Published online Jul 25, 2015. doi: 10.4239/wjd.v6.i8.1009
Peer-review started: August 27, 2014
First decision: December 17, 2014
Revised: January 8, 2015
Accepted: May 26, 2015
Article in press: May 27, 2015
Published online: July 25, 2015
Respiratory failure complicating the course of diabetic ketoacidosis (DKA) is a source of increased morbidity and mortality. Detection of respiratory failure in DKA requires focused clinical monitoring, careful interpretation of arterial blood gases, and investigation for conditions that can affect adversely the respiration. Conditions that compromise respiratory function caused by DKA can be detected at presentation but are usually more prevalent during treatment. These conditions include deficits of potassium, magnesium and phosphate and hydrostatic or non-hydrostatic pulmonary edema. Conditions not caused by DKA that can worsen respiratory function under the added stress of DKA include infections of the respiratory system, pre-existing respiratory or neuromuscular disease and miscellaneous other conditions. Prompt recognition and management of the conditions that can lead to respiratory failure in DKA may prevent respiratory failure and improve mortality from DKA.
Core tip: Despite progress in its management, diabetic ketoacidosis (DKA) continues to cause significant morbidity and mortality. One of the conditions aggravating the course of DKA and causing several deaths is respiratory failure, which can be detected at presentation or, more frequently during the course of treatment of DKA. Several risk factors for respiratory failure in DKA are preventable. Early recognition and management of these risk factors, as well as early recognition of respiratory failure have the potential to improve both morbidity and mortality resulting from DKA.
- Citation: Konstantinov NK, Rohrscheib M, Agaba EI, Dorin RI, Murata GH, Tzamaloukas AH. Respiratory failure in diabetic ketoacidosis. World J Diabetes 2015; 6(8): 1009-1023
- URL: https://www.wjgnet.com/1948-9358/full/v6/i8/1009.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i8.1009
Ketoacidosis in subjects with type 1, or less frequently, type 2 diabetes mellitus remains a potentially life-threatening diabetic manifestation. The subject has justifiably attracted attention in the literature. Sequential reviews[1-9] have documented important changes in the clinical concepts that are related to diabetic ketoacidosis (DKA) and its management. A large number of case series of DKA have addressed various aspects of its clinical presentation and management. For this review, we selected representative studies focused on management, outcome, age differences, gender differences, associated morbid conditions, ethnicity and prominent clinical and laboratory features[10-35].
In recognition of the complexity of treatment, the recommendation to provide this care in intensive care units was made more than 50 years ago[36]. Severe DKA is treated in intensive care units today[31]. Evidence-based guidelines for the diagnosis and management of DKA have been published and frequently revised in North America[37,38] and Europe[39]. Losses of fluids and electrolytes, which are important causes of morbidity and mortality in DKA, vary greatly between patients. Quantitative methods estimating individual losses and guiding their replacement have also been reported[40,41].
The outcomes of DKA have improved with new methods of insulin administration[42] and adherence to guidelines[43-46]. The aim of treatment is to minimize mortality and prevent sequelae. One study documented that the target of zero mortality is feasible[42]. However, mortality from DKA, although reduced progressively in the early decades after the employment of insulin treatment[1], remains high. Up to fifty plus years ago, mortality from DKA was between 3% and 10%[1,16]. A recent review reported a death rate from hyperglycemic crises of 7.5% in the United States, with greater mortality from hyperglycemic hyperosmolar state (HHS) than from DKA[9]. Reported mortality from DKA varies among age groups and countries. In various academic medical centers, death rate from DKA was < 1%[26,27] and < 2% among adult patients younger than 65 years[24] in the United States, 0.4% in Japanese children without and 4.7% with coma[23], 6.5% in adult Mexican patients[25], 4.1% in adult Israeli patients[34] without differences between men and women[29], 5.8% in adult Thai patients[30], 3.6% in adult Nigerian patients[32], around 13% in Indian children[33,47], and 22% in American patients older than 65 years[24]. In an autopsy study, DKA was identified as a major cause of death in diabetic patients[48].
One general observation impacting the outcome of DKA is that its management is not always optimal. Several reports documented varying degrees of non-adherence to guidelines[49-51], despite their proven effectiveness. The last of these reports also documented an increasing prevalence of DKA[51]. In addition to adherence to guidelines, efforts to reduce mortality from DKA should focus on individual causes of death. Causes of death in DKA were analyzed in several studies[23,25,27,30-34,52-55]. Cerebral edema and sepsis were the two most common causes. In a study from Greece reporting a 12.9% death rate, multivariate analysis identified the following as predictors of mortality from DKA: co-morbidities, severe acidemia at presentation (arterial blood pH < 7.0), high dose of insulin and persistence of hyperglycemia, and the development of coma or fever during treatment[54]. Another study from Indonesia reporting 40% death rate identified coma plus high serum lactate levels (> 4 mmol/L) as poor prognostic factors[55].
In this review, we analyzed the causes, mechanisms, management and prevention of respiratory failure which is one of the causes of death in DKA. Respiratory or cardiorespiratory deaths were reported in several series of DKA[25,27,31,34,47]. Respiratory failure may either be recognized at presentation or, more frequently, develop during the course of treatment of DKA. The main purpose of the review is to underline the diagnosis, pathogenesis, management and, in particular, prevention of respiratory failure in DKA through proper management.
The key features establishing the diagnosis of DKA are the presence of metabolic acidosis and large amounts of ketones (acetone, acetoacetic acid, beta-hydroxyburyric acid) in serum and urine. Hyperglycemia may be absent in some patients complicating the diagnosis of DKA[56]. DKA should be differentiated from other conditions producing increased ketone formation including alcoholic ketoacidosis and starvation ketosis. Lactic acidosis from sepsis or hypovolemia is another type of metabolic acidosis which occurs frequently during the course of both DKA and HHS that should be differentiated from DKA. Serum lactate level should be measured in all patients with hyperglycemia and metabolic acidosis.
Assessment of the severity of DKA is primarily based on the degree of the acid-base disturbance. The most recent version of the United States guidelines for hyperglycemic crises in adults[38] set three levels of DKA severity: mild, moderate and severe. Criteria common to all three categories included plasma glucose > 250 mg/dL, positive serum and urine ketone test, and variable levels of effective serum osmolality, calculated as [2 × (serum sodium) + (serum glucose.mg/dL)/18]. The criteria that differed between the three categories were arterial blood pH (mild: 7.25-7.30, moderate: 7.00 to < 7.25, severe: < 7.00), plasma bicarbonate ([HCO3]p, mild: 15-18 mEq/L, moderate: 10 to < 15 mEq/L, severe: < 10 mEq/L), serum anion gap calculated as [serum sodium – (serum chloride + serum bicarbonate)] (mild: > 10 mEq/L, moderate: > 12 mEq/L, severe: > 12 mEq/L) and mental status (mild: alert, moderate: alert/drowsy, severe: stupor/coma). The European guidelines for DKA in children[39] classify the severity of DKA only by the magnitude of metabolic acidosis (mild: venous pH < 7.30, [HCO3]p: < 15 mEq/L. Moderate: pH < 7.20, [HCO3]p: 10 mEq/L. Severe: pH < 7.10, [HCO3]p: < 5 mEq/L).
In addition to its use in the diagnosis of DKA and establishing its severity, blood gas analysis provides two objective criteria for assessing the presence and severity of respiratory failure complicating DKA. The universal feature of respiratory failure is hypoxemia. Arterial blood PO2 (PaO2) at room air should be evaluated in all patients presenting with DKA. The second parameter of arterial blood gases that allows detection of respiratory failure in DKA is the arterial PCO2 (PaCO2). The application of PaCO2 in the detection of respiratory failure complicating DKA merits some discussion.
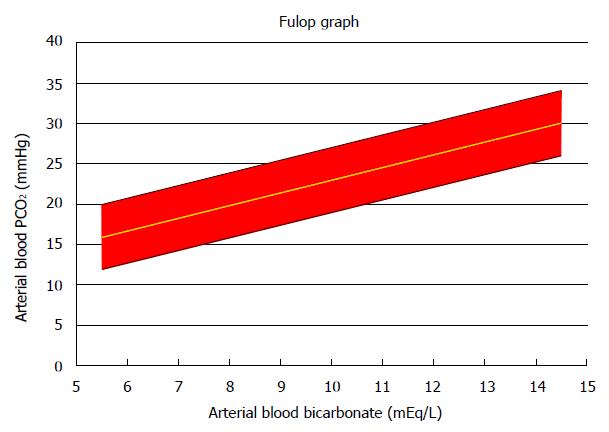
It has been known for a long time that there is a predictable alveolar ventilatory compensation, within narrow limits, to a given degree of metabolic acidosis[57-59]. In its simplest form, this compensation is expressed as PaCO2 as a function of [HCO3]p. The ventilatory response to metabolic acidosis was analyzed by Fulop[60] in a series of 27 episodes of DKA uncomplicated by lactic acidosis or other acid-base disturbances. Fulop derived the following regression equation, which is almost identical to the original Winters equation[57] that was derived from several types of metabolic acidosis: PaCO2, mmHg = 7.27 + 1.57 × ([HCO3]p, mEq/L])
Figure 1 shows the 95% confidence area defined by Fulop’s study. Fulop’s diagram should be used to evaluate every case of DKA. This diagram may assist in the detection of associated other primary acid-base disorders, for example metabolic alkalosis from vomiting[61]. In addition, its use is critical for the detection of respiratory abnormalities accompanying the DKA. Fulop’s diagram allows detection of an associated primary respiratory alkalosis. In this instance, the measured PaCO2 is below the corresponding low 95% confidence limit in the Fulop diagram. Primary respiratory alkalosis is not rare in DKA[62,63]. Detection of primary respiratory alkalosis in a patient with DKA has great importance because it often provides a clue for the presence of sepsis[62] which is the underlying cause of DKA in many instances[37-39], respiratory distress secondary to cerebral edema, and other causes of respiratory alkalosis.
The second critical use of Fulop’s diagram is in the detection of respiratory failure complicating DKA. In this case the PaCO2 value is higher than the corresponding upper limit for PaCO2 in the Fulop diagram. Such a finding should lead to a systematic search for potential causes of respiratory failure and frequent monitoring of the respiratory status of the patient including arterial blood gases.
Detection of respiratory failure in DKA is not based only on PaO2 and PaCO2 values. A diligent search for coexisting conditions adversely affecting the respiratory function has also great importance. Respiratory failure is more often encountered during treatment of DKA than at its presentation, as will be shown later. Consequently, vigilance for any clinical or laboratory clue suggesting development of respiratory failure should be maintained throughout the treatment of DKA.
One potential pitfall of Fulop’s equation is that it may not compute the respiratory response to profound degrees of DKA with accuracy. Soubani et al[64] suggested that there are limits for hyperventilation resulting from metabolic acidosis or sepsis. Subsequently, Guh et al[65] derived different regression equations of PaCO2 on [HCO3]p in DKA patients with arterial pH > 7.10 (PaCO2 = 6.6 + 1.65 × [HCO3]p) and those with arterial pH ≤ 7.10 (PaCO2 = 2.88 + 3.18 × [HCO3]p). The regression equation for DKA with moderate acidemia was similar to Fulop’s equation. The Guh equation for severe acidemia appears to differ from Fulop’s equation. Indeed, there are substantial differences between the two equations for [HCO3]p values that are not extremely low. For example, for [HCO3]p equal to 10 mEq/L, Fulop’s equation calculates an appropriate PaCO2 of 23 mmHg (95%CI: 23-27 mmHg). From the Henderson-Hasselbach equation, the resulting arterial pH value is 7.24 (95%CI: 7.19-7.34). The corresponding values obtained from the Guh equation for severe acidemia are PaCO2 34.0 (95%CI: 30.6-37.4) mmHg, and arterial pH 7.09 (95%CI: 7.05-7.14). However, the differences between the two equations are trivial in cases of profound acidosis. For example, we used the same equations to calculate the ventilatory responses and arterial pH values for [HCO3]p equal to 3 mEq/L: from Fulop’s equation, the values were PaCO2 12.0 (95%CI: 8-16) mmHg; arterial pH 7.02 (95%CI: 6.89-7.19); from Guh’s equation, the values were PaCO2 12.4 (96%CI: 9.0-15.8) mmHg; arterial pH 7.01 (95%CI: 6.90-7.14). We suggest that Fulop’s diagram is appropriate for evaluating the alveolar ventilation in profound DKA. In addition, vigilance for other clues of potential respiratory failure (hypoxemia, history of pulmonary or neuromuscular disease, clinical monitoring of the respiratory system) should be enhanced in this instance.
Metabolic acid-base parameters (pH, plasma bicarbonate concentration) were found to be comparable between venous and arterial blood samples in uncomplicated cases of DKA[66-68]. However, venous blood gas determination is not appropriate for the detection of respiratory failure in the course of DKA[66] for two reasons: The first reason is that venous blood measurements cannot detect abnormalities in the partial pressure of oxygen caused by ventilatory failure, especially in states of hypotension and tissue hypoperfusion. The second reason is that the regression equations used for detection of inadequate ventilatory response to DKA were developed in arterial blood. Transcutaneous monitoring of carbon dioxide has been proposed as a means of monitoring the treatment of DKA[69]. Determining the accuracy of this technique in identifying respiratory failure in DKA will require further research.
The diagnosis of respiratory failure during the course of DKA can be greatly facilitated by a systematic search for risk factors that have been identified to specifically complicate the course of DKA. Clinicians should also be alert for pre-existing respiratory insufficiency and for conditions that can cause respiratory failure independently of DKA (e.g., severe hypothyroidism). The next section addresses risk factors for respiratory insufficiency associated with DKA. Detailed discussion of all causes of respiratory failure is beyond the scope of this review.
Table 1 shows these factors. The first two categories listed in this table (depletion of primarily intracellular ions and development of pulmonary edema) are direct consequences of hyperglycemia and DKA. The last two categories (infections of the respiratory tract and miscellaneous risk factors) include conditions that may lead to DKA but are not caused by it.
| Depletion of primarily intracellular ions |
| Potassium |
| Magnesium |
| Phosphate |
| Pulmonary edema |
| Hydrostatic (cardiogenic) |
| Non-hydrostatic (adult respiratory distress syndrome) |
| Respiratory tract infections |
| Pneumonia |
| Infections of the airways |
| Miscellaneous conditions |
| Neuromuscular disease |
| Non-infectious diseases of the respiratory tract |
| Other |
Potassium, magnesium and phosphate are ions with primary intracellular distribution that are depleted as a consequence of DKA. Depletion of these ions has severe, but preventable, clinical consequences. Clinical manifestations relevant to this report are muscle weakness that can culminate in respiratory failure and cardiac dysrhythmias that may affect myocardial function. If appropriate replacement is not done, the depletion of these ions is more frequent and profound during treatment than at presentation with DKA[70]. A major aim of the treatment of DKA is to address the deficits of these ions. The mechanisms of deficit and their clinical consequences are discussed below. The mechanisms of potassium deficiency and of changes in serum potassium concentration in DKA will be discussed in some detail. Similar mechanisms create the abnormalities in the other two ions.
Potassium: There are disturbances in internal and external potassium balance in DKA. During development of DKA, the internal imbalance is caused by movement of potassium from the intracellular into the extracellular compartment causing hyperkalemia. The external imbalance is attributed to the fact that DKA causes losses of body potassium causing hypokalemia. The losses can be profound and are usually accentuated during treatment. In Martin’s report[70], hyperkalemia was present in 39% of the DKA cases at presentation and in 4% of the cases after 12 h of treatment, while hypokalemia was present in 18% of the DKA cases at presentation and 63% of the cases after 12 h of treatment. The incidence of hypokalemia at presentation of DKA may be affected by factors independent of DKA, such as previous gastrointestinal loss of potassium or diuretic use[71]. In published reports of DKA, the frequency of hypokalemia at presentation varied between 0[17] and 36.7%[13]. A recent study found hypokalemia at presentation in 5% of patients with DKA[72].
Balance studies during development or treatment of DKA documented the abnormalities in external and internal potassium balance caused by DKA[73-79]. Potassium is lost in the urine during development of DKA because of osmotic diuresis caused by glycosuria. Urinary excretion of ketoacids oblicates the loss in the urine of equivalent amounts of cations, particularly sodium and potassium. The contribution of ketonuria to the urinary potassium loss has not been studied in DKA, to our knowledge. Nevertheless, the loss of potassium in DKA is often large. Patients with previously undiagnosed type 1 diabetes who present with DKA after protracted polyuria may have life threatening potassium deficits[41].
Despite the urine losses, large numbers of patients exhibit hyperkalemia at presentation with DKA[70,80,81], because of transfer of potassium from the intracellular into the extracellular compartment. The mechanisms of this transfer have been studied extensively. The most important underlying mechanism is absence of insulin action, which has direct and indirect effects on internal potassium balance. Directly, inhibition of basal insulin secretion causes loss of intracellular potassium[82] and hyperkalemia. Insulin causes hyperpolarization of the cell membranes and potassium entry into the cytoplasm[83] through an increase in the sites of the alpha-2 subunit of the sodium-potassium ATPase of the cell membranes[84]. The effect of insulin on cellular potassium uptake is dissociated from that on cellular glucose uptake[85].
Indirectly, absence of insulin action causes hypertonicity (elevated serum effective osmolarity) through both extracellular accumulation of solute (glucose) and osmotic diuresis, which causes loss of water in excess of monovalent cations[41]. Hypertonicity leads to transfer of water and intracellular solutes, particularly potassium, into the extracellular compartment[86]. Hyperkalemia will result in this case even if the state of hypertonicity has no effect on the transport mechanisms of cell membranes for potassium or on the electrical potential difference across the cell membrane[87]. Contraction of the extracellular volume as a result of osmotic diuresis tends to concentrate extracellular solutes including potassium and constitutes another source of hyperkalemia in DKA[41].
The hyperkalemic effects of the disrupted internal potassium balance described so far are encountered in DKA and all other hyperglycemic syndromes. The question whether metabolic acidosis has additional hyperkalemic effects in DKA has been a matter of controversy[88-90]. Two lines of research provide support for an added hyperkalemic effect of acidosis in DKA: Multivariate analysis in clinical studies identified arterial pH as a predictor of serum potassium level, in addition to serum glucose[91,92]. The other line of evidence is the recent discovery that acidosis affects potassium distribution across cell membranes though alterations in cellular membrane transporters[93].
For patients on maintenance dialysis, the hyperglycemic effects on internal potassium balance are almost completely unopposed because of absent or minimal osmotic diuresis. Studies of hyperglycemic syndromes in this group have provided support for an additional hyperkalemic effect of DKA when serum glucose concentration and effective osmolarity are comparable between DKA and HHS[94-99]. Finally, we are unaware of studies showing that the catabolic state induced by acidosis causes the release of cellular potassium into the extracellular compartment and contributes to the hyperkalemia. Nevertheless, there is sufficient evidence to support the concept of an independent hyperkalemic effect of DKA that is added to the other hyperkalemic effects of hyperglycemia. This further complicates the evaluation of potassium deficits in DKA.
Treatment of DKA leads to substantial declines in serum potassium concentration, even when large amounts of potassium are infused[100-107]. Multiple mechanisms contribute to the hypokalemic effect of treatment. These include a direct effect of insulin on cellular potassium uptake[83], correction of the hyperglycemic hypertonicity[96], dilution of extracellular potassium due to infusion of large volumes of fluids and continuing losses of potassium through the urine or the gastrointestinal tract.
Urinary potassium losses during treatment of DKA merit attention because they often do not constitute a treatment focus as they should. Urinary losses of potassium in DKA are accentuated by coexistent states of hyperaldosteronism[108]. Insulin has an effect similar to aldosterone on renal transport mechanisms of sodium and potassium and its administration to patients with DKA causes excessive renal potassium losses[109]. The other mechanism of excessive potassium losses during treatment of DKA is ongoing osmotic diuresis while serum glucose remains elevated[41]. Improvement of the renal circulation as fluid deficits are corrected has the potential of worsening potassium losses through osmotic diuresis.
Clinical consequences of hypokalemia associated with DKA were reported first in 1946 in a seminal paper by Holler[110] who observed a patient who developed hypokalemia and respiratory failure during treatment of DKA and whose respiratory failure resolved after infusion of potassium salts. The significance of Holler’s report was stressed in a more recent report[111]. Subsequently, a series of articles reported severe clinical manifestations secondary to hypokalemia developing or worsening during treatment of DKA[112-135]. The majority of the reported cases exhibited varying degree of respiratory failure. In several patients, respiratory failure was associated with severe cardiac manifestations and/or profound and generalized muscle weakness. Death occurred in some cases[122,129].
The management of DKA should adhere to guidelines that recommend the administration of intravenous potassium salts to patients presenting with DKA and hypokalemia and initiation of insulin infusion only after serum potassium has reached values > 3.3 mEq/L[38]. Serum potassium concentration should be monitored during treatment in all patients with DKA. In DKA patients presenting with hypotension, extreme hyperglycemia and hypokalemia, urine volume and urine potassium concentration should also be monitored during treatment in order to guide, along with serum potassium, changes in the rate of infusion of potassium salts[41]. Measuring potassium levels with the apparatus used for blood gas determination is not appropriate because these levels may vary substantially from simultaneous serum potassium determinations[136]. Finally, electrocardiographic changes may indicate changes in serum potassium during the course of DKA[137,138]. Monitoring of electrocardiogram to prevent inappropriate administration of potassium salts to patients with DKA has been proposed[137]. However, dissociation of plasma potassium concentration and electrocardiographic abnormalities in a patient on DKA has been reported[139]. Monitoring of serum potassium during the course of treatment of DKA should be primarily based on frequent determinations of serum potassium concentration. Electrocardiographic monitoring should be used as a guide for management of life-threatening hyperkalemia and for timely detection of dysrhythmias complicating the treatment of DKA.
Magnesium: In DKA body magnesium deficits through urinary losses are routinely encountered and are the consequence of absence of insulin[140]. However, magnesium exit from the cells may cause hypermagnesemia, which is frequent at presentation with DKA. The magnesium defect is unmasked during treatment. In Martin’s study[70], hypomagnesemia was recorded in 7% of the cases at presentation with DKA and 55% of the cases after 12 h of treatment, while hypermagnesemia was found in 68% of the cases at presentation and 21% of the cases after 12 h of treatment.
In one reported case, profound hypomagnesemia caused respiratory failure and asystole, and cardiac function recovered after cardiopulmonary resuscitation and infusion of a large bolus of magnesium salts[141]. A small number of patients with DKA and severe hypomagnesemia were subsequently reported[142-144]. The development of hypomagnesemia during treatment of DKA was linked to infusion of potassium phosphate[142,144]. Aldosterone promotes urinary magnesium losses[145]. Magnesium loss in the urine during treatment of DKA may be increased because of the state of secondary hyperaldosteronism discussed in the subsection on potassium.
An important consequence of magnesium deficiency is that it causes excessive urinary losses of potassium and phosphate[146]. It is difficult to replete potassium stores when there are large magnesium deficits[147]. In addition to its direct and indirect effects on respiration, magnesium deficits have major effects on both cardiac contractility and rhythm. Insulin, along with its effects on cellular uptake of potassium and nutrients, increases intracellular free magnesium concentration in myocardial cells[148].
Magnesium deficit should be anticipated in patients with DKA. Serum magnesium concentration should be measured at presentation with DKA and should be monitored during treatment. Magnesium replacement should be guided by serum magnesium levels in this state.
Phosphate: Changes induced by DKA on both external and internal phosphate balances are similar to those of the balances of potassium and magnesium. Hyperglycemic osmotic diuresis causes urinary losses of phosphate, while metabolic acidosis causes shifts of phosphate from the intracellular into the extracellular compartment[149]. Insulin causes cellular phosphate uptake and a decrease in serum phosphate concentration[150,151]. The insulin-mediated decrease in serum phosphate concentration may be accentuated by dilution through intravenous replacement fluids and by continuing urinary losses. In Martin’s study[70], hypophosphatemia was found in 11% of the cases at presentation with DKA and 71% of the cases after 12 h of treatment, while hyperphosphatemia was detected in 90% of the cases at presentation and was not detected after 12 h of treatment.
Severe hypophosphatemia has multiple adverse consequences[149]. Oxygen delivery to peripheral tissues is impaired by the depletion of red cell 2, 3 diphosphoglycerate (2,3-DPG), which causes a shift of the oxygen dissociation curve to the left thus impeding oxygen release. Depletion of high-energy phosphate compounds in muscles secondary to phosphate deficits causes muscle weakness and rhabdomyolysis, dysrhythmias, myocardial dysfunction and seizures[149,152,153].
A number of cases of development of severe hypophosphatemia with varying degrees of respiratory failure during the treatment of DKA have been reported[154-161]. Rhabdomyolysis was present in one patient[161]. A recent report found that the severity of metabolic acidosis at presentation affects the degree of hypophosphatemia during treatment of DKA[162]. Monitoring of serum phosphate should guide the replacement of phosphate deficit. Phosphate replacement was shown to be effective in preventing the development of severe hypophosphatemia in this instance[163,164]. However, phosphate infusion has not been shown to improve the outcome of DKA in prospective studies[38] and may have adverse consequences including hypocalcemia and hypomagnesemia[142,143]. Phosphate should be replaced during treatment of DKA only if serum phosphate levels are low. The guidelines suggest rates of infusion of potassium phosphate and other ions[38]. The critical measure during treatment of DKA consists of close monitoring of the patient’s clinical status and all serum components that are replaced[41].
The second category of direct consequences of DKA is the development of pulmonary edema. Arterial blood gases are necessary for evaluation of its severity and to guide its treatment. Oxygen administration is guided by the degree of hypoxemia, which is universal in patients with pulmonary edema[165]. Abnormalities of PaCO2 accompany the hypoxemia in the majority of the cases[166]. Respiratory alkalosis, triggered by the hypoxemia, is frequent in pulmonary edema. Eucapnia and respiratory acidosis are also present in substantial numbers of patients with acute pulmonary edema[165]. “Normal” or elevated values of PaCO2 in patients with pulmonary edema indicate inadequate respiratory response to hypoxemia and should be considered as indicators of the severity of this condition. Elevated PaCO2 levels have been reported in a small number of patients with end-stage renal disease and extreme hyperglycemia without DKA[63]. Two varieties of pulmonary edema in DKA have been are recognized, a hydrostatic form attributed to elevated pulmonary venous pressure and a form that develops because of increased pulmonary capillary permeability.
Hydrostatic pulmonary edema in DKA: Hydrostatic pulmonary edema is usually diagnosed at presentation with DKA or severe hyperglycemia without DKA and is corrected during the treatment of these syndromes. The sequence of pulmonary edema at presentation with severe hyperglycemia with or without DKA and its correction with insulin administration has been reported in patients with advanced renal failure[166-170]. Development of circulatory overload and hydrostatic pulmonary edema in these patients was initially attributed to the acute shift of a substantial volume of fluid from the intracellular into the extracellular compartment. This volume shift is an osmotic consequence of solute accumulation in the extracellular compartment during development of hyperglycemia. Correction of hyperglycemia with insulin administration shifts fluid back into cells[166].
The magnitude of osmotic translocation of fluid between the two major body fluid compartments that is secondary to hyperglycemia should affect the severity of the ensuing circulatory overload. This magnitude is affected by two main factors: The first and most obvious factor is degree of hyperglycemia. The volume of the osmotic fluid transfer increases as the serum glucose level increases in the same episode of hyperglycemia. The second factor affecting the volume of fluid transferred from the intracellular into the extracellular compartment during development of hyperglycemia is the baseline status of the extracellular volume. For the same degree of hyperglycemia, patients with preexisting peripheral edema develop larger osmotic fluid transfers than those without edema and the same baseline intracellular volume[171,172]. Insulin administration without any other therapeutic measures has led to correction of the pulmonary edema in the reported cases[166-170]. However, other measures, including mechanical ventilation and emergency ultrafiltration may be required in some patients.
The development of extracellular volume expansion may not be the only cause of hydrostatic pulmonary edema in DKA. This syndrome has been reported in DKA patients without advanced renal failure, who usually have volume deficits at presentation[173,174]. This suggests that the development of DKA may be due to factors other than extracellular volume expansion in some cases. In one instance, DKA was diagnosed during treatment of high altitude pulmonary edema[173]. Recovery required treatment of both conditions. It is not clear which condition appeared first.
In another case, hydrostatic pulmonary edema developed during treatment of DKA in a 9-year-old child[175]. Serum troponin levels were elevated and echocardiography showed segmental myocardial dysfunction when pulmonary edema was diagnosed. Repeated cardiac echocardiography was normal 6 d later. This case report illustrates the potential of DKA to cause acutely myocardial dysfunction. This dysfunction could be secondary to excessive fluid replacement. Another cause of myocardial dysfunction in DKA is absence of insulin. Insulin has inotropic effects in subjects with type 1 diabetes[175], subjects with type 2 diabetes[176] and normal controls[176]. It is unclear whether the resolution of pulmonary edema at presentation results from a correction of the extracellular volume excess or a direct action of insulin on myocardial contractility.
Non-hydrostatic pulmonary edema in DKA: Diabetes mellitus may affect the structure and function of the lungs, in addition to other target organs. Histological changes in the lungs of diabetic patients involve the wall of the alveoli and the pulmonary capillaries, while the most consistent functional changes include reduced lung volumes, reduced pulmonary elastic recoil, and reduced capillary lung volume leading to impaired diffusion capacity[177]. Respiratory function in these patients is apparently preserved under normal conditions, but their lung reserves are reduced and can cause clinical lung dysfunction under stressful conditions including volume overload[178]. The development of non-hydrostatic pulmonary edema [adult respiratory distress syndrome (ARDS)] in DKA may be related to the effects of stress on diabetic lungs.
Characteristically, ARDS is not present initially, but develops during the course of treatment of DKA. ARDS appears to be a more frequent and severe complication of DKA than hydrostatic pulmonary edema. A number of publications reported patients who developed ARDS during treatment of DKA[179-192]. ARDS developing during treatment of DKA may lead to death[192]. The severity of ARDS complicating the treatment of DKA is underlined by its association with cerebral edema.
DKA is one of the major causes of cerebral edema[193]. Cerebral edema usually develops during treatment of DKA[194] and is a major cause of mortality and long-term neurological sequelae[195]. Simultaneous development of cerebral edema and ARDS has been reported in several publications[28,196-203]. Research efforts have addressed factors that affect fluid transfers across blood capillary membranes of the brain and lungs during treatment of DKA. Early studies focused on Starling forces controlling fluid exchanges across capillary membranes. Infusion of large volumes of crystalloid solutions leads to increase in the capillary hydrostatic pressure and to dilution of serum proteins and decrease in the colloid osmotic pressure of the serum. Decreased serum colloid osmotic pressure was identified as a risk factor for ARDS during treatment of DKA[204-208].
Decreased serum colloid osmotic pressure may lead to increased fluid transfer from the intravascular into the interstitial space of various tissues including the lungs where it will cause respiratory distress. However, serum colloid osmotic pressure is not a key determinant of fluid transfers between interstitial fluid and intracellular compartment. Efforts to identify risk factors for the development of both ARDS and cerebral edema in DKA have been focused on altered capillary membrane permeability and changes in the serum effective osmolarity (tonicity). Increased pulmonary capillary permeability during treatment of ARDS was found in early reports[209,210]. The potential explanations include activation of lymphocytes[211] and release of cytokines, particularly interleukin-1 (IL-1)[212-215], the serum levels of which are much higher during treatment of DKA than at its presentation. These findings have linked the development of cerebral edema and ARDS in patients with DKA.
A potent driver of fluid exchanges between the extracellular and intracellular compartments is the tonicity of the extracellular compartment, which changes during treatment of DKA. Current strategy for preventing cerebral edema and ARDS consists of careful infusion of crystalloid solutions. Care should be exercised when selecting their volume and tonicity[41]. Replacement of volume deficits should be guided by the clinical picture: Deficits causing severe clinical manifestations should be replaced promptly with isotonic solutions. Monitoring of the clinical signs of hypovolemia (hypotension, tachycardia, low urine output) should guide the rate of infusion, which should be slowed down when these signs are corrected. Clinical monitoring is critical for adequate volume replacement and prevention of overshooting.
Hypertonicity is common in hyperglycemic syndromes and can be severe. Tonicity changes may play a role in the development of cerebral edema during treatment of DKA[203]. Tonicity changes have, in general, a substantially larger effect on intracellular volume than extracellular volume changes. Unlike volume deficits hypertonicity should be corrected slowly during treatment of DKA. The guidelines for management of hyperglycemic crises recommend an hourly decline in serum glucose concentration between 50 and 65 mg/dL[37], which corresponds to a decrease in serum effective osmolarity of between 1.2 and 2.8 mOsm/L[41]. An hourly rate of decrease in effective osmolarity ≤ 3 mOsm/L during treatment of DKA is desirable. Whether measures addressing lymphocyte function and cytokine production can be effective in preventing cerebral edema and ARDS during treatment of DKA are topics for future research.
Infections are known to be a major category of conditions precipitating DKA. A systematic search for infections is warranted in all patients presenting with DKA. This search is complicated by the similarity between symptoms of infection and those of DKA (malaise, abdominal distress, dyspnea, etc.) and by finding elevated white blood cell counts in both conditions. In addition, infections in the respiratory tract have the potential of causing respiratory failure in patients with DKA. Examples of respiratory tract infections reported to cause respiratory failure include pneumonia secondary to Streptococcus pneumoniae[134,216], Legionella pneumonia[217], Klebsiella pneumonia[218], community-acquired pneumonia[219], influenza[220], pulmonary zygomycosis[221], mucormycosis[222-225], candidiasis[224] and coccidiomycosis[226]. The list of respiratory tract infections that can cause respiratory failure during the course of DKA is, in all probability, much larger.
A complicating feature of DKA associated with lung infections is that severe volume deficits may mask the clinical and radiographic manifestations of pneumonia, which blossom after hydration[134]. This scenario represents one condition in which evaluation of the respiratory compensation to DKA in the arterial blood gases can offer an early sign of the presence of a condition complicating the DKA with the potential of respiratory failure[134].
Pneumonia associated with hyperglycemic symptoms is an independent predictor of short-term (28 d) mortality in both DKA and HHS[227]. Early diagnosis and management of pulmonary infections associated with DKA offers the promise of reducing this mortality.
DKA may develop in patients with other serious medical conditions. Regardless of whether these conditions had an etiologic relationship with DKA or not, DKA aggravates their course. For example, development of respiratory stress from DKA during the course of conditions potentially causing respiratory failure, such as pre-existing neuromuscular or pulmonary disease, should intensify the monitoring of the respiratory function.
Preexisting neuromuscular disease: DKA with respiratory failure has been reported in patients with Guillain-Barré syndrome[228,229] and one of the mitochondrial myopathies, the Kearns-Sayre syndrome[230]. Mitochondrial myopathies are associated with a high incidence of diabetes mellitus. Kearns-Sayre syndrome is characterized by progressive external ophthalmoplegia and cardiac conduction defects as its primary clinical manifestations. Patients with this syndrome exhibit multiple endocrine disorders including diabetes mellitus requiring insulin in about 15% of the cases[231]. This condition exemplifies the potential severity of DKA in the course of a neuromuscular disease. It is probable that patients with other types of mitochondrial syndromes causing myopathy develop DKA with respiratory failure. The reported case of DKA with respiratory failure in a patient with Kearns-Sayre syndrome had a fatal outcome[230].
Preexisting conditions of the respiratory system: We found reports of respiratory failure during the course of DKA in a patient with tracheal stenosis[232] and another patient with central venous catheter thrombosis[233]. DKA creates a severe stress on the respiratory function that has the potential to aggravate chronic lung disease. There is a paucity of studies addressing the effects of DKA on respiratory function in patients with chronic lung disease and on the outcomes of DKA in this setting.
Other associated conditions: The development of acute kidney injury during the course of DKA may place greater demands on respiratory function. Two reports addressed respiratory distress in patients with DKA and acute renal failure[234,235]. As previously noted, there is a great need for studies of DKA in patients with conditions potentially affecting the respiratory function (e.g., drugs, severe hypothyroidism, etc.).
Respiratory failure developing during the course of DKA worsens its prognosis and is preventable in most instances. Prevention of respiratory failure has the potential to reduce mortality from DKA. Prevention and early detection of respiratory failure, by close monitoring of the clinical status and timely use and appropriate interpretation of arterial blood gases, have the potential of both decreasing mortality from DKA and preventing the debilitating somatic and psychiatric sequelae of prolonged mechanical ventilation[134].
P- Reviewer: Kawai H S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Felig P. Diabetic ketoacidosis. N Engl J Med. 1974;290:1360-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Alberti KG, Nattrass M. Severe diabetic ketoacidosis. Med Clin North Am. 1978;62:799-814. [PubMed] [Cited in This Article: ] |
| 3. | Kreisberg RA. Diabetic ketoacidosis: new concepts and trends in pathogenesis and treatment. Ann Intern Med. 1978;88:681-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Engl J Med. 1983;309:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 166] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Schade DS, Eaton RP. Diabetic ketoacidosis--pathogenesis, prevention and therapy. Clin Endocrinol Metab. 1983;12:321-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006;35:725-751, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Rosenbloom AL. The management of diabetic ketoacidosis in children. Diabetes Ther. 2010;1:103-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | Maletkovic J, Drexler A. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2013;42:677-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 10. | Harwood R. Diabetic acidosis; results of treatment in 67 consecutive cases. N Engl J Med. 1951;245:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Cohen AS, Vance VK, Runyan JW, Hurwitz D. Diabetic acidosis: an evaluation of the cause, course and therapy of 73 cases. Ann Intern Med. 1960;52:55-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 49] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Shaw CE, Hurwitz GE, Schmukler M, Brager SH, Bessman SP. A clinical and laboratory study of insulin dosage in diabetic acidosis: comparison with small and large doses. Diabetes. 1962;11:23-30. [PubMed] [Cited in This Article: ] |
| 13. | Bortz WH, Spoont S. Diabetic acidosis, a transition. Pa Med. 1967;70:47-50. [PubMed] [Cited in This Article: ] |
| 14. | Whang R, Avasthi PS. Water and electrolyte disturbances associated with diabetic ketoacidosis. Rocky Mt Med J. 1967;64:45-49. [PubMed] [Cited in This Article: ] |
| 15. | Beigelman PM, Martin HE, Miller LV, Grant WJ. Severe diabetic ketoacidosis. JAMA. 1969;210:1082-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Beigelman PM. Severe diabetic ketoacidosis (diabetic “coma”). 482 episodes in 257 patients; experience of three years. Diabetes. 1971;20:490-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 117] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Gerich JE, Martin MM, Recant L. Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Diabetes. 1971;20:228-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 165] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Assal JP, Aoki TT, Manzano FM, Kozak GP. Metabolic effects of sodium bicarbonate in management of diabetic ketoacidosis. Diabetes. 1974;23:405-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Keller U, Berger W, Ritz R, Truog P. Course and prognosis of 86 episodes of diabetic coma. A five year experience with a uniform schedule of treatment. Diabetologia. 1975;11:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Oh MS, Carroll HJ, Goldstein DA, Fein IA. Hyperchloremic acidosis during the recovery phase of diabetic ketosis. Ann Intern Med. 1978;89:925-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Adrogué HJ, Wilson H, Boyd AE, Suki WN, Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N Engl J Med. 1982;307:1603-1610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 207] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Adrogué HJ, Eknoyan G, Suki WK. Diabetic ketoacidosis: role of the kidney in the acid-base homeostasis re-evaluated. Kidney Int. 1984;25:591-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Coma at the onset of young insulin-dependent diabetes in Japan. The results of a nationwide survey. Japan and Pittsburgh Childhood Diabetes Research Groups. Diabetes. 1985;34:1241-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc. 1992;40:1100-1104. [PubMed] [Cited in This Article: ] |
| 25. | Gómez Díaz RA, Rivera Moscoso R, Ramos Rodríguez R, Reza Albarrán A, Gómez-Pérez FJ, Rull J. Diabetic ketoacidosis in adults: clinical and laboratory features. Arch Med Res. 1996;27:177-181. [PubMed] [Cited in This Article: ] |
| 26. | Warner EA, Greene GS, Buchsbaum MS, Cooper DS, Robinson BE. Diabetic ketoacidosis associated with cocaine use. Arch Intern Med. 1998;158:1799-1802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997;157:669-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Hanas R, Lindgren F, Lindblad B. Diabetic ketoacidosis and cerebral oedema in Sweden--a 2-year paediatric population study. Diabet Med. 2007;24:1080-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Barski L, Harman-Boehm I, Nevzorov R, Rabaev E, Zektser M, Jotkowitz AB, Zeller L, Shleyfer E, Almog Y. Gender-related differences in clinical characteristics and outcomes in patients with diabetic ketoacidosis. Gend Med. 2011;8:372-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Anthanont P, Khawcharoenporn T, Tharavanij T. Incidences and outcomes of hyperglycemic crises: a 5-year study in a tertiary care center in Thailand. J Med Assoc Thai. 2012;95:995-1002. [PubMed] [Cited in This Article: ] |
| 31. | Barski L, Nevzorov R, Rabaev E, Jotkowitz A, Harman-Boehm I, Zektser M, Zeller L, Shleyfer E, Almog Y. Diabetic ketoacidosis: clinical characteristics, precipitating factors and outcomes of care. Isr Med Assoc J. 2012;14:299-303. [PubMed] [Cited in This Article: ] |
| 32. | Edo AE. Clinical profile and outcomes of adult patients with hyperglycemic emergencies managed at a tertiary care hospital in Nigeria. Niger Med J. 2012;53:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Kanwal SK, Bando A, Kumar V. Clinical profile of diabetic ketoacidosis in Indian children. Indian J Pediatr. 2012;79:901-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Barski L, Nevzorov R, Harman-Boehm I, Jotkowitz A, Rabaev E, Zektser M, Zeller L, Shleyfer E, Almog Y. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am J Med Sci. 2013;345:326-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 35. | Popli S, Sun Y, Tang HL, Kjellstrand CM, Tzamaloukas AH, Ing TS. Acidosis and coma in adult diabetic maintenance dialysis patients with extreme hyperglycemia. Int Urol Nephrol. 2013;45:1687-1692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Soler NG, Bennett MA, FitzGerald MG, Malins JM. Intensive care in the management of diabetic ketoacidosis. Lancet. 1973;1:951-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 264] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 38. | Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1032] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 39. | Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, Glaser NS, Hanas R, Hintz RL, Levitsky LL. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113:e133-e140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Bartoli E, Bergamasco L, Castello L, Sainaghi PP. Methods for the quantitative assessment of electrolyte disturbances in hyperglycaemia. Nutr Metab Cardiovasc Dis. 2009;19:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 41. | Tzamaloukas AH, Sun Y, Konstantinov NK, Ing TS, Dorin RI, Malhotra D, Murata GH, Shapiro JI. Principles of quantitative fluid and cation replacement in extreme hyperglycemia. Cureus. 2013;5:e110. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Wagner A, Risse A, Brill HL, Wienhausen-Wilke V, Rottmann M, Sondern K, Angelkort B. Therapy of severe diabetic ketoacidosis. Zero-mortality under very-low-dose insulin application. Diabetes Care. 1999;22:674-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Wallace TM, Matthews DR. Recent advances in the monitoring and management of diabetic ketoacidosis. QJM. 2004;97:773-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Ilag LL, Kronick S, Ernst RD, Grondin L, Alaniz C, Liu L, Herman WH. Impact of a critical pathway on inpatient management of diabetic ketoacidosis. Diabetes Res Clin Pract. 2003;62:23-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Volkova NB, Fletcher CC, Tevendale RW, Munyaradzi SM, Elliot S, Peterson MW. Impact of a multidisciplinary approach to guideline implementation in diabetic ketoacidosis. Am J Med Qual. 2008;23:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Thuzar M, Malabu UH, Tisdell B, Sangla KS. Use of a standardised diabetic ketoacidosis management protocol improved clinical outcomes. Diabetes Res Clin Pract. 2014;104:e8-e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Jayashree M, Singhi S. Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country. Pediatr Crit Care Med. 2004;5:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33:189-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Singh RK, Perros P, Frier BM. Hospital management of diabetic ketoacidosis: are clinical guidelines implemented effectively? Diabet Med. 1997;14:482-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 50. | Glaser NS, Kuppermann N, Yee CK, Schwartz DL, Styne DM. Variation in the management of pediatric diabetic ketoacidosis by specialty training. Arch Pediatr Adolesc Med. 1997;151:1125-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Jervis A, Champion S, Figg G, Langley J, Adams GG. Prevalence of diabetes ketoacidosis rises and still no strict treatment adherence. Curr Diabetes Rev. 2013;9:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 52. | Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Daneman D. Diabetes-related mortality. A pediatrician’s view. Diabetes Care. 2001;24:801-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Efstathiou SP, Tsiakou AG, Tsioulos DI, Zacharos ID, Mitromaras AG, Mastorantonakis SE, Panagiotou TN, Mountokalakis TD. A mortality prediction model in diabetic ketoacidosis. Clin Endocrinol (Oxf). 2002;57:595-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Suwarto S, Sutrisna B, Waspadji S, Pohan HT. Predictors of five days mortality in diabetic ketoacidosis patients: a prospective cohort study. Acta Med Indones. 2014;46:18-23. [PubMed] [Cited in This Article: ] |
| 56. | Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J. 1973;2:578-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 187] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 57. | Albert MS, Dell RB, Winters RW. Quantitative displacement of acid-base equilibrium in metabolic acidosis. Ann Intern Med. 1967;66:312-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 125] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Fulop M. The ventilatory response in severe metabolic acidosis. Clin Sci Mol Med. 1976;50:367-373. [PubMed] [Cited in This Article: ] |
| 59. | Verdon F, van Melle G, Perret C. Respiratory response to acute metabolic acidosis. Bull Eur Physiopathol Respir. 1981;17:223-235. [PubMed] [Cited in This Article: ] |
| 60. | Fulop M. The ventilatory response in uncomplicated diabetic ketoacidosis. Crit Care Med. 1977;5:190-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Cronin JW, Kroop SF, Diamond J, Rolla AR. Alkalemia in diabetic ketoacidosis. Am J Med. 1984;77:192-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Elisaf MS, Tsatsoulis AA, Katopodis KP, Siamopoulos KC. Acid-base and electrolyte disturbances in patients with diabetic ketoacidosis. Diabetes Res Clin Pract. 1996;34:23-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Tzamaloukas AH, Avasthi PS. Acid-base disorders in hyperglycemia of insulin-dependent diabetic patients on chronic dialysis. J Diabet Complications. 1988;2:75-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Soubani AO, Khan FA. Hyperventilation in sepsis and acidosis. What is the limit? Chest. 1994;105:1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Guh JY, Lai YH, Yu LK, Shin SJ, Tsai JH. Evaluation of ventilatory responses in severe acidemia in diabetic ketoacidosis. Am J Nephrol. 1997;17:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Kelly AM. The case for venous rather than arterial blood gases in diabetic ketoacidosis. Emerg Med Australas. 2006;18:64-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Bilan N, Behbahan AG, Khosroshahi AJ. Validity of venous blood gas analysis for diagnosis of acid-base imbalance in children admitted to pediatric intensive care unit. World J Pediatr. 2008;4:114-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Herrington WG, Nye HJ, Hammersley MS, Watkinson PJ. Are arterial and venous samples clinically equivalent for the estimation of pH, serum bicarbonate and potassium concentration in critically ill patients? Diabet Med. 2012;29:32-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 69. | McBride ME, Berkenbosch JW, Tobias JD. Transcutaneous carbon dioxide monitoring during diabetic ketoacidosis in children and adolescents. Paediatr Anaesth. 2004;14:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Martin HE, Smith K, Wilson ML. The fluid and electrolyte therapy of severe diabetic acidosis and ketosis; a study of twenty-nine episodes (twenty-six patients). Am J Med. 1958;24:376-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 71. | Alberti KG, Hockaday TD. Thiazides and hypokalaemia in diabetic ketoacidosis. Postgrad Med J. 1973;49:29-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Arora S, Cheng D, Wyler B, Menchine M. Prevalence of hypokalemia in ED patients with diabetic ketoacidosis. Am J Emerg Med. 2012;30:481-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME. On diabetic acidosis: A Detailed Study of Electrolyte Balances Following the Withdrawal and Reestablishment of Insulin Therapy. J Clin Invest. 1933;12:297-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 74. | Butler AM, Talbot NB. Metabolic studies in diabetic coma. Trans Assoc Am Physicians. 1947;60:102-109. [PubMed] [Cited in This Article: ] |
| 75. | Martin HE, Wertman M. Serum potassium, magnesium, and calcium levels in diabetic acidosis. J Clin Invest. 1947;26:217-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Danowski TS, Peters JH, Rathbun JC, Quashnock JM, Greenman L. Studies in diabetic acidosis and coma, with particular emphasis on the retention of administered potassium. J Clin Invest. 1949;28:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Greenman L, Mateer FM, Gow RC, Peters JH, Danowski TS. Some observations on the development of hypokaliemia during therapy of diabetic acidosis in juvenile and young adult subjects. J Clin Invest. 1949;28:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Seldin DW, Tarail R. The metabolism of glucose and electrolytes in diabetic acidosis. J Clin Invest. 1950;29:552-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 79. | Nabarro JD, Spencer AG, Stowers JM. Metabolic studies in severe diabetic ketosis. Q J Med. 1952;21:225-248. [PubMed] [Cited in This Article: ] |
| 80. | Sinden RH, Tullis JL, Root HF. Serum potassium levels in diabetic coma. N Engl J Med. 1949;240:502-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 81. | Fulop M. Hyperkalemia in diabetic ketoacidosis. Am J Med Sci. 1990;299:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | DeFronzo RA, Sherwin RS, Dillingham M, Hendler R, Tamborlane WV, Felig P. Influence of basal insulin and glucagon secretion on potassium and sodium metabolism. Studies with somatostatin in normal dogs and in normal and diabetic human beings. J Clin Invest. 1978;61:472-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 143] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Zierler KL. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol. 1959;197:515-523. [PubMed] [Cited in This Article: ] |
| 84. | Marette A, Krischer J, Lavoie L, Ackerley C, Carpentier JL, Klip A. Insulin increases the Na(+)-K(+)-ATPase alpha 2-subunit in the surface of rat skeletal muscle: morphological evidence. Am J Physiol. 1993;265:C1716-C1722. [PubMed] [Cited in This Article: ] |
| 85. | Nguyen TQ, Maalouf NM, Sakhaee K, Moe OW. Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol. 2011;6:1533-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Makoff DL, Da Silva JA, Rosenbaum BJ. On the mechanism of hyperkalaemia due to hyperosmotic expansion with saline or mannitol. Clin Sci. 1971;41:383-393. [PubMed] [Cited in This Article: ] |
| 87. | Tzamaloukas AH, Ing TS, Elisaf MS, Raj DS, Siamopoulos KC, Rohrscheib M, Murata GH. Abnormalities of serum potassium concentration in dialysis-associated hyperglycemia and their correction with insulin: a unique clinical/physiologic exercise in internal potassium balance. Int Urol Nephrol. 2010;42:1015-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Fulop M. Serum potassium in lactic acidosis and ketoacidosis. N Engl J Med. 1979;300:1087-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Adrogué HJ, Madias NE. Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med. 1981;71:456-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 191] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Adrogué HJ, Madias NE. PCO2 and [K+]p in metabolic acidosis: certainty for the first and uncertainty for the other. J Am Soc Nephrol. 2004;15:1667-1668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 91. | Adrogué HJ, Lederer ED, Suki WN, Eknoyan G. Determinants of plasma potassium levels in diabetic ketoacidosis. Medicine (Baltimore). 1986;65:163-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Van Gaal LF, De Leeuw IH, Bekaert JL. Diabetic ketoacidosis-induced hyperkalemia. Prevalence and possible origin. Intensive Care Med. 1986;12:416-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22:1981-1989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 94. | Tzamaloukas AH, Avasthi PS. Serum potassium concentration in hyperglycemia of diabetes mellitus with long-term dialysis. West J Med. 1987;146:571-575. [PubMed] [Cited in This Article: ] |
| 95. | Rohrscheib M, Tzamaloukas AH, Ing TS, Siamopoulos KC, Elisaf MS, Murata HG. Serum potassium concentration in hyperglycemia of chronic dialysis. Adv Perit Dial. 2005;21:102-105. [PubMed] [Cited in This Article: ] |
| 96. | Tzamaloukas AH, Rohrscheib M, Ing TS, Siamopoulos KC, Qualls C, Elisaf MS, Vanderjagt DJ, Spalding CT. Serum potassium and acid-base parameters in severe dialysis-associated hyperglycemia treated with insulin therapy. Int J Artif Organs. 2005;28:229-236. [PubMed] [Cited in This Article: ] |
| 97. | Tzamaloukas AH, Ing TS, Elisaf MS, Raj DS, Siamopoulos KC, Rohrscheib M, Murata GH. Abnormalities of serum potassium concentration in dialysis-associated hyperglycemia and their correction with insulin: review of published reports. Int Urol Nephrol. 2011;43:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 98. | Gupta A, Rohrscheib M, Tzamaloukas AH. Extreme hyperglycemia with ketoacidosis and hyperkalemia in a patient on chronic hemodialysis. Hemodial Int. 2008;12 Suppl 2:S43-S47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Konstantinov NK, Sun Y, Vigil D, Agaba EI, Servilla KS, Murata GH, Tzamaloukas AH. Hyperkalemia in two patients with diabetes mellitus and chronic kidney disease and the role of disrupted internal potassium balance. Cureus. 2014;6:e162. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Clementsen HJ. Potassium therapy. A break with tradition. Lancet. 1962;2:175-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Seftel HC, Kew MC. Early and intensive potassium replacement in diabetic acidosis. Diabetes. 1966;15:694-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 102. | Abramson E, Arky R. Diabetic acidosis with initial hypokalemia. Therapeutic implications. JAMA. 1966;196:401-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Pullen H, Doig A, Lambie AT. Intensive intravenous potassium replacement therapy. Lancet. 1967;2:809-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Lindsay R, McFarlane L. Potassium balance during treatment of diabetic acidosis. Lancet. 1972;2:1309-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 105. | Mach RS. Potassium balance during treatment of diabetic ketoacidosis. Lancet. 1972;2:930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 106. | Soler NG, Bennett MA, Dixon K, FitzGerald MG, Malins JM. Potassium balance during treatment of diabetic ketoacidosis with special reference to the use of bicarbonate. Lancet. 1972;2:665-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Beigelman PM. Potassium in severe diabetic ketoacidosis. Am J Med. 1973;54:419-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Kalambokis G, Tsatsoulis A, Economou G, Tsianos EV. A case of insulin edema with inappropriate hyperaldosteronism. J Endocrinol Invest. 2004;27:957-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 109. | Carlotti AP, St George-Hyslop C, Bohn D, Halperin ML. Hypokalemia during treatment of diabetic ketoacidosis: clinical evidence for an aldosterone-like action of insulin. J Pediatr. 2013;163:207-212.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 110. | Holler JW. Potassium deficiency occurring during the treatment of diabetic acidosis. J Am Med Assoc. 1946;131:1186-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 111. | Tattersall RB. A paper which changed clinical practice (slowly). Jacob Holler on potassium deficiency in diabetic acidosis (1946). Diabet Med. 1999;16:978-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Frenkel M, Groen J, Willebrands AF. Low serum potassium level during recovery from diabetic coma with special reference to its cardiovascular manifestations. Arch Intern Med (Chic). 1947;80:728-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Nicholson WM, Branning WS. Potassium deficiency in diabetic acidosis. J Am Med Assoc. 1947;134:1292-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Tuynman PE, Wilhelm SK. Potassium deficiency associated with diabetic acidosis. Ann Intern Med. 1948;29:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 115. | Fischer DS, Nichol BA. Intraventricular conduction defect and respiratory tract paralysis in diabetic ketoacidosis. Am J Med. 1963;35:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 116. | Joshi RC, Mathew M. Diabetic ketosis presenting as hypokalaemic muscular quadriplegia. (A case report). J Assoc Physicians India. 1966;14:675-677. [PubMed] [Cited in This Article: ] |
| 117. | Bergen SS. Extreme hypokalemia during treatment of diabetic ketoacidosis. N Y State J Med. 1967;67:2267-2271. [PubMed] [Cited in This Article: ] |
| 118. | Patel AN. [Hypokalemic quadriparesis as a presenting manifestation of diabetic ketoacidosis]. Indian J Med Sci. 1968;22:633-635. [PubMed] [Cited in This Article: ] |
| 119. | Kiraly JF, Becker CE, Williams HE. Diabetic ketoacidosis. A review of cases at a university medical center. Calif Med. 1970;112:1-9. [PubMed] [Cited in This Article: ] |
| 120. | Hodgin UG. Acute hypokalemia occurring during the treatment of diabetic acidosis. Rocky Mt Med J. 1971;68:46-48. [PubMed] [Cited in This Article: ] |
| 121. | Tillman CR. Hypokalemic hypoventilation complicating severe diabetic ketoacidosis. South Med J. 1980;73:231-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 122. | Jewett JF. Committee on maternal welfare. Diabetes and cardiac arrest. N Engl J Med. 1973;288:737-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 123. | Dorin RI, Crapo LM. Hypokalemic respiratory arrest in diabetic ketoacidosis. JAMA. 1987;257:1517-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 124. | Leventhal RI, Goldman JM. Immediate plasma potassium levels in treating diabetic ketoacidosis. Arch Intern Med. 1987;147:1501-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 125. | Krentz AJ, Ryder RE. Hypokalemia-induced respiratory failure complicating treatment of diabetic ketoacidosis. J Diabetes Complications. 1994;8:55-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Murthy K, Harrington JT, Siegel RD. Profound hypokalemia in diabetic ketoacidosis: a therapeutic challenge. Endocr Pract. 2005;11:331-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Segar WE. Clinical quiz. Diabetic ketoacidosis with severe dehydration, cerebral edema, and acute hypokalemia. Pediatr Nephrol. 1991;5:99-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Inokuchi R, Matsumoto A, Odajima H, Shinohara K. Fulminant type 1 diabetes mellitus. BMJ Case Rep. 2012;2012:pii: bcr2012006560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 129. | Abdulaziz S, Dabbagh O, Al Daker MO, Hassan I. Hypokalaemia and refractory asystole complicating diabetic ketoacidosis, lessons for prevention. BMJ Case Rep. 2012;2012:pii: bcr-2012-007312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Amalnath DS, Dutta TK. Hypokalemic paralysis as the presenting manifestation of diabetes in two patients. Indian J Endocrinol Metab. 2013;17:1127-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 131. | Kapitan KS. Ventilatory failure. Can you sustain what you need? Ann Am Thorac Soc. 2013;10:396-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Szrama J, Smuszkiewicz P. Acid-base disorder analysis during diabetic ketoacidosis using the Stewart approach--a case report. Anaesthesiol Intensive Ther. 2013;45:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Wirth CD, Leitner C, Perrig M. Bilateral posterior ischaemic optic neuropathy after severe diabetic ketoacidosis, cardiopulmonary resuscitation and respiratory failure. BMJ Case Rep. 2013;2013:pii: bcr2012008291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Regmi A, Konstantinov NK, Agaba EI, Rohrscheib M, Dorin RI, Tzamaloukas AH. Respiratory failure in the course of treatment of diabetic ketoacidosis. Clinical Diabetes. 2014;32:28-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | Vishnu VY, Kattadimmal A, Rao SA, Kadhiravan T. Sporadic hypokalemic paralysis caused by osmotic diuresis in diabetes mellitus. J Clin Neurosci. 2014;21:1267-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 136. | Fu P, Douros G, Kelly AM. Does potassium concentration measured on blood gas analysis agree with serum potassium in patients with diabetic ketoacidosis? Emerg Med Australas. 2004;16:280-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Malone JI, Brodsky SJ. The value of electrocardiogram monitoring in diabetic ketoacidosis. Diabetes Care. 1980;3:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Harrower AD, Campbell IW, Ewing DJ, Murray A, Neilson JM, Clarke BF. The value of continuous ECG monitoring during treatment of diabetic ketoacidosis. A computer study. Acta Diabetol Lat. 1978;15:88-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 139. | Stenzel KH, Dougherty JC, Scherr L, Lubash GD. Diabetic keto acidosis. dissociation of plasma potassium levels and electrocardiographic abnormalities. JAMA. 1964;187:372-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 140. | Wacker WE, Parisi AF. Magnesium metabolism. N Engl J Med. 1968;278:712-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 135] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 141. | McMullen JK. Asystole and hypomagnesaemia during recovery from diabetic ketoacidosis. Br Med J. 1977;1:690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Winter RJ, Harris CJ, Phillips LS, Green OC. Diabetic ketoacidosis. Induction of hypocalcemia and hypomagnesemia by phosphate therapy. Am J Med. 1979;67:897-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Zipf WB, Bacon GE, Spencer ML, Kelch RP, Hopwood NJ, Hawker CD. Hypocalcemia, hypomagnesemia, and transient hypoparathyroidism during therapy with potassium phosphate in diabetic ketoacidosis. Diabetes Care. 1979;2:265-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 144. | Casey JH. Diabetic ketoacidosis and magnesium deficiency. Med J Aust. 1981;1:92. [PubMed] [Cited in This Article: ] |
| 145. | Horton R, Biglieri EG. Effect of aldosterone on the metabolism of magnesium. J Clin Endocrinol Metab. 1962;22:1187-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 159] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 146. | Whang R, Welt LG. Observations in experimental magnesium depletion. J Clin Invest. 1963;42:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 147. | Whang R, Whang DD, Ryan MP. Refractory potassium repletion. A consequence of magnesium deficiency. Arch Intern Med. 1992;152:40-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Amano T, Matsubara T, Watanabe J, Nakayama S, Hotta N. Insulin modulation of intracellular free magnesium in heart: involvement of protein kinase C. Br J Pharmacol. 2000;130:731-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 149. | Knochel JP. The pathophysiology and clinical characteristics of severe hypophosphatemia. Arch Intern Med. 1977;137:203-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 266] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 150. | Riley MS, Schade DS, Eaton RP. Effects of insulin infusion on plasma phosphate in diabetic patients. Metabolism. 1979;28:191-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Kebler R, McDonald FD, Cadnapaphornchai P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am J Med. 1985;79:571-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Shiber JR, Mattu A. Serum phosphate abnormalities in the emergency department. J Emerg Med. 2002;23:395-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 153. | Ditzel J, Lervang HH. Disturbance of inorganic phosphate metabolism in diabetes mellitus: clinical manifestations of phosphorus-depletion syndrome during recovery from diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2010;3:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 154. | Hasselstrøm L, Wimberley PD, Nielsen VG. Hypophosphatemia and acute respiratory failure in a diabetic patient. Intensive Care Med. 1986;12:429-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Ravenscroft AJ, Valentine JM, Knappett PA. Severe hypophosphataemia and insulin resistance in diabetic ketoacidosis. Anaesthesia. 1999;54:198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 156. | Liu PY, Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc. 2004;67:355-359. [PubMed] [Cited in This Article: ] |
| 157. | Mégarbane B, Guerrier G, Blancher A, Meas T, Guillausseau PJ, Baud FJ. A possible hypophosphatemia-induced, life-threatening encephalopathy in diabetic ketoacidosis: a case report. Am J Med Sci. 2007;333:384-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 158. | de Oliveira Iglesias SB, Pons Leite H, de Carvalho WB. Hypophosphatemia-induced seizure in a child with diabetic ketoacidosis. Pediatr Emerg Care. 2009;25:859-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | Osuka A, Matsuoka T, Idoguchi K. Is this the worst outcome of metabolic syndrome? Hypophosphatemia and resulting cardiac arrest during the treatment of diabetic ketoacidosis with hypertriglyceridemia. Intern Med. 2009;48:1391-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 160. | Spicek-Macan J, Hodoba N, Kolarić N, Nikolić I, Majerić-Kogler V, Popović-Grle S. Immeasurable levels of serum phosphate--an unidentified cause of respiratory failure in a diabetic patient. Coll Antropol. 2010;34:1457-1460. [PubMed] [Cited in This Article: ] |
| 161. | Kutlu AO, Kara C, Cetinkaya S. Rhabdomyolysis without detectable myoglobulinuria due to severe hypophosphatemia in diabetic ketoacidosis. Pediatr Emerg Care. 2011;27:537-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 162. | Shen T, Braude S. Changes in serum phosphate during treatment of diabetic ketoacidosis: predictive significance of severity of acidosis on presentation. Intern Med J. 2012;42:1347-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 163. | Keller U, Berger W. Prevention of hypophosphatemia by phosphate infusion during treatment of diabetic ketoacidosis and hyperosmolar coma. Diabetes. 1980;29:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Wilson HK, Keuer SP, Lea AS, Boyd AE, Eknoyan G. Phosphate therapy in diabetic ketoacidosis. Arch Intern Med. 1982;142:517-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 165. | Aberman A, Fulop M. The metabolic and respiratory acidosis of acute pulmonary edema. Ann Intern Med. 1972;76:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 166. | Axelrod L. Response of congestive heart failure to correction of hyperglycemia in the presence of diabetic nephropathy. N Engl J Med. 1975;293:1243-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 167. | Kaldany A, Curt GA, Estes NM, Weinrauch LA, Christlieb AR, D’Elia JA. Reversible acute pulmonary edema due to uncontrolled hyperglycemia in diabetic individuals with renal failure. Diabetes Care. 1982;5:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Tzamaloukas AH, Levinstone AR, Gardner KD. Hyperglycemia in advanced renal failure: sodium and water metabolism. Nephron. 1982;31:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Catalano C, Fabbian F, Di Landro D. Acute pulmonary oedema occurring in association with diabetic ketoacidosis in a diabetic patient with chronic renal failure. Nephrol Dial Transplant. 1998;13:491-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 170. | Blicker J, Herd AM, Talbot J. Diabetic ketoacidosis in the dialysis-dependent patient: two case reports and recommendations for treatment. CJEM. 2004;6:281-284. [PubMed] [Cited in This Article: ] |
| 171. | Tzamaloukas AH, Ing TS, Siamopoulos KC, Rohrscheib M, Elisaf MS, Raj DS, Murata GH. Body fluid abnormalities in severe hyperglycemia in patients on chronic dialysis: theoretical analysis. J Diabetes Complications. 2007;21:374-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 172. | Tzamaloukas AH, Ing TS, Siamopoulos KC, Rohrscheib M, Elisaf MS, Raj DS, Murata GH. Body fluid abnormalities in severe hyperglycemia in patients on chronic dialysis: review of published reports. J Diabetes Complications. 2008;22:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 173. | Ahmad FM. Diabetic ketoacidosis in an undiagnosed diabetic precipitated by high altitude pulmonary edema. High Alt Med Biol. 2006;7:84-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 174. | Roberts KD, Levin DL. Diabetic ketoacidosis, respiratory distress and myocardial dysfunction. BMJ Case Rep. 2009;2009:pii: bcr01.2009.1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 175. | Schwingshandl J, Ward C, Silink M, Sholler G. Echocardiographic load-independent indices of contractility in children and adolescents with type I diabetes: effect of metabolic control and insulin on left ventricular performance. Pediatr Cardiol. 1995;16:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 176. | Sasso FC, Carbonara O, Cozzolino D, Rambaldi P, Mansi L, Torella D, Gentile S, Turco S, Torella R, Salvatore T. Effects of insulin-glucose infusion on left ventricular function at rest and during dynamic exercise in healthy subjects and noninsulin dependent diabetic patients: a radionuclide ventriculographic study. J Am Coll Cardiol. 2000;36:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 177. | Sandler M. Is the lung a ‘target organ’ in diabetes mellitus? Arch Intern Med. 1990;150:1385-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Hsia CC, Raskin P. Lung function changes related to diabetes mellitus. Diabetes Technol Ther. 2007;9 Suppl 1:S73-S82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 179. | Hayes TM, Woods CJ. Unexpected death during treatment of uncomplicated diabetic ketoacidosis. Br Med J. 1968;4:32-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 180. | Pleet AB. “Shock lung” syndrome following diabetic ketoacidosis; treatment with heparin. Chest. 1973;63:434-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 181. | Russell J, Follansbee S, Matthay M. Adult respiratory distress syndrome complicating diabetic ketoacidosis. West J Med. 1981;135:148-150. [PubMed] [Cited in This Article: ] |
| 182. | Carroll P, Matz R. Adult respiratory distress syndrome complicating severely uncontrolled diabetes mellitus: report of nine cases and a review of the literature. Diabetes Care. 1982;5:574-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 183. | Gérard A, Schooneman F, Guine JM, Roche G, Canton P, Dureux JB, Janot C, Streiff F. Treatment by plasma exchange of a patient with hyperlipidemia and diabetic ketoacidosis with lesional pulmonary edema and acute pancreatitis. Vox Sang. 1982;43:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 184. | Brun-Buisson CJ, Bonnet F, Bergeret S, Lemaire F, Rapin M. Recurrent high-permeability pulmonary edema associated with diabetic ketoacidosis. Crit Care Med. 1985;13:55-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Botha J, van Niekerk DJ, Rossouw DJ, Stewart RI. The adult respiratory distress syndrome in association with diabetic keto-acidosis. A case report. S Afr Med J. 1987;71:535-536. [PubMed] [Cited in This Article: ] |
| 186. | Breidbart S, Singer L, St Louis Y, Saenger P. Adult respiratory distress syndrome in an adolescent with diabetic ketoacidosis. J Pediatr. 1987;111:736-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 187. | Fouet P, Hilpert F, Wysocki M, Zarka D, Margent P. [Non-cardiogenic pulmonary edema associated with severe diabetic ketoacidosis]. Ann Fr Anesth Reanim. 1987;6:127-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 188. | Fernandes Júnior CJ, Hidal JT, Barbas CS, Akamine N, Knobel E. Noncardiogenic pulmonary edema complicating diabetic ketoacidosis. Endocr Pract. 1996;2:379-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 189. | Holsclaw DS, Torcato B. Acute pulmonary edema in juvenile diabetic ketoacidosis. Pediatr Pulmonol. 1997;24:438-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 190. | Jain S, Kahn CB, Pella J, Myers JR. Noncardiogenic pulmonary edema in a patient with diabetic ketoacidosis. Diabetes Care. 1997;20:1922-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 191. | Hoffman WH, Locksmith JP, Burton EM, Hobbs E, Passmore GG, Pearson-Shaver AL, Deane DA, Beaudreau M, Bassali RW. Interstitial pulmonary edema in children and adolescents with diabetic ketoacidosis. J Diabetes Complications. 1998;12:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 192. | Terano T, Fukuda K, Nakamura M, Takiguchi Y, Sakai Y, Hirai A. Diabetic ketoacidosis associated with recurrent pulmonary edema and rhabdomyolysis in a patient with Turner’s syndrome. Intern Med. 2001;40:418-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 193. | Adeva MM, Souto G, Donapetry C, Portals M, Rodriguez A, Lamas D. Brain edema in diseases of different etiology. Neurochem Int. 2012;61:166-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 194. | Rosenbloom AL, Riley WJ, Weber FT, Malone JI, Donnelly WH. Cerebral edema complicating diabetic ketoacidosis in childhood. J Pediatr. 1980;96:357-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 195. | Cameron FJ, Scratch SE, Nadebaum C, Northam EA, Koves I, Jennings J, Finney K, Neil JJ, Wellard RM, Mackay M. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37:1554-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 196. | Taubin H, Matz R. Cerebral edema, diabetes insipidus, and sudden death during the treatment of diabetic ketoacidosis. Diabetes. 1968;17:108-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 197. | Lufkin EG, Reagan TJ, Doan DH. Acute cerebral dysfunction in diabetic ketoacidosis: survivial followed by panhypopituitarism. Metabolism. 1977;26:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 198. | Young MC. Simultaneous acute cerebral and pulmonary edema complicating diabetic ketoacidosis. Diabetes Care. 1995;18:1288-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 199. | Mahoney CP, Vlcek BW, DelAguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol. 1999;21:721-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 200. | McIntyre EA, Abraha HD, Perros P, Sherwood RA. Serum S-100beta protein is a potential biochemical marker for cerebral oedema complicating severe diabetic ketoacidosis. Diabet Med. 2000;17:807-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 201. | Dixon AN, Jude EB, Banerjee AK, Bain SC. Simultaneous pulmonary and cerebral oedema, and multiple CNS infarctions as complications of diabetic ketoacidosis: a case report. Diabet Med. 2006;23:571-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 202. | Edge JA, Jakes RW, Roy Y, Hawkins M, Winter D, Ford-Adams ME, Murphy NP, Bergomi A, Widmer B, Dunger DB. The UK case-control study of cerebral oedema complicating diabetic ketoacidosis in children. Diabetologia. 2006;49:2002-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 203. | Haringhuizen A, Tjan DH, Grool A, van Vugt R, van Zante AR. Fatal cerebral oedema in adult diabetic ketoacidosis. Neth J Med. 2010;68:35-37. [PubMed] [Cited in This Article: ] |
| 204. | Fein IA, Rachow EC, Sprung CL, Grodman R. Relation of colloid osmotic pressure to arterial hypoxemia and cerebral edema during crystalloid volume loading of patients with diabetic ketoacidosis. Ann Intern Med. 1982;96:570-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 68] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 205. | Leonard RC, Asplin C, McCormick CV, Hockaday TD. Acute respiratory distress in diabetic ketoacidosis: possible contribution of low colloid osmotic pressure. Br Med J (Clin Res Ed). 1983;286:760-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 206. | Hillman KM. Acute respiratory distress in diabetic ketoacidosis. Br Med J (Clin Res Ed). 1983;286:1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 207. | Oswald GA, Corcoran S, Yudkin JS. Changes in pulmonary venous pressure and albumin concentration during treatment of severe diabetic decompensation. Diabetes Res. 1987;4:91-94. [PubMed] [Cited in This Article: ] |
| 208. | Laggner AN, Lenz K, Kleinberger G, Sommer G, Druml W, Schneeweiss B. Influence of fluid replacement on extravascular lung water (EVLW) in patients with diabetic ketoacidosis. Intensive Care Med. 1988;14:201-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 209. | Powner D, Snyder JV, Grenvik A. Altered pulmonary capillary permeability complicating recovery from diabetic ketoacidosis. Chest. 1975;68:253-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 210. | Sprung CL, Rackow EC, Fein IA. Pulmonary edema; a complication of diabetic ketoacidosis. Chest. 1980;77:687-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 211. | Hoffman WH, Helman SW, Passmore G. Acute activation of peripheral lymphocytes during treatment of diabetic ketoacidosis. J Diabetes Complications. 2001;15:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 212. | Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol. 2003;108:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 213. | Eisenhut M. Interleukin-1 and the constellation of pulmonary oedema, and cerebral infarctions and oedema in diabetic ketoacidosis. Diabet Med. 2006;23:1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 214. | Karavanaki K, Karanika E, Georga S, Bartzeliotou A, Tsouvalas M, Konstantopoulos I, Fotinou A, Papassotiriou I, Karayianni C. Cytokine response to diabetic ketoacidosis (DKA) in children with type 1 diabetes (T1DM). Endocr J. 2011;58:1045-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 215. | Karavanaki K, Kakleas K, Georga S, Bartzeliotou A, Mavropoulos G, Tsouvalas M, Vogiatzi A, Papassotiriou I, Karayianni C. Plasma high sensitivity C-reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clin Biochem. 2012;45:1383-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 216. | Yokoyama T, Sakamoto T, Shida N, Shimada T, Kaku N, Aizawa H, Oizumi K. Bacteremic and leukopenic pneumococcal pneumonia: successful treatment with antibiotics, pulse steroid, and continuous hemodiafiltration. J Infect Chemother. 2002;8:247-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 217. | Ikeda T, Iriki T, Mori I, Tamiya S, Usijima M, Kiyama T. [A case of Legionella pneumonia with diabetic ketoacidosis rescued despite the development of acute renal failure and ARDS]. Nihon Kokyuki Gakkai Zasshi. 2009;47:491-495. [PubMed] [Cited in This Article: ] |
| 218. | Distel C, Jacobson S, Tille PM. Alcohol induced diabetic ketoacidosis exacerbated by an acute respiratory infection with Klebsiella pneumoniae. Clin Lab Sci. 2013;26:68-71. [PubMed] [Cited in This Article: ] |
| 219. | Christodoulidou M, Selmi F. Severe diabetic ketoacidosis leading to cardiac failure, pulmonary oedema and spinal cord oedema resulting in tetraplegia. BMJ Case Rep. 2012;2012:pii: bcr2012006769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 220. | Watkins PJ, Soler NG, Fitzgerald MG, Malins JM. Diabetic ketoacidosis during the influenza epidemic. Br Med J. 1970;4:89-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 221. | Hansen LA, Prakash UB, Colby TV. Pulