Published online Dec 15, 2016. doi: 10.4239/wjd.v7.i20.605
Peer-review started: July 1, 2016
First decision: September 5, 2016
Revised: September 16, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 15, 2016
To examine the efficacy of three extraction techniques: Soxhlet-extraction (SE), cold-maceration (CM) and microwave-assisted-extraction (MAE) using 80% methanol as solvent.
The study was performed on each of 50 g of Vernonia amygdalina (VA) and Occimum gratissimum (OG) leaves respectively. The percentage yield, duration of extraction, volume of solvent used, qualitative and quantitative phytoconstituents present was compared. The biological activities (hypoglycemic effect) were investigated using albino wistar rat model of diabetes mellitus (n = 36) with a combined dose (1:1) of the two plants leaf extracts (250 mg/kg b.w.) from the three methods. The extracts were administered orally, once daily for 21 d.
In this report, the percentage VA extract yield from MAE was highest (20.9% ± 1.05%) within 39 min using 250 mL of solvent, when compared to the CM (14.35% ± 0.28%) within 4320 min using 900 mL of solvent and SE (15.75% ± 0.71%) within 265 min using 500 mL of solvent. The percentage differences in OG extract yield between: MAE vs SE was 41.05%; MAE vs CM was 46.81% and SE vs CM was 9.77%. The qualitative chemical analysis of the two plants showed no difference in the various phytoconstituents tested, but differs quantitatively in the amount of the individual phytoconstituents, as MAE had significantly high yield (P > 0.05) on phenolics, saponins and tannins. SE technique gave significantly high yield (P > 0.05) on alkaloid, while CM gave significant high yield on flavonoids. The extracts from CM exhibited a significantly (P > 0.05) better hypoglycemic activity within the first 14-d of treatment (43.3% ± 3.62%) when compared to MAE (36.5% ± 0.08%) and SE methods (33.3% ± 1.60%). However, the percentage hypoglycemic activity, 21 d post-treatment with 250 mg/kg b.w. extract from MAE was 72.6% ± 1.03% and it was more comparable to 10 mg/kg b.w. glibenclamide treated group (75.0% ± 0.73%), unlike the SE (69.5% ± 0.71%) and CM (69.1% ± 1.03%).
CM technique produces extract with better hypoglycemic activity, whereas; MAE is a better option for high yield of phytoconstituents using less solvent within a short time.
Core tip: Extraction of active phytoconstituents from medicinal plants rely mostly on the use of appropriate extraction method. Different extraction techniques affect the yield and biological activity of phytocomponents. In this study, we observed that microwave assisted extraction produces significantly higher overall extract yield as well as in phenolic, saponin and tannin content. Cold maceration and soxhlet extraction produced higher flavonoid and alkaloid yield respectively. Maceration extracts exhibited significantly better hypoglycemic activities in diabetic rats compared to extracts from soxhlet and microwave assisted extraction. This study reveals that the choice of extraction protocol should depend primarily on the purpose of interest.
- Citation: Okoduwa SIR, Umar IA, James DB, Inuwa HM, Habila JD. Evaluation of extraction protocols for anti-diabetic phytochemical substances from medicinal plants. World J Diabetes 2016; 7(20): 605-614
- URL: https://www.wjgnet.com/1948-9358/full/v7/i20/605.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i20.605
Diabetes mellitus (DM) is one of the most common non-communicable diseases globally, affecting the quality of human life of all ages across the world[1,2]. The disease has become a global public health problem affecting the socio-economic status of the individual[3]. It is an age long, serious heterogeneous metabolic disorder characterized by hyperglycemia and glucose intolerance, due to endogenous insulin deficiency, impaired effectiveness of insulin action, or both[4]. With DM the body cannot regulate the amount of sugar in the blood. This leads to increased glucose in the body that causes deregulation of the metabolism, often accompanied by glycosuria, polydipsia, and polyuria[4].
According to International Diabetic Foundation report, every 6 s, a person dies from diabetes[5]. In 2013, 5.0 million deaths were recorded across the globe with a prevalence of 8.3%[5]. A total of 415 million people are affected with diabetes, worldwide as at December, 2015. The figure is estimated to rise above 642 million by 2040[5]. In Nigeria, 3921500 cases have been reported as at 2013 with a prevalence rate of 4.99%. This alarming rate calls for urgency to find better treatment and novel prevention strategies for the disease.
Several hypoglycemic drugs are available for managing diabetes since it is incurable but they suffer from generally inadequate efficacy and number of serious adverse effects[6,7]. Hence, the shift to the use of plant source, a new hopeful approach that has long been authenticated by World Health Organization in its general assembly[8].
Plants are the major source of potential therapeutic agents worldwide. The uses of Plants for therapeutic purposes have been recorded to be as long as history. Plants which contain substances that could be used for medicinal purposes or which are precursors for the synthesis of useful drugs are considered as therapeutic plants[9]. According to the report of Farnsworth and Soejarto[10], there are between 35000 and 70000 plant species that have been used for medicinal purposes in the world[10]. The bioactive components present in plants can only be utilized in disease treatment/management after being extracted with suitable solvent and prepared into substances such as ointment, cream, gel, moisturizer, pills and so on[11,12].
Extraction is the partitioning of therapeutically dynamic segments of plant utilizing suitably selective solvents through standard methods[12,13]. The basis of all extraction in medicinal plant research is to isolate the dissolvable plant metabolites, excluding the insoluble cell marc. The preliminary unrefined extracts obtained utilizing these strategies contain complex blend of numerous plant metabolites, for example, alkaloids, glycosides, phenolics, terpenoids and flavonoids[12,14,15]. Some bioactive components present in plants are either heat sensitive or solvent specific hence the quality and composition of the extracts as well as their biological activities are affected by the type of extraction procedure. To get the most astounding biological efficacy and yield of plant extract, it is important to consider these limitations and utilize a standardized method for a specific bioactive molecule[14,16].
The needs of standardized extraction methodology for unrefined medications are to accomplish the remedially craved part and to dispose of the inactive portion by treatment with a specific solvent called menstruum. The extract consequently obtained might be prepared for use as a therapeutic agent in the form of tinctures and fluid extracts, it may be further processed to be fused in any dosage form such as tablets or capsules, or it might be fractionated to seclude singular synthetic substances, for example, vincristine, hyoscine and ajmalicine which are modem drugs. For that reason, standardization of extraction methodologies contributes fundamentally to the final nature of the medicinal drug[13,15].
However, with the expanding interest for natural therapeutic products and nutraceuticals for healthcare everywhere throughout the world, producers of medicinal plant extracts are in continuous search for the most suitable extraction strategy keeping in mind the end goal to produce extracts of characterized quality with minimal variability from batch to batch. Conventional extraction is generally carried-out using reflux, maceration, soxhlet and distillation techniques. These techniques which have been utilized for a long time are extremely tedious and require generally a lot of solvents. Extraction utilizing non-routine techniques, for instance, microwave techniques can produce high yield, within a shorter time utilizing a smaller amount of solvents[17,18]. Among the different customary and routine extraction systems, Soxhlet extraction has been the most generally utilized. Unfortunately, there is paucity of literature on the best extraction method for a specific bioactive molecule. The evaluation on some of these methods by most previous investigators focuses on either the yield or duration of extraction, without considering the effects of the protocol on the various bioactive entities that works in a synergic manner.
Soxhlet extraction serves not just as a method for extraction of phyto-constituents but additionally as a reference to look at more current extraction procedures. Previously, it has been suggested that the microwave-assisted-extraction (MAE), a present day extraction method is a superior method for extricating phyto-components from plants[19]. Several reports on the usefulness of the MAE as it concerns medicinal plants have been published[20-24].
Vernonia amygdalina (VA) commonly known as bitter leaf and Occimum gratissimum (OG) generally refers to as scent leaf, have been reported to have anti-diabetic properties[25]. The efficacy of the combined use of both plants with respect to diabetes has been documented[26]. Also, their hypoglycemic activities have been attributed to the presence of flavonoids, alkaloids and saponins among others[27-29]. It is therefore imperative to examine the effect of different extraction methods on their biological activities.
From our insight, the extraction of phytoconstituents from VA and OG using MAE strategy has not yet been accounted for. These two plants were chosen as a reference point for other therapeutic plants owing to the fact that their anti-diabetic potentials have been established in recent publications[26-31]. Therefore, this study evaluated three extraction technologies, viz: MAE, Soxhlet extraction and cold maceration with an aim to present a comparison among the distinctive strategies utilized for extraction of hypoglycemic bioactive constituents from medicinal plants. The primary goal of the research is to give a successful and effective, straightforward, safe and less time consuming with maximal yield strategy for extricating specific bioactive parts from therapeutic plants.
Fresh specimen of VA and OG leaves were harvested in the month of May, 2015 from a local farm in Samaru, Zaria, Kaduna State, Nigeria. The plant samples were identified and authenticated by the Herbarium unit of the Department of Biological Science, Ahmadu Bello University, Zaria, Nigeria. A voucher specimen number 1166 and 1285 were deposited for VA and OG respectively. The leaves of the plants sample were dried under shade at room temperature to constant weights for seven days. The dried samples were then pulverized into powder using a laboratory milling machine (Thomas-Wiley Laboratory mill Model 4, United States). The powders were preserved in clean plastic containers, kept away from light, heat and moisture until use.
All the chemicals and reagents used were of analytical reagent grade.
The research was conducted between May and December, 2015.
A conventional microwave oven (2450 MHz, Toshiba, and Tokyo, Japan) with variable power up to 1000 watts, a time controller, beam reflector and a stirring device was used.
Two conventional extraction techniques namely, Soxhlet and cold maceration were used in comparison with a new modern technology, the MAE technique.
Exhaustive Soxhlet extraction was performed using classical apparatus with accurately weighed 50 g of the powdered leaf samples of VA and OG respectively. Extraction was performed with 80% methanol as the extraction solvent. After extraction, the methanol solvent was evaporated by concentrating under vacuum with rotary evaporator (Senco Rotary Evaporator, Model RE 801) at 40 °C under reduced pressure. The solvent free methanol extract was thereafter evaluated.
Maceration was carried out in a closed conical flask for 72 h. In both case 50 g powdered VA and OG leaf sample and 80% methanol as the extraction solvent were used. The suspension after maceration was centrifuged and the supernatant evaporated under reduce pressure. The solvent free methanol extracts obtained were similarly evaluated.
Accurately weighed 50 g of the homogeneous powder leaf samples was mixed with 60 mL, 80% methanol. After allowing a preleaching time of 5 min the suspension was irradiated with microwave at optimized conditions[32-34]. The samples were treated under microwave irradiation in an intermittent way, i.e., Irradiation: cooling: irradiation. The microwave irradiation time was set at three minutes and cooling time of five minutes was allowed. After 5 repeats, the samples were centrifuged at 4000 rpm and the supernatant evaporated under pressure. The dried residue was evaluated accordingly.
The percentage extraction yield (w/w) by the three extraction methods was calculated using the formula: Percentage extraction yield for plant extract = [mass of extract (g)/mass of plant sample (g)] × 100
Aliquots of the extracts were stored in screwed cap vials at 4 °C-8 °C until further use. The extracts were re-dissolved in distilled water when required and given orally through gastric intubations.
Standard protocols were used in detecting the phyto-chemical constituents present in the two plants samples[35,36]. Tannins according the method describe by Markkar et al[37], Saponins as described by Bruneton[38], Alkaloids as described by Harbone[39], Flavonoids as described by Bohm et al[40].
To test biological activities of the plant extract from the different extraction techniques, thirty six albino wistar rats (150-200 g of either sex) fed with rat pellet diet (Grand Cereals Ltd, Nigeria) and water ad libitum were used. Animals were first acclimatized for two weeks before used. The study was conducted at the Research and Development Laboratory of Nigerian Institute of Leather and Science Technology (NILEST), Zaria Nigeria. The anti-hyperglycemic effect of the extracts obtained from the three extraction methods were assessed using rat model of DM. The experimental protocol was approved by the Institutional Animal Ethic Committee. All experimental protocol was in conformity with the institutional guidelines that are in compliance with National and International Laws and Guidelines for Care and Use of Laboratory Animals in Biomedical Research. The rules and regulations in accordance to the Ethical Committee directive were strictly followed.
The rat model of diabetes used for this study was developed as followed. First, the rats were fasted overnight after which they were given a single intra-peritoneal injection (ip) of 55 mg/kg b.w. of streptozotocin (STZ) (Adooq Bioscience, LLC, United States) dissolved in 0.1 mL fresh cold citrate buffer pH 4.5.
Confirmation of diabetes was done 72 h after STZ induction, using a One Touch Glucometer (Lifescan Inc 1995 Milpas, California, United States). Blood samples were obtained from the tail puncture of the rats. Animals with fasting blood glucose ≥ 200 mg/dL, after 10 d of STZ induction were considered diabetic and included in the study as diabetic animals[30].
Thirty six rats were divided into 6 groups of 6 rats per group. The treatments were as follows: (1) diabetic rats treated with extracts from cold maceration 250 mg/kg b.w.; (2) diabetic rats treated with extracts from soxhlet extraction (SEE) 250 mg/kg b.w.; (3) diabetic rats treated with extracts from MAE 250 mg/kg; (4) diabetic rats as positive control treated with standard drug (10 mg/kg b.w, glibenclamide); (5) diabetic rats as negative control. No treatment was given; and (6) non-diabetic rats as standard control (no induction, no treatment).
The extracts were administered orally, once daily for 21 d using combined dose (1:1) of the two plants leaf extracts (250 mg/kg b.w.)
Blood sample was withdrawn from the tail vein and tested using glucose test strips and glucometer (On-Call Plus, Acon Laboratories Ins, United States) after an overnight fast.
The results obtained were expressed as mean ± SD where applicable. The data were analyzed using analysis of variance and significant differences among means were determined by Duncan’s multiple range test at P < 0.05 using Statistical Package for Social Sciences software version 20 for windows.
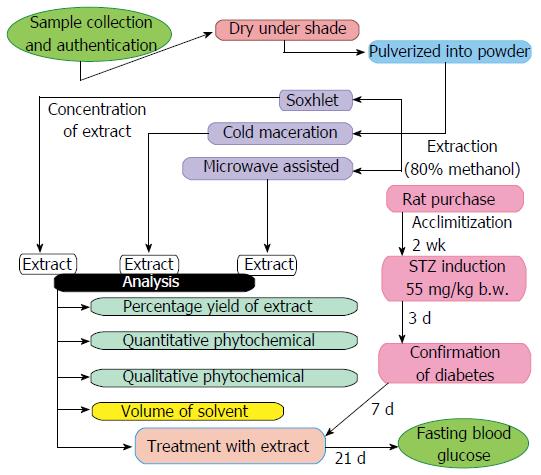
The efficacy of three extraction methods were compared by evaluating the anti-hyperglycemic effects of the two medicinal plants (VA and OG) leaf extracts. A flow chart illustrating the experimental design in detail is presented in Figure 1. The experiments were performed using the same quantity of plant samples (50 g each) and the biological activities were analyzed using rat model of diabetes with the same dose of extract (250 mg/kg body weight). A comparison of the extraction of the medicinal plants (VA and OG) using conventional microwaves, soxhlet extractor and cold maceration are shown in Table 1.
| Vernonia amygdalina | Ocimum gratissimum | |||||
| Parameter | MAE | Soxhlet | Maceration | MAE | Soxhlet | Maceration |
| Sample (g) | 50 | 50 | 50 | 50 | 50 | 50 |
| Solvent volume (mL) | 250 | 500 | 900 | 250 | 500 | 900 |
| Extraction time (min) | 39 | 265 | 4320 | 39 | 255 | 4320 |
| Recovery yield (g) | 10.45 ± 0.53b | 7.88 ± 0.35a | 7.18 ± 0.14a | 9.55 ± 0.84b | 5.63 ± 0.21a | 5.08 ± 0.32a |
| Percentage recovered (%) | 20.90 ± 1.05b | 15.75 ± 0.71a | 14.35 ± 0.28a | 19.10 ± 1.67b | 11.25 ± 0.24a | 10.15 ± 0.65a |
The yield of MAE extracts from VA for 39 min were higher (20.90% ± 1.05%) than that of soxhlet extract (15.75% ± 0.71%) for 265 min and that of maceration extract (14.35% + 0.28%) for 4320 min. Similarly, the yield of microwave extracts from OG (19.10% ± 1.67%) for 39 min were higher than that of soxhlet extract (11.25% ± 0.42%) for 255 min and that of maceration extract (10.15% ± 0.65%) for 4320 min. Regarding the extraction time, our result show that MAE appeared to be the fastest method since the extraction could be achieved within minutes. The MAE also consumed the least amount of solvent 250 mL when compared to the other methods, 500 mL and 900 mL for soxhlet and cold maceration respectively (Table 1).
The percentage differences in the recovery yield between two specific extraction methods are shown in the Table 2. It was observed that for VA, the difference in percentage recovery yield between MAE vs soxhlet extraction method was 24.59%; but with OG it was 41.05%. The difference in percentage yield between MAE vs cold maceration method was 31.29% for VA and 46.81% for OG. Although there were no significant differences between the soxhlet and cold maceration method for both plants, the percentage difference was 8.88% and 9.77 for VA and OG respectively (Table 2).
| Vernonia amygdalina | Ocimum gratissimum | ||
| MAE | Soxhlet | Maceration | |
| MAE | 41.05% | 46.81% | |
| Soxhlet | 24.59% | 9.77% | |
| Maceration | 31.29% | 8.88% | |
The results from the present study showed that there was no difference between the qualitative phytoconstituents obtained by the various extraction technologies under investigation (Table 3).
| S/N | Constituents | Maceration | Soxhlet | Microwave | |||
| VA | OG | VA | OG | VA | OG | ||
| 1 | Carbohydrates | + | + | + | + | + | + |
| 2 | Anthraquinones | - | - | - | - | - | - |
| 3 | Glycosides | + | + | + | + | + | + |
| 4 | Cardiac glycosides | + | + | + | + | + | + |
| 5 | Saponins | + | + | + | + | + | + |
| 6 | Steroids | + | + | + | + | + | + |
| 7 | Triterpenes | + | + | + | + | + | + |
| 8 | Tannins | + | + | + | + | + | + |
| 9 | Flavonoids | + | + | + | + | + | + |
| 10 | Alkaloid | + | + | + | + | + | + |
However, the quantitative chemical analysis (Table 4) revealed a statistically higher yield in alkaloid from the soxhlet extraction when compared to the MAE and cold maceration for VA. But there was no significant difference in the alkaloid yield between the soxhlet and MAE method for OG. The cold maceration technology recorded the highest yield in flavonoid in both plant when compared to the MAE and soxhlet extraction method. The difference was statistically significant (P < 0.05). The MAE had the highest yield in phenolics, saponins and tannins from the two plants studied when compared to the conventional extraction techniques (soxhlet and cold maceration). However, there was no significant difference between MAE and soxhlet in the yield of tannin from VA.
| Vernonia amygdalina (mg/100 g) | Ocimum gratissimum (mg/100 g) | |||||
| MAE | Soxhlet | Maceration | MAE | Soxhlet | Maceration | |
| Alkaloid | 4.0 ± 0.65a | 7.0 ± 0.57b | 5.0 ± 0.46a | 6.5 ± 0.75b | 7.0 ± 0.15b | 5.0 ± 0.90a |
| Flavonoid | 13.0 ± 0.35b | 9.0 ± 0.50a | 15.0 ± 0.21c | 10.0 ± 0.35b | 8.0 ± 0.18a | 12.0 ± 0.25c |
| Phenolic | 17.5 ± 0.25c | 15.0 ± 0.21b | 12.0 ± 0.70a | 15.0 ± 0.22c | 14.0 ± 0.21b | 11.0 ± 0.65a |
| Saponin | 4.5 ± 0.45b | 3.0 ± 0.30a | 2.0 ± 0.75a | 6.1 ± 0.75b | 4.0 ± 0.92a | 5.0 ± 0.20a |
| Tannin | 14.0 ± 0.55b | 13.0 ± 0.50b | 10.0 ± 0.25a | 12.0 ± 0.18b | 9.0 ± 0.24a | 9.1 ± 0.25a |
Soxhlet, cold maceration and MAE extracts of VA and OG were tested and compared for anti-diabetic activities. All the extracts exhibited comparable anti-diabetic activities with that of standard drug (glibenclamide) under the same dose rate of 250 mg/kg body weight tested according to the method of Abdulazeez et al[26]. The cold maceration extract exhibited a better hypoglycemic effect within the first 7 (17.9%) and 14 (43.2%) d of treatment when compared to others. But the hypoglycemic activity of extract obtained from MAE was more comparable to the standard drug glibenclamide in reducing the blood glucose of the animals after 28 d post induction (21 d treatment).
The extract from MAE exhibited the least hypoglycemic effect within the first 7 day of treatment. There was no significant difference between the hypoglycemic effects of extracts obtained from soxhlet and the cold maceration method at the end of the 21 d (Tables 5 and 6).
| Group | Before induction | 7 d after induction | Days after treatment | ||
| 7th | 14th | 21st | |||
| CME | 79.4 ± 4.5 | 344.6 ± 10.3 | 282.8 ± 17.6 | 195.8 ± 18.3 | 106.5 ± 6.7 |
| SEE | 85.3 ± 3.2 | 396.8 ± 15.7 | 342.5 ± 14.7 | 264.9 ± 16.8 | 121.1 ± 10.2 |
| MAE | 82.7 ± 5.1 | 373.3 ± 13.6 | 331.3 ± 11.4 | 237.1 ± 8.9 | 102.4 ± 7.6 |
| PC | 91.6 ± 4.5 | 389.4 ± 11.9 | 359.0 ± 12.6 | 266.3 ± 13.2 | 97.3 ± 5.8 |
| NC | 80.6 ± 3.8 | 365.5 ± 9.8 | 395.1 ± 16.3 | 419.6 ± 10.2 | 448.4 ± 17.4 |
| HC | 83.2 ± 6.2 | 84.8 ± 4.7 | 83.9 ± 7.8 | 84.7 ± 6.5 | 84.2 ± 7.1 |
| Group | Initial blood glucose on day 0 (mg/dL) | Percentage change after treatment (%) | ||
| Day 7 | Day 14 | Day 21 | ||
| CME | 344.6 ± 10.3 | 18.0 ± 2.66e | 43.3 ± 3.62e | 69.1 ± 1.03c |
| SEE | 396.8 ± 15.7 | 13.7 ± 0.29d | 33.3 ± 1.60c,d | 69.5 ± 0.71c |
| MAE | 373.3 ± 13.6 | 11.2 ± 0.18c,d | 36.5 ± 0.08d | 72.6 ± 1.03d |
| PC | 389.4 ± 11.9 | 7.8 ± 0.42c | 31.6 ± 1.30c | 75.0 ± 0.73d |
| NC | 365.5 ± 9.8 | -8.1 ± 1.57a | -14.8 ± 0.29a | -22.7 ± 1.47a |
| HC | 84.8 ± 4.7 | 1.4 ± 3.96b | 0.1 ± 2.12b | 0.7 ± 2.88b |
Developing nations are rich in therapeutic plants at the same time, because of trouble in getting reliably effective extraction equipments; esteem expansion to this rich bioresources are difficult. Usually and prevalently in extremely poor nations, the advancements utilized are improper and not efficient. The crucial setback is identified with the nature of the products. Primitive extraction techniques don’t promise a steady and top notch reliable quality and, sometimes, unseemly innovations and techniques result in creating defiled products which has low market value[41]. The present study, evaluated three different technologies for extraction of hypoglycemic compounds from medicinal plants, with a view to ascertain the best option for the isolation of specific bioactive entity.
In the study it was noticed that Soxhlet extraction and cold maceration spent longer time for complete extraction of same quantity of the plant sample studied. Also, the amount of the solvent utilized in MAE was less, proving that, MAE is indeed truly economical. Comparable results were reported by Vongsangnak et al[42], when contrasting routine extraction procedures with MAE throughout the isolation of saponins from cell culture of Panax notoginseng and analgesic substances from the roots of Ximenia americana[20]. Besides, microwave illumination technique has been reported to be extremely quick, dependable for making of Schiff bases[21]. Possible explanation observed in the various methods of extraction could be due to differences in rate of chemical reaction. For instance, in soxhlet extraction approach, the applied heat supplies the activation energy needed to rupture the plant tissues in order to release its phytoconstituents. Whereas in MAE, the activation energy is achieved due to oscillatory wave generated by the system and by the ionic conduction and dipole rotation of the molecules of the plant sample. This brings about increase in the cell pressure, thereby rupturing to release its contents on attaining its elastic limit. The heat in MAE is internally generated by the molecules unlike in soxhlet whereby the heat is externally generated. In cold maceration, the release of phytoconstituents from plant matrix is due to differences in ionic solvent concentration gradients, hence more solvent is require to create a positively dynamic concentration gradients. This account for the higher volume of solvent utilized by the cold maceration as observed in the present study. In a nutshell, with MAE, the plant cell tissues are effectively broken and separated to release phytoconstituents with ease, hence the greater yield as recorded.
The result of the quantitative phytochemical screening within the limit of the analyzed components in the present study suggests that the phytoconstituents of the studied plants were not destroyed since the results obtained showed that the same phytoconstituents tested in all the extracts from the different procedures were the same. This finding is in agreement with earlier reports of Mandal et al[19], that, the plant components obtained from MAE are neither decomposed nor oxidized under optimized conditions. Several phytochemicals have been found to give an increase in their extractive yields when compared to their yields on subjection to conventional extraction techniques[21]. Chan et al[22] also recorded a higher yield in the extraction of anti-diabetic ingredient from herbal plant. In their experiment using 5 g sample with 150 mL solvent recorded a yield of 1.63 mg/g sample in 5 min using MAE as against 0.47 mg/g in 3 h obtained with soxhlet extraction technology. In a related study for extraction of caffeine and polyphenols from leaves of green tea, MAE achieved higher extraction yield within 4 min than any extraction methods at room temperature for 20 h[43]. Ginsenosides extraction yield from ginseng root was obtained in 15 min using focused MAE technique which was also better than other conventional solvent extraction technologies for 10 h[24,44]. The higher yield obtained from MAE and soxhlet extraction when compared to the cold maceration may be attributed to the heat exchange and mass transfer.
In soxhlet extraction, the heat exchange and the mass transfer are restricting variables contrast with MAE, where heat exchange happens from the focal point of the specimens to the external colder environment, and volumetric warming impact prompts a speedier ascend in temperature. Additionally, the interior warming of in situ water inside of the plant material expands the plant cells and prompts the burst of the plant tissues[45]. This may be the explanation behind the higher extraction of phytochemicals from MAE when compared to soxhlet and cold maceration extracts observed in the present study. It is very obvious from these results, that microwave extraction represents a promising substitute for extracting hypoglycemic compounds from natural substrate.
The hypoglycemic activity of the 250 mg/kg b.w. cold maceration extract was significantly better than others within the first 14 day of treatment. Surprisingly after 21 d treatment, the blood glucose lowering capacity of the MAE extract was almost the same with that of 10 mg/kg b.w. glibenclamide. The percentage change in blood glucose by MAE extract after the experimental duration of 21 d treatment was 72.6% whereas that obtained from the standard drug glibenclamide was 75.0%. The higher yield of alkaloid by soxhlet extraction may account for its better efficacy when compared to the cold maceration in the present study. Saponin, alkaloid and flavonoid are known to play a significant role in anti-diabetic action[27-29].
MAE had the highest yield in three different phytoconstituents viz phenolics, saponins and tannins. This suggests that microwave methanol extract could be used even at 250 mg/kg b.w. to complement currently available oral hypoglycemic drugs. The results suggest that microwave technology is a viable means for extracting valuable anti-diabetic components from medicinal plants. The reason for the observed differences in hypoglycemic activities by the various extract within the first 14 d and after the 21 d of experimental treatment could be due to the concentrations of the phyto-chemicals which vary in the extracts. It could also be possible that the different phytochemical exhibiting hypoglycemic effect have different rate of reaction in reducing the blood glucose level.
The main advantages of MAE over the conventional extraction techniques is that it reduces solvent consumption, it has a shorter operational time, modestly high recoveries, decent reproducibility and negligible specimen control for extraction process[46]. Several biologically active compounds have been extracted by application of MAE, such as extraction of azadiractine related limonoids from Azadirachta indica seed kernels[47], extraction of artemisinin from Artemisia annua[48] and ginsenosides extraction from roots of Panax ginseng[44], quercetin from herbal plant[22]. According to Pan et al[49], antioxidant activity of phenolic substances extricated from the peel of Dimocarpus Longan utilizing MAE was better than that of Soxhlet extraction. Besides, MAE of curcumin from Curcuma longa showed a better results and a higher extraction yield with noteworthy diminishing in the extraction time when compared to that of Soxhlet extraction, maceration and stirring extraction[50]. In the present study, there was higher yield in phenolics, saponins and tannins from both plants-VA and OG. This implies that MAE is a better technology for the extraction of these phytoconstituents. The soxhlet extraction technology showed a higher yield in alkaloid whereas maceration technology was best for the extraction of flavonoids.
We find the use of MAE leads to very fast extraction rate with high value of phytoconstituents compared to soxhlet and cold maceration technique. The findings obtained from the present research showed that the choice of extraction technology should be based primarily on the phytochemical entity of interest. For instance, with respect to the result from the present research, the use of soxhlet extraction technology would be recommended when alkaloid is the main phytoconstituent of interest, whereas, the cold maceration would be preferred for extraction of flavonoids. However, since there was no apparent destruction of any bioactive components by the extraction technologies studied, the MAE is recommended as the most suitable technology for routine extraction processes because it is faster, utilizes relatively less amount of solvent and saves more time. Nevertheless, since extraction efficiency differs from efficacy, no single method can be rated as best for extracting all forms of phyto-components. So, a further study on the isolation of the precise bioactive component(s) and its structural elucidation is recommended to ascertain the best technology for obtaining pure bioactive hypoglycemic compound(s) from medicinal plants.
The authors say thanks to the management of SIRONigeria Global Limited, Abuja for providing the basic logistics for the research. The Director and Staff of R and D, NILEST, Zaria for their technical assistance throughout the period of the research.
Although several methods are available for extraction of active phytoconstituents from medicinal plants. The technique that produces higher yield of specific phytoconstituents has not been reported. The biological activities of these active phytoconstituents are known to be affected by the extraction protocol employed. To address these issues, this study examines three different procedures of extracting anti-diabetic substances from medicinal plants and evaluated their hypoglycemic activities.
There is active research in the field of investigation of a more effective extraction technique of active ingredient from medicinal plant. Hence, it is imperative to ascertain which extraction protocol produces a significant amount of specific phytoconstituents.
The hypoglycemic activity of anti-diabetic plant is revealed in this study to be subject to the extraction method employed. For the first time, extraction method producing significantly high yield of specific phytoconstituents is presented.
Since the biological efficacy of phytoconstituents is subject to the extraction protocol employed, the use of an appropriate extraction technique with respect to a specific active ingredient would enhance the process of drug development.
Phytoconstituents are chemical compounds that occur naturally in plants. Some of which are responsible for the medicinal effect of the plant.
The authors provided the complete review of this issue. This manuscript provides the updated evidence to the readers.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liao KF, Papanas N S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Okoduwa SI, Umar IA, Ibrahim S, Bello F, Habila N. Age-dependent alteration of antioxidant defense system in hypertensive and type-2 diabetes patients. J Diabetes Metab Disord. 2015;14:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Cheng Y, Malik U, Chang S. The risk factors of diabetic nephropathy in Taiwan, including old age, hypertension and aspirin therapy. Int J Diabetes Dev C. 2013;33 Suppl 2:128. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Okoduwa SI, Umar IA, Ibrahim S, Bello F, Ndidi US. Socio-economic status of patients with type 2 diabetes and hypertension attending the Ahmadu Bello University Teaching Hospital, Zaria, North-West Nigeria. Glob J Health Sci. 2015;7:280-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2986] [Cited by in F6Publishing: 3131] [Article Influence: 313.1] [Reference Citation Analysis (15)] |
| 5. | International Diabetes Federation. IDF Diabetes Atlas, 7th ed. Brussels, Belgium: International Diabetes Federation 2015; Available from: http://www.diabetesatlas.org. [Cited in This Article: ] |
| 6. | Fowler MJ. Diabetes Treatment, Part 2: Oral agents for glycemic management. Clinical Diabetes. 2007;25:131-134. [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9344] [Cited by in F6Publishing: 8746] [Article Influence: 437.3] [Reference Citation Analysis (1)] |
| 8. | Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7:45-58. [PubMed] [Cited in This Article: ] |
| 9. | Fagbohun ED, Asare, RR and Egbebi, AO. Chemical composition and antimicrobial activities of Urena lobata L. (Malvaceae). J Med Plants Res. 2012;6 Suppl 12:2256-2260. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Farnsworth NR, Soejarto , DD . Global Importance of Medicinal Plants. In: Akerele O, Heywood V, Synge H editors. The Conservation of Medicinal Plants Cambridge University Press, Cambridge, United Kingdom 1991; 25-51. [DOI] [Cited in This Article: ] |
| 11. | Handa SS. An Overview of Extraction Techniques for Medicinal and Aromatic Plants. In: Handa et al., (eds): Extraction technologies for medicinal and aromatic plants, 1st ed, no. 66. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology. Trieste, 2008: 21-25. . [Cited in This Article: ] |
| 12. | Azwanida NN. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med Aromat Plants. 2015;4:196. [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction Technologies for Medicinal and Aromatic Plants, 1st ed, no. 66. Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology. Trieste, 2008. . [Cited in This Article: ] |
| 14. | Prabhu KS, Lobo R, Shirwaikar AA, Shirwaikar A. Ocimum gratissimum: A Review of its Chemical, Pharmacological and Ethnomedicinal Properties. TOALTMED. 2009;1:1-15. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 2011;1 Suppl 1:98-106. [Cited in This Article: ] |
| 16. | Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial property of natural compounds of plant origin. Current methods and future trend. Afr J Biotechnol. 2008;7 Suppl 12:1797-1806. [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Hijazi A, Bandar H, Rammal H, Hachem A, Saad Z, Badran B. Techniques for the Extraction of Bioactive Compounds from Lebanese Urtica dioica. AJPCT. 2013;1:Suppl 6: 507-513. [Cited in This Article: ] |
| 18. | Chemat F, Tomao V, Virot M. In: Otles, S. (Ed.), Handbook of Food Analysis Instruments. Ultrasound-Assisted Extraction in Food Analysis. CRC Press, 2008: 85-94. [Cited in This Article: ] |
| 19. | Mandal V, Mohan Y, Hemalatha S. Review Article Microwave Assisted Extraction an Innovative and Promising Extraction Tool for Medicinal Plant Research, Pharmacognosy Review. PHCOG Rev. 2007;7-18. [Cited in This Article: ] |
| 20. | Kenmogne SB, Ngassoum M, Tchatchueng JB, Vardamides JC, Dongmo . Microwave Assisted Extraction of Analgesic Compounds of the Root of Ximenia americana (Olacaceae). RJCS. 2014;4 Suppl 7:7-10. [Cited in This Article: ] |
| 21. | Savalia RV, Patel AP, Trivedi PT, Gohel HR, Khetani DB. Rapid and Economic Synthesis of Schiff Base of Salicylaldehyde by Microwave Irradiation. RJCS. 2013;3 Suppl 10:69-76. [Cited in This Article: ] |
| 22. | Chan CH, Yusoff R, Ngoh GC, Kung FW. Extraction of anti-diabtetic active ingredient, quercetin from herbal plant using microwave-assisted extraction (MAE) technique, International conference on Materials for Advanced Technologies. SUNTEC Singapore, 2011: KK-PO2-5. . [DOI] [Cited in This Article: ] |
| 23. | Tatke PA, Jirge , SS , Shukla TA. An extraction procedure of scopoletin from Convovuulus plaricuulis (Shankhapushpi). JMAPS. 2010;31:126-126. [Cited in This Article: ] |
| 24. | Dhobi M, Mandal V, Hemalatha S. Optimization of microwave assisted extraction of bioactive flavonolignan silybinin. J Chem and Metrl. 2009;3:Suppl 1: 13-23. [Cited in This Article: ] |
| 25. | Mohammed YT, Okasha MA, Magaji RA, Yaro AH. Effects of aqueous leaves extract of Ocimum gratissimum on blood glucose levels of streptozotocin-induced diabetic wistar rats. Afr J Biotechnol. 2007;6 Suppl 18:2087-2090. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Abdulazeez MA, Ibrahim K, Bulus K, Babvoshia HB, Abdullahi Y. Effect of combined use of Ocimum gratissimum and Vernonia amygdalina extract on the activity of angiotensin converting enzyme, hypolipidemic and antioxidant parameters in streptozotocin-induced diabetic rats. AJBR. 2013;7 Suppl 9:165-173. [Cited in This Article: ] |
| 27. | Taoying Z, Denghong L, xingyuan L, Yunbo . Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J Med Plants Res. 2009;3 Suppl 4:290-293. [Cited in This Article: ] |
| 28. | Day C, Cartwright T, Provost J, Bailey CJ. Hypoglycaemic effect of Momordica charantia extracts. Planta Med. 1990;56:426-429. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 894] [Cited by in F6Publishing: 705] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 30. | Okon UA, Owo DU, Udokang NE, Udobang JA, Ekpenyong CE. Oral Administration of Aqueous Leaf Extract of Ocimum Gratissimum Ameliorates Polyphagia, Polydipsia and Weight Loss in Streptozotocin-Induced Diabetic Rats. AJMSM. 2012;2 Suppl 3:45-49. [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Modu S, Adeboye AE, Maisaratu A, Mubi BM. Studies on the administration of Vernonia amygdalina Del. (Bitter leaf) and glucophage on blood glucose level of alloxan - Induced diabetic rats. IJMPAM. 2013;1 Suppl 1:013-019. [Cited in This Article: ] |
| 32. | Proestos C, Komaitis M. Application of microwave assisted extraction to the fast extraction of plant phenolic compounds. LWT-Food Sci Technol. 2008;41 Suppl 4:652-659. [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Gharekhani M, Rafiee Z, Ghorbani M, Jafari SM. Open vessel microwave system for extraction of analytes from medicine plants. Iran Patent. 2009;59321. [Cited in This Article: ] |
| 34. | Asghari J, Ondruschka B, Mazaheritehrani M. Extraction of bioactive chemical compounds from the medicinal Asian plants by microwave irradiation. J Med Plants Res. 2011;5 Suppl 4:495-506. [Cited in This Article: ] |
| 35. | Evans WA. Plants in African traditional medicines. An over view. In Trease and Evans Pharmacognosy (15th ed). India: Saunders in Print Elsevier 2005; 448-491. [Cited in This Article: ] |
| 36. | Harbone JB. Methods of extraction and isolation. In Phytochemical Methods. London: Chapman and Hall 1998; 60-66. [Cited in This Article: ] |
| 37. | Markkar AOA, Goodchild AV. Quantification of tannins. A laboratory manual. Aleppo Syria: International Centre for Agriculture Research in the dry areas. (ICARDA), Aleppo, Syria 1996; 55. [Cited in This Article: ] |
| 38. | Bruneton J. Pharmacognosy, phytochemistry, Medicinal plants. Hamshire, UK: Intercept 1999; 385-386. [Cited in This Article: ] |
| 39. | Harbone JB. Phytochemistry Methods: a guide to modern techniques of plants analysis. London: Chapman and Hall 1973; 267-270. [Cited in This Article: ] |
| 40. | Bohm BA, Koupai-Abyazani . Flavonoids and condensed tannins from leaves of Hawaiian Vacinium reticulatum and V. calycinum (ericaceae). Pacific Science. 1994;48:458-463. [Cited in This Article: ] |
| 41. | Fermeglia M. Role of Process Simulation in Extraction Technologies for Medicinal and Aromatic Plants. In: Extraction technologies for medicinal and aromatic plants 2008; . [Cited in This Article: ] |
| 42. | Vongsangnak W, Gua J, Chauvatcharin S, Zhong , JJ . Toward Efficient Extraction of Notogingseng Saponins from Cultured Cells of Notoginseng. Biochem Eng J. 2004;18:115-120. [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. 2003;42 Suppl 2:129-133. [DOI] [Cited in This Article: ] |
| 44. | Shu YY, Ko MY and Chang YS. Microwave assisted extraction of ginsenosides from ginseng root. Microchem J. 2003;74 Suppl 2:131-139. [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Tatke P, Jaiswal Y. An overview of Microwave Assisted Extraction and its Applications in Herbal Drugs Research. Research Journal of Medicinal Plant. 2011;5 Suppl 1:21-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Odabas HI, Koca I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind Crop Prod. 2016;91:114-124. [DOI] [Cited in This Article: ] |
| 47. | Dai J, Yaylayan , V , Raghavan G, Pare J. Extraction and colorimetric determination of azadirachtin related limonoids in neem seed kernel. J Agr Food Chem. 1999;47:3738-3742. [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Hao JY, Han W, Huang SD, Xue BY, Deng X. Microwave assisted extraction of artemisinin from Artemisia annua L. Sep Purif Technol. 2002;28 Suppl 3:191-196. [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji X, Zang J, Huang F. Antioxidant activity of microwave assisted extract of longan (Dimorcarpus logan) peel. Food Chem. 2008;106 Suppl 3:1264-1270. [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Mandal V, Mohan Y, Hemalatha S. Microwave assisted extraction of curcumin by sample-solvent dual heating mechanism using Taguchi L9 orthogonal design. J Pharmaceut Biomed. 2008;46:322-327. [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |