Published online Sep 15, 2016. doi: 10.4239/wjd.v7.i17.354
Peer-review started: March 30, 2016
First decision: May 17, 2016
Revised: July 2, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: September 15, 2016
To achieve good metabolic control in diabetes and keep long term, a combination of changes in lifestyle and pharmacological treatment is necessary. Achieving near-normal glycated hemoglobin significantly, decreases risk of macrovascular and microvascular complications. At present there are different treatments, both oral and injectable, available for the treatment of type 2 diabetes mellitus (T2DM). Treatment algorithms designed to reduce the development or progression of the complications of diabetes emphasizes the need for good glycaemic control. The aim of this review is to perform an update on the benefits and limitations of different drugs, both current and future, for the treatment of T2DM. Initial intervention should focus on lifestyle changes. Moreover, changes in lifestyle have proven to be beneficial, but for many patients is a complication keep long term. Physicians should be familiar with the different types of existing drugs for the treatment of diabetes and select the most effective, safe and better tolerated by patients. Metformin remains the first choice of treatment for most patients. Other alternative or second-line treatment options should be individualized depending on the characteristics of each patient. This article reviews the treatments available for patients with T2DM, with an emphasis on agents introduced within the last decade.
Core tip: To achieve good metabolic control in diabetes and keep long term, a combination of changes in lifestyle and pharmacological treatment is necessary. Physicians should be familiar with the different types of existing drugs for the treatment of diabetes and select the most effective, safe and better tolerated by patients. This article reviews current and future treatments for patients with type 2 diabetes mellitus, its use in clinical practice and in special situations such as kidney failure and elderly patient, with an emphasis on agents introduced within the last decade.
- Citation: Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes 2016; 7(17): 354-395
- URL: https://www.wjgnet.com/1948-9358/full/v7/i17/354.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i17.354
Type 2 diabetes mellitus (T2DM) is a disease that affects more than 400 million people around the world. In 2040, there will be more than 640 million people with diabetes worldwide[1]. The prevalence of T2DM is expected to double within the next 20 years, due to the increase of the age, obesity and the number of ethnic groups of high risk in the population[2], with significant increases in cardiovascular disease[3], end-stage renal disease (ESRD)[4], retinopathy and neuropathy. Additionally, to achieve good metabolic control in diabetes and keep long term, a combination of changes in lifestyle and pharmacological treatment is necessary. Achieving near-normal glycated hemoglobin (HbA1c) significantly decreases risk of macrovascular and microvascular complications[4]. However, only about 50% of diabetic patients reach their HbA1c target[5]. Algorithms for the treatment of diabetes highlight the need for good glycaemic control to reduce the development or progression of diabetes complications. In recent years has increased the number hypoglycaemic agents available for the treatment of T2DM. A recent position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) on a patient-centered approach in the management of patients with T2DM[6] gives an overview on how different conditions and co-morbidities may influence the choice of different hypoglycaemic agents. The ADA/EASD suggests that initial intervention should focus on lifestyle changes. Moreover, changes in lifestyle have proven to be beneficial[7], but for many patients is a complication keep long term, due to differing experiences or perceptions[8]. In general, drug therapy includes not only initial hypoglycaemic agents, but other intensification strategies to maintain glycaemic control over time, often requiring several drugs with different mechanisms of action[9]. Physicians should be familiar with the different types of existing drugs for the treatment of diabetes and select the most effective, safe and better tolerated by patients.
This article reviews current and future treatments for patients with T2DM, its use in clinical practice and in special situations such as kidney failure and elderly patient, with an emphasis on agents introduced within the last decade. The aim of this review is to perform an update on the benefits and limitations of different drugs, both current and future, for the treatment of T2DM.
Dietary intake and physical exercise are the two main determinants of the energy balance[10], and they are considered as a basic base in the treatment of patients with diabetes. Adequate rest is also very important for maintaining energy levels and well-being, and all patients should be advised to sleep approximately 7 h per night[9]. Evidence supports an association of 6 to 9 h of sleep per night with a reduction in cardiometabolic risk factors[11], whereas sleep deprivation aggravates insulin resistance, hypertension, hyperglycaemia, and dyslipidaemia[12]. On the other hand, a screening of patients with suspected obstructive sleep apnoea should be performed, and refer them to a sleep specialist for evaluation and treatment[9].
Although the pharmacological options are each time more extensive and they offer more therapeutics possibilities, especially in the T2DM, the interventions in the life style are essentials in the approach of these patients and they are needed to get the therapeutics goals[13].
When nutritional intervention is contemplated, the co-morbidities that can coexist in a diabetic patient also have to be considered. The recommendations on dietary aspects can contribute to achieve the desired blood glucose, blood pressure, lipid profile and weight[10,14], as well as improve sleep apnoea, depression and quality of life related to health; in addition, it has been observed that the incidence of urinary incontinence in women is reduced[15-18].
Numerous randomized controlled trials have demonstrated the metabolic benefits of nutritional recommendations in reducing HbA1c; being variables the results got depending mainly on the length of the disease[19,20].
Energetic contribution: Total caloric intake diet will depend on several factors, being determining the presence of overweight or obesity. Body mass index (BMI) is a tool commonly utilized in clinical practice to classify patients and it is calculated by the following equation: [weight (kg)/height (m2)] (Table 1).
| Body mass index (kg/m2) | |
| Normal weight | 18.5-24.9 |
| Overweight grade 1 | 25-26.9 |
| Overweight grade 2 | 27-29.9 |
| Obesity grade 1 | 30-34.9 |
| Obesity grade 2 | 35-39.9 |
| Obesity grade 3 (morbid) | 40-49.9 |
| Obesity grade 4 (extreme) | ≥ 50 |
Most T2DM patients have some degree of overweight or obesity[21]. It has been connected to insulin resistance and defects in insulin secretion. These alterations favour the appearance and worsening of diabetes[22], so in these cases in addition to an adequate distribution of macro and micronutrients, we should look for as a main objective a weight reduction by reducing the caloric intake. To achieve this objective, it has been proposed that the caloric intake of the diet prescribed to a diabetic patient with obesity should contain between 500 and 1000 kcal less of its energy needs[23]. This weight reduction will improve the insulin sensitivity, being a favourable factor to improve the glycaemic control parameters[24]. In the case of patients for whom there is no excess weight, the diet should be isocaloric.
There are different formulas for calculating baseline energy needs of people (Table 2). To these basal needs, a factor depending on the physical activity must be added. The randomized trial LOOK AHEAD, showed that weight loss after an intervention in lifestyles, improve blood pressure, and blood glucose control and lipid profile[25], especially in patients with a recent diagnosis of disease[3]. When this study was prolonged, it was found that intensive nutritional intervention did not provide an improvement in the rate of cardiovascular events or weight loss when it is compared against a standard nutritional intervention[26].
| Harris-Benedict equation1 |
| Males: BMR (kcal/d) = 66 + 13.7 × weight (kg) + 5 × height (cm) - 6.8 × age |
| Females: BMR (kcal/d) = 655 + 9.6 × weight (kg) + 1.8 × height (cm) - 4.7 × age |
| Mifflin St Jeor equation2 |
| Males: BMR (kcal/d) = 10 × weight (kg) + 6.25 × height (cm) - 5 × age + 5 |
| Females: BMR (kcal/d) = 10 × weight (kg) + 6.25 × height (cm) - 5 × age - 161 |
Macronutrient distribution: There is not enough evidence to suggest an ideal percentage in the distribution of carbohydrates, lipids and proteins. There are several studies that have sought to distribute the best ratio macronutrients without finding valid results, and several dietary patterns that have been analysed as the Mediterranean diet, vegetarian or vegan diet, Dietary Approaches to Stop Hypertension (DASH), low-fat diet and low carbohydrates diet observing a modest effectiveness of managing diabetes. The benefits happen only when they are accompanied by a lose weight so more studies are needed[27].
Carbohydrates: Although there is no consensus on the percentage of carbohydrates that people with diabetes should eat, it has been shown that the amount and the type of carbohydrates are the main determinants for glycaemic control. Counting carbohydrates has proven to be very important in all patients. It allows a better adjustment of the postprandial blood glucose for those who take insulin. With this method, patients consumed a known amount of carbohydrates divided among different meals and calculated it in grams of carbohydrates per portion (Table 3). This type of measurement is more important in patients with basal-bolus treatment or with continuous insulin infusion[28].
| GI: Observed increase in blood glucose after eating 50 g of a food, compared with the observed increase after intake of 50 g of white bread or glucose |
| Glycaemic load: GI × total amount of carbohydrates (grams) of the usual food portion |
| Carbohydrates portion: amount of food containing 10 g of carbohydrates |
It is preferable that the intake of carbohydrates comes from products such as fruits, vegetables, legumes, whole grains and dairy vs those involve the added contribution of salt, fat or simple sugars[10].
Index and glycaemic load: There is large confusion in the interpretation about the effect of the diet with low glycaemic index and there is not unanimity in the results of the different studies. Even though these diets are recommended by some associations because there are studies in which have been observed a better glycaemic control when it is compared above all with high glycaemic index food[29], there are articles that have questioned this assertion. They based this divergence on: The different definition of glycaemic index, they do not take into account the fiber contribution, and the different glycaemic response to the same food in different individuals. They consider that cannot be determinate that the observed effect is exclusively due to the food’s glycaemic load[30] (Table 3).
Fiber: Dietary fiber intake, especially the fiber that provide the natural resources, has shown that improve the control of cardiovascular risk factors, and improved the glycaemic control, turning into a lower risk of cardiovascular mortality in people with diabetes[27,31]. However, some studies have shown that the effect on diabetes has a modest significance and it is achieved with high amounts of fiber a day but this is far away from a real consumption in daily life (greater than 50 g/d)[32].
Generally, and taking into account the modest beneficial effects on cardiovascular risk factors, in diabetic patients is suggested a consumption of fiber and whole grains at least similar to that recommended for the general population; about 25 g/d for women, and 38 g/d for men or 14 g per 1000 kcal[28].
Sucrose and fructose: Contrary to what one might think sucrose intakes of 10%-35% of total energy do not have a negative effect on glycaemic or lipid responses when sucrose is substituted for isocaloric amounts of starch[33]. Consume free fructose (naturally occurring from foods such as fruit) did not get worsen the glycaemic control more than other forms of sugar, although it should avoid further intake of 12% of daily calories[28]. Restriction is advised of these sugars in the diet to avoid excessive caloric intake that can contribute to weight gain if are taken in large quantities. Moreover, sugary drinks contain large amounts of fast absorbing carbohydrates and have demonstrated a cardiovascular risk and diabetes increase in the healthy population that consumes them. Especially harmful when are sweetened with fructose free. Although there are not many studies in diabetic patients, there is no reason to think they will not have the same consequences. Therefore, the consumption of these drinks is contraindicated[34].
Non caloric sweeteners: Opposite of natural simple sugars there are sweeteners with lower calorific value. Most are artificial. They do not have caloric contribution, except aspartame (containing 4 kcal/g), and do not increase blood glucose. These sweeteners can be used by diabetic patients. If they are employed to replace glucose, bring the benefit of reducing the kilocalories in the diet[35].
Proteins: It is interesting to make a differentiation between diabetic patients with and without kidney disease. In people without kidney disease, protein intake usually recommended is between 15%-20%; however, reviewing scientific studies no firm conclusion could be reached with respect to this issue. In the literature we can find different randomized clinical trials faced on this issue results. On the one hand there are studies that demonstrate that if 28%-40% of the energy of the diet is taken as proteins there is an improvement of the HbA1c, triglycerides, total cholesterol and/or LDL cholesterol[36], while others studies have not shown a benefit in any of these aspects[37]. In patients with kidney disease, whether if we refer to micro or macroalbuminuria, reducing protein intake below the usual has been undergone various tests and meta-analysis and the evidence has not shown that improve glycaemic control, cardiovascular risk factors or renal disease progression following low-protein diets[27]. With regard to the origin of proteins, there is no difference between animal and vegetable origin in relation to proteinuria[28].
Finally, the proteins in patients with T2DM, although they do not have effect on blood glucose control itself, seems to increase the insulin response so it is not advisable to use proteins in situations of hypoglycaemia.
Fat: Epidemiological studies have related fats with the risk of developing obesity and cardiovascular risk[38]. As in the rest of immediate principles there is no optimal fat proportion and, as a general rule, the recommendations for the general population (between 20%-35%) are applied for diabetic patient, paying special attention if the patient is overweight, then the percentage should be at the lower limits. Despite these recommendations, diabetic patients often take more fat than the recommended[39].
We can distinguish between saturated and unsaturated fats (monounsaturated and polyunsaturated). In addition, has to be specified that trans fatty acids may be a type of unsaturated fat but with harmful effects on the body for its different structure. Distinguish between these types is important because it has been demonstrated that the quality is more relevant than the amount of fat consumed.
There are few studies in diabetic patients about consumption of saturated fatty acids or cholesterol; in this regard the recommendations for patients with diabetes are the same as for the general population: A contribution of saturated fat < 10%, with a minimum intake of trans fatty acids and with a contribution of cholesterol < 300 mg/dL[10] preferably choosing monounsaturated and polyunsaturated fatty acids (including omega-3 fatty acids). Some studies, that have studied the Mediterranean dietary pattern, have demonstrated that monounsaturated fatty acids can improve cardiovascular risk factors and glycaemic control[40], especially if they are replaced with saturated fatty acids.
Omega-3 fatty acids: Although there are unlike results, in general we cannot say that omega-3 supplements have shown clear cardiovascular benefit[41]. However, consumption of products high in omega-3 can be positive in preventing cardiovascular disease[42].
Alcohol: Alcohol should be drunk in moderation and it should not exceed one serving per day for women, or two servings per day in the case of men. To avoid excess of energy when they are consumed, this contribution must be exchanged for other products. This moderate consumption does not harm the glycaemic control but rather in some studies has been found the contrary, with moderation can improve glycaemic control and reduce cardiovascular events.
Despite the above facts, it is very important to note that alcoholic beverages may contribute to the appearance of late hypoglycaemia especially in patients in treatment with hypoglycaemic drugs, so we should warn the patient to pay attention to any symptoms of hypoglycaemia[28].
Sodium: The recommendation for the general population to reduce sodium intake to less than 2300 mg/d shall also apply to patients with diabetes mellitus. When these also have hypertension, which is very common, reduced sodium intake should be individualized[43].
Specific supplements: The potential benefits of dietary supplements for diabetic patients with various specific nutrients have been subjected to trials. In despite of this, reliable data has not been observed to confirm benefits in glycaemic control supplementing because of supplement the diet with antioxidants as vitamin and carotenes, micronutrients such as chromium or other herbs. The recommendations of vitamins and minerals are not different from the general population, they are provided by a varied diet[38].
The physical activity and exercise are one of the basic strategies in the treatment of diabetes. Promoting exercise, within a specific plan, provides in general terms multiple benefits: Increased insulin sensitivity in tissues, improvement of glycaemic control[44], benefits in lipid profile and blood pressure, maintenance or weight loss, cardiovascular benefits, better quality of life, psychological well-being and improvement of depression[10].
Benefits of glycaemic control: In some studies it has observed a significant decrease in HbA1c in patients with T2DM who do exercise. The difference in the degree of improvement observed in the different studies will depend on the characteristics of the patient and the type of training, thus, it is more effective when training programs are based on aerobic exercises of programs based on muscle strength in isolation[45].
Other benefits: The physical exercise also brings improvement in other metabolic parameters. It helps control cardiovascular risk factors (dyslipidaemia, hypertension, weight maintenance, psychological benefits, reduces mortality, improvement cardiorespiratory fitness and peripheral neuropathy[10,45].
Types of exercise: Both aerobic and resistance exercises have demonstrated benefits in people with diabetes through increased glucose uptake and decreased insulin resistance.
Though aerobic exercise in isolation seems to get better benefits that resistance exercise[45], in patients with diabetes is recommended the combination of both types because the effect is greater than if each one is performed in isolation[46,47].
This type of training has been traditionally recommended for patients with T2DM. A frequency of at least 3 d per week is recommended, preferably if it can be increased to 5 d with no more than two consecutive days between periods of activity, because the increase of the sensitivity and the glucose tolerance is maintained for about 12-24 h. It should be done with moderate intensity which is 40%-60% of maximum aerobic capacity. This corresponds to 55%-69% of maximum heart rate according to age (maximum heart rate = 220-age)[47]. Another method for measuring the intensity can be the subjective perception of the effort that assigns values to 20 points according to the patient judgment about the activity performed (Table 4). A moderate-intensity exercise can also be an activity that can be conducted while maintaining an uninterrupted conversation.
| Intensity | % oxygen consumption | % maximum heart rate1 | Subjective perceived exertion |
| Very light | < 20 | < 35 | < 10 |
| Light | 20-39 | 35-54 | 10-11 |
| Moderate | 40-59 | 55-69 | 12-13 |
| High | 60-84 | 70-89 | 14-16 |
| Very high | > 85 | > 90 | 17-19 |
| Maximum | 100 | 100 | 20 |
The effect of exercise in T2DM is clearly related to the volume done, thus, in different societies, it is recommended at least a minimum of 150 min per week[43,47]. Despite following the same recommendations, it has recently published a review where it is expounded that shorter performance exercises, with reference to the accumulated time during the week, keeps some benefit although this is less[48].
This type of exercise should be performed 2-3 times a week on non-consecutive days. For optimal gains in strength and insulin action, training should be moderate (50% of 1 repetition maximum) or vigorous (75%-80% of 1 repetition maximum). Each session should include from 5 to 10 exercises involving the use of large muscle groups. Ten to fifteen repetitions of each exercise (30-45 s) have to be made. Between each series should be left between 1-2 min for the recovery. Supervision by a professional can ensure an appropriate enforcement and progression of the exercise that optimized the benefits and reduce the risk of complications[47].
Although they have not demonstrated benefits in glycaemic control, these exercises are also recommended and can be very useful in older patients with T2DM[49].
Unstructured physical activity: It is also recommended to advise patients to increase energy expenditure in activities of daily life. It requires an increase of unstructured physical activity (walking more in the day, climb the stairs...)[50].
Prescription of a specific plan: Exercise should be prescribed individually for each patient and taking into accounts the characteristics of the person. Initially, the guidelines should recommend a slow progression and, if it is necessary, the patient has to start with low volumes of work. Recommendations should take into account the type of diabetes and the treatment utilized, the possibility that patients have diabetic foot, retinopathy, neuropathy, nephropathy or some degree of cardiovascular risk[49]. Training plans that are supervised by professionals have proved to be more effective as this study have demonstrated. In it, is compared a supervised program against a general advice, and although in both an increase in physical activity is observed, some better effects in HbA1c and cardiovascular risk factors in the supervised group have been seen[51].
Before starting the exercise would be advisable to pre-clinical evaluation, paying special attention to physical ability, complications of diabetes and comorbidities that constrain the realization of physical activity. For patients at high cardiovascular risk or for those who start high-intensity exercises, the ADA recommends performing an effort test with a grade of recommendation C[47].
Exercise and diabetes complications: The presence of diabetes complications involves a number of considerations at the time of writing prescriptions of physical exercise in these patients.
The physical exercise has proved benefits in reducing the appearance of peripheral neuropathy[52]. When it is already present, it is recommended to avoid exercises that cause impacts of repetition in the lower extremities and especially in patients with foot ulcers and wounds[53]. Furthermore, recent studies have demonstrated that moderate intensity walking do not increase the risk of ulcers.
In respect of the weight-bearing exercises, it can be performed while there are no ulcers or foot lesions. In any case it should pay attention and examine the feet and always wear suitable shoes.
The presence of retinopathy advises against the practice of physical activities that increase intrathoracic pressure (Valsalva manoeuvre), or high-intensity exercises by the risk of retinal detachment or intravitreal haemorrhage. The exercises with low and moderate intensity (walking, swimming...) are perfectly authorized and they can be done safely. Contact exercises like boxing should be avoided because of the risk of impact[50].
Exercise for diabetic patients is beneficial at any stage of renal function. In epidemiological studies it has been shown to improve renal function. Promotes muscle strengthening in case of kidney failure that helps to counteract sarcopenia, and improves various parameters in patients on dialysis, so with supervision and restraint exercise is recommended and although they have been transient increases in microalbuminuria with sessions of exercise (because of increasing blood pressure) is not considered as a marker of persistent microalbuminuria[50].
Physical activity has many beneficial cardiovascular effects but must take into account some considerations when there is vascular disease. Patients with diabetes that present a moderate or high cardiovascular risk should be included in supervised cardiac rehabilitation programs, because exist an association with mortality. In addition, during the exercise there is an increased activity of the sympathetic nervous system and catecholamines and decrease vagal tone[47,50].
In people with peripheral arterial disease benefits from the practice of sports aerobics and resistance also exist because of the improvement of the mobility, functional capacity, pain tolerance and quality of life[47].
Moderate physical exercise can improve the autonomic nervous system both in patients with autonomic neuropathy and those who do not have it[54], however it may represent a prescription limitation because it may favour silent ischemia, doubling mortality, impairing exercise tolerance and decreasing the maximum heart rate and thus a prior cardiovascular study is recommended[55].
Hyperglycaemia: In T2DM is very strange developing a true insulin deficiency, as in type 1 diabetic, so if the patient feels well is not necessary to postpone the exercise by hyperglycaemia, although they must ensure an adequate hydration state[56].
In non-diabetic person with aerobic exercise the increase of the glucose uptake is offset with similar increase of the hepatic glucose, but in diabetic person the muscle uptake is greater than the liver’s production although the risk of hypoglycaemia is minimal if hypoglycaemic drugs are not taken[47]. However, if in addition to the effect of exercise add up the effects of hypoglycaemic drugs, we recommend a series of precautions mainly based on carbohydrate intake and adjust drug doses. If the levels before exercise are less than 100 mg/dL should take a supplement of 15 g of carbohydrates before exercise. This measure should only be recommended if blood glucose lowering drugs (secretagogues or insulin) are taken. If the control is with other drugs, supplements are not required if the exercise is less than an hour[56]. It is important to note that regardless of the initial levels, if the exercise is prolonged a monitoring could be required and also intakes over the same period.
Before physical activity, to prevent the appearance of hypoglycaemia during exercise, doses of drugs such as insulin secretagogues or insulin (especially the latter) can be decreased. These measures can be associated with dietary measures mentioned above. During the hours after exercise glucose needs increase, so after exercise delayed hypoglycaemia can happens. This hypoglycaemia should be expected and may require reducing the dose of drugs after exercise and/or increase the intake after it[47].
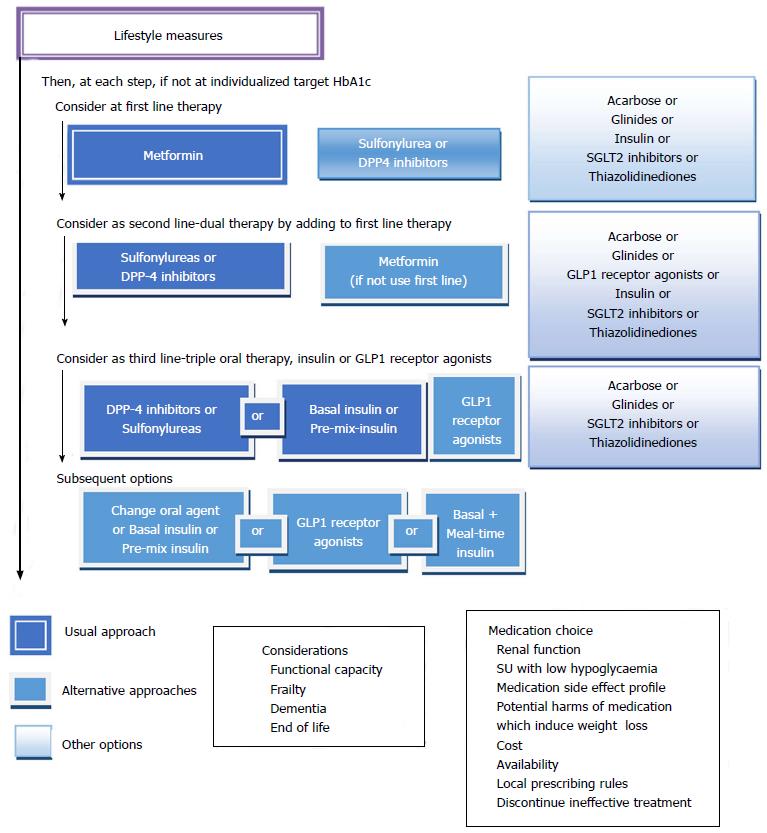
Metformin is considered the agent of first line for treatment of T2DM, in the absence of contraindications[6,13,57].
Mechanism of action[58]: Metformin can change the composition of gut microbiota[59] and activate mucosal AMP-activated protein-kinase (AMPK) that maintain the integrity of the intestinal barrier. These effects, in combination with the activation of AMPK[60] in hepatocytes appear to be the mechanism by which metformin decrease lipopolysaccharide (LPS) levels in circulation and in the liver.
After being delivered to the liver from the intestines, metformin can inhibit gluconeogenesis through four different mechanisms[61]: (1) by activating hepatic AMPK through liver-kinase B1 and decreased energy charge (9, 10); (2) through the inhibition of glucagon-induced cAMP production by blocking adenylcyclase (11); (3) in high concentrations (5 mmol/L) inhibit NADH coenzyme Q oxidoreductase (complex I) in the mitochondrial electron transport chain (12) to reduce ATP levels and increase AMP/ATP ratio. This increased ratio should activate AMPK; and (4) the inhibition of mitochondrial glycerol phosphate dehydrogenase (mG3PDH)[58], will affect transport of NADH from the cytoplasm into mitochondrion, suppressing gluconeogenesis process from lactate.
Also, metformin works through the Peutz-Jeghers protein LKB1. LKB1 is a tumour suppressor, and activation of AMPK through LKB1[62] may play a role in inhibiting cell growth.
Indications and contraindications: Metformin is the drug of first-line for many patients with T2DM. It decreases fasting blood glucose by approximately 20% and HbA1c by 1.5%. It can be given in combination with sulfonylureas, glinides, alpha-glucosidase inhibitors, insulin, thiazolidinediones (TZD), glucagon-like peptide-1 receptor agonist (RA-GLP1), dipeptidylpeptidase 4 inhibitors (iDPP4), and sodium-glucose co-transporter 2 inhibitors (iSGLT2). Metformin is contraindicated in patients with factors that predispose to lactic acidosis. The predisposing factors are: A renal function damaged, concomitant liver disease or excessive alcohol intake, unstable or acute heart failure and personal history of lactic acidosis.
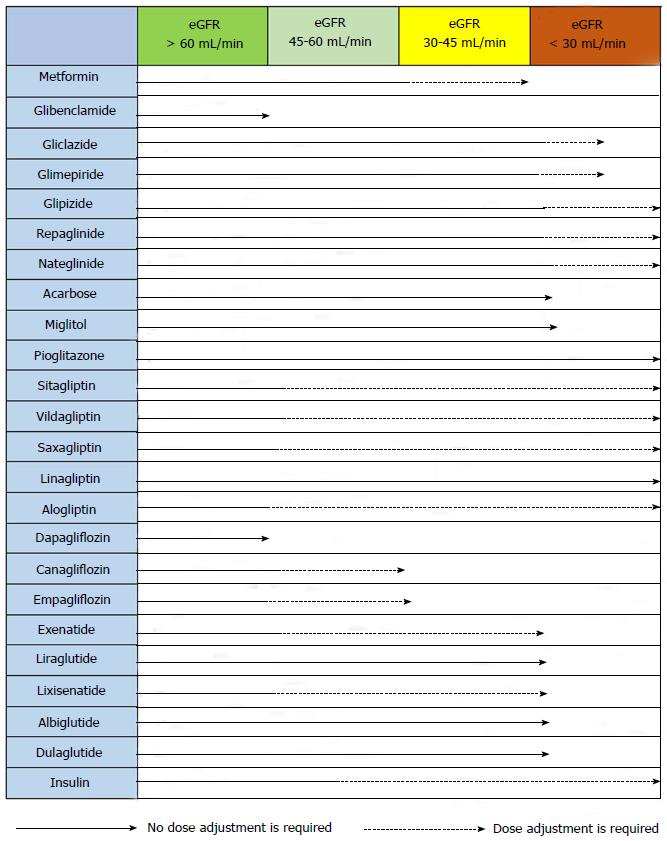
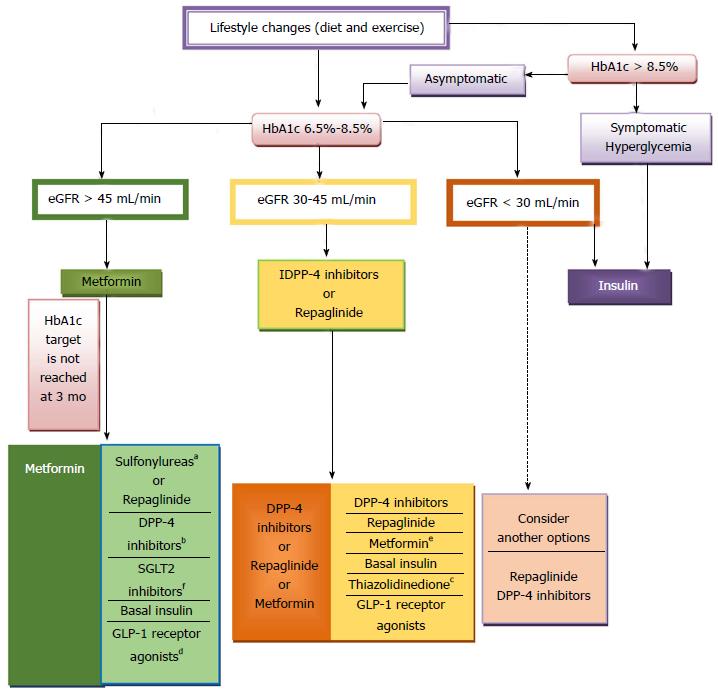
The precise serum creatinine and estimated glomerular filtration rate (eGFR) limits for the use of metformin remain uncertain. In the metformin prescribing information is contraindicated when creatinine level is above 1.4 mg/dL in woman and 1.5 mg/dL in men, and with eGFR < 60 mL/min. However, in observational studies of T2DM patients and eGFR 45-60 mL/min, improved clinical outcomes have been reported. Nowadays[63-65], in patients with eGFR above 45 mL/min, metformin can be utilized. The absolute contraindication is with GFR < 30 mL/min. With eGFR 30-45 mL/min, in clinical practice, currently we reduce metformin dose by a half. It is very important to advise patients with eGFR 30-60 mL/min to stop taking metformin if they develop any condition associated with dehydration, sepsis or hypoxemia. Also metformin should be stopped prior to intravenous iodinated contrast.
Side effects: The most frequents are gastrointestinal, such as anorexia, nausea, abdominal discomfort and diarrhoea; they are usually mild and transient. Also, metformin reduces intestinal absorption of vitamin B12.
Less common is lactic acidosis. In a review[66] of 347 randomized trials and prospective cohort studies, there were no cases of lactic acidosis. However, is very important because of the high case-fatality rate. Predisposing factors are all situations that predispose to hypoperfusion and hypoxemia (sepsis, heart failure, dehydration, acute or progressive renal impairment).
Cardiovascular effects: Metformin does not have adverse cardiovascular effects, and it appears to decrease cardiovascular events as we saw in UKPDS, and during the post-interventional observation period of the UKPDS, in which reductions in the risk of macrovascular complications were maintained in the metformin group.
Metformin also has a lipid-lowering activity, and it result in a decrease in free fatty acid concentration, serum triglyceride, small decrease in LDL cholesterol and a modest increase in HDL cholesterol.
Cancer incidence: Observational data suggest that metformin decreases cancer incidence[67,68]. In different meta-analyses in T2DM patients, use of metformin compared with non-use or with use of other diabetes treatment, was related with a reduced risk of all cancers and lower cancer mortality[69,70]. The majority of the trials were not designed to explore cancer outcomes, so we must be prudent in the interpretation of their results.
Sulfonylureas and meglitinides or glinides (insulin secretagogues) are two different classes of oral hypoglycaemic drugs but they have a common mechanism of action, and both stimulate pancreatic beta cells to release insulin.
Sulfonylureas are a classic first or second-line therapy for patients with T2DM[71], and since their introduction to clinical practice in the 1950s they have been widely utilized[72]. They are utilized as a reference to compare the efficacy and safety of other hypoglycaemic drugs excluding insulin.
Meglitinides stimulate insulin release through similar mechanisms but they have a different subunit binding site, with a more rapid absorption and more rapid stimulus to insulin secretion. However they require more frequent dosing[73].
Mechanism of action: Both sulfonylureas and glinides base their mechanism of action in increasing insulin secretion, which is regulated by ATP-sensitive potassium channels (KATP potassium channel) located in the membrane of pancreatic beta cells[74]. Although the receptor’s binding site is different for sulfonylureas and glinides, they both induce channel closure and cell depolarization leading to an increase in cytoplasmic calcium level and consequently insulin secretion[37].
Pharmacokinetics: Differences in pharmacokinetic and binding properties of insulin secretagogues result in the specific responses that each drug produces. Sulfonylureas can be divided into first- and second-generation agents. Glyburide (known as glibenclamide in Europe), glipizide, gliclazide and glimepiride are second-generation sulfonylureas[57]. New generation agents are more potent and have fewer adverse effects[37]. Although second-generation sulfonylureas are equally effective, there are differences in absorption, metabolism, and duration of action as well as in effective dose; for example, glyburide has active metabolites that can prolong his action.
There are two different glinides: Repaglinide and nateglinide. Repaglinide is a member of the meglitinide family different from the sulfonylurea. Nateglinide is a derivate of phenylalanine and it is structurally difference from sulfonylureas and meglitinide. They both cause less hypoglycaemia and less weight gain due to their shorter half-life and a different sulfonylureas receptor binding site, leading to faster absorption and a more rapid stimulus to insulin secretion[37].
As a result of their pharmacokinetics, the major effect of sulfonylureas is the reduction of fasting plasma glucose concentrations, whereas meglitinides mainly reduce postprandial glucose[75].
Advantages and effectiveness: Sulfonylureas and meglitinides can be effective when employed as monotherapy, or in combination with other oral hypoglycaemic drugs or insulin. Sulfonylureas are the most cost-effective glucose-lowering agents, have been on the market for a long time[37], and are widely utilized because of their long term efficacy and safety history, low cost and extensive clinical trial data demonstrating good glucose-lowering efficacy[76,77]. The glucose-lowering effectiveness is said to be high for sulfonylureas (expected HbA1c reduction 1.0%-1.5%) and generally lower for meglitinides (0.5%-1.0%)[9,57].
In the Consensus of ADA/EASD 2015 sulfonylureas and glinides appear as an alternative to metformin when metformin is contraindicated or not tolerated, and they represent an alternate treatment option in double and triple therapy[57], whereas in the Consensus of the American Association of Clinical Endocrinologist (AACE) 2016, sulfonylureas and glinides appear as the last alternative both in monotherapy and combined treatment[9].
Side effects: Loss of efficacy, hypoglycaemia and weight gain represent the main problems related to the use of these drugs.
Over time insulin secretagogues lose effectiveness (secondary failure), caused by an exacerbation of islet dysfunction with beta cell failure[78,79]. As a result, the percentage of patients maintaining adequate glycaemic control decreases progressively. Although this effect may also be related to disease progression, it has shown an increase in secondary failure than other agents[80].
Weight gain can be via many of the same mechanisms that are triggered by insulin therapy, and it has been observed in different studies[81,82]. However, metformin might counter the weight gain effect when used in combination[81,83]. Different generations of sulfonylureas have shown to cause weight gain and its magnitude appears to correlate with the propensity to cause hypoglycaemia. It may also occur with meglitinides as they have similar profiles[76], but it seems to occur in a lesser extent due to their short action[78].
Hypoglycaemia is the most common adverse effect[83,84], especially with long-acting sulfonylureas (such as glyburide/glimepiride)[85]. New generation sulfonylureas have shown to have a significantly lower risk of hypoglycaemia. Meglitinides generally have less risk of hypoglycaemia[37], thus being useful for individuals in whom the goal of avoiding hypoglycaemic events is important.
The risk factors for hypoglycaemia are inconsistent eating patterns in older individuals (meglitinides can be useful in these patients), malnutrition, alcohol ingestion, renal insufficiency, hepatic failure, hypothyroidism or drug interactions[86,87]. The risk of hypoglycaemia, as well as considerations of the risk-to benefit-relationship, is particularly relevant in older individuals where results from trials have suggested that aggressive control may not have significant benefits and may present some risk[6].
Cardiovascular disease: Sulfonylureas have been associated with increased cardiovascular risk, especially when it comes to glyburide/glibenclamide. Some studies[88,89] support this association, which can be explained by the interference with ischemic preconditioning, a protective autoregulatory mechanism in the heart. However, other studies like UKPDS, ADVANCE and ACCORD and many meta-analyses failed to proof an increased risk in cardiovascular mortality or morbidity[76]. Therefore, it remains unclear whether sulfonylureas are associated with an increased cardiovascular risk but as glibenclamide may indeed be when compared with other sulfonylureas, clinicians should consider possible differences in risk of mortality if a sulfonylurea is to be utilized.
Other considerations: Most insulin secretagogues undergo significant renal clearance except for meglitinides, and the risk of hypoglycaemia is higher in patients who have chronic kidney disease (CKD) especially with glyburide/glibenclamide which has a prolonged duration of action and active metabolites[58]. In patients with liver disease, sulfonylurea is not specifically contraindicated and meglitinides can also be employed. When liver disease is severe, insulin secretagogues have an increased risk of hypoglycaemia and should be avoided[57,90].
Sulfonylureas have several drug-drug interactions as they are metabolized by cytochrome p450[84]. Repaglinide with gemfibrozil is contraindicated because of its higher risk of hypoglycaemia.
There are three currently available agents, acarbose, miglitol and voglibose[37]. Their properties are different from other antidiabetics owing to its unique mode of action. Acarbose has been used for over 20 years in the treatment of hyperglycaemia[91].
The alpha-glucosidase inhibitors reduce postprandial triglycerides but their effect on LDL and HDL cholesterol levels and fasting triglycerides is insignificant and inconsistent[75,92]. Alpha-glucosidase inhibitors rarely induce hypoglycaemia, because these agents do not stimulate insulin release, and do not significantly affect body weight[82].
Acarbose has demonstrated to have beneficial effects by reducing the risk of cardiovascular disease and slowing the progression to diabetes in patients with impaired glucose tolerance[93,94].
Mechanism of action: Alpha-glucosidases are enzyme complexes located in the brush border membrane of the small intestine and hydrolyse oligosaccharides into monosaccharides[95]. Alpha-glucosidases inhibitors are structurally similar to natural oligosaccharides with higher affinity for alpha-glucosidases[91], and they produce a reversible inhibition of membrane-bound intestinal alpha-glucoside hydrolase enzymes. This cause delayed carbohydrate absorption and digestion, and results in a reduction in postprandial hyperglycaemia. The undigested carbohydrates in the lower parts of the small intestine increase plasma RA-GLP1 levels[95]. Because reduced blood glucose concentrations, alpha-glucosidase inhibitors do not enhance insulin secretion[91,95].
Efficacy: In general, alpha-glucosidase inhibitors have modest HbA1c lowering effects. In the Consensus of ADA/EASD 2015, alpha-glucosidase inhibitors are not included in the algorithm due to their lower efficacy and limiting side effects compared to other options[57], whereas in the Consensus of AACE 2016, alpha-glucosidase inhibitors appear only before sulfonylureas and glinides as monotherapy and combined treatment[9].
Side effects: The side effects are mainly gastrointestinal and include flatulence, diarrhoea and abdominal pain. These symptoms are usually mild, but they may reduce compliance and they are the most common reason for discontinuation treatment[94,95]. These symptoms occur when undigested carbohydrates arrive to the colon and as a result, there is a fermentation by bacteria in the large bowel and intestinal gas production[91]. For this reason, they are contraindicated in patients with chronic intestinal disorders associated with impaired digestion or absorption, and with conditions that may worsen when an intestinal gas increase appears (hernias, intestinal obstruction and intestinal ulcers). Treatment should be discontinued immediately if there is or is suspected ileus or sub ileus. To maximize the potential for these agents to be well tolerated, start with a low dose and increase slowly[37].
Alpha-glucosidase inhibitors are not recommended for patients with creatinine clearance < 25 mL/min and they can produce asymptomatic elevation of liver enzymes, for this it is necessary a control of liver enzymes[96]. In hypoglycaemia (when it is associated with sulfonylureas, glinides and insulin), like inhibitors of α-glucosidase delay absorption and digestion of sucrose, patients must take glucose.
Two TZD are currently available in United States: Rosiglitazone and pioglitazone. In Europe, since 2010, rosiglitazone was suspended by the European Medicines Agency, based on the overall risks of rosiglitazone exceed their benefits. French and Germany Medicines Agencies also discontinued pioglitazone in 2011.
Mechanism of action: TZD increase insulin sensitivity by acting on muscle, adipose tissue and liver to increase glucose utilization and decrease glucose production. TZD bind to peroxisome proliferator-activated receptors (PPARs). PPAR-γ is found predominantly in central nervous system, macrophages, vascular endothelium, adipose tissue and pancreatic beta-cells. The concentration of PPAR gamma is increased in the skeletal muscle of obese and diabetic patients[97]. In the central nervous system PPAR-gamma activation mediates weight gain by stimulating increased feeding[98]; this is, in part, the reason for weight gain associated with TZD.
PPAR-alpha is found predominantly in liver, skeletal muscle, heart and vascular walls. Rosiglitazone is purely PPAR-gamma agonist, while pioglitazone has also some PPAR-alpha effects; therefore they have different effects on lipids. Pioglitazone produces a more favourable lipid profile: LDL-cholesterol remained constant during treatment while rosiglitazone raises them; in addition decreased more triglyceride levels than rosiglitazone. HDL-cholesterol increased more or less 10% with both of them.
TZD also may improve blood glucose levels by preserving pancreatic beta-cell function. They are probably similar in efficacy to metformin in monotherapy but we don’t usually choose them because of their adverse effects and cost. Also, they are effective in combination therapy, but again, we typically prefer combination with other drugs with less adverse effects. TZD should not be given to diabetic patients with a history of heart failure or low bone mass.
The ratio between benefit and risk at cardiovascular system of rosiglitazone and pioglitazone remains unclear. Meta-analyses and observational studies (RECORD study, BARI 2D, PROactive trial) suggest caution with rosiglitazone use and also with pioglitazone.
Weight gain: The weight gain is the result of diverse mechanisms as: Fluid retention, the activation of PPAR-γ in the central nervous system (which increases feeding) and the up regulation of genes that facilitate adipocyte lipid storage (in part weight gain may be also a result from the proliferation of new adipocytes[99]). It’s time and dose dependent.
Heart failure: PPAR-γ is more abundant in the collecting tubules of the nephron; the PPAR-gamma stimulation (induced by TZD treatment) activate sodium reabsorption in the luminal membrane of the collecting tubule cells[100], leading to a fluid retention that may lead to the precipitation of heart failure or worsening it. Peripheral oedema occurs in 4%-6% of patients in treatment with TZD, and this percentage is higher in patients with heart failure history. Because of the risk of heart failure the American Heart Association and the ADA published a consensus statement in 2003[101].
Because of their mechanism of action (they improve blood glucose by increasing insulin sensitivity) TZD monotherapy cause hypoglycaemia less frequently than sulfonylureas or insulin.
In preclinical studies pioglitazone increased bladder tumours in rats. Latter the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) saws more cases of bladder cancer: 14 vs 5, in the treatment group[102]. In an analysis of an ongoing 10-years observational study, there wasn’t a significant association between pioglitazone and cancer[103], but the risk of bladder cancer was significantly increased in those with the longest exposure and highest cumulative dose. Using data from the Adverse Even Reporting System of the United States FDA, again risk of bladder cancer was higher with pioglitazone[104]. Because of these in 2011 German and French Medicines Agencies suspended the use of pioglitazone.
