobic barrier and permeability of membrane protein components, and provides an opportunity to group morphologically similar RBCs into age populations. Young red cells possess the highest resistance to acid hemolysis and take erythrograms correctly. The aging of RBCs is accompanied by a gradual decrease of their resistance to acidic hemolysis, which is reflected in an erythrogram shift to the left. Its level reveals the functional-metabolic state of the cell membrane and undergoes changes not only due to cell aging but also as a result of physicochemical modifications and destabilization of membrane components under various pathologies[105].
Results of the investigation of RBCs’ resistance to acid hemolysis are described in our previous paper[96]. Development of DM in rats is accompanied by the shift of erythrogram to the left, reduction of hemolysis time, the accelerated peak of hemolysis and higher percentage of hemolyzed RBCs at the peak’s point. A decrease in red cell resistance to acid hemolysis indicates not only the aging of the cells but also reveals the possibility of their membrane destruction during formation and maturation.
Administration of SCMP of medicinal mushrooms to the control group did not cause changes in the parameters of hemolysis. However, in diabetic rats this administration led to the return of the above mentioned parameters to control values. Obtained results indicate the redistribution of RBC populations towards the growth of full functional cell numbers, which confirms a corrective membrane protective effect produced by the selected mushrooms[96].
Reduction of the RBCs’ number and an increased content of HbA1c lead to a violation in tissue oxygenation and the development of hypoxia. The body launches compensatory reactions aimed at restoring homeostasis[105]. One such reaction under conditions of DM is an intensification of erythropoiesis that can be assessed by the determination of number and daily production of reticulocytes - precursors of mature RBCs. Our study showed that the development of diabetes intensified erythropoiesis in rats evidenced by the increased number of reticulocytes in the bloodstream and their daily production[96]. A. brasiliensis treatment of control animals led to significant increases of the precursors’ number and their production. At the same time, there were no changes of these indices detected in the G. lucidum treated group[90]. In diabetic rats, administration of medicinal mushrooms’ SCMP did not affect erythropoiesis effectiveness, i.e., elevated levels remained[96].
The reduced levels of blood glucose and content of glycosylated hemoglobin after the course of medicinal mushroom mycelium powders administration were shown on the model of rats with streptozotocin-induced DM. It is believed that the major bioactive compounds of A. brasiliensis are polysaccharides and protein-polysaccharide complexes containing beta-glucans[81,106]. The effect of beta-glucans to reduce blood glucose could be mediated possibly by delaying stomach emptying, so that dietary glucose is absorbed more gradually[105]. Tapola et al[107] showed that after ingestion of oats (with high content of beta-glucans), the blood glucose levels were lower at 15, 30 and 45 min but higher at 90 min after 12.5 g glucose loading. Thus, the peak level is much more smoothed and the shape of the plasma glucose response curve is much flatter[107]. Another possible mechanism for beta-glucans is mediated by a signal pathway through activation of PI3K/Akt in which activity decreases under DM[108]. Beta-glucans interact with a specific receptor and, through messenger molecules [e.g., SYk kinase[109]], activate the PI3-kinase pathway. Bioactive compounds may also include oligopeptides. Recently described oligopeptide of A. brasiliensis is rich in proline, lysine, and phenylalanine and has antioxidant activity[110]. Also, A. brasiliensis contains low-molecular weight compounds such as tocopherol, ergosterol, phenols, etc.[111,112] and metal ions[85,113]. These compounds are included in different regulated mechanisms responsible for inhibition of free radicals formation and the development of oxidative stress. Microelements promote the function of the antioxidant defense system. They include beta-carotine, vitamin C, vitamin E (the vitamin E family includes both tocopherols and tocotrienols, but alpha-tocopherol is predominant and the most active form). Water-soluble molecules, such as vitamin C, are powerful radical scavengers in the aqueous phase of cytoplasm, while lipid-soluble molecules (such as vitamin E and beta-carotene) are antioxidants in the lipid phase. Metal ions (e.g., selenium, copper, zinc, manganese, etc.), besides scavenger properties, are in active sites of enzymes of the antioxidant system[114]. In addition, three phenols - gallic acid, lilac acid, and pirogallol, which have pronounced antioxidant activities, were identified in A. brasiliensis[111]. Such a protective effect, in turn, improves functioning of residual pancreas beta cells and improves insulin secretion[85].
The major active components of G. lucidum, which have antidiabetic properties, are polysaccharides and triterpenoids[90]. The therapeutic effect of polysaccharides consists of: The facilitation of glucose uptake by muscle cells through activation of PI3K and AMPK-dependent metabolic pathways[115]; regulation of the expression of key enzymes in the conversion of glucose (e.g., liver glucokinase, phosphofructokinase, glucose 6-phosphate dehydrogenase, etc.)[116,117]; and an increase of insulin content in blood plasma[68]. Triterpenoids show aldose reductase and α-glucosidase inhibitory properties that correlate with glucose metabolism and diabetic complications[118]. Another mechanism of the protective effect of this mushroom is the scavengering of free radicals and thus “neutralize” the negative impact of oxidative stress (on β-cells of the pancreas, in particular), which is one of the etiological causes of diabetes development[88].
In addition, medicinal mushrooms are rich in nucleotides and nucleozides, e.g., adenosine, which (in the same way as A1 activators of purinergic receptors) shows cytoprotective action in cardiovascular and central nervous systems by activating adenosine receptors of the cell surface. Activation of these receptors, in turn, activates antioxidant enzymes. This occurs via the protein kinase C, which phosphorylates those enzymes or intermediates that contribute to their activation[119].
Despite the great efforts made in the field of DM treatment, this disease is still one of the major problem of humanity. Physical and biochemical changes of blood cells, that occurred due to metabolic disorders, lead to the development of different kinds of angiopathies and the worsening of patients’ health. Medicinal mushrooms A. brasiliensis and G. lucidum, due to their composition and biological properties, are potential sources of new biopreparations for the prevention and treatment of DM. SCMP of medicinal mushrooms has a pronounced hypoglycemic effect manifesting in the normalization of blood glucose levels and content of glycosylated hemoglobin. Also, mycelium powders promote weight gain of rats with STZ-induced diabetes confirming the general restorative role for the body. The selected mushrooms showed the normalizing effect on the indices of leukocyte formula under DM type 1. However, they do not show a significant impact on the number of leukocytes in healthy animals, which indicate no adverse physiological effects of their action. The action of the medicinal mushrooms under diabetes is aimed at the normalization of a ratio between protein-regulators of apoptosis (p53 and Bcl-2) in white blood cells, as well as the reduction of the apoptotic index. These changes indicate an inhibitory effect of the selected mushrooms on programmed cell death, which is significantly increased under DM. Administration of medicinal mushrooms A. brasiliensis and G. lucidum leads to restoration of RBCs’ number and their resistance to acid hemolysis indicating the anti-anemic and membrane protective impacts.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aureliano M, Gao C, Mitra A, Nayak BS, Zhao JB S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization. WHO/NCD/NCS/99.2 1999; 59. [Cited in This Article: ] |
| 2. | Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation 2013; Available from: http://www.idf.org/diabetesatlas. [Cited in This Article: ] |
| 3. | Alam F, Islam MA, Kamal MA, Gan SH. Updates on Managing Type 2 Diabetes Mellitus with Natural Products: Towards Antidiabetic Drug Development. Curr Med Chem. 2016; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 4. | Kahn CR, Gordon С, Weir MD, George L, King MD, Alan M, Jacobson MD, Alan C, Moses MD, Robert J, Smith MD. Joslin’s Diabetes Mellitus. Boston: Lippincott Williams & Wilkins 2004; 1224. [Cited in This Article: ] |
| 5. | Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ. 2011;343:d5364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:pii: a007641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | IDF Diabetes Atlas. 3rd ed. Brussels: International Diabetes Federation 2006; Available from: http://www.idf.org/diabetesatlas. [Cited in This Article: ] |
| 8. | Tuch B, Dunlop M, Proietto J. Diabetes research: A guide for postgraduates. Harwood: Harwood Academic Publishers 2004; 128. [Cited in This Article: ] |
| 9. | Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1462] [Cited by in F6Publishing: 1456] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 10. | Kawasaki E, Abiru N, Eguchi K. Prevention of type 1 diabetes: from the view point of beta cell damage. Diabetes Res Clin Pract. 2004;66 Suppl 1:S27-S32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Pak IV. Modern ideas about the etiology and pathogenesis of insulin-dependent diabetes mellitus. Vestnik KRSU. 2012;12:130-133 (in Russian). [Cited in This Article: ] |
| 13. | Desouky OS. Rheological and electrical behavior of erythrocytes in patients with diabetes mellitus. Rom J Biophys. 2009;19:239-250. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gordon-Smith T. Structure and function of red and white blood cells. Medicine. 2013;41:193-199. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Bodnar TP, Kozinec GI. Morphofunctional state of peripheral blood erythrocytes in late vascular complications of type 2 diabetes mellitus (literature review). Clin Lab Diagn. 2002;12:22-34 (in Ukrainian). [Cited in This Article: ] |
| 16. | Sailaja YR, Baskar R, Srinivas Rao CS, Saralakumari D. Membrane lipids and protein-bound carbohydrates status during the maturation of reticulocytes to erythrocytes in type 2 diabetics. Clin Chim Acta. 2004;341:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Vahalkar GS, Haldankar VA. RBC membrane composition in insulin dependent diabetes mellitus in context of oxidative stress. Indian J Clin Biochem. 2008;23:223-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Venerando B, Fiorilli A, Croci G, Tringali C, Goi G, Mazzanti L, Curatola G, Segalini G, Massaccesi L, Lombardo A. Acidic and neutral sialidase in the erythrocyte membrane of type 2 diabetic patients. Blood. 2002;99:1064-1070. [PubMed] [Cited in This Article: ] |
| 19. | Samaja M, Melotti D, Carenini A, Pozza G. Glycosylated haemoglobins and the oxygen affinity of whole blood. Diabetologia. 1982;23:399-402. [PubMed] [Cited in This Article: ] |
| 20. | Choi JW, Nahm CH, Lee MH. Relationships of Fetal-Type Erythropoiesis versus Nitric Oxide Production and Glycated Hemoglobin Levels in Diabetics. Ann Clin Lab Sci. 2011;41:224-228. [PubMed] [Cited in This Article: ] |
| 21. | Owusu BY, Stapley R, Honavar J, Patel RP. Effects of erythrocyte aging on nitric oxide and nitrite metabolism. Antioxid Redox Signal. 2013;19:1198-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Jubelin BC, Gierman JL. Erythrocytes may synthesize their own nitric oxide. Am J Hypertens. 1996;9:1214-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kang ES, Ford K, Grokulsky G, Wang YB, Chiang TM, Acchiardo SR. Normal circulating adult human red blood cells contain inactive NOS proteins. J Lab Clin Med. 2000;135:444-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kosiakova HV, Hula NM. [The N-stearoylethanolamine effect on the NO-synthase way of nitrogen oxide formation and phospholipid composition of erythrocyte membranes in rats with streptozotocine diabetes]. Ukr Biokhim Zh (1999). 2007;79:53-59. [PubMed] [Cited in This Article: ] |
| 25. | Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozüyaman B. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943-2951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 26. | Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2014;2:251-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Halpin ST, Anderson KB, Vogel PA, Spence DM. The red blood cell and nitric oxide: derived, stimulated, or both? The Open Nitric Oxide Journal. 2011;3:8-15. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047-1060. [PubMed] [Cited in This Article: ] |
| 31. | Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol. 2012;302:H159-H166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Burke T. Part 3: Nitric oxide metabolism in diabetic patients. 2009. Available from: http://www.anodynetherapy.ca/clinical.html. [Cited in This Article: ] |
| 33. | Vydyborets SV. Changes in red blood cells under condition of diabetes. Med Case. 1996;2:11-14 (in Ukrainian). [Cited in This Article: ] |
| 34. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8502] [Cited by in F6Publishing: 8379] [Article Influence: 465.5] [Reference Citation Analysis (0)] |
| 35. | Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1916] [Cited by in F6Publishing: 1830] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 36. | McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 2001;38:113-122. [PubMed] [Cited in This Article: ] |
| 37. | Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1-9. [PubMed] [Cited in This Article: ] |
| 38. | Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997;46 Suppl 2:S19-S25. [PubMed] [Cited in This Article: ] |
| 39. | Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med. 2003;34:1563-1574. [PubMed] [Cited in This Article: ] |
| 40. | Pandey KB, Rizvi SI. Biomarkers of oxidative stress in red blood cells. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:131-136. [PubMed] [Cited in This Article: ] |
| 41. | Pandey KB, Rizvi SI. Resveratrol may protect plasma proteins from oxidation under conditions of oxidative stress in vitro. J Braz Chem Soc. 2010;21:909-913. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 43. | Isaji Y, Okochi M, Horio F, Honda H. Use of erythrocyte adhesion assay to predict the risk of diabetic complications. Biochem Eng J. 2009;43:178-184. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Flood RG, Chiang VW. Rate and prediction of infection in children with diabetic ketoacidosis. Am J Emerg Med. 2001;19:270-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Graves DT, Naguib G, Lu H, Leone C, Hsue H, Krall E. Inflammation is more persistent in type 1 diabetic mice. J Dent Res. 2005;84:324-328. [PubMed] [Cited in This Article: ] |
| 46. | Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256-260. [PubMed] [Cited in This Article: ] |
| 47. | Fajka-Boja R, Hidvégi M, Shoenfeld Y, Ion G, Demydenko D, Tömösközi-Farkas R, Vizler C, Telekes A, Resetar A, Monostori E. Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class I proteins in tumor T and B cell lines. Int J Oncol. 2002;20:563-570. [PubMed] [Cited in This Article: ] |
| 48. | Zdioruk M, Brodyak І, Barska M, Sybirna N. Sialic acid-containing glycoproteins are the marker molecules that determine the leukocyte functional states under diabetes mellitus. Sepsis. 2011;4:47-55. [Cited in This Article: ] |
| 49. | Sybirna N, Brodjak I, Barska M. Lectin-induced aggregation of neutrophil granulocytes in patients with diabetes mellitus type 1. Laboratornaya diagnostika. 2004;3:57-61 (in Ukrainian). [Cited in This Article: ] |
| 50. | Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in diabetic retinopathy. Curr Diabetes Rev. 2007;3:3-14. [PubMed] [Cited in This Article: ] |
| 51. | Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44:1973-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6145] [Cited by in F6Publishing: 5936] [Article Influence: 258.1] [Reference Citation Analysis (0)] |
| 53. | Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241-S245. [PubMed] [Cited in This Article: ] |
| 54. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4561] [Cited by in F6Publishing: 4188] [Article Influence: 246.4] [Reference Citation Analysis (0)] |
| 55. | Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Grinevich IV, Kamyshny AM. Influence of experimental diabetes mellitus on expression of protein-regulators of spleen apoptosis. Morfologіya. 2010;4:19-23 (in Russian). [Cited in This Article: ] |
| 57. | Lu Q, Zhai Y, Cheng Q, Liu Y, Gao X, Zhang T, Wei Y, Zhang F, Yin X. The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp Physiol. 2013;98:934-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423-R430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344-351. [PubMed] [Cited in This Article: ] |
| 60. | Lagranha CJ, Senna SM, de Lima TM, Silva E, Doi SQ, Curi R, Pithon-Curi TC. Beneficial effect of glutamine on exercise-induced apoptosis of rat neutrophils. Med Sci Sports Exerc. 2004;36:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1430] [Cited by in F6Publishing: 1480] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 62. | Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98:2239-2247. [PubMed] [Cited in This Article: ] |
| 63. | Clemente-Casares X, Tsai S, Huang C, Santamaria P. Antigen-specific therapeutic approaches in Type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 737] [Cited by in F6Publishing: 729] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 65. | Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr Polym. 2016;148:86-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 66. | Liday C. Overview of the guidelines and evidence for the pharmacologic management of type 2 diabetes mellitus. Pharmacotherapy. 2011;31:37S-43S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab. 2012;14 Suppl 1:9-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Mohammed A, Adelaiye AB, Abubakar MS, Abdurahman EM. Effects of aqueous extract of Ganoderma lucidum on blood glucose levels of normoglycemic and alloxan-induced diabetic Wistar rats. J Med Plant Res. 2007;1:34-37. [Cited in This Article: ] |
| 69. | Chatterjee A, Acharya K. Include mushroom in daily diet - a strategy for better hepatic health. Food Reviews International 2015. . [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Chatterjee B, Patel T. Edible mushroom - a nutritious food improving human health. Int J Clin and Biomed Res. 2016;2:34-37. [Cited in This Article: ] |
| 71. | Wasser SP. Medicinal mushroom science: history, current status, future trends, and unsolved problems. Int J Med Mushrooms. 2010;12:1-16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 72. | Chang ST, Wasser SP. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int J Med Mushrooms. 2012;14:95-134. [PubMed] [Cited in This Article: ] |
| 73. | Money NP. Are mushrooms medicinal? Fungal Biol. 2016;120:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Van Griensven LJ. Culinarymedicinal mushrooms: must action be taken. Int J Med Mushrooms. 2009;11:281-286. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Lo HC, Wasser SP. Medicinal mushrooms for glycemic control in diabetes mellitus: history, current status, future perspectives, and unsolved problems (review). Int J Med Mushrooms. 2011;13:401-426. [PubMed] [Cited in This Article: ] |
| 76. | Wasser SP. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed J. 2014;37:345-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 77. | De Silva D, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fung Diversity. 2012;56:1-29. [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Smiderle FR, Olsen LM, Ruthes AC, Czelusniak PA, Santana-Filho AP, Sassaki GL, Gorin PA, Iacomini M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohyd Polym. 2012;87:368-376. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Friedman M. Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods. 2016;5:80. [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 80. | Dai YC, Zhou LW, Cui BK, Chen YQ, Decock C. Current advances in Phellinus sensu lato: medicinal species, functions, metabolites and mechanisms. Appl Microbiol Biotechnol. 2010;87:1587-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Firenzuoli F, Gori L, Lombardo G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid Based Complement Alternat Med. 2008;5:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Di Naso FC, de Mello RN, Bona S, Dias AS, Porawski M, Ferraz Ade B, Richter MF, Marroni NP. Effect of Agaricus blazei Murill on the pulmonary tissue of animals with streptozotocin-induced diabetes. Exp Diabetes Res. 2010;2010:543926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Oh TW, Kim YA, Jang WJ, Byeon JI, Ryu CH, Kim JO, Ha YL. Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. J Agric Food Chem. 2010;58:4113-4119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Hsu CH, Liao YL, Lin SC, Hwang KC, Chou P. The mushroom Agaricus Blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: a randomized, double-blinded, and placebo-controlled clinical trial. J Altern Complement Med. 2007;13:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Niwa A, Tajiri T, Higashino H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J Clin Biochem Nutr. 2011;48:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Hikino H, Konno C, Mirin Y, Hayashi T. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med. 1985;51:339-340. [PubMed] [Cited in This Article: ] |
| 87. | Zhang HN, He JH, Yuan L, Lin ZB. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73:2307-2319. [PubMed] [Cited in This Article: ] |
| 88. | Jia J, Zhang X, Hu YS, Wu Y, Wang QZ, Li NN, Guo QC, Dong XC. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009;115:32-36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 89. | Xiao C, Wu Q, Zhang J, Xie Y, Cai W, Tan J. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 downregulated hepatic glucose regulatory enzymes in diabetic mice. J Ethnopharmacology. 2017;196:47-57. [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 90. | Seto SW, Lam TY, Tam HL, Au AL, Chan SW, Wu JH, Yu PH, Leung GP, Ngai SM, Yeung JH. Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine. 2009;16:426-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Meng G, Zhu H, Yang S, Wu F, Zheng H, Chen E, Xu J. Attenuating effects of Ganoderma lucidum polysaccharides on myocardial collagen cross-linking relates to advanced glycation end product and antioxidant enzymes in high-fat-diet and streptozotocin-induced diabetic rats. Carbohyd Polym. 2011;84:180-185. [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Cheng CR, Yue QX, Wu ZY, Song XY, Tao SJ, Wu XH, Xu PP, Liu X, Guan SH, Guo DA. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry. 2010;71:1579-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 93. | Teng BS, Wang CD, Yang HJ, Wu JS, Zhang D, Zheng M, Fan ZH, Pan D, Zhou P. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J Agric Food Chem. 2011;59:6492-6500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Lee IK, Yun BS. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot (Tokyo). 2011;64:349-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 95. | Lima CU, Cordova CO, Nóbrega Ode T, Funghetto SS, Karnikowski MG. Does the Agaricus blazei Murill mushroom have properties that affect the immune system? An integrative review. J Med Food. 2011;14:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Vitak TY, Wasser SP, Nevo E, Sybirna NO. The Effect of the Medicinal Mushrooms Agaricus brasiliensis and Ganoderma lucidum (Higher Basidiomycetes) on the Erythron System in Normal and Streptozotocin-Induced Diabetic Rats. Int J Med Mushrooms. 2015;17:277-286. [PubMed] [Cited in This Article: ] |
| 97. | Tahmasebpour N, Dehghan G, Feizi MAH, Esmaeili HA. Variation in body weight and some hematological parameters in streptozotocin-induced diabetic rats, treated with Teurcium orientale. Pharmacology OnLine (PhOL). 2013;3:32-36. [Cited in This Article: ] |
| 98. | Yurkiv B, Wasser SP, Nevo E, Sybirna NO. The Effect of Agaricus brasiliensis and Ganoderma lucidum Medicinal Mushroom Administration on the L-arginine/Nitric Oxide System and Rat Leukocyte Apoptosis in Experimental Type 1 Diabetes Mellitus. Int J Med Mushrooms. 2015;17:339-350. [PubMed] [Cited in This Article: ] |
| 99. | Gouni-Berthold I, Krone W. Diabetic ketoacidosis and hyperosmolar hyperglycemic state. Med Klin (Munich). 2006;101 Suppl 1:100-105. [PubMed] [Cited in This Article: ] |
| 100. | Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93:1541-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 101. | Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res. 2012;159:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 102. | Moussa SA. Biophysical changes in red blood cells and hemoglobin components of diabetic patients. J Gen Eng Biotechnol. 2007;5:27-32. [Cited in This Article: ] |
| 103. | Lawen A. Apoptosis-an introduction. Bioessays. 2003;25:888-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386-401. [PubMed] [Cited in This Article: ] |
| 105. | Ferents IV, Brodiak IV, Liuta MIa, Kulachkovs’kyĭ OR, Sybirna NO. [Effects of decarboxylated L-arginine on morphofunctional characteristics of the erythron in experimental diabetes mellitus in rats]. Fiziol Zh. 2014;60:70-79. [PubMed] [Cited in This Article: ] |
| 106. | Henriques GS, Helm CV, Busato AP, Simeone MLF. Lipid profile and glycemic response of rats fed on a semi-purified diet supplemented with Agaricus brasiliensis mushroom. Acta Scientiarum. Health Sciences, Maringá. 2016;38:71-79 Available from: http://www.alice.cnptia.embrapa.br/handle/doc/1047204. [Cited in This Article: ] |
| 107. | Tapola N, Karvonen H, Niskanen L, Mikola M, Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2005;15:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Chen J, Raymond K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc Health Risk Manag. 2008;4:1265-1272. [PubMed] [Cited in This Article: ] |
| 109. | Li B, Allendorf DJ, Hansen R, Marroquin J, Ding C, Cramer DE, Yan J. Yeast beta-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J Immunol. 2006;177:1661-1669. [PubMed] [Cited in This Article: ] |
| 110. | Zhang YR, Wang DW, Zhang YY, Liu TT, Li Y. Preparation, characterization and properties of Agaricus blazei murill oligopeptide. Chem J Chinese U. 2009;30:293-296. [Cited in This Article: ] |
| 111. | Carvajal AE, Koehnlein EA, Soares AA, Eler GJ, Nakashima AT, Bracht A. Peralta RM. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT Food Sci Technology. 2012;46:493-499. [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 112. | de Miranda AM, Rossoni Júnior JV, Souza E Silva L, Dos Santos RC, Silva ME, Pedrosa ML. Agaricus brasiliensis (sun mushroom) affects the expression of genes related to cholesterol homeostasis. Eur J Nutr. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Cohen N, Cohen J, Asatiani MD, Varshney VK, Yu HT, Yang YC, Li YH, Mau JL, Wasser SP. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int J Med Mushrooms. 2014;16:273-291. [PubMed] [Cited in This Article: ] |
| 114. | Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 438] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 115. | Jung KH, Ha E, Kim MJ, Uhm YK, Kim HK, Hong SJ, Chung JH, Yim SV. Ganoderma lucidum extract stimulates glucose uptake in L6 rat skeletal muscle cells. Acta Biochim Pol. 2006;53:597-601. [PubMed] [Cited in This Article: ] |
| 116. | McCormack JG, Westergaard N, Kristiansen M, Brand CL, Lau J. Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy. Curr Pharm Des. 2001;7:1451-1474. [PubMed] [Cited in This Article: ] |
| 117. | Agius L. New hepatic targets for glycaemic control in diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21:587-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 118. | Ma HT, Hsieh JF, Chen ST. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry. 2015;114:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 119. | Ramkumar V, Nie Z, Rybak LP, Maggirwar SB. Adenosine, antioxidant enzymes and cytoprotection. Trends Pharmacol Sci. 1995;16:283-285. [PubMed] [Cited in This Article: ] |