Decrease bone density and increase fracture risk. The activation of PPAR-gamma has been demonstrated to down regulate components of the IGF-1 system, and IGF-1 is an important regulator of osteoblast proliferation and differentiation[105]. The absolute increase in risk fracture seems to be small and occurred with both of them, rosiglitazone and pioglitazone; the fractures are more frequently in the distal upper or lower extremities. These treatments should not be utilized in women with low bone density or with risk factors for fracture.
Troglitazone suspended its commercialization due of severe hepatocellular injury[106]. FDA currently recommends periodic monitoring of liver function in patients in treatment with rosiglitazone or pioglitazone.
The incretin agents (GLP1 and GIP), secreted by intestine L cells, increase insulin secretion and inhibit glucagon in response to nutrient inputs. The glucoregulatory effects of incretins are the basis for treatment with inhibitors of DPP4 in patients with T2DM. Agents that inhibit DPP4, an enzyme that rapidly inactivates incretins, increase active levels of these hormones and, in doing so, improve islet function and glycaemic control in T2DM.
iDPP4 are used as monotherapy in patients inadequately controlled by diet and exercise, and dual therapy in combination with metformin, TZDs and insulin. iDPP4 are well tolerated; they have a low risk of producing hypoglycaemia, and maintain the patient’s weight. We have five iDPP: Sitagliptin, Vildagliptin, Saxagliptin, Linagliptin and Alogliptin.
Sitagliptin: Sitagliptin, which is approved for the treatment of T2DM in many countries, can be employed alone or dual therapy with sulfonylurea, metformin or TZD or third therapy. The normal dose of sitagliptin is 100 mg once daily; half dose is utilized in patients with an eGFR 30-50 mL/min, and quarter dose in those with an eGFR < 30 mL/min[107].
Monotherapy with this drug there are multiple studies, with significant reduction in HbA1c. The results of a study with sitagliptin monotherapy for 18 wk were: HbA1c significantly decreased with sitagliptin 100 and 200 mg compared to placebo (low HbA1c vs placebo: -0.48% and -0.60% respectively). Sitagliptin also significantly reduced fasting blood glucose vs placebo. Patients with baseline HbA1c higher (> or = 9%) had greater reductions in HbA1c subtracted sitagliptin placebo (-1.20% for 100 mg and -1.04% in the case of 200 mg) than those with HbA1c < 8% (-0.44% and -0.33%, respectively) or > or = 8% to 8.9% (-0.61% and -0.39%, respectively). Sitagliptin had a neutral effect on body weight[108].
In dual therapy studies the results confirm that sitagliptin was as effective as glipizide in patients inadequately controlled with metformin. In one of them the following results were found a year: From a mean baseline of 7.5%, HbA1c changes from baseline were -0.67% at week 52 in both groups, confirming non-inferiority. The proportions achieving an HbA1c < 7% were 63% (sitagliptin) and 59% (glipizide). Fasting plasma glucose changes from baseline were -0.56 mmol/L (-10.0 mg/dL) and -0.42 mmol/L (-7.5 mg/dL) for sitagliptin and glipizide, respectively[109]. With sitagliptin were observed less hypoglycaemia and less weight gain than with glipizide.
Vildagliptin: This is an iDPP4 which FDA was not approved so that is not being used in the United States. The usual dose is 50 mg twice daily when utilized as monotherapy, with metformin, or with a TZD, and 50 mg once daily (in the morning) when utilized with a sulfonylurea. No dose adjustment is necessary in patients with mild renal impairment (creatinine clearance ≥ 50 mL/min). In patients with moderate or severe renal impairment, the dose is 50 mg once daily.
In some studies comparing the efficacy and safety of vildagliptin compared with placebo target the treatment difference (vildagliptin-placebo) in adjusted mean change (AM Delta) ± SE in HbA1c from baseline to endpoint it was -0.7% ± 0.1% (P < 0.001) and -1.1% ± 0.1% (P < 0.001) in patients receiving 50 or 100 mg of vildagliptin, respectively. The difference between treatments in the Delta GPA (GPA) was -0.8 ± 0.3 mmol/L (P = 0.003) and -1.7 ± 0.3 mmol/L (P < 0.001) in patients receiving 50 or 100 mg of vildagliptin, respectively[110].
Saxagliptin: Saxagliptin is approved as a drug for home treatment of T2DM or dual therapy for patients not controlled with a sulfonylurea, metformin or TZD. The dose is 2.5 or 5 mg of saxagliptin once daily. The dose of 2.5 mg is recommended for patients with an eGFR ≤ 50 mL/min and patients taking drugs inhibitors of cytochrome P450 3A4/5 (e.g., ketoconazole), Saxagliptin monotherapy is effective, achieving reductions in HbA1c of 0.5 in naive patients vs placebo[111,112]. There are studies with saxagliptin (2.5, 5 and 10 mg) in dual therapy with metformin showed a statistically significant adjusted mean HbA1c decrease from baseline to week 24 compared to placebo (-0.59%, -0.69%, and -0.58% vs +0.13%; all P < 0.0001)[113]. There are also studies showing the efficacy of sitagliptin in combination with sulfonylureas and TZD.
Linagliptin: The dose of linagliptin is 5 mg once daily. It is eliminated mainly through the enterohepatic system so it is not necessary to adjust the dose in patients with renal or hepatic impairment. Inducers of CYP3A4 or P-glycoprotein (e.g., rifampicin) may reduce the effectiveness of this agent. In patients receiving these drugs should avoid the use of linagliptin.
In a monotherapy study vs placebo, linagliptin achieved a reduction in HbA1c of 0.44% against rising 0.25% with placebo in 6 mo[114]. In a 24 wk study in triple therapy in patients treated with metformin and sulfonylureas that was added linagliptin or placebo, appeared a reduction in HbA1c of 0.72% in the group with linagliptin vs 0.1% in the group with placebo[115].
Alogliptin: The usual dose of alogliptin is 25 mg once daily, with dose reductions to 12.5 mg once daily in patients with creatinine clearance between 30 and 60 mL/min and to 6.25 mg daily in patients with creatinine clearance < 30 mL/min or undergoing dialysis[116].
In a study to twelve weeks in patients treated with metformin with poor control of their diabetes, alogliptin group achieved a reduction in HbA1c of 0.64% compared to an increase of 0.22% in the placebo group[117]. In another 26 wk studies, with alogliptin (12.5 or 25 mg once a day) vs placebo in patients with poorly controlled T2DM on a stable dose of glyburide (n = 500) or insulin (alone or in combination with metformin, n = 390) there were greater reductions in HbA1c in the alogliptin groups (mean change in HbA1c from baseline -0.39, -0.53 and +0.01 percentage points for the 12.5, 25 mg, and placebo groups, respectively, in the glyburide trial, and -0.63, -0.71 and -0.13 percentage points, respectively, in the insulin trial)[118,119].
Side effects: These drugs are considered very safe since both the risk of hypoglycaemia and other adverse effects are rare. All of them at increased risk of hypoglycaemia in combination with sulfonylureas or insulin. In comparative studies have not observed any significant differences between them in the risk of hypoglycaemia. With vildagliptin and alogliptin have been reported cases of hepatic dysfunction unusually still advisable to monitor liver enzymes during the first three months of treatment. If an increase in transaminases of three times the upper limit of normal or greater persists, the drugs should be discontinued.
At present, there is insufficient data to know whether there is a causal relationship between acute pancreatitis and iDPP4[120-123]. They should be discontinued in patients with persistent severe abdominal pain. In patients with pancreatitis should not start these drugs, or if there is a history of this disease.
Commonly reported side effects include headache, nasopharyngitis, and upper respiratory tract infection[124,125]. Some, but not all, studies have reported a slight increased risk of gastrointestinal side effects with sitagliptin[108,109,126].
Cardiovascular effects: Sitagliptin, saxagliptin and alogliptin have been studied for cardiovascular safety. They are TECOS, SAVOR-TIMI and EXAMINE studies respectively, with thousands of patients at high cardiovascular risk with a median follow up of 18 to 36 m.
In the TECOS study with sitagliptin 14735 patients with T2DM and established cardiovascular disease (history of major diseases of the coronary artery, ischemic cerebrovascular disease or peripheral arterial atherosclerotic disease) were randomized a group with sitagliptin and one with placebo, plus other diabetes medications (mainly metformin, sulfonylurea, insulin)[127]. After three years, the primary cardiovascular combined outcome (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina) was observed in a similar proportion of diabetics (11.4% and 11.6% in the sitagliptin and placebo group’s human resources, respectively, 0.98; 95%CI: 0.89-1.08). There was no significant difference in any of the individual components of the composite endpoint or the rate of hospitalization for heart failure (3.1% in each group).
In the test with saxagliptin (SAVOR-TIMI), 16492 patients with T2DM and either a history of cardiovascular disease or multiple risk factors for vascular disease were randomized to the branch of saxagliptin or placebo, and other medicines for diabetes (such as metformin, sulfonylureas, insulin). After a two-year follow-up, the first target (combination of cardiovascular death, nonfatal ischemic stroke or nonfatal myocardial infarction) appeared in a similar number of diabetics in proportion, 7.3% and 7.2% in the saxagliptin and placebo, respectively; hazard ratio (HR) 1.00, 95%CI: 0.89-1.12[128]. Significantly more patients in the field of saxagliptin were hospitalized for heart failure (3.5% vs 2.8%; HR = 1.27, 95%CI: 1.07-1.51). It stresses significantly the hospitalization for heart failure in the saxagliptin study[129] increase. However, the possible association between heart failure and iDPP4 has been linked to other epidemiological data and claims data[130,131].
In the EXAMINE trial alogliptin, 5380 patients with T2DM and either an acute myocardial infarction or unstable angina requiring recent hospitalization were randomized to alogliptin or placebo, along with other antidiabetic (mainly metformin, sulfonylureas, insulin)[132]. At 18 mo follow-up, the primary composite endpoint including cardiovascular death, nonfatal stroke, or nonfatal myocardial appeared in a very similar proportion of patients (11.3% and 11.8% in the branches of alogliptin and placebo respectively; HR 0.96, 95% of the unilateral CI: 1.16). In a post hoc analysis of the data, there was no significant difference in the rate of hospitalization for heart failure (3.1% and 2.9% in the branches of alogliptin and placebo, respectively; HR = 1.07, 95%CI: 0.79-1.46)[133].
iSGLT2 inhibit renal reabsorption of glucose, increase its excretion and reduce hyperglycaemia in patients with T2DM. Therefore, reducing the reabsorption of glucose by inhibition of SGLT2 is a new way to treat T2DM. The increase in glucosuria and diuresis produced results in a reduction in weight and blood pressure[134].
Kidneys from healthy people filter approximately 180 g of glucose each day through renal glomerulus and reabsorbed in the then proximal convoluted tubule. This is possible by passive and active co-carriers which are known as glucose transporter (GLUT) and SGLT[135] conveyors. There are two types of SGLT; SGLT1 located mainly in the small intestine and the kidney proximal convoluted tubule, and SGLT2 located only in the proximal tubule (segment 1 and 2), that are responsible for about 90% of glucose reabsorption[7]. The other 10% of the glucose is reabsorbed by SGLT1 in segment 3. SGLT2 inhibitors block the SGLT2 transporter in the proximal tubule, to lower glucose reabsorption and increase its excretion in the urine. Glucose is excreted in the urine and plasma levels are reduced by improving glycaemia figures plasma[136-138]. It is an independent mechanism of insulin, there is low risk for hypoglycaemia, and no risk of fatigue or overstimulation of the beta cells[139]. Due to its mode of action is based on normal glomerular-tubular function; the iSGLT2 efficiency is lower in patients with renal failure[140]. The three most representative drugs family iSGLT2 are: Dapagliflozin, canagliflozin and empagliflozin.
Dapagliflozin: Dapagliflozin was the first iSGLT2 employee, and has many published data from clinical trials. In phase 3 trials comparing placebo for 24 wk and dapagliflozin (2.5, 5 and 10 mg once daily) used alone or added to metformin[141], pioglitazone[142], glimepiride[143] or insulin[144] was observed that HbA1c and fasting plasma glucose in patients with T2DM was reduced. In tests longer-term (102 wk) added to metformin, dapagliflozin resulted in a sustained decrease in HbA1c, glucose fasting blood glucose and weight without increasing the risk of hypoglycaemia in patients with T2DM not controlled on metformin alone[145]. The initial decrease in HbA1c observed at 24 wk with both doses of dapagliflozin (5 or 10 mg) added to metformin was maintained at 102 wk, and was superior to placebo (-0.58%, -0.78% and 0.02% against). Also the low fasting plasma glucose with both doses of dapagliflozin, remained and was higher than placebo (-1.47 mmol/L and -1.36 mmol/L vs -0.58 mmol/L). This drug has studies which compared with patients whose hyperglycaemia glipizide was poorly controlled by metformin[146]. After 52 wk, a drop in HbA1c starting from the baseline of -0.52% is target with dapagliflozin (≤ 10 mg/d) and glipizide (≤ 20 mg/d). Weight reduction was greater with dapagliflozin (-3.2 kg) vs glipizide (+1.4 kg). Dapagliflozin (≤ 10 mg/d) in T2DM patients was non-inferior to glipizide (≤ 20 mg/d) in reduction of HbA1c at 52 wk (both -0.52%). At 4 years the HbA1c reduction is attenuated in both groups, but more in the glipizide vs dapagliflozin (+0.2% vs -0.1%). There were differences in weight change, with weight loss in the dapagliflozin group vs weight gain in the glipizide group (-3.95 kg vs +1.12 kg). In the dapagliflozin group decreases the mean average of systolic blood pressure, but did no change in the glipizide group (difference: -3.7 mmHg)[146].
Canagliflozin: Canagliflozin was the first of this family of drugs approved by the FDA and began its commercialization in March 2013 for use in T2DM. It is an effective drug in monotherapy and after 26 wk of treatment with canagliflozin 100 mg and 300 mg once daily significantly reduced HbA1c (-0.77% and -1.03% respectively) in patients with T2DM not controlled with diet and exercise compared to placebo (0.14%, P < 0.001)[147]. Also, significantly reduced fasting blood glucose, -27 mg/dL to -34 mg/dL with both doses of canagliflozin (placebo = 9 mg/dL, P < 0.001). Get for this reason a larger number of patients in target HbA1c < 7.0% compared to placebo (44.5% to 62.4% vs 20.6%; P < 0.001). At week 52 in the double therapy, 300 mg canagliflozin under more HbA1c that sitagliptin (-0.73%, -0.88%, -0.73%, respectively)[148]. Data reduction in body weight with canagliflozin 100 and 300 mg vs placebo at week 26 were -3.7, -4.2, -1.2 kg, respectively (P < 0.001) and vs sitagliptin at week 52 were -3.8, - 4.2, -1.3 kg, respectively (P < 0.001). Also, in combination therapy, improved canagliflozin reducing body weight, HbA1c, and tolerance was better than in diabetics treated with metformin plus sulfonylurea more than 52 wk[149]. At week 26, HbA1c decreased significantly with canagliflozin 100 and 300 mg vs placebo (-0.85%, -1.06%, -0.13%; P < 0.001); this improvement was maintained at week 52 (-0.74%, -0.96%, +0.01%). Both doses of canagliflozin (100 mg/d and 300 mg/d) showed non-inferiority in HbA1c reduction (-0.82% and -0.93%) compared to glimepiride for 52 wk of treatment in diabetic subjects treated with metformin. Canagliflozin 300 mg/d was more effective than glimepiride in decreasing HbA1c, and both doses of canagliflozin were higher than glimepiride in lowering body weight (-3.7 kg to 100 mg/d, -4.0 kg with 300 mg/d vs + 0.7 kg with glimepiride)[149]. Data from this study, objectified to 104 wk, showed that reductions in HbA1c remained with canagliflozin 100 and 300 mg and glimepiride vs placebo at week 104 (-0.65%, -0.55% and -0.76%), and both canagliflozin dose were better than glimepiride in weight reduction (-4.1 kg with 100 mg/d, -4.2 kg with 300 mg/d vs + 0.9 kg with glimepiride)[150].
Empagliflozin: Empagliflozin is a drug that has eight multinational clinical trials, including a very important safety trial of cardiovascular risk. Data empagliflozin 12 wk at doses 5-25 mg/d are increased excretion of glucose and a decrease of fasting blood glucose (-31.1 mg/dL. at 25 mg vs an increase +0.8 mg/dL. placebo), HbA1c (-0.63% vs 25 mg vs an increase of +0.09%) and body weight (-2.0 kg to 25 mg vs -0.8 kg) in T2DM[151]. Both doses of empagliflozin (10 mg or 25 mg daily) added to metformin received greater reductions in HbA1c vs sitagliptin (-0.34% to -0.63% vs -0.40%) and these were maintained for 90 wk. The fasting glucose reduction was also higher after 90 wk of treatment with two doses of empaglifozin against sitagliptin (-21 mg/dL and -32 mg/dL vs -16 mg/dL), and these effects were maintained over the treatment period[152]. The weight was reduced from the baseline of -2.2 to -4.0 kg with empaglifozin, -1.3 kg with metformin, and sitagliptin -0.4 kg after 90 wk[153]. In a randomized, double-blind empagliflozin (10, 25 mg) or placebo add-on to basal insulin for 78 wk; compared with placebo, 10 and 25 mg/d of empagliflozin significantly lower body weight (-2.2 kg, -2.0 kg, and +0.7 kg respectively), and decreased HbA1c (-0.48%, -0.64%, and -0.02%, respectively), and systolic blood pressure (-4.1 mmHg, -2.4 mmHg, and +0.1 mmHg, respectively)[154]. Therefore, a long-term empagliflozin is an effective treatment for patients with T2DM.
Pleiotropic effects: iSGLT2 achieve a decrease in body weight between 1-5 kg medium[155]. Weight loss is greater if, in addition, the use of these drugs able to decrease the dose of insulin. Patients fastest achieve greater weight reduction[156]. The results of studies over 4 years in T2DM patients treated with dapagliflozin vs glimepiride, both in combination with metformin, showed a reduction of 3.65 kg in the dapagliflozin group compared with the branch of glimepiride that gained an average of 0.73 kg[155]. There has been demonstrated in multiple studies that the loss of weight produced by these medicaments is principally secondary to a loss of fat mass (especially visceral abnormal fat) and not due to a volume depletion. Also, one has found a reduction of the abdominal perimeter[154]. In studies with canagliflozin it was observed that the 0.66% reduction in body weight was fat mass, and 0.33% was lean body mass. The association of iSGLT2 with anti-diabetic drugs that increase the weight (pioglitazone, insulin) can get minimize this gain[144]. iSGLT2 also reduce the systolic (-1.66 mmHg to -6.9 mmHg) and diastolic (-0.88 mmHg to -3.5 mmHg) blood pressure. This decrease occurs because the initial osmotic diuresis, and subsequent inhibition of the renin-angiotensin system[157], and the decrease is independent from the levels of glucose or from the weight of the patients. Also the effects on blood pressure were not dose-dependent and were not accompanied by any notable changes in heart rate or increases in hypotension and/or syncope[158,159]. Some analysis from phase IIb studies with empagliflozin revealed even greater decreases in systolic blood pressure of 13.4 mmHg to 17 mmHg amongst a subgroup of patients with a baseline systolic blood pressure > 140 mmHg compared to the overall population. In a study of dapagliflozin it was that the effects on blood pressure were more important in patients with a baseline systolic blood pressure > 140 mmHg.
It‘s not clear the effect of these medicaments on the lipid profile. The same results do not exist with all the iSGLT2. In some studies are lipid-friendly and in others are lipid-neutral drugs. Canagliflozin, for example, increases HDL cholesterol by 7.1%, LDL cholesterol by 7.1%, and reduces triglycerides by 2.3%, over 52 and 104 wk[160]. These modifications in lipid profiles were not observed with other iSGLT2 such dapagliflozin[161].
This new drugs also have a paper reducing the serum uric acid levels. They can decrease the levels in a range from -5.9% to -17.8% with the effect sustained for 2 years[162].
Finally, SGLT2 is associated with glomerular hyperfiltration; thus blockade of SGLT2 has potential nephroprotective action[163].
Side effects: The iSGLT2 has a similar incidence of adverse events in clinical trials which are given with other oral antidiabetic agents. The overall incidence of adverse events moves between 57.3% to 83.0%, and serious adverse events is between 1.0% and 12.6%[155].
Increased glucosuria produces the urogenital tract infections that are the most common side effects of these drugs[164], especially in women and uncircumcised men. Genital mycotic infections in women were vulvovaginal candidiasis, vulvitis, vulvovaginitis, and vulvovaginal mycotic infection. In male patients balanitis and balanoposthitis occur. In trials with dapagliflozin 2.5, 5 and 10 mg doses, the incidence of urogenital tract infections was 4.1%; 5.7% and 4.8% depending on the dose of the drug vs 0.9% in placebo patients[165].
Another adverse effects of these agents also derived from his mechanism of action is the orthostatic hypotension and the volume depletion. These drugs are associated with an osmotic diuresis that can produce it. In randomized controlled trials the occurrence of these side effects was very low (< 3%)[166]. The extra diuresis experienced per day does not cause nicturia[167].
iSGLT2 have a non-insulin based mechanism and because of that the risk of hypoglycaemia is minimal with them. This risk can increase in therapy combined with sulfonylureas or insulin.
The use of iSGLT2 is associated with changes in bone turnover markers, with reduction in bone formation without changes in bone mineral density. There are long-term studies do not confirm these changes related to skeletal system[150,154]. A 2-year study with dapagliflozin, no objective changes in bone turnover markers compared with placebo when combined with metformin[162].
There have been reports of euglycaemic ketoacidosis in some patients treated with iSGLT2[168]. They are studying the mechanisms by which this complication may occur. This is frames ketoacidosis with blood glucose levels < 200 mg/dL. The possible cause of the euglycaemic ketoacidosis can be attributed to the recent use of insulin, reducing calorie intake, alcohol abuse, chronic liver disease and glycogen storage disorders[169].
Cardiovascular effects: All iSGLT2 have launched important studies of cardiovascular safety. It has now ended with empagliflozin conducted with promising results for this therapeutic group.
EMPA-REG is an international prospective, placebo-controlled trial of empagliflozin cardiovascular outcomes in patients with T2DM and know cardiovascular disease. In the trial he managed to reach the main objective of non-inferiority and also showed, after 3.1 years of median follow-up, the superiority of empagliflozin group (10 or 25 mg/d) vs placebo in what with respect to the primary composite cardiovascular endpoint (-14%), hospitalizations for heart failure (-35%), cardiovascular mortality (-38%) and mortality from all causes (-32%, each P < 0.001). The decrease in mortality appeared from early stages (< 6 mo) and referred to all subgroups, without any apparent heterogeneity. These reductions in mortality appear to be related to the diuretic and natriuretic effect of empagliflozin, and not with concomitant reductions in HbA1c, body weight, blood pressure, waist circumference and serum uric acid levels in the field of empagliflozin respect to placebo. Tolerance and safety of empagliflozin was good, objectifying only a moderate increase in benign genital fungal infections, adverse event known iSGLT2[170].
Human GLP1 is secreted in response to food intake and stimulates insulin release[171]. Two incretins have been identified: GLP1, which is produced and released mainly by L-cells located in the distal ileum and GIP, which is secreted by enteroendocrine K-cells in the proximal gut.
GLP1 treatment in T2DM patients increased insulin secretion glucose dependent and decrease secretion of glucagon, slowed gastric emptying, raised satiety, and reduce food intake[172]. GLP1 also protect against myocardial ischemia[173,174]. In blood vessels promotes endothelium-independent artery relaxation protecting against endothelial dysfunction. Also have effect in protecting renal function by increasing diuresis and natriuresis[175,176]. All of these actions allow lower blood pressure and have positive effects on cardiovascular risk markers such as plasminogen activator inhibitor and brain natriuretic peptide.
The use of GLP1 therapy is limited by its rapid breakdown by DPP4; it has a short half-life: 1-2 min. Multiple RA-GLP1 have been developed with the physiological effects of GLP1 and an extended duration of action. RA-GLP1 agonists have proven efficacy for lowering HbA1c, fasting plasma glucose, body weight and systolic blood pressure, with a reduced risk of hypoglycaemia[6]. EASD/ADA and AACE guidelines recommended their use in combination with metformin, or as triple therapy in combination with metformin, sulfonylureas, TZD or insulin[9,42].
RA-GLP1 are classified by their duration in short-acting or long-acting. Short acting RA-GLP1 are exenatide twice daily and lixisenatide; their provide short-lived GLP1 receptor activation; tend to have a more accentuated effect on postprandial hyperglycaemia and gastric emptying and less effect on fasting glucose. Long acting RA-GLP1 are liraglutide, once-weekly formulation of exenatide Exenatide LAR), albiglutide and dulaglutide; they activate the GLP1 receptor continuously, compared with short-acting effect on gastric emptying and postprandial glucose. Exenatide, exenatide LAR and lixisenatide derived from the exendin-4 molecule, a peptide with a 53% homology with human GLP1[177-179]. Liraglutide, albiglutide and dulaglutide are 97%, 95% and 90% identity.
Exenatide: Exenatide was the first RA-GLP1 to be approved for glycaemic control. Is a synthetic 39-amino acid peptide identical to the exendine-4 molecule isolated from salivary glands of the Gila monster; shares approximately 53% homology with native GLP1. The usual dose is 5-10 μg twice-daily subcutaneous injection.
Exenatide in monotherapy lowered HbA1c by 0.7%-0.9% and fasting plasma glucose by 17.5-18.7 mg/dL. The efficacy and safety of exenatide has been proved in several clinical studies[180-183]. Up to 46% of patients treated with exenatide achieved HbA1c ≤ 7% objective compared with up to 13% of placebo group. Moreover, mean change in body weight from baseline was greater in the exenatide group (-1.6 to -2.8 kg) than in the placebo group (-0.3 to -0.9 kg)[180-182]. When compared exenatide with insulin glargine or biphasic insulin aspart in patients with T2DM not controlled with oral agents, there were similar reductions in HbA1c in the exenatide and insulin groups (approximately -1.0%) suggesting non-inferiority of exenatide compared to insulin in relation to HbA1c reduction[184,185]. Exenatide showed weight loss and reduction in postprandial glycaemia compared with any insulin therapy, and lower rate of nocturnal hypoglycaemia compared with insulin glargine. In the glargine comparison study, insulin was titrated based upon achieving a target fasting glucose level < 100 mg/dL (5.6 mmol/L).
Lixisenatide: Lixisenatide is a RA-GLP1 that shares some structural elements with exendin-4. Compared with native GLP1, it has a prolonged half-life (2.7 to 4.3 h). Is available in Europe, not in United States, for use in combination with oral agents or insulin; is not considered a first-line therapy. Is available in a prefilled pen containing 14 doses of 10 or 20 mcg of lixisenatide. The initial dose is 10 mcg subcutaneously once daily within one hour prior to any meal of the day; after 2 wk the dose can be increased to 20 mcg.
Lixisenatide has been studied as monotherapy and in combination with one or two oral agents (metformin, pioglitazone, sulfonylureas). In a 24-wk double-blind trial of lixisenatide 20 mcg once daily vs placebo in 680 T2DM patients inadequately controlled with metformin (mean HbA1c 8.1%), the mean reduction in HbA1c was significantly greater with lixisenatide (-0.9% vs -0.4%)[186], and in another 24-wk no inferiority trial of once-daily subcutaneous lixisenatide 20 mcg once daily vs exenatide 10 mcg twice daily in 634 T2DM patients inadequately controlled with metformin alone (mean baseline HbA1c 8%), lixisenatide was no inferior to exenatide (mean change HbA1c -0.79% vs -0.96% with exenatide)[187].
Lixisenatide has been also used in combination with basal insulin therapy[188-190]. In a 24-wk double-blind trial, in 495 patients with T2DM not controlled with insulin glargine and metformin (mean HbA1c 8.4%), HbA1c reduction was significantly greater in the lixisenatide group compared to placebo (-0.6% vs -0.3%).
Liraglutide: Liraglutide is a human RA-GLP1, obtained through modifications of the human GLP1, with a large half life, which is administered once a day. Is available for use as monotherapy (adjunct to life style changes) or in combination with oral agents and basal insulin in adults with T2DM. The initial dose is 0.6 mg once daily subcutaneously the first week; and after the dose should be increased to 1.2 mg; and if HbA1c remain above the goal range the dose can be increased to 1.8 mg. It can be administered at any time of the day, with or without meals.
In clinical studies, administration of liraglutide (0.6-1.8 mg/d), alone or added to other antidiabetics agents, resulted in a reduction in HbA1c between 0.6%-1.6%. In a 52-wk trial of monotherapy with liraglutide (1.2 or 1.8 mg) vs glimepiride (8 mg) in 746 patients with recently diagnosed T2DM, the proportions of patients achieving an HbA1c ≤ 7% were 43%, 51% and 28%, respectively. Reductions in HbA1c were significantly greater with liraglutide 1.2 and 1.8 mg (-0.84% and -1.14% vs -0.51% with glimepiride). In addition, the HbA1c reduction with liraglutide (1.8 mg) was higher than that with other doses[191]. In another 26-wk double-blind trial, 413 T2DM patients not controlled with basal insulin and metformin were randomly assigned to exchange basal insulin with insulin degludec or insulin degludec plus liraglutide; all patients continued metformin[192]. The reduction in HbA1c was significantly greater in the degludec-liraglutide group (treatment difference -1.1%). The mean reduction in weight with degludec-liraglutide was 2.7 kg vs no change with degludec alone.
Exenatide LAR: Administration of exenatide LAR was proved more effective than highest dose of exenatide twice-daily[193,194], sitagliptin and pioglitazone[195], and insulin glargine[196] in T2DM patients treated with oral hypoglycaemic agents. Is available for use as adjunct to lifestyle changes to improve glycaemic control in T2DM. The usual dose is 2 mg subcutaneously once weekly at any time of the day with or without meals.
Albiglutide: It is a RA-GLP1 with a half-life of five to seven days, which allows once-weekly administration. It is available for use as monotherapy or in combination with oral agents or basal insulin. Is available in prefilled pen that contain a powder (30 or 50 mg), and a diluent to make a solution that is injected subcutaneously once weekly. The initial dose is 30 mg, and if after 6-8 wk blood glucose remain above the goal, the dose can be increased to 50 mg.
Albiglutide has been studied as monotherapy and in combination with one or two oral agents (metformin, pioglitazone, sulfonylureas and insulin). As examples: In a one-year trial of albiglutide vs insulin glargine in 779 T2DM patients inadequately controlled with metformin (with or without a sulfonylurea), the mean HbA1c reduced from 8.28% to 7.62% in the albiglutide group and from 8.36% to 7.55% in the glargine group[197]. Albiglutide met its pre-specified non-inferiority margin; however the comparison should be interpreted with caution because the dose of glargine was not systematically up titrated. Glargine was significantly more effective than albiglutide in reducing fasting blood sugar. In another two-year trial of weekly albiglutide vs daily sitagliptin, daily glimepiride, and weekly placebo in patients with T2DM inadequately controlled with metformin (mean HbA1c 9.1% to 8.2%), the reduction in HbA1c from baseline among the four groups was -0.6%, -0.3%, -0.4%, and +0.3%, respectively[198]. Although statistically significant, the mean reduction in HbA1c from baseline in the albiglutide group compared with the sitagliptin and glimepiride groups was small and of uncertain clinical relevance.
Dulaglutide: It is the last RA-GLP1 in appear. It has a structure that gives it the properties of slow absorption and reduced renal clearance rate. It is available for use as monotherapy or in combination with oral agents or insulin, in a ready-mixed pen at dose of 0.75 mg in monotherapy once weekly or 1.5 mg in combination, once weekly.
It has been compared with other antiabetic agents such metformin, iDPP4, insulin and other RA-GLP1, with a reduction in HbA1c ranging from -0.78% to -1.51%. In a 52-wk trial of weekly dulaglutide (0.75 or 1.5 mg weekly) vs sitagliptin in 1098 T2DM patients not controlled with metformin, the reduction in mean HbA1c was significantly greater with either dose of dulaglutide (mean HbA1c reduced from 8.2% to 7.3% with dulaglutide 0.75 mg weekly, from 8.1% to 7.0% with dulaglutide 1.5 mg weekly, and from 8% to 7.6% with sitagliptin)[199]. The mean change in body weight was significantly better with dulaglutide (-2.6 kg and -3 kg vs -1.53 kg with sitagliptin).
Precautions and side effects: All RA-GLP-1 should not be used in patients with history of pancreatitis and are not approved for use in T1DM. Exenatide and lixisenatide should not be utilized in patients with an eGFR < 30 mL/min and with severe gastrointestinal disease. Liraglutide, albiglutide and dulaglutide should not be used in patients with personal or family history of medullary thyroid cancer or multiple endocrine neoplasia 2A or 2B.
The mayor side effect are gastrointestinal, particularly nausea, vomiting and diarrhoea. It appears with lower frequency with exenatide LAR or lixisenatide than exenatide twice daily; albiglutide had lower rates than liraglutide and liraglutide and dulaglutide are similar. The risk of hypoglycaemic events is small, and may occur when RA-GLP-1 is given in conjunction with other treatments that cause hypoglycaemia, e.g., basal insulin, sulfonylureas.
Injection site reactions are more common with RA-GLP-1 than with insulin. Between RA-GLP1 are more common with exenatide LAR and with albiglutide. These reactions can be abscess, cellulitis and necrosis with or without subcutaneous nodules. Antibodies to RA-GLP1 may occur. In the majority of patients, the titre of antibodies decreases over time and does not affect glycaemic control. In a meta-analysis of 17 trials, the proportion of patients with antibodies against RA-GLP1 was higher in the albiglutide group compared with placebo (6.4% albiglutide 30 mg weekly vs 2% with placebo)[200].
Head-to-head comparisons of RA-GLP1: They have been published 9 phase III clinical trials, comparing different pairs of RA-GLP1[201]. One of them is with taspoglutide: T-emerge 2; we are not going to include it in the present review because it development was halted because of serious hypersensitivity reactions and gastrointestinal adverse events.
DURATION-1[193]: Exenatide twice daily vs exenatide LAR. Duration: 30 wk. Inclusion criteria: ≥ 16 years, therapy with lifestyle changes, or with 1-2 oral agents (metformin, sulfonylureas and/or TZD), HbA1c 7.1%-11.0%, fasting plasma glucose < 16 mmol/L, and body mass index (BMI) 25-45 kg/m2.
DURATION-5[194]: Exenatide twice daily vs exenatide LAR. Duration 24 wk. Inclusion criteria: ≥ 18 years, therapy with lifestyle changes, or with metformin, sulfonylureas, TZD or a combination, HbA1c 7.1%-11.0%, fasting plasma glucose < 15.5 mmol/L, and BMI 25-45 kg/m2.
DURATION-6[202]: Exenatide LAR vs liraglutide once daily. Duration 26 wk. Inclusion criteria: ≥ 18 years, therapy with lifestyle changes and oral agents (metformin, sulfonylureas, metformin + sulfonylureas or metformin + pioglitazone), HbA1c 7.1%-11.0%, and BMI ≤ 45 kg/m2 and stable body weight.
LEAD-6[203]: Exenatide twice daily vs liraglutide once daily. Duration 26 wk. Inclusion criteria: 18-80 years, treated with metformin, sulfonylureas or both, HbA1c 7.0%-11.0%, and BMI ≤ 45 kg/m2.
GetGoal-X[188]: Exenatide twice daily vs lixisenatide once daily. Duration 24 wk. Inclusion criteria: 21-84 year, therapy with metformin, and HbA1c 7.0%-11.0%.
HARMONY 7[204]: Albiglutide once weekly vs liraglutide once daily. Duration 32 wk. Inclusion criteria: ≥ 18 years, therapy with metformin, sulfonylureas, TZD or a combination, HbA1c 7.0%-10.0%, and BMI 20-45 kg/m2.
AWARD-6[205]: Dulaglutide once weekly vs liraglutide once daily. Duration 26 wk. Inclusion criteria: ≥ 18 years, therapy with metformin, and HbA1c 7.0%-10.0%.
Kapitza et al[206]: Lixisenatide once daily vs liraglutide once daily. Duration 28 wk. Inclusion criteria: 37-74 years, therapy with metformin, and HbA1c 6.5%-9.0%.
Effects on HbA1c: In the DURATION-1 and DURATION-5 exenatide LAR produced more consistent and greater reductions in HbA1c than exenatide twice daily. In the GetGoal-X exenatide twice daily showed greater HbA1c reduction than lixisenatide. Liraglutide in LEAD-6 and DURATION-6 reach greater HbA1c reductions tan exenatide twice daily or exenatide LAR, and in HARMONY 7 shows also greater reductions tan albiglutide. Liraglutide and dulaglutide did not differ in AWARD-6 study.
Effects on weight: It varies among RA-GLP1 and studies. In DURATION-1 and DURATION-5, there were no significant differences in weight loss between the two exenatide preparations. In LEAD-6, liraglutide and exenatide twice daily loss similar weight as in GetGoal-X study, between exenatide twice daily and lixisenatide, the difference was non-significant. Only in AWARD-6 and in the study by Kapitza et al[206], liraglutide revealed significantly greater reductions than dulaglutide and lixisenatide.
Cardiovascular effects: Improvements in both systolic and diastolic blood pressure have been reported in clinical trials; however in these head-to-head trials there were no statistically significant differences between treatments. Increases in resting heart rate have been reported. With exenatide twice-daily the heart rate increases, but it is lower than with exenatide LAR or liraglutide; dulaglutide is similar to liraglutide. With lixisenatide and albiglutide has not shown an increase in heart rate.
Insulin is utilized in the treatment of patients with all types of diabetes[207]. Human insulin preparations (NPH and regular insulin) do not imitate endogenous insulin secretion (basal and postprandial). Then, insulin analogues (aspart, lispro, glulisine, detemir, glargine, degludec and U-300) were developed. They have increased the flexibility and efficacy of diabetes management. The very rapid-acting insulin analogs have both: Faster and shorter duration of action than regular insulin for pre-meal coverage, while the long-acting analogs have a longer duration of action allowing once-daily dosing; also shows less day-to-day variability[208] (Table 5).
| Insulin type | Onset of action | Peak effect | Duration of action |
| Lispro, aspart, glulisine | 5 to 15 min | 45 to 75 min | 2 to 4 h |
| Regular | About 30 min | 2 to 4 h | 5 to 8 h |
| NPH | About 2 h | 4 to 12 h | 18 to 28 h |
| Insulin glargine | About 2 h | No peak | 20 to 24 h |
| Insulin detemir | About 2 h | No peak | 6 to 24 h1 |
| NPL | About 2 h | Six hours | 15 h |
| Insulin degludec | About 2 h | No peak | > 40 h |
| Insulin U-300 | About 2 h | No peak | > 36 h |
Insulin preparations: Long-acting insulins (glargine and detemir), and ultra-long-acting insulins (degludec and Glargine U-300) can be combined with rapid-acting insulins (aspart, lispro or glulisine) in basal bolus therapy.
Insulin glargine and human insulin are the same except for a substitution of glycine for asparagine in position A21 and by the addition of two arginine molecules in the B-chain of the insulin molecule[209]. These modifications originate a change in the pH such that, after administration, glargine precipitates in the subcutaneous tissue making hexamers, which delays absorption and extends duration of action. Glargine has a duration of action that usually lasts 24 h. Glargine cannot be mixed with rapid-acting insulins as the kinetics of both the rapid acting insulin and glargine and will be modified.
Insulin detemir is another insulin analog developed by removing a threonine an acylating a lysine with 14-carbon fatty acid; the fatty acid side chain allows albumin binding and results in prolongation of action. Clinical trials in patients with type 1 diabetes have suggested that twice-per-day injections may be necessary to achieve acceptable basal rate coverage and optimal glycaemic control[210]. In T2DM, where endogenous insulin secretion may mask any deficiencies in basal insulin, the data are less clear. Nevertheless the duration of action is dose-dependent; at higher doses mean duration of action is longer. Detemir cannot be mixed with rapid-acting insulins.
When glargine and detemir are administered in high doses, both show a peak on pharmacokinetic and pharmacodynamics profile[211]; also there is still interindividual variability and low doses are insufficient to cover a 24-h period[212]. Therefore, new ultra-long insulins were developed: Degludec, glargine U300 and LY2605541 (PEGylated Lispro). Lilly had discontinued the development of the last one because of hepatic lipid accumulation.
Insulin degludec is a modified B chain analogue that forms hexamers and di-hexamers when is administrated. Compared with other long-acting insulins (glargine and detemir), the insulin degludec profile is flatter with a half life greater than 25 h, and action that exceeds 42 h, which results in a reduction of confirmed and nocturnal hypoglycaemias[213]. Glargine U300 is the same glargine molecule concentrated three times; so it has the same mechanism to slow its absorption as insulin glargine.
At present there are no head-to-head comparisons of insulin degludec and Glargine U-300. We are going to analyse clinical trials of both of them but comparisons between them should not be make because the studies are different: for example hypoglycaemia definition use in degludec is plasma glucose threshold of 3.1 mmol/L (55.85 mg/dL) and in Glargine U-300 is 3.9 mmol/L (70.26 mg/dL).
Clinical trials in T2DM: BEGIN basal-bolus type 2 study is a 52-wk, randomised, treat-to-target, parallel-group, open-label, non-inferiority trial. Compared the efficacy and safety of once-daily insulin degludec with once-daily insulin glargine in a basal-bolus regimen with mealtime insulin aspart, with or without metformin, pioglitazone, or both in participants with T2DM[214]. After 1 year, HbA1c decreased by 1.1% in the degludec group and 1.2% in the glargine group. Rates of overall confirmed hypoglycaemia (plasma glucose < 55 mg/dL) were lower with degludec, as well as rates of confirmed nocturnal hypoglycaemia. These results were maintained in a 26-wk extension of this study with fewer hypoglycaemic episodes (24% overall reduction and 31% confirmed nocturnal episodes reduction)[215].
BEGIN Once Long was a 1 year phase 3 trial with type 2 insulin naive patients not controlled with oral hypoglycaemic agents. Again insulin degludec shows non inferiority in reducing HbA1c, and demonstrate a lower rate of nocturnal hypoglycaemia compared with glargine[216].
EDITION 1, 2 and 3[217-219] evaluated Gla-U300 in T2DM patients through 6 mo. The primary endpoint in the three studies was meeting the non-inferiority criterion in reduction HbA1c levels; which is confirmed in all studies; and the secondary endpoint was the percentage of patients with one or more confirmed or several nocturnal hypoglycaemias between week 9 and month 6. In EDITION 1 fewer patients reported one or more confirmed (< 3.9 mmol/L or < 70 mg/dL) or severe nocturnal hypoglycaemic events between week 9 and month 6 with Gla-U300 [36% vs 46% with Gla-U100; relative risk 0.79 (95%CI: 0.67-0.93); P < 0.005]. In EDITION 2, again the percentage of patients with nocturnal hypoglycaemia was lower in those with Gla-U300 than with Gla-U100 with a risk reduction of 23%. In EDITION 3, the percentage of patients with nocturnal hypoglycaemia was statistically similar in patients with Gla-U300 and Gla-U100.
In conclusion, insulin degludec showed similar efficacy in reducing HbA1c to insulin glargine, with a decreased risk of confirmed and nocturnal hypoglycaemia.
Colesevelam is a bile acid sequestrant that reduces LDL cholesterol in patients with hypercholesterolemia.
Mechanism of action: Possibly colesevelam interferes glucose absorption at gastrointestinal level.
Efficacy: In T2DM patients not controlled, colesevelam added to treatment of oral hypoglycaemic agents or insulin resulted in a reduction of HbA1c levels of 0.5%[220-222].
Side effects: nausea, constipation and dyspepsia are frequent side effects. Also increases triglyceride concentrations by approximately 20%. We do not recommend colesevelam to treat T2DM patients due the modest glucose-lowering effectiveness, expense, and limited clinical experience.
Bromocriptine is a dopamine agonist that has been used for the treatment of hyperprolactinemia and Parkinson disease.
Mechanism of action: The mechanism of action in reducing blood glucose is unknown. A quick release formulation of bromocriptine (Cycloset) was approved by the FDA for the treatment of T2DM[223].
Efficacy: In short-term clinical trials in T2DM patients, bromocriptine (up to 4.8 mg daily) as monotherapy or added to sulfonylureas reduce HbA1c compared with placebo in 0.4%-0.5%[223,224].
Side effects: Nausea, vomiting and headache[225] are frequent side effects. We do not recommend bromocriptine to treat T2DM patients due its glucose lowering effect and very frequent side effects.
Pramlintide is an amylin analog that is administered by mealtime subcutaneous injection. It is available for use for both T1 and insulin-treated T2DM; is only be used in patients also taking prandial insulin. Pramlintide replicates amylin actions and controls glucose without causing weight gain.
Mechanism of action: Pramlintide control postprandial blood glucose levels by slowing gastric emptying, promoting satiety, and reducing the postprandial glucagon increase in patients with diabetes[226]. The effects are glucose-dependent. Pramlintide does not cause hypoglycaemia in the absence of therapies that may cause hypoglycaemia. Supraphysiologic doses of pramlintide do not provoke hypoglycaemia in normal subjects, and pramlintide does not interfere with recovery from insulin-induced hypoglycaemia[227].
Efficacy: There are several randomized controlled trials in T2DM that shows its efficacy; for example when added pramlintide to existing insulin therapy with or without a sulfonylurea or metformin, reductions in HbA1c (mean 0.62%) and weight (1.4 kg) were seen with 120 mcg but not 90 mcg of pramlintide given twice daily[228]. In a 24-wk trial or without oral agents had similar glycaemic efficacy as the addition of premeal rapid acting insulin analogs (HbA1c reduction of approximately 1%)[229]. Patients randomly assigned to pramlintide maintained their weight, whereas those assigned to rapid acting insulin gained weight (mean 4.7 kg). Pramlintide was associated with fewer hypoglycaemic events compared with prandial insulin. In addition to modest reductions in HbA1c and body weight, pramlintide has been associated with reductions in postprandial glucose excursions and in surrogate markers of cardiovascular risk and oxidative stress[230,231].
Side effects: The most frequent side effect is nausea and generally dissipates by four weeks. Pramlintide should not be administered to patients with severe hypoglycaemia unawareness. Pramlintide should only be administered before meals that contain at least 250 calories or 30 g of carbohydrates. The recommended initial dose for T2DM is 60 mcg, titrated upward as tolerated to 120 mcg with each meal.
Elderly people with diabetes have a risk of developing macrovascular and microvascular complications, similar to that of younger patients with diabetes. In addition, they have a higher rate of lower limb amputations, and other complications than any other age group[232,233]; and those ≥ 75 years have a higher rate of most complications than those between 65 and 74 years. Older people > 75 years have a significant increase in death by hypoglycaemia, and visits to the emergency room for hypoglycaemia, compared to the general population with diabetes[234].
Therefore, older people with diabetes have a number of characteristics that will influence their treatment, such as[235]: (1) presence of high co-morbidities; (2) presence of cognitive and functional impairment (falls); (3) polypharmacy; (4) visual and hearing impairment; (5) decreased physical activity; (6) high risk of hypoglycaemia; (7) common situations of social isolation and dependence. Depression; (8) nutrition-related problems; and (9) heterogeneity in terms of clinical presentation of the diabetes (diabetes duration, co-morbidities, functional status, life expectancy).
Based on all the above, the treatment of diabetes in the elderly people should achieve the following objectives: (1) to avoid disability, ensuring the best quality of life; (2) to avoid side effects of treatment, especially the most associated with impaired quality of life such as hypoglycaemia and falls; and (3) to have a global vision of the patient, introducing competitive risks in the decision-making process.
The initial treatment of T2DM in elderly patients is similar to that of younger patients, and includes changes in the lifestyle, with weight reduction, although most of elderly patients with T2DM will need drug treatment throughout his life.
Changes in lifestyle are very important in the treatment of diabetes at any age, but they deserve special considerations for the elderly. In the Diabetes Prevention Program, people < 60 years of age improved their glycaemic control over time, due in part to better adapt to changes in lifestyle, compared with other younger age groups[236,237].
Nutritional needs: Although calorie needs decrease with age, macronutrient needs will be similar throughout adulthood. Older people with diabetes are at risk of malnutrition from anorexia, altered taste and smell, difficulty swallowing, oral and dental problems, and functional alterations; major difficulties in the preparation and consumption of food. The Mini Nutritional Assessment, a questionnaire designed to detect malnutrition, is very easy to use and has proved useful in diabetics elderly patients[238].
Nutritional recommendations should take into account the customs of the patients, their preferences and their personal goals and skills. When the regular intake does not meet the nutritional needs, a number of modifications, such as recommending fewer meals but more frequent, change the texture of foods, fortifying common foods, or add nutritional supplements between meals will be necessary. Overweight and obesity are common among the elderly. BMI is not useful in some older people due to changes in body composition with age[239]. Sarcopenia can occur in either overweight or underweight elderly. Moreover, obesity is often accompanied by decreased physical activity and increased frailty[240]. The unintentional weight loss in overweight or obese older people could worsen sarcopenia, bone mineral density and nutritional deficits[241,242]. Strategies that combine physical activity with nutritional therapy in older patients with diabetes, will lead to improved physical performance and a reduction of cardiometabolic risk[240,241].
The caloric intake in the elderly should be between 25 and 35 kcal/kg per day[243]. Protein should provide 15%-20% of total calories, fat 30% maximum, avoiding saturated fats and trans fats, and promoting the consumption of monounsaturated fats and omega 3 fatty acids, and carbohydrates 50%-55% based on complex carbohydrates. A dietary fiber intake of about 14 g/1000 kcal is recommended, and they may also require calcium and vitamin D and vitamin B12 supplements. Fluid intake should be 30 mL/kg per day, with a minimum intake of 1500 mL/d, which may be increased in situations such as fever, infections, high temperatures, or excessive losses in urine and feces; or decreased in case of advanced renal insufficiency, or in states of fluid retention such as heart failure and liver cirrhosis[243].
Physical activity: In older people with diabetes, muscle mass and strength decrease with age, worsening by complications of diabetes, co-morbidities and hospitalizations. People with diabetes of long duration and high levels of HbA1c, have less muscle strength per unit of muscle mass, that people without diabetes of similar age and BMI, and that people with diabetes of short duration and better glycaemic control[244]. Increased physical activity will improve the functional status of the elderly with or without diabetes[245]. In the elderly, mild physical activity is related with increased physical health and psychosocial well-being[246], so that in these people with diabetes, healthy, it is recommended to perform the same exercise as other adults with diabetes[42]. Older patients with poorer health, will benefit even a modest increase in physical activity. Finally, patients at risk of falls should be referred to a physiotherapist for muscle and exercise balance.
Older patients have an increased risk of adverse events related to drugs due to pharmacokinetic changes as decreased renal elimination, and pharmacodynamics changes, age related, such as increased sensitivity to certain medications, which can affect at their disposal. These changes may result in an increased risk of hypoglycaemia, the need to reduce the dose of certain medicines and monitor renal function to minimize adverse effects[247,248]. It is important to select drugs with a strong benefit/risk ratio, to provide efficacy, persistence and safety of treatment. Usually, in older people with diabetes is recommended to start treatment with antidiabetic at low doses, and titrate the dose progressively according to response, without reaching the maximum dose, due to the risk of increased side effects without increasing efficiency[249].
Knowledge of the advantages and disadvantages of each family of antidiabetic drugs will help clinicians individualize treatment of elderly patients with T2DM[6].
Metformin: Metformin remains the drug of choice for first-line treatment of T2DM in any age group, including the elderly. Its low risk of hypoglycaemia, its potential benefits in patients with stable cardiovascular disease[250] or heart failure, and its low cost, makes it a beneficial drug for older people. However its side effects such as gastrointestinal intolerance, vitamin B12 deficiency and weight loss, do not recommend its use in frailty patients. Although the risk of lactic acidosis is minimal, it is recommended to monitor renal function frequently, reduce the dose if the eGFR is between 30-60 mL/min[242], and do not use it with eGFR < 30 mL/min[249-251]. Moreover, metformin should not be used in situations of tissue hypoxia, acute intercurrent disease, respiratory failure, acute heart failure, hepatic failure, administration of iodinated contrast, and risk of functional renal impairment (vomiting, diarrhoea). It is recommended to start with a low dose of 425 mg/d and titrate up to 1700 mg/d maximum, because with higher doses does not increase efficiency but increases side effects.
Sulfonylureas: They are also cheaper drugs, but due to its high risk of hypoglycaemia, should be utilized carefully in elderly. Hypoglycaemia appears more frequently with long-acting sulfonylureas such as chlorpropamide, glibenclamide and glimepiride, especially in older adults who develop severe and prolonged hypoglycaemia. We must stop using long-acting sulfonylureas in older adults[252], being preferable the use of shorter-acting sulfonylureas such as gliclazide and glipizide[253]. Circumstances that influence the occurrence of sulfonylureas-induced hypoglycaemia in the elderly are: (1) after exercise; (2) missed meals, eat poorly, without meal time, or abuse alcohol; (3) existence of impaired renal or cardiac function or intercurrent gastrointestinal disease; (4) after being in the hospital[254]; and (5) by associating salicylates, sulfonamides, fibric acid derivates such as gemfibrozil, and warfarin[255].
On the other hand, these drugs produce weight gain, and its use is limited in renal failure because of the high risk of hypoglycaemia. Furthermore, the large amount of drug interactions, interfere their use in the elderly.
Meglitinides: Repaglinide and nateglinide are designed to control postprandial glycaemia, so that its duration is short and they require more frequent administrations with meals than sulfonylureas. Moreover, are more expensive, which limits its use in older people, especially in patients with polypharmacy. They lead a lower risk of hypoglycaemia than sulfonylureas[256], especially in patients who do not a set meal schedule[72], but they have a similar risk for weight gain. In addition, repaglinide, for its mainly biliary elimination, can be utilized in patients with moderate or advanced renal impairment[257], and could be utilized as first-line in patients with impaired renal function when they are intolerant to metformin and sulfonylureas, or are contraindicated. It should not be associated with drugs that act by activating or inhibiting cytochrome P450, such as gemfibrozil, because of the high risk of hypoglycaemia.
Alpha-glycosidase inhibitors: Acarbose and miglitol are drugs that are intended to control postprandial blood glucose, with low risk of hypoglycemia, which are theoretically attractive to treat older people[258]. However gastrointestinal effects, low efficiency, more frequent daily doses, and cost limit their use. They can alter the levels of digoxin and acenocumarol.
Thiazolidinediones: Although TZD do not increase the risk of hypoglycaemia, and pioglitazone may be beneficial in patients in secondary prevention[259], the high cost and side effects that induce as weight gain, macular oedema, fluid retention, increased risk of heart failure and bone fractures, and possible risk of bladder cancer[260], limit their use in the elderly[261].
DPP-4 inhibitors: iDPP4 inhibitors are once-a-day oral agents which can be used safely in elderly patients. They are very beneficial agents for the treatment of T2DM in the elderly since they control both basal and postprandial hyperglycaemia, with good tolerability, low risk of hypoglycaemia, and without significant drug interactions, or weight gain. These agents do not require dose adjustment in patients with advanced age. Although vildagliptin has demonstrated efficacy and safety in patients ≥ 75 years[262], data safety in these patients is very limited. Linagliptin do not require dose adjustment in patients with renal impairment; vildagliptin at doses of 50 mg/d can be employed at any degree of renal failure; saxagliptin half-dose (2.5 mg/d) can be used in ESRD; and sitagliptin dose should be adjusted to the degree of renal insufficiency: 50 mg/d if the eGFR is between 30-50 mL/min, and 25 mg/d if < 30 mL/min. Finally, vildagliptin requires monitoring of liver function.
iSGLT2: The iSGLT2 dapagliflozin, canagliflozin and empagliflozin, represent a new class of oral hypoglycaemic agents that increase the urinary excretion of glucose. This effect results in lower blood glucose levels in an insulin-independent manner, with a lower risk of hypoglycaemia, as well as mild diuresis[263]. The increase in glycosuria and diuresis produced, results in a reduction in weight and blood pressure. Because of these actions can be very attractive in the treatment of T2DM in the elderly[264,265]. However should not be utilized with an eGFR < 60 mL/min. Moreover, by inducing osmotic diuresis may increase the risk of dehydration, electrolyte abnormalities and weight loss that could limit its use in frail elderly patients. A common side effect of iSGLT2 is an increased incidence of genital and urinary infections, so they must be used with caution in elderly patients at increased risk of developing these infections or those with urinary incontinence[266].
RA-GLP1: The RA-GLP1 exenatide, liraglutide, lixisenatide, albiglutide and dulaglutide, will control both basal and postprandial hyperglycaemia with a low risk of hypoglycaemia. The drug-related effects such as nausea, vomiting, decreased appetite and weight loss can be a problem for frail elderly patients; however may be an option in those not vulnerable, obese elderly patients with good performance status where weight loss is a priority[200], as in those with knee osteoarthritis, sleep apnoea syndrome, hypoventilation, etc. Its use is not recommended in patients with an eGFR < 50 mL/min. There is little experience in patients ≥ 75 years, and its high cost and subcutaneous administration will limit its use in older patients.
Insulin: Insulin treatment can be utilized to achieve the goals of glycaemic control in selected older patients with T2DM, with similar efficacy and risk of hypoglycaemia than in younger patients. Before prescribing insulin in elderly subjects, we should think about the risk of hypoglycaemia related with this agent. The use of multiple daily injections of insulin, or by continuous subcutaneous insulin infusion in healthy elderly patients (mean age 66 years), has proven to be effective with a low rate of hypoglycaemia[267]. Also, the addition of long-acting insulin in elderly patients with T2DM (mean age 69 years) was as effective in achieving the HbA1c goals, without increased rate of hypoglycaemia, than in younger people (mean age 53 years)[268]. However there are few publications on the use of these insulin regimens in patients ≥ 75 years or in elderly patients with several co-morbidities, and/or a functional limitation. Visual or manual dexterity problems can be difficult to insulin therapy in some older patients. The use of insulin delivery devices will facilitate this work, selecting the one that best suits the skills and abilities of the patient. The risk of hypoglycaemia and weight gain will be lower with the use of insulin analogues compared to human insulins, and are preferred in elderly, despite their higher cost[208,269], especially if there is a high risk of hypoglycaemia as in the frail or institutionalized elderly. Also, when necessary, the insulin analogs are preferable to short regular human insulin, due to its lower rate of hypoglycaemia[270]. Insulinization, especially in frail elderly, should start with a single daily dose of long-acting insulin (0.1-0.2 IU/kg), lower than in younger patients, to avoid hypoglycaemia. Figure 1 shows the International Diabetes Federation Global Guidelines for managing older people with T2DM[271].
Before choosing a hypoglycaemic agent, we must consider the existence of an impairment renal function (Figure 2). Management of T2DM in patients with renal impairment is a complex process that requires a comprehensive approach. Clinicians must be aware that as renal function worsens, abnormalities in glucose homeostasis develop, affecting secretion, clearance, and peripheral tissue sensitivity to insulin[272]. CKD diagnosis adds risk factors for hypoglycaemia to those already present in patients with diabetes due to accumulation of uremic toxins, which lead to lower hepatic and renal insulin degradation, and also as a result of decreased renal gluconeogenesis, uremic malnutrition, and deficient catecholamine release[273]. Some of the additional factors are altered drug metabolism, drug-drug interactions, albuminuria, autonomic neuropathy, anorexia, malnutrition, infections, problems linked to dialysis, related cardiac and hepatic disease, and impaired renal glucose release[274,275]. On the other hand, both hypoglycaemia and CKD are related with increased morbidity and mortality from cardiovascular disease[276-278]. Many drugs are available for treatment of T2DM. Although all drugs can be utilized in patients with mild renal impairment[6,279], therapeutic choices for patients with moderate to severe CKD and ESRD are reduced, since drug or metabolite accumulation may occur due to a reduced GFR resulting in increasing side effects. In this case, some drugs are not recommended, while others can be used with dose adjustment.
Metformin: The incidence of lactic acidosis in the setting of metformin therapy is low, and the drug is not necessarily responsible when lactic acidosis occurs in patients taking this medication[65]. Although drug levels are higher in those with kidney disease, levels are still maintained largely within the therapeutic range[280,281] and lactate levels are not substantially increased when metformin is utilized in those with reduced GFR[282-285]. The recommendations for use of metformin based on eGFR are shown in Figure 3[247]. However, the main problem for metformin treatment in CKD patients is the prevention of intoxication. Dosage guidelines for CKD patients have recently been published[286]. These recommend the following maximum daily doses related to creatinine clearance: 3 g (120 mL/min); 2 g (60 mL/min); 1 g (30 mL/min); 500 mg (15 mL/min). Moreover, Lipska et al[247] have proposed a possible approach to metformin prescribing in the setting of CKD. The physician contemplating metformin treatment in a CKD patient should also address other problems. He should be advised to temporarily cease therapy if he develops sudden weight loss or acute illness, particularly if accompanied by vomiting and diarrhoea. X-ray contrast can occasionally cause acute renal insufficiency. In accordance with recent guidelines[287], patients with an eGFR < 45 mL/min should stop metformin 48 h before contrast investigations, and restart 48 h after. Other contraindications, e.g., liver disease and pregnancy, remain.
Sulfonylureas: Sulfonylureas can cause unregulated insulin release and lead to severe hypoglycaemia that can be particularly serious in the presence of CKD[288], due to the accumulation of active metabolites. Long-acting sulfonylureas like glyburide and chlorpropamide are more notorious for causing hypoglycaemia[289]. Shorter-acting sulfonylureas as glimepiride, glipizide and gliclazide agents are relatively safe and preferred in patients with CKD[290]. Major therapeutic considerations of sulfonylureas in patients with CKD and diabetes are[279,291-293]: (1) Glibenclamide should be prescribed with caution in patients with an eGFR 60-90 mL/min, and cannot be used in patients with an eGFR < 60 mL/min; (2) Glimepiride can be utilized in patients with an eGFR of < 60 mL/min, and dosage adjustment is required if the eGFR is < 30 mL/min. Begin at 1 mg daily or switch to another drug if the eGFR is < 15 mL/min; (3) Gliclazide is less than 1% excreted unchanged by the kidneys and does not have active metabolites[294]. It is recommended in subjects with an eGFR of 30-60 mL/min, need to reduce dose if the eGFR is < 30 mL/min, and it’s not recommended if the eGFR is < 15 mL/min; and (4) Glipizide does not increase hypoglycaemia in patients with CKD. Can be utilized in all stages of CKD with caution and with dose reduction.
Meglitinides: Both repaglinide and nateglinide are primarily metabolized in the liver, and generally, dose adjustment is not required for either of these agents. Therefore, their risk of hypoglycaemia is lower, and they are more effective for postprandial glycaemic control. Thus, at first, they may be employed in patients with CKD, without dose adjustment[295]. Repaglinide is mostly metabolised by the liver and could therefore be utilized in patients with low renal function, although some dose adjustment is required[296]. Nateglinide is rapidly degraded by the liver to mostly inactive or weakly active metabolites which are eliminated in the urine[297], also so can be considered patients with poor renal function, again with dose reduction. In conclusion, repaglinide and nateglinide can be prescribed in all stages of CKD with caution and dose reduction is necessary if the eGFR is < 30 mL/min[279,291,297,298].
Alpha-glucosidase inhibitors: As only less than 2% of an oral dose of acarbose was absorbed as active drug, patients with an eGFR < 25 mL/min attained increases about fivefold higher for peak plasma concentration of acarbose and six fold higher for AUC values than subjects with normal renal function[95]. Miglitol is systematically absorbed but is no metabolized, and is rapidly eliminated by renal excretion as unchanged drug[299]. Patients with an eGFR < 25 mL/min taking miglitol 25 mg three times daily showed a twofold increase in miglitol plasma levels when compared with patients with an eGFR > 60 mL/min[300]. Voglibose, an alpha-glucosidase inhibitor only commercialized in Japan, has no renal excretion, and two studies showed that it can be safely utilized in diabetic patients on haemodialysis, in combination with pioglitazone or mitiglinide[300,301]. In conclusion, alpha-glucosidase inhibitors acarbose and miglitol cannot be used if the eGFR is < 25 mL/min or the serum creatinine level is > 2 mg/dL[279,291,293,302], while voglibose can be used in all stages of CKD including haemodialysis[300,301].
Thiazolidinediones: Pioglitazone and rosiglitazone (only available in United States) are mainly metabolized in the liver and although a significant amount of active metabolites are eliminated in the urine; there is no need dose adjustment for either of these agents for patients with CKD[303]. However, both TZD cause fluid retention and increase the risk of heart failure, a problem that may be worse in patients with CKD. Although no dose adjustment in patients with CKD stages 3 to 5 is recommended[290], its use in patients with CKD should be balanced with the possibility of worsening of fluid retention and fractures, the latter particularly in patients with underlying bone disease[290,304,305].
DPP-4 inhibitors: iDPP4 are effective at lowering HbA1c in T2DM patients with moderate to severe renal impairment[304]. All iDPP4 differ in their renal excretion and therefore should be handled differently in patients with impairment renal function. Results from dedicated pharmacokinetics studies in subjects with various degrees of renal impairment suggest that the daily doses of all iDPP4 except linagliptin should be adjusted according to eGFR[305]. Several studies have demonstrated that the glucose-lowering efficacy is maintained while a good safety profile when reduced doses of these gliptins are utilized in patients with renal impairment[306-309]. On the other hand, linagliptin not require any dose adjustment in case of renal impairment, because is mainly excreted by the biliary route[310], and can be used in patients with all degrees of CKD[311]. Sitagliptin is largely excreted unchanged in the urine (87%) or via the feces (13%). No dose adjustment is necessary in patients with an eGFR > 50 mL/min, and can be utilized with dose reduction in patients with moderate to severe renal impairment[279,291,292,312]. The dose should be reduced by half in patients with an eGFR 30-50 mL/min, and a quarter in those with an eGFR < 30 mL/min or requiring dialysis. Around 80% of vildagliptin dose is metabolised mostly in the kidneys into non-active metabolites which are then renally excreted (85%) or recovered in the feces (15%)[313]. Vildagliptin not need dose adjustment in patients whose eGFR is > 50 mL/min and with caution in those with ESRD. The dose should be reduced by half in patients with moderate to severe renal impairment[279,291,292]. Saxagliptin is metabolised mainly in the liver to an active metabolite that is renally excreted, with approximately 20% of a dose being recovered unchanged in the urine and 20%-50% as metabolites[314]. No dose adjustment is required for patients with an eGFR > 50 mL/min, whereas the dose should be reduced by half in patients with moderate or severe renal impairment[279,291,292]. Vildagliptin can not be utilized in those on renal replacement therapy. Linagliptin is excreted almost entirely unchanged in bile, and its elimination is essentially via the feces[315]. No dose adjustment is required in patients with any stage of CKD[279,291,292] including, with caution, those requiring renal replacement therapy[316,317]. Alogliptin does not suffer appreciable metabolism and around 80% is eliminated unchanged in urine[318]. No dose adjustment is required for patients with an eGFR > 50 mL/min. Alogliptin dose adjustments are recommended for patients with moderate to severe renal impairment, including those with ESRD requiring dialysis. The dose should be reduced by half in patients with an eGFR 30-50 mL/min, and a quarter in patients with an eGFR < 30 mL/min or ESRD[279].
Sodium-glucose co-transporter 2 inhibitors: iSGLT2 decrease plasma glucose concentration by inhibiting the reabsorption of glucose by the kidney, which in turn, is a function of plasma glucose concentration and GFR. Because these agents rely on GFR to increase urinary glucose excretion, they are expected to have a decreased effect as kidney function declines. Studies examining the efficacy of iSGLT2 inhibition in patients with diabetes have been reported for a number of iSGLT2 including canagliflozin[319], dapagliflozin[320], empaglifozin[321] and ipragliflozin[322]. As expected, the efficacy of iSGLT2 decreases as kidneys function decreases[320-322]. Although renal function does not seem to be affected[141], its use in patients with moderate to severe CKD is not recommended. Dapagliflozin is not recommended if the eGFR is < 60 mL/min. In patients with an eGFR < 60 mL/min., canagliflozin and empagliflozin should not be initiated, but they may be continued in patients already taking the medications. Patients with an eGFR of 45-60 mL/min should be of the lower doses once a day, and both medications are contraindicated in patients with an eGFR < 45 mL/min, or on dialysis[139].
RA-GLP1: Due to the effect of these agents on gastric emptying, side effects are mainly gastrointestinal: Nausea, vomiting and diarrhoea. These gastrointestinal side effects with recurrent vomiting will lead to dehydration and secondary acute renal failure[323]. Exenatide is extensively renally eliminated by glomerular filtration and undergoes degradation by the kidneys to small, inactive peptide fragments[324]. There is reduced clearance in people with renal impairment[325]. Exenatide can be utilized in patients with and eGFR > 50 mL/min, whereas cannot be used in patients with an eGFR < 30 mL/min. In subjects with an eGFR of 30-60 mL/min, exenatide should only be employed with great caution and a lower doses[279,291,302]. Liraglutide is metabolised in a similar manner to large proteins, and its shows no reduced clearance in patients with renal impairment, and undergoes only minimal renal excretion[326]. No dose adjustment is required in subjects with an eGFR > 30 mL/min. Limited data are available in patients with an eGFR < 30 mL/min and ESRD, and should not be used in these populations[326]. As a peptide, lixisenatide is eliminated through glomerular filtration, followed by tubular reabsorption and subsequent metabolic degradation. No dose adjustment is recommended for patients with an eGFR > 50 mL/min, but as there is limited therapeutic experience in patients with an eGFR 30-50 mL/min, lixisenatide should be utilized with caution and is contraindicated in those with an eGFR < 30 mL/min and with ESRD[327]. Albiglutide is a protein, so the expected metabolic pathway is degradation to small peptides and amino acids by ubiquitous proteolytic enzymes. No dose adjustment is necessary in subjects with an eGFR > 30 mL/min. Limited data are available in subjects with an eGFR < 30 mL/min and should be used with caution in these populations[328]. Finally, dulaglutide is presumed to be degraded into its component amino acids by general protein pathways. No dose adjustment is recommended in subjects with renal impairment including ESRD. Limited data are available in patients with an eGFR < 30 mL/min, and should be employed with caution[329].
Insulin: Insulin is generally considered to be safe in patients with a reduced kidney function. Because of their low levels of degradation, insulin prolongs its half life when there is an impairment in kidney function[330]. As a result the risk of hypoglycaemic events is 5 times higher than in subjects without impairment renal function[331]. Almost 50% of circulating insulin is cleared by the kidney via glomerular filtration and subsequent luminal reabsorption of insulin by proximal tubular cells by means of endocytosis, or via diffusion of insulin from peritubular capillaries and subsequent binding of insulin to the contraluminal membranes of tubular cells. In insulin-treated T2DM patients, insulin doses should be reduced by 25% when the eGFR is between 10-50 mL/min, and by 50% when the eGFR is < 10 mL/min[332,333]. As for human insulin, the pharmacokinetic/pharmacodynamic profiles for insulin analogs may be influenced by many variables including renal function, although the available data are rather scarce[334]. Reduction of initial glargine/glulisine insulin weight-based dosing in hospitalized patients with T2DM and renal impairment reduced the frequency of hypoglycaemia by 50% without compromising the control of hyperglycaemia[335]. Short-acting insulin analog can also be utilized in haemodialysis patients with T2DM[336].
Figure 3 shows the therapeutic algorithm for the treatment of patients with T2DM and CKD, proposed by the Spanish Working Group, sponsored by several scientific societies[337].
Bariatric surgery could be an alternative in the treatment of obesity. Candidates for bariatric surgery are patients with a morbid obesity or those with a BMI > 35 kg/m2 who also have co-morbidities, such as hypertension, T2DM or obstructive sleep apnoea. After this surgery, it was observed a metabolic response leading to decrease blood glucose with improvements or remission of diabetes. Moreover, bariatric surgery also improves the metabolic status, improving lipid profile and hypertension, thus decreasing cardiovascular risk[338].
The improvement in glycaemic control, has been observed before the achievement of clinically significant weight loss. Although there are no consistent theories to explain the early improvement in T2DM after surgery, it seems a direct consequence of gastrointestinal anatomy restructuring that produces hormonal change and decreases food intake with an acute negative calorie balance[339]. This supports the idea that “metabolic surgery” is a definition more appropriate, and it refers a bariatric surgery in patients with less grade obesity than those who are traditionally eligible for bariatric surgery[338]. Despite this, it is necessary more investigation for known entirely the relationship between metabolic effects of bariatric surgery in overweight and in patients with obesity class I.
Unfortunately, all anti-diabetic agents have adverse effects, and are expensive. Therefore, the investigation of novel antidiabetic regimens, with less adverse effects and cheaper, is a major challenge for researchers.
Natural products containing high polyphenol levels as blackberries, red grapes, apricots, eggplant, coffee, cocoa and green tea can regulate glucose metabolism through different paths, such as restoring beta-cell integrity, enhancing insulin releasing activity, and increasing cellular glucose uptake, which can improve insulin resistance[340].
A new smart insulin patch has been created. It is a thin square covered with more than 100 tiny needles. The patch made of biocompatible materials works fast and it’s easy to use. The patch consists of small painless needles that are packed together with insulin and glucose-sensitive enzymes in microscopic storage units. The patch releases these enzymes when blood glucose increases. In a mouse model, patch administration showed reduced glucose levels up to 9 h[341]. It is suggested that the patch could have a longer effect in diabetic humans since humans are more sensitive to insulin than mice.
GLP1 and GIP are the two main incretin hormones that are released from the intestine in response to food intake. Both hormones stimulate glucose-dependent insulin secretion. Evidence from animal studies suggests that anti-obesity efficacy of GLP1 can be enhanced by co-administration with the incretin hormone GIP. Finan et al[342] showed that an acylated version of GLP1 and GIP dual agonist, reduced weight (-18.8% vs -8.8%, P < 0.001), food intake (P < 0.05), fat mass (P < 0.001) and blood glucose (P < 0.05), compared to liraglutide. Also showed increases in plasma insulin and C-peptide more pronounced that liraglutide (P < 0.001 for both). No differences in improved glycaemic control between these co-agonists and liraglutide were found. In T2DM patients they found a dose-dependent reductions of HbA1c, being -0.53% in patients treated with 4 mg of the dual agonist, and -1.11% in those treated with 30 mg, compared with placebo (-0.16%). The pharmacokinetics and pharmacodynamics results of co-activation of GLP1 and GIP receptors[343] are considered as a promising new strategy for the treatment of obese T2DM patients, to prolong the activity of GLP1 and GIP dual agonists, and for the future development of a possible once-weekly GLP-1 and GIP dual agonists drug candidate for the treatment of T2DM.
GLP1 and Glucagon receptor dual agonism: Glucagon and GLP1 have distinct receptors that are also structurally related[344]. Glucagon stimulates gluconeogenesis and glycogenolysis in the liver, raising blood glucose levels; while GLP1 reduce blood glucose levels by increasing insulin synthesis and secretion in the pancreas[345]. Administration of oxyntomodulin, a GLP1 receptor/glucagon receptor dual agonist peptide, to rodents[346-348] and humans[349,350], resulted in a improvement of glucose metabolism by decreasing food intake and body weight, and increasing energy expenditure, more pronounced than those reported by GLP1. Moreover, weekly administration of PEGylated peptides reduced adiposity and improved glucose tolerance in diet-induced obese mice[351], and sustained GLP1/glucagon dual agonism reverses obesity in diet-induce obese mice[352]. These co-agonist compounds also normalized glucagon, glucose and lipid metabolism and reduced liver steatosis, and is a novel therapeutic approach to the treatment of obesity in patients with T2DM.
GLP1 receptor agonist and Glucagon receptor antagonism activity: GLP1/Glucagon hybrid peptides, a dual acting peptide that bind both receptors, for diabetes (DAPD) have been reported previously[344], and more recently have been identified in vitro[353]. Administration of PEGylated DAPD in mice, showed a decrease in blood glucose by increasing insulin secretion GLP1-induced, and a rise in fasting glucagon levels following a glucagon challenge[354]. Moreover, unlike RA-GLP1, does not inhibit gastrointestinal motility and has not adverse events at this level.
The combination of GLP1 mimetics with basal insulin reduced the risk of hypoglycaemia and weight gain induced for intensive insulin regimens in T2DM patients. Preliminary evidence suggests that the addition of a basal insulin to a GLP1 mimetic with or without oral therapy, provide improvements in basal and postprandial glucose control, with less weight gain, reduced risk of hypoglycaemia and increased satisfaction[188-190,355-358]. Data from the DUAL I extension (insulin-naïve patients not controlled with oral hypoglycaemic agents) and DUAL II (patients not controlled on basal insulin plus oral hypoglycaemic agents) randomized trials, the novel fixed combination of insulin degludec and liraglutide (IDegLira), effectively lowered HbA1c across a range of measures, implying suitability for patients with either early or advanced T2DM[359]. LixiLan is a new once-daily single injection fixed-ratio combination of lixisenatide, and insulin glargine. Results from the Lixilan-L trial, showed that LixiLan successfully met the primary study endpoint of demonstrating a statistically superior reduction in HbA1c compared with insulin glargine[360].
G protein-coupled receptor 119 (GPR119) agonists is a G protein-coupled receptor that is expressed predominantly in the pancreas and gastrointestinal tract in rodents and humans, as well as in the brain in rodents[361]. Activation of the receptor showed a reduction in food intake and body weight gain in rats[361]. GPR119 has also been shown to regulate incretin and insulinsecretion[362-364]. New agents acting on this receptor have been suggested as novel treatments for obesity and diabetes[361,363,365].
It is worth pointing out the potential advantages that could be obtained by the co-administration of a GPR119 agonist and a iDPP4. The role of these additional hormonal agents will required to clarify in the further study[366].
Currently, RA-GLP1s are available only as injectables, either once daily or once weekly. Semaglutide is a long-acting RA-GLP1 that is also being developed as a once-weekly injectable. An oral semaglutide version leading to higher solubility and protection from enzymatic degradation is also being developed.
The phase 2 study[367] enrolled 632 adults with T2DM of 6 to 7 years duration, managed with lifestyle with or without metformin, and HbA1c 7.0% to 9.5% (mean, 7.9%). They were randomized to oral semaglutide in doses of 2.5, 5, 10, 20 or 40 mg once daily, or to placebo, or to open-label injected once-weekly 1.0-mg semaglutide. Patients started at 2.5 or 5 mg once daily and the higher-dose groups were titrated up at 4-wk intervals. The primary endpoint was change in HbA1c from baseline to week 26.
At 26 wk, mean HbA1c decreased dose-dependently with oral semaglutide, with drops ranging from 0.7% with 2.5 mg to 1.9% with 40 mg. Subcutaneous once-weekly semaglutide also produced a 1.9% drop in HbA1c, while the placebo group experienced a decrease of only 0.3% (P = 0.07 for 2.5 mg vs placebo, P < 0.0001 for other doses). For all the groups taking 5-mg oral semaglutide or higher doses, more than 80% of the patients achieved HbA1c values less than 7%, and the groups treated with 10-mg dose or more achieved mean HbA1c less than 6.5%. Fasting plasma glucose also dropped significantly, from a baseline of 170 mg/dL, with reductions ranging from 17 mg/dL with 2.5 mg to 51 mg/dL for the other oral doses (P = 0.08 for 2.5 mg, P < 0.0001 for other doses) and a reduction of 56 mg/dL with 1.0-mg subcutaneous semaglutide vs 1 mg/dL with placebo.
The proportion of patients achieving 5% or more weight loss was 21% to 71% in the oral group and 66% in subcutaneous group, compared with 13% in the placebo group.
None of the adverse events were considered serious and all were reported as mild to moderate in severity. Increases in lipase levels were greater in the oral and subcutaneous semaglutide groups, compared with placebo.
Based on these data, oral semaglutide is now being studied in a large phase 3 trial[368].
Oral administration of insulin is a novel treatment to improve glycaemic control in patients with T2DM. Oral insulin has a more physiological action than parenteral insulin. Due to its first pass through the liver, it reduces glycogenolysis, hepatic glucose production, and the risk of hypoglycaemia, compared with parenteral insulin. Currently, the data available in human trials suggest that could be a novel approach to the treatment of diabetes[369,370].
There are several oral insulins in development: Short-acting insulins as ORMD-0801 (Oramed) and Capsulin (Diabetology) in phase 2 studies, and the IN-105 (Biocon) in phase 3 studies; and basal insulins, such as the OI287GT (NN1956) (NovoNordisk).
Sotagliflozin is a dual inhibitor of SGLT1 and SGLT2 with approximately 20-fold selectivity for SGLT2 over SGLT1[371]. Animal pharmacology studies showed that sotagliflozin produced increased urinary glucose excretion, delivery of glucose to the caecum, increased postprandial GLP1 and peptide YY release, that were related with significant reductions in postprandial glucose[372,373]. Sotagliflozin was evaluated in patients with T2DM not controlled with metformin[372]. Sotagliflozin reduced fasting plasma glucose and HbA1c with a modest urinary glucose excretion, compared with selective iSGLT2. The high glycaemic efficacy observed with only modest urinary glucose excretion suggested that clinically relevant gastrointestinal SGLT1 inhibition was present. Phase 1 and phase 2 studies have identified special opportunities for synergy with iDPP-4 for treatment of patients with T2DM and renal impairment.
Technosphere insulin, a new inhaled insulin represent an alternative to bolus insulin injections, but can be used concomitantly with basal insulin injections. Its hypoglycaemic effect is less than the rapid-acting insulin, but has less hypoglycaemias[374]. Major adverse effects are respiratory, with cough being the most prominent, and there is a small decrease in the forced expiratory volume in 1 s (FEV1) with technosphere insulin, consistent, no progressive, and reversible; so that patients must receive pulmonary function test periodically throughout therapy. Should be utilized with caution in patients who smoke and is contraindicated in patients with chronic lung disease.
New chitosan formulations of xanthine derivatives (CS-6, CS-7) have been synthesized as antidiabetic and antioxidant treatments. Formulations of chitosan 6 (CS-6) have shown to reduce blood glucose levels by 59.3%, with a recorded 4.53% HbA1c level[375]. These effects were more intense than the induced by pioglitazone (114.5 mg/dL vs 148.5 mg/dL), when used as standard antidiabetic medication. These results have shown the potential application of chitosan formulations of Xanthine 6 derivates (CS-6) in the treatment of diabetes mellitus.
Recent studies have shown the dynamic role of zinc, an insulin mimetic, as a “cellular second messenger” in glucose homeostasis and in the control of insulin signaling[376]. Synthesis, secretion and insulin action are dependent on zinc and transporters. This suggests that zinc plays a role, previously not identified, where changes in the state of zinc over time can affect the activity of insulin. This is a novel area of investigation, and introduces a new class of useful drugs for diabetes pharmacotherapy.
Imeglimin is the first of the family of agents called “glimins” and, more specifically, is a tetrahydrotriazene compound[377]. Laboratory studies[377,378] have shown that acts on impaired glucose uptake by muscle tissue, excessive hepatic gluconeogenesis, and increased apoptosis of beta cells. Imeglimin is still in development and human studies are limited. The few human studies recently published[377,379-381] show that reduces HbA1c and fasting glucose similar to sitagliptin and metformin, with a low incidence of side effects, especially hypoglycaemia. Currently, there is an ongoing trial that evaluated the safety and efficacy of imeglimin with insulin therapy or compared directly with insulin in patients newly diagnosed or treated with oral monotherapy, whose results have not yet been published[382]. Imeglimin seems to be a promising antidiabetic agent as monotherapy in the treatment of T2DM.
Recent studies reported a possible role of the G protein coupled receptor 40 (GPR40), also known as FFAR 1, in the regulation of beta-cell function[383]. It was reported that chronic treatment of male zucker diabetic fatty (ZDF) rats (insulin resistant model with elevated blood glucose and FFAs levels) with CNX-011-67 (GPR40 agonist) increased insulin secretion, decreased blood glucose and reduced beta-cell apoptosis without affecting body weight[384]. From this study data it appears that CNX-011-67 could have the potential to provide good and durable glycaemic control in T2DM patients. Another study provided evidence that activation of GPR40 with CNX-011-67 stimulates glucose metabolism, improve glucose responsiveness and enhances insulin secretion, with the hope that pharmacological activation of GPR40 will prove beneficial for the treatment of T2DM[384]. TAK-875, a novel highly selective, orally bioavailable GPR40 agonist, significantly improved glycaemic control in patients with T2DM with a minimum risk of hypoglycaemia. The outcomes show that activation of FFAR1 is a viable therapeutic target for the treatment of T2DM[385]. According to current data it can be appreciated that beta-cell failure could be delayed or prevented by attaining and maintaining good glycaemic control. It is theoretically possible to inhibit multiple mechanisms by blocking the pathways leading to beta-cell apoptosis, and this is a challenge for the future.
Finally, in vivo studies, administration of hot water extracts of Salacia chinensis to diet-fed KK-Ay mice, resulted in a significant reduction in the basal and postprandial blood glucose and HbA1c levels; with an improvement of glucose tolerance[386]. The active components, salacinol, kotalanol, and neokotalanol inhibited human α-glucosidases as potently as they inhibited rat small intestinal α-glucosidase. The results suggest that these sulfoniums can be good candidates as new type of anti-diabetic agents.
While lifestyle modifications and metformin are the cornerstone of the initial management of T2DM, there is an increasing array of second and third-line pharmacological agents for this condition. At present there are different families of oral and injectable drugs, available for the treatment of T2DM. These include sulfonylureas, meglitinides, insulin, TZD and alpha-glucosidase inhibitors, and recently with the addition of RA-GLP1 receptor agonists, iDPP4 and iSGLT2. Moreover, insulin analogues that better simulate endogenous insulin secretion have been developed. Metformin remains the first choice of treatment for most patients. Other alternative or second-line treatment options should be individualized taking into consideration patient characteristics as degree of hyperglycaemia, presence of co-morbidities, and patient preference and ability to access treatments; and properties of the treatment such effectiveness and durability of lowering blood glucose, risk of hypoglycaemia, effectiveness in reducing diabetes complications, effect on body weight, side effects and contraindications. Although it does not appear that in the near future cure diabetes, novel safety and effective agents that will improve the quality of life of T2DM patients, are developing.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): E
P- Reviewer: Ali O, Chogtu B, Georgescu A, García-Mayor RV, Lee TS, Swierczynski J, Takebayashi K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | International Diabetes Foundation. Diabetes: facts and figures [accessed 2016 Mar 22]. Available from: http://www.idf.org/WDD15-guide/facts-and-figures.html. [Cited in This Article: ] |
| 2. | DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1365] [Cited by in F6Publishing: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 379] [Article Influence: 23.7] [Reference Citation Analysis (2)] |
| 4. | Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 527] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | del Cañizo-Gómez FJ, Moreira-Andrés MN. Cardiovascular risk factors in patients with type 2 diabetes. Do we follow the guidelines? Diabetes Res Clin Pract. 2004;65:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1872] [Cited by in F6Publishing: 1834] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 7. | Bagnasco A, Di Giacomo P, Da Rin Della Mora R, Catania G, Turci C, Rocco G, Sasso L. Factors influencing self-management in patients with type 2 diabetes: a quantitative systematic review protocol. J Adv Nurs. 2014;70:187-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Shen H, Edwards H, Courtney M, McDowell J, Wei J. Barriers and facilitators to diabetes self-management: perspectives of older community dwellers and health professionals in China. Int J Nurs Pract. 2013;19:627-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm--2016 executive summary. Endocr Pract. 2016;22:84-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 320] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 10. | National Diabetes Education Program (NDEP). Guiding principles for the care of people with or at risk for diabetes [accessed 2016 Jan]. Available from: http://www.niddk.nih.gov. [Cited in This Article: ] |
| 11. | Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 12. | McNeil J, Doucet É, Chaput JP. Inadequate sleep as a contributor to obesity and type 2 diabetes. Can J Diabetes. 2013;37:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2272] [Cited by in F6Publishing: 2220] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 14. | Esposito K, Maiorino MI, Ciotola M, Di Palo C, Scognamiglio P, Gicchino M, Petrizzo M, Saccomanno F, Beneduce F, Ceriello A. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Faulconbridge LF, Wadden TA, Rubin RR, Wing RR, Walkup MP, Fabricatore AN, Coday M, Van Dorsten B, Mount DL, Ewing LJ. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity (Silver Spring). 2012;20:783-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619-1626. [PubMed] [Cited in This Article: ] |
| 17. | Phelan S, Kanaya AM, Subak LL, Hogan PE, Espeland MA, Wing RR, Burgio KL, DiLillo V, Gorin AA, West DS. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol. 2012;187:939-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Franz MJ, Boucher JL, Green-Pastors J, Powers MA. Evidence-based nutrition practice guidelines for diabetes and scope and standards of practice. J Am Diet Assoc. 2008;108:S52-S58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, Jackson N, Fitzsimons K, Bright J, Coulman K. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999-2006. Obes Surg. 2011;21:351-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2726] [Cited by in F6Publishing: 2865] [Article Influence: 159.2] [Reference Citation Analysis (0)] |
| 23. | Gargallo Fernández Manuel M, Breton Lesmes I, Basulto Marset J, Quiles Izquierdo J, Formiguera Sala X, Salas-Salvadó J. Evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults (FESNAD-SEEDO consensus document). The role of diet in obesity treatment (III/III). Nutr Hosp. 2012;27:833-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 24. | Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab. 2003;16:277-283. [PubMed] [Cited in This Article: ] |
| 25. | Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1097] [Cited by in F6Publishing: 1019] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 26. | Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1843] [Cited by in F6Publishing: 1756] [Article Influence: 159.6] [Reference Citation Analysis (0)] |
| 27. | Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, Yancy WS. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012;35:434-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 28. | Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 29. | Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009;CD006296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Franz MJ. Diabetes mellitus nutrition therapy: beyond the glycemic index. Arch Intern Med. 2012;172:1660-1661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Burger KN, Beulens JW, van der Schouw YT, Sluijs I, Spijkerman AM, Sluik D, Boeing H, Kaaks R, Teucher B, Dethlefsen C. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS One. 2012;7:e43127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Post RE, Mainous AG, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Sanz París A, Boj Carceller D, Melchor Lacleta I, Albero Gamboa R. Sugar and diabetes: international recommendations. Nutr Hosp. 2013;28 Suppl 4:72-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 34. | Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1178] [Cited by in F6Publishing: 1147] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 35. | Serra-Majem L, Riobó Serván P, Belmonte Cortés S, Anadón Navarro A, Aranceta Bartrina J, Franco Vargas E, García-Closas R, Gómez-Candela C, Herrero Sancho E, La Vecchia C. Chinchón declaration; decalogue on low- and no-calorie sweeteners (LNCS). Nutr Hosp. 2014;29:719-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 36. | Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734-741. [PubMed] [Cited in This Article: ] |
| 37. | Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33:969-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Disorders of Carbohydrate and Metabolism. Williams Textbook of Endocrinology 12th edition. New York: USA Press 2011; 1413-1414. [Cited in This Article: ] |
| 39. | Vitolins MZ, Anderson AM, Delahanty L, Raynor H, Miller GD, Mobley C, Reeves R, Yamamoto M, Champagne C, Wing RR. Action for Health in Diabetes (Look AHEAD) trial: baseline evaluation of selected nutrients and food group intake. J Am Diet Assoc. 2009;109:1367-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3157] [Cited by in F6Publishing: 2746] [Article Influence: 249.6] [Reference Citation Analysis (0)] |
| 41. | Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 675] [Cited by in F6Publishing: 647] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 42. | Karlström BE, Järvi AE, Byberg L, Berglund LG, Vessby BO. Fatty fish in the diet of patients with type 2 diabetes: comparison of the metabolic effects of foods rich in n-3 and n-6 fatty acids. Am J Clin Nutr. 2011;94:26-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39 Suppl 1:S4-S5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 45. | Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 46. | Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 735] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 47. | Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147-e167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 851] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 48. | Armstrong MJ, Sigal RJ. Exercise as Medicine: Key Concepts in Discussing Physical Activity with Patients who have Type 2 Diabetes. Can J Diabetes. 2015;39 Suppl 5:S129-S133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Exercise prescription for patients with type 2 diabetes-a synthesis of international recommendations: narrative review. Br J Sports Med. 2015;pii:bjsports-2015-094895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Duclos M, Oppert JM, Verges B, Coliche V, Gautier JF, Guezennec Y, Reach G, Strauch G. Physical activity and type 2 diabetes. Recommandations of the SFD (Francophone Diabetes Society) diabetes and physical activity working group. Diabetes Metab. 2013;39:205-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170:1794-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 52. | Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 632] [Cited by in F6Publishing: 565] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 54. | Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med. 2008;42:47-54. [PubMed] [Cited in This Article: ] |
| 55. | Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 769] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 56. | Sigal RJ, Armstrong MJ, Colby P, Kenny GP, Plotnikoff RC, Reichert SM, Riddell MC. Physical activity and diabetes. Can J Diabetes. 2013;37 Suppl 1:S40-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2563] [Cited by in F6Publishing: 2524] [Article Influence: 210.3] [Reference Citation Analysis (2)] |
| 58. | An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol. 2016;228:R97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 59. | Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1031] [Cited by in F6Publishing: 1056] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 60. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3802] [Cited by in F6Publishing: 3995] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 61. | Song R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care. 2016;39:187-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 62. | Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 615] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 63. | Lalau JD, Arnouts P, Sharif A, De Broe ME. Metformin and other antidiabetic agents in renal failure patients. Kidney Int. 2015;87:308-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 64. | Lu WR, Defilippi J, Braun A. Unleash metformin: reconsideration of the contraindication in patients with renal impairment. Ann Pharmacother. 2013;47:1488-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668-2675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 365] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 66. | Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;CD002967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 782] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 68. | Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 69. | Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 419] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 70. | Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18:1248-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Eldor R, Raz I. Diabetes therapy--focus on Asia: second-line therapy debate: insulin/secretagogues. Diabetes Metab Res Rev. 2012;28 Suppl 2:85-89. [PubMed] [Cited in This Article: ] |
| 72. | Genuth S. Should sulfonylureas remain an acceptable first-line add-on to metformin therapy in patients with type 2 diabetes? No, it’s time to move on! Diabetes Care. 2015;38:170-175. [PubMed] [Cited in This Article: ] |
| 73. | Gerich J, Raskin P, Jean-Louis L, Purkayastha D, Baron MA. PRESERVE-beta: two-year efficacy and safety of initial combination therapy with nateglinide or glyburide plus metformin. Diabetes Care. 2005;28:2093-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K(ATP) channels. Curr Pharm Des. 2005;11:2699-2716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288-2296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 76. | Lau DC, Teoh H. Impact of Current and Emerging Glucose-Lowering Drugs on Body Weight in Type 2 Diabetes. Can J Diabetes. 2015;39 Suppl 5:S148-S154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5314] [Cited by in F6Publishing: 4985] [Article Influence: 311.6] [Reference Citation Analysis (0)] |
| 78. | Takahashi A, Nagashima K, Hamasaki A, Kuwamura N, Kawasaki Y, Ikeda H, Yamada Y, Inagaki N, Seino Y. Sulfonylurea and glinide reduce insulin content, functional expression of K(ATP) channels, and accelerate apoptotic beta-cell death in the chronic phase. Diabetes Res Clin Pract. 2007;77:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 80. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2205] [Cited by in F6Publishing: 2041] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 81. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14327] [Cited by in F6Publishing: 12338] [Article Influence: 474.5] [Reference Citation Analysis (0)] |
| 82. | McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, Dahl M. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35-e48. [PubMed] [Cited in This Article: ] |
| 83. | Lim PC, Chong CP. What’s next after metformin? focus on sulphonylurea: add-on or combination therapy. Pharm Pract (Granada). 2015;13:606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Scott LJ. Repaglinide: a review of its use in type 2 diabetes mellitus. Drugs. 2012;72:249-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 881] [Cited by in F6Publishing: 780] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 87. | International Hypoglycaemia Study Group. Minimizing Hypoglycemia in Diabetes. Diabetes Care. 2015;38:1583-1591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 88. | Morgan CL, Mukherjee J, Jenkins-Jones S, Holden SE, Currie CJ. Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: a retrospective, observational study. Diabetes Obes Metab. 2014;16:957-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Jørgensen CH, Gislason GH, Andersson C, Ahlehoff O, Charlot M, Schramm TK, Vaag A, Abildstrøm SZ, Torp-Pedersen C, Hansen PR. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention--a retrospective nationwide cohort study. Cardiovasc Diabetol. 2010;9:54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Kalra S, Gupta Y. Sulfonylureas. J Pak Med Assoc. 2015;65:101-104. [PubMed] [Cited in This Article: ] |
| 91. | Rosak C, Mertes G. Critical evaluation of the role of acarbose in the treatment of diabetes: patient considerations. Diabetes Metab Syndr Obes. 2012;5:357-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 92. | van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 93. | Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP-NIDDM Trial: an international study on the efficacy of an alpha-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to Prevent Non-Insulin-Dependent Diabetes Mellitus. Diabetes Care. 1998;21:1720-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 94. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1103] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 95. | Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12:57-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Kao CC, Wu PC, Wu CH, Chen LK, Chen HH, Wu MS, Wu VC. Risk of liver injury after α-glucosidase inhibitor therapy in advanced chronic kidney disease patients. Sci Rep. 2016;6:18996. [PubMed] [Cited in This Article: ] |
| 97. | Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. PPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes. 1997;46:1230-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med. 2011;17:623-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 99. | Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004;27:1660-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 100. | Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 101. | Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941-2948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 102. | Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 103. | Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 104. | Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 105. | Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007;148:903-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 106. | Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 296] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 107. | Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, Gottesdiener K, Wagner J, Herman GA. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30:1862-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 108. | Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564-2571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 109. | Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 499] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 110. | Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 111. | Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 112. | Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 113. | DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, Chen RS. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 114. | Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle HJ, Dugi KA. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13:258-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 265] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 115. | Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 116. | Fujii Y, Abe M, Higuchi T, Mizuno M, Suzuki H, Matsumoto S, Ito M, Maruyama N, Okada K, Soma M. The dipeptidyl peptidase-4 inhibitor alogliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Expert Opin Pharmacother. 2013;14:259-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. Diabetes Obes Metab. 2012;14:927-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 119. | Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 120. | Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 121. | Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 122. | Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 123. | Thomsen RW, Pedersen L, Møller N, Kahlert J, Beck-Nielsen H, Sørensen HT. Incretin-based therapy and risk of acute pancreatitis: a nationwide population-based case-control study. Diabetes Care. 2015;38:1089-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 124. | Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 858] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 125. | Gooßen K, Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2012;14:1061-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 126. | Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 525] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 127. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1831] [Cited by in F6Publishing: 1764] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 128. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2548] [Cited by in F6Publishing: 2470] [Article Influence: 224.5] [Reference Citation Analysis (0)] |
| 129. | Bhatt DL, Cavender MA. Do dipeptidyl peptidase-4 inhibitors increase the risk of heart failure? JACC Heart Fail. 2014;2:583-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 130. | Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:356-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 131. | Clifton P. Do dipeptidyl peptidase IV (DPP-IV) inhibitors cause heart failure? Clin Ther. 2014;36:2072-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 132. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1927] [Cited by in F6Publishing: 1827] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 133. | Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 561] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 134. | Weir MR. The kidney and type 2 diabetes mellitus: therapeutic implications of SGLT2 inhibitors. Postgrad Med. 2016;128:290-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 135. | Kalra S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 2014;5:355-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 136. | Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10-F18. [PubMed] [Cited in This Article: ] |
| 137. | Lee YJ, Lee YJ, Han HJ. Regulatory mechanisms of Na(+)/glucose cotransporters in renal proximal tubule cells. Kidney Int Suppl. 2007;S27-S35. [PubMed] [Cited in This Article: ] |
| 138. | Hummel CS, Lu C, Loo DD, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300:C14-C21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 139. | Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 140. | Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53:875-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 141. | Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223-2233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 142. | Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 143. | Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 144. | Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 145. | Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 146. | Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 147. | Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 480] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 148. | Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582-2592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 149. | Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 150. | Leiter LA, Langslet G, Cefalu WT, Yoon KH, Arias P, Xie J, Balis D, Milligton D, Vercruysse F, Carnovatchel W. Canagliflozin demonstrates durable glycemic improvements over 104 weeks compared with glimepiride in subjects with type 2 diabetes mellitus on metformin. Can J Diabetes. 2013;37:S27. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 151. | Ferrannini E, Seman LJ, Seewaldt-Becker E. The potent and highly selective sodium-glucose co-transporter (SGLT-2) inhibitor BI10773 is safe and efficacious as monotherapy in patients with type 2 diabetes mellitus. 46th Ann Mtg of the European Association for the Study of Diabetes (EASD), Stockholm, 20-24 Sep 2010. Diabetologia. 2010;53:S351. [Cited in This Article: ] |
| 152. | Woerle H, Ferrannini E, Berk A, Hantel S, Pinnetti S, Broedl U. Safety and Efficacy of Empagliflozin as Monotherapy or Add-On to Metformin in a 78-Week Open-Label Extension Study in Patients with Type 2 Diabetes. Presented at 72nd American Diabetes Association Scientific Sessions. Philadelphia, PA, USA, June 8-12. 2012;. [Cited in This Article: ] |
| 153. | Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, Broedl UC. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015-4021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 154. | Rosenstock J, Jelaska A, Wang F, Kim G, Broedl U, Woerle HJ, Bajaj HS. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated type 2 diabetes. Can J Diabetes. 2013;37:S32. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Rosenwasser RF, Sultan S, Sutton D, Choksi R, Epstein BJ. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes. 2013;6:453-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 156. | Dapagliflozin [summary of product characteristics] Middlesex. United Kingdom: Bristol-Myers Squibb/AstraZeneca, 2013. [Cited in This Article: ] |
| 157. | Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 158. | Foote C, Perkovic V, Neal B. Effects of SGLT2 inhibitors on cardiovascular outcomes. Diab Vasc Dis Res. 2012;9:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 159. | List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 493] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 160. | Sha S, Devineni D, Ghosh A, Polidori D, Hompesch M, Arnolds S, Morrow L, Spitzer H, Demarest K, Rothenberg P. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One. 2014;9:e105638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 161. | Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217-2224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 550] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 162. | Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 163. | Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, Cella C, Ferrari S, Stucchi N, Parvanova A. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061-2068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 164. | Sarnoski-Brocavich S, Hilas O. Canagliflozin (invokana), a novel oral agent for type-2 diabetes. P T. 2013;38:656-666. [PubMed] [Cited in This Article: ] |
| 165. | Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 166. | Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 167. | Kalra S, Baruah MP, Sahay R. Medication counselling with sodium glucose transporter 2 inhibitor therapy. Indian J Endocrinol Metab. 2014;18:597-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 168. | Modi A, Agrawal A, Morgan F. Euglycemic Diabetic Ketoacidosis. Curr Diabetes Rev. 2016; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 169. | Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 170. | Scheen AJ. EMPA-REG OUTCOME: Empagliflozin reduces mortality in patients with type 2 diabetes at high cardiovascular risk. Rev Med Liege. 2015;70:583-589. [PubMed] [Cited in This Article: ] |
| 171. | Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 562] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 172. | Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 173. | Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 481] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 174. | Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Rydén L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 175. | Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125-1135. [PubMed] [Cited in This Article: ] |
| 176. | Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055-3061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 177. | Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650-19655. [PubMed] [Cited in This Article: ] |
| 178. | DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011;13:1145-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 179. | Quianzon CCL, Shomal ME. Lixisenatide-once-daily glucagón-like peptide-1 receptor agonist in the management of type 2 diabetes. Eur Endocrinol. 2012;8:12-17. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 180. | Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 955] [Cited by in F6Publishing: 900] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 181. | DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1122] [Cited by in F6Publishing: 1040] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 182. | Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 961] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 183. | Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 184. | Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 543] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 185. | Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 186. | Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013;36:2543-2550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 187. | Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36:2945-2951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 188. | Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, Ping L, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013;36:2497-2503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 189. | Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, Ping L, Ye J, Rosenstock J. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36:2489-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 190. | Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14:910-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 191. | Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 761] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 192. | Buse JB, Vilsbøll T, Thurman J, Blevins TC, Langbakke IH, Bøttcher SG, Rodbard HW. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926-2933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 193. | Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 790] [Cited by in F6Publishing: 751] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 194. | Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, Trautmann M, Porter L. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 195. | Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 196. | Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 197. | Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, Pratley R. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 198. | Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141-2148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 199. | Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37:2149-2158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 200. | Shyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;CD006423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3035] [Cited by in F6Publishing: 2438] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 201. | Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 202. | Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 203. | Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 1057] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 204. | Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart M. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 205. | Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 206. | Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin-Méry A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15:642-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 207. | Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: What progress have we made? Diabetes Metab Res Rev. 2014;30:104-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 208. | Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;CD005613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 209. | Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23:644-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 314] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 210. | Sanches AC, Correr CJ, Venson R, Pontarolo R. Revisiting the efficacy of long-acting insulin analogues on adults with type 1 diabetes using mixed-treatment comparisons. Diabetes Res Clin Pract. 2011;94:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 211. | Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 212. | Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with Type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23:879-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 213. | Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 214. | Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, Rosenstock J, Endahl LA, Francisco AM, Hollander P. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498-1507. [PubMed] [Cited in This Article: ] |
| 215. | Hollander P, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, Rosenstock J, Hansen CT, Niemeyer M, Garber AJ. Insulin degludec improves long-term glycaemic control similarly to insulin glargine but with fewer hypoglycaemic episodes in patients with advanced type 2 diabetes on basal-bolus insulin therapy. Diabetes Obes Metab. 2015;17:202-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 216. | Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, Endahl L, Mathieu C. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464-2471. [PubMed] [Cited in This Article: ] |
| 217. | Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755-2762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 218. | Yki-Järvinen H, Bergenstal R, Ziemen M, Wardecki M, Muehlen-Bartmer I, Boelle E, Riddle MC. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235-3243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 219. | Bolli GB, Riddle MC, Bergenstal RM, Ziemen M, Sestakauskas K, Goyeau H, Home PD. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 220. | Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2012;12:CD009361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 221. | Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 222. | Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 223. | Bromocriptine (Cycloset) for type 2 diabetes. Med Lett Drugs Ther. 2010;52:97-98. [PubMed] [Cited in This Article: ] |
| 224. | Cincotta AH, Meier AH. Bromocriptine improves glycaemic control and serum lipid profile in obese Type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8:1683-1707. [PubMed] [Cited in This Article: ] |
| 225. | Gaziano JM, Cincotta AH, O’Connor CM, Ezrokhi M, Rutty D, Ma ZJ, Scranton RE. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33:1503-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 226. | Kong MF, Stubbs TA, King P, Macdonald IA, Lambourne JE, Blackshaw PE, Perkins AC, Tattersall RB. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia. 1998;41:577-583. [PubMed] [Cited in This Article: ] |
| 227. | Heise T, Heinemann L, Heller S, Weyer C, Wang Y, Strobel S, Kolterman O, Maggs D. Effect of pramlintide on symptom, catecholamine, and glucagon responses to hypoglycemia in healthy subjects. Metabolism. 2004;53:1227-1232. [PubMed] [Cited in This Article: ] |
| 228. | Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, Weyer C, Kolterman OG. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784-790. [PubMed] [Cited in This Article: ] |
| 229. | Riddle M, Pencek R, Charenkavanich S, Lutz K, Wilhelm K, Porter L. Randomized comparison of pramlintide or mealtime insulin added to basal insulin treatment for patients with type 2 diabetes. Diabetes Care. 2009;32:1577-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 230. | Ceriello A, Lush CW, Darsow T, Piconi L, Corgnali M, Nanayakkara N, Frias JP, Maggs D. Pramlintide reduced markers of oxidative stress in the postprandial period in patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24:103-108. [PubMed] [Cited in This Article: ] |
| 231. | Wysham C, Lush C, Zhang B, Maier H, Wilhelm K. Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:79-85. [PubMed] [Cited in This Article: ] |
| 232. | Li Y, Burrows NR, Gregg EW, Albright A, Geiss LS. Declining rates of hospitalization for nontraumatic lower-extremity amputation in the diabetic population aged 40 years or older: U.S., 1988-2008. Diabetes Care. 2012;35:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 233. | Centers for Disease Control and Prevention. Diabetes public health resource. Available from: http://www.cdc.gov/diabetes. [Cited in This Article: ] |
| 234. | Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342-2356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 364] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 235. | Gómez Huelgas R, Díez-Espino J, Formiga F, Lafita Tejedor J, Rodríguez Mañas L, González-Sarmiento E, Menéndez E, Sangrós J. Treatment of type 2 diabetes in the elderly. Med Clin (Barc). 2013;140:134.e1-134.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 236. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13206] [Cited by in F6Publishing: 11844] [Article Influence: 538.4] [Reference Citation Analysis (0)] |
| 237. | Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 424] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 238. | Miller CK, Edwards L, Kissling G, Sanville L. Nutrition education improves metabolic outcomes among older adults with diabetes mellitus: results from a randomized controlled trial. Prev Med. 2002;34:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 239. | Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 240. | Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 241. | Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 242. | Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453-1456. [PubMed] [Cited in This Article: ] |
| 243. | Vega Piñero B. Aspectos diferenciales de la nutrición en los pacientes ancianos con diabetes. Av Diabetol. 2010;26:307-313. [Cited in This Article: ] |
| 244. | Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 500] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 245. | Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 710] [Cited by in F6Publishing: 690] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 246. | Buman MP, Hekler EB, Haskell WL, Pruitt L, Conway TL, Cain KL, Sallis JF, Saelens BE, Frank LD, King AC. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 247. | Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 248. | Shorr RI, Ray WA, Daugherty JR, Griffin MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc. 1996;44:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 249. | Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 250. | Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC, Goto S, Ravaud P, Marre M, Porath A, Bhatt DL. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 251. | National Institute for Health and Care Excellence. Type 2 diabetes in adults: management: December 2015 NICE guidelines. Available from: https://www.nice.org.uk/guidance/ng28. [Cited in This Article: ] |
| 252. | Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4759] [Cited by in F6Publishing: 4690] [Article Influence: 293.1] [Reference Citation Analysis (0)] |
| 253. | The American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1696] [Cited by in F6Publishing: 1721] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 254. | Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 255. | Bressler R, Johnson DG. Oral antidiabetic drug use in the elderly. Drugs Aging. 1996;9:418-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 256. | Papa G, Fedele V, Rizzo MR, Fioravanti M, Leotta C, Solerte SB, Purrello F, Paolisso G. Safety of type 2 diabetes treatment with repaglinide compared with glibenclamide in elderly people: A randomized, open-label, two-period, cross-over trial. Diabetes Care. 2006;29:1918-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 257. | Bloomgarden Z, Drexler A. What role will ‘gliptins’ play in glycemic control? Cleve Clin J Med. 2008;75:305-310. [PubMed] [Cited in This Article: ] |
| 258. | Hsieh CJ. Acarbose reduces the risk of pre-lunch hypoglycemia in elderly people with diabetes eating rice porridge for breakfast. Diabetes Res Clin Pract. 2010;89:e66-e68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 259. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3109] [Cited by in F6Publishing: 2873] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 260. | Available from: http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2011/NI-MUH_13-2011.htm. [Cited in This Article: ] |
| 261. | Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006;66:85-109. [PubMed] [Cited in This Article: ] |
| 262. | Schweizer A, Dejager S, Foley JE, Shao Q, Kothny W. Clinical experience with vildagliptin in the management of type 2 diabetes in a patient population ≥75 years: a pooled analysis from a database of clinical trials. Diabetes Obes Metab. 2011;13:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 263. | McEwen A, Mc Kay GA, Fisher M. Drugs for diabetes. Part 8: SGLT2 inhibitors. Br J Cardiol. 2012;19:26-29. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 264. | Demaris KM, White JR. Dapagliflozin, an SGLT2 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49:289-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 265. | Elmore LK, Baggett S, Kyle JA, Skelley JW. A review of the efficacy and safety of canagliflozin in elderly patients with type 2 diabetes. Consult Pharm. 2014;29:335-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 266. | Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 267. | Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, Plunkett CD, LaPorte FB, Burke R, Brown MB. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28:1568-1573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 268. | Lee P, Chang A, Blaum C, Vlajnic A, Gao L, Halter J. Comparison of safety and efficacy of insulin glargine and neutral protamine hagedorn insulin in older adults with type 2 diabetes mellitus: results from a pooled analysis. J Am Geriatr Soc. 2012;60:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 269. | Home PD, Fritsche A, Schinzel S, Massi-Benedetti M. Meta-analysis of individual patient data to assess the risk of hypoglycaemia in people with type 2 diabetes using NPH insulin or insulin glargine. Diabetes Obes Metab. 2010;12:772-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 270. | Siebenhofer A, Plank J, Berghold A, Narath M, Gfrerer R, Pieber TR. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev. 2004;CD003287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 271. | International Diabetes Federation. Global Guidelines for managing older people with type 2 diabetes (Glucose control, management and targets. 2015;35) Available from: http://www.idf.org. [Cited in This Article: ] |
| 272. | Garg R, Williams ME. Diabetes management in the kidney patient. Med Clin North Am. 2013;97:135-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 273. | Nogueira C, Souto SB, Vinha E, Braga DC, Carvalho D. Oral glucose lowering drugs in type 2 diabetic patients with chronic kidney disease. Hormones (Athens). 2013;12:483-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 274. | Alsahli M, Gerich JE. Hypoglycemia in Patients with Diabetes and Renal Disease. J Clin Med. 2015;4:948-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 275. | Torffvit O, Lindqvist A, Agardh CD, Pahlm O. The association between diabetic nephropathy and autonomic nerve function in type 1 diabetic patients. Scand J Clin Lab Invest. 1997;57:183-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 276. | Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 686] [Cited by in F6Publishing: 678] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 277. | Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R, Bonfigli AR, Esposito K, Giugliano D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61:2993-2997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 278. | Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1067] [Cited by in F6Publishing: 1044] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 279. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 829] [Cited by in F6Publishing: 873] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 280. | Sambol NC, Chiang J, Lin ET, Goodman AM, Liu CY, Benet LZ, Cogan MG. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35:1094-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 281. | Frid A, Sterner GN, Löndahl M, Wiklander C, Cato A, Vinge E, Andersson A. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33:1291-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 282. | Liu F, Lu JX, Tang JL, Li L, Lu HJ, Hou XH, Jia WP, Xiang KS. Relationship of plasma creatinine and lactic acid in type 2 diabetic patients without renal dysfunction. Chin Med J (Engl). 2009;122:2547-2553. [PubMed] [Cited in This Article: ] |
| 283. | Lin YC, Lin LY, Wang HF, Lin HD. Fasting plasma lactate concentrations in ambulatory elderly patients with type 2 diabetes receiving metformin therapy: a retrospective cross-sectional study. J Chin Med Assoc. 2010;73:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 284. | Lim VC, Sum CF, Chan ES, Yeoh LY, Lee YM, Lim SC. Lactate levels in Asian patients with type 2 diabetes mellitus on metformin and its association with dose of metformin and renal function. Int J Clin Pract. 2007;61:1829-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 285. | Duong JK, Roberts DM, Furlong TJ, Kumar SS, Greenfield JR, Kirkpatrick CM, Graham GG, Williams KM, Day RO. Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab. 2012;14:963-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 286. | Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet. 2013;52:373-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 287. | Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement O. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527-2541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 288. | Schejter YD, Turvall E, Ackerman Z. Characteristics of patients with sulphonurea-induced hypoglycemia. J Am Med Dir Assoc. 2012;13:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 289. | Holstein A, Plaschke A, Hammer C, Egberts EH. Characteristics and time course of severe glimepiride- versus glibenclamide-induced hypoglycaemia. Eur J Clin Pharmacol. 2003;59:91-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 290. | Rosenkranz B, Profozic V, Metelko Z, Mrzljak V, Lange C, Malerczyk V. Pharmacokinetics and safety of glimepiride at clinically effective doses in diabetic patients with renal impairment. Diabetologia. 1996;39:1617-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 291. | Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association. 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada.Chronic kidney disease in diabetes. Can J Diabetes. 2013;37:S129-S136. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 292. | Zanchi A, Lehmann R, Philippe J. Antidiabetic drugs and kidney disease--recommendations of the Swiss Society for Endocrinology and Diabetology. Swiss Med Wkly. 2012;142:w13629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 293. | Scheen AJ. Pharmacokinetic considerations for the treatment of diabetes in patients with chronic kidney disease. Expert Opin Drug Metab Toxicol. 2013;9:529-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 294. | Sarkar A, Tiwari A, Bhasin PS, Mitra M. Pharmacological and Pharmaceutical Profile of Gliclazide: A Review. J App Pharmaceut Sci. 2011;1:11-19. [Cited in This Article: ] |
| 295. | Schumacher S, Abbasi I, Weise D, Hatorp V, Sattler K, Sieber J, Hasslacher C. Single- and multiple-dose pharmacokinetics of repaglinide in patients with type 2 diabetes and renal impairment. Eur J Clin Pharmacol. 2001;57:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 296. | Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet. 2002;41:471-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 297. | McLeod JF. Clinical pharmacokinetics of nateglinide: a rapidly-absorbed, short-acting insulinotropic agent. Clin Pharmacokinet. 2004;43:97-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 298. | Inoue T, Shibahara N, Miyagawa K, Itahana R, Izumi M, Nakanishi T, Takamitsu Y. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol. 2003;60:90-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 299. | Scott LJ, Spencer CM. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs. 2000;59:521-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 303] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 300. | Abe M, Kikuchi F, Kaizu K, Matsumoto K. Combination therapy of pioglitazone with voglibose improves glycemic control safely and rapidly in Japanese type 2-diabetic patients on hemodialysis. Clin Nephrol. 2007;68:287-294. [PubMed] [Cited in This Article: ] |
| 301. | Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K. Combination therapy with mitiglinide and voglibose improves glycemic control in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother. 2010;11:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 302. | Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 303. | Budde K, Neumayer HH, Fritsche L, Sulowicz W, Stompôr T, Eckland D. The pharmacokinetics of pioglitazone in patients with impaired renal function. Br J Clin Pharmacol. 2003;55:368-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 304. | Cheng D, Fei Y, Liu Y, Li J, Chen Y, Wang X, Wang N. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS One. 2014;9:e111543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 305. | Mikhail N. Use of dipeptidyl peptidase-4 inhibitors for the treatment of patients with type 2 diabetes mellitus and chronic kidney disease. Postgrad Med. 2012;124:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 306. | Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, Schützer KM. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract. 2011;65:1230-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 307. | Chan JC, Scott R, Arjona Ferreira JC, Sheng D, Gonzalez E, Davies MJ, Stein PP, Kaufman KD, Amatruda JM, Williams-Herman D. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10:545-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 308. | Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 309. | Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab. 2012;14:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 310. | Scheen AJ. Linagliptin for the treatment of type 2 diabetes (pharmacokinetic evaluation). Expert Opin Drug Metab Toxicol. 2011;7:1561-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 311. | McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, Woerle HJ. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 312. | Herman GA, Stevens C, Van Dyck K, Bergman A, Yi B, De Smet M, Snyder K, Hilliard D, Tanen M, Tanaka W. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 358] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 313. | He YL. Clinical pharmacokinetics and pharmacodynamics of vildagliptin. Clin Pharmacokinet. 2012;51:147-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 314. | Fura A, Khanna A, Vyas V, Koplowitz B, Chang SY, Caporuscio C, Boulton DW, Christopher LJ, Chadwick KD, Hamann LG. Pharmacokinetics of the dipeptidyl peptidase 4 inhibitor saxagliptin in rats, dogs, and monkeys and clinical projections. Drug Metab Dispos. 2009;37:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 315. | Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51:411-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 316. | Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes Metab. 2011;13:939-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 317. | Barnett AH. Linagliptin: a novel dipeptidyl peptidase 4 inhibitor with a unique place in therapy. Adv Ther. 2011;28:447-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 318. | Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 319. | Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 320. | Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 464] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 321. | Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 322. | Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013;36:1260-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 323. | Weise WJ, Sivanandy MS, Block CA, Comi RJ. Exenatide-associated ischemic renal failure. Diabetes Care. 2009;32:e22-e23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 324. | Copley K, McCowen K, Hiles R, Nielsen LL, Young A, Parkes DG. Investigation of exenatide elimination and its in vivo and in vitro degradation. Curr Drug Metab. 2006;7:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 325. | Linnebjerg H, Kothare PA, Park S, Mace K, Reddy S, Mitchell M, Lins R. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:568-569. [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 326. | Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 327. | Leiter LA, Gómez-Huelgas R, Ambos A, Arteaga JM, Marchesini G, Nikonova E, Shestakova M, Stager W, Tambascia M, Hanefeld M. Lixisenatide is Effective and Well Tolerated in Patients with Type 2 Diabetes Mellitus and Renal Impairment. Can J Diabetes. 2014;38:S10-S11. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 328. | Young MA, Wald JA, Matthews JE, Yang F, Reinhardt RR. Effect of renal impairment on the pharmacokinetics, efficacy, and safety of albiglutide. Postgrad Med. 2014;126:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 329. | Kuritzky L, Umpierrez G, Ekoé JM, Mancillas-Adame L, Landó LF. Safety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetes. Postgrad Med. 2014;126:60-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 330. | Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 178] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 331. | Mühlhauser I, Toth G, Sawicki PT, Berger M. Severe hypoglycemia in type I diabetic patients with impaired kidney function. Diabetes Care. 1991;14:344-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 332. | Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab. 2000;26 Suppl 4:73-85. [PubMed] [Cited in This Article: ] |
| 333. | Reilly JB, Berns JS. Selection and dosing of medications for management of diabetes in patients with advanced kidney disease. Semin Dial. 2010;23:163-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 334. | Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med. 2011;4:827-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 335. | Baldwin D, Zander J, Munoz C, Raghu P, DeLange-Hudec S, Lee H, Emanuele MA, Glossop V, Smallwood K, Molitch M. A randomized trial of two weight-based doses of insulin glargine and glulisine in hospitalized subjects with type 2 diabetes and renal insufficiency. Diabetes Care. 2012;35:1970-1974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 336. | Ersoy A, Ersoy C, Altinay T. Insulin analogue usage in a haemodialysis patient with type 2 diabetes mellitus. Nephrol Dial Transplant. 2006;21:553-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 337. | Gómez-Huelgas R, Martínez-Castelao A, Artola S, Górriz JL, Menéndez E. Treatment of type 2 diabetes mellitus in patients with chronic kidney disease. Grupo de Trabajo para el Documento de Consenso sobre el tratamiento de la diabetes tipo 2 en el paciente con enfermedad renal crónica. Med Clin (Barc). 2014;142:85.e1-85.10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 338. | Frühbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11:465-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 339. | Singh AK, Singh R, Kota SK. Bariatric surgery and diabetes remission: Who would have thought it? Indian J Endocrinol Metab. 2015;19:563-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 340. | Solayman M, Ali Y, Alam F, Islam MA, Alam N, Khalil MI, Gan SH. Polyphenols: Potential Future Arsenals in the Treatment of Diabetes. Curr Pharm Des. 2016;22:549-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 341. | Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112:8260-8265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 503] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 342. | Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, Kirchner H, Holland J, Hembree J, Raver C. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 343. | Deryabina MA, Daugaard JR, Knudsen CB, Shelton PT, Fog JU, Jessen L, Noerregaard P. Pharmacokinetics and pharmacodynamics of GLP-1-GIP receptor dual agonist peptides: from once-daily to once-weekly. Boston: American Diabetes Association (ADA) 2015; June 05-09. [Cited in This Article: ] |
| 344. | Hjorth SA, Adelhorst K, Pedersen BB, Kirk O, Schwartz TW. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J Biol Chem. 1994;269:30121-30124. [PubMed] [Cited in This Article: ] |
| 345. | Orskov C. Glucagon-like peptide-1, a new hormone of the entero-insular axis. Diabetologia. 1992;35:701-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 346. | Parlevliet ET, Heijboer AC, Schröder-van der Elst JP, Havekes LM, Romijn JA, Pijl H, Corssmit EP. Oxyntomodulin ameliorates glucose intolerance in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2008;294:E142-E147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 347. | Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244-4250. [PubMed] [Cited in This Article: ] |
| 348. | Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 349. | Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390-2395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 350. | Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond). 2006;30:1729-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 351. | Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 442] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 352. | Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 353. | Pan CQ, Buxton JM, Yung SL, Tom I, Yang L, Chen H, MacDougall M, Bell A, Claus TH, Clairmont KB. Design of a long acting peptide functioning as both a glucagon-like peptide-1 receptor agonist and a glucagon receptor antagonist. J Biol Chem. 2006;281:12506-12515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 354. | Claus TH, Pan CQ, Buxton JM, Yang L, Reynolds JC, Barucci N, Burns M, Ortiz AA, Roczniak S, Livingston JN. Dual-acting peptide with prolonged glucagon-like peptide-1 receptor agonist and glucagon receptor antagonist activity for the treatment of type 2 diabetes. J Endocrinol. 2007;192:371-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 355. | Arnolds S, Dellweg S, Clair J, Dain MP, Nauck MA, Rave K, Kapitza C. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 356. | Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 386] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 357. | Rosenstock J, Ahrén B, Chow F. Once-weekly GLP-1 receptor agonist albiglutide vs titrated prandial lispro added on to titrated basal glargine in type 2 diabetes (T2D) uncontrolled on glargine plus oral agents: similar glycemic control with weight loss and less hypoglycemia. Diabetes. 2012;61:A15. [Cited in This Article: ] |
| 358. | Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31:1511-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 359. | Rodbard HW, Buse JB, Woo V, Vilsbøll T, Langbakke IH, Kvist K, Gough SC. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab. 2016;18:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 360. | Lixiland Clinical Development Program: Assessing a fixed-ratio combination of insulin glargine (100 units/ml) and Lixisenatide. Sanofi Diabetes at 51st Annual Meeting, September 14-18, 2015, Stockholm. Available from: http://www.drugs.com/nda/lyxumia_150914.html. [Cited in This Article: ] |
| 361. | Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 362. | Ning Y, O’Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol. 2008;155:1056-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 363. | Swaminath G. Fatty acid binding receptors and their physiological role in type 2 diabetes. Arch Pharm (Weinheim). 2008;341:753-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 364. | Lan H, Vassileva G, Corona A, Liu L, Baker H, Golovko A, Abbondanzo SJ, Hu W, Yang S, Ning Y. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol. 2009;201:219-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 365. | Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153 Suppl 1:S76-S81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 366. | Dhayal S, Morgan NG. The significance of GPR119 agonists as a future treatment for type 2 diabetes. Drug News Perspect. 2010;23:418-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 367. | Novo Nordisk A/S, ClinicalTrials. Multiple Dose Trial Examining Dose Range, Escalation and Efficacy of Oral Semaglutide in Subjects With Type 2 Diabetes. ClinicalTrials . gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016; May 26] Available from: https://clinicaltrials.gov/ct2/show/NCT01923181 NLM Identifier: NCT01923181. [Cited in This Article: ] |
| 368. | Novo Nordisk A/S. Efficacy and Long-term Safety of Oral Semaglutide Versus Sitagliptin in Subjects With Type 2 Diabetes (PIONEER 3) [accessed 2016 May 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT02607865 NLM Identifier: NCT02607865. [Cited in This Article: ] |
| 369. | Khedkar A, Iyer H, Anand A, Verma M, Krishnamurthy S, Savale S, Atignal A. A dose range finding study of novel oral insulin (IN-105) under fed conditions in type 2 diabetes mellitus subjects. Diabetes Obes Metab. 2010;12:659-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 370. | Eldor R, Arbit E, Corcos A, Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8:e59524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 371. | Zambrowicz B, Freiman J, Brown PM, Frazier KS, Turnage A, Bronner J, Ruff D, Shadoan M, Banks P, Mseeh F. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012;92:158-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 372. | Powell DR, Smith M, Greer J, Harris A, Zhao S, DaCosta C, Mseeh F, Shadoan MK, Sands A, Zambrowicz B. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 373. | Powell DR, DaCosta CM, Smith M, Doree D, Harris A, Buhring L, Heydorn W, Nouraldeen A, Xiong W, Yalamanchili P. Effect of LX4211 on glucose homeostasis and body composition in preclinical models. J Pharmacol Exp Ther. 2014;350:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 374. | Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother. 2015;49:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 375. | Lupascu FG, Dash M, Samal SK, Dubruel P, Lupusoru CE, Lupusoru RV, Dragostin O, Profire L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. Eur J Pharm Sci. 2015;77:122-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 376. | Myers SA. Zinc transporters and zinc signaling: new insights into their role in type 2 diabetes. Int J Endocrinol. 2015;2015:167503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 377. | Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14:852-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 378. | Vial G, Chauvin MA, Bendridi N, Durand A, Meugnier E, Madec AM, Bernoud-Hubac N, Pais de Barros JP, Fontaine É, Acquaviva C, Hallakou-Bozec S, Bolze S, Vidal H, Rieusset J. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64:2254-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 379. | Pacini G, Mari A, Fouqueray P, Bolze S, Roden M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:541-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 380. | Fouqueray P, Pirags V, Inzucchi SE, Bailey CJ, Schernthaner G, Diamant M, Lebovitz HE. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2013;36:565-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 381. | Fouqueray P, Pirags V, Diamant M, Schernthaner G, Lebovitz HE, Inzucchi SE, Bailey CJ. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37:1924-1930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 382. | Poxel SA, ClinicalTrials. A study of the efficacy and safety of 4 doses of imeglimin after 24 weeks of treatment in subjects with type 2 diabetes. ClinicalTrials . gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016; Mar 22] Available from: https://clinicaltrials.gov/ct2/show/NCT01951235 NLM Identifier: NCT01951235. [Cited in This Article: ] |
| 383. | Gowda N, Dandu A, Singh J, Biswas S, Raghav V, Lakshmi MN, Shilpa PC, Sunil V, Reddy A, Sadasivuni M. Treatment with CNX-011-67, a novel GPR40 agonist, delays onset and progression of diabetes and improves beta cell preservation and function in male ZDF rats. BMC Pharmacol Toxicol. 2013;14:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 384. | Sunil V, Verma MK, Oommen AM, Sadasivuni M, Singh J, Vijayraghav DN, Chandravanshi B, Shetty J, Biswas S, Dandu A. CNX-011-67, a novel GPR40 agonist, enhances glucose responsiveness, insulin secretion and islet insulin content in n-STZ rats and in islets from type 2 diabetic patients. BMC Pharmacol Toxicol. 2014;15:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 385. | Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 386. | Morikawa T, Akaki J, Ninomiya K, Kinouchi E, Tanabe G, Pongpiriyadacha Y, Yoshikawa M, Muraoka O. Salacinol and related analogs: new leads for type 2 diabetes therapeutic candidates from the Thai traditional natural medicine Salacia chinensis. Nutrients. 2015;7:1480-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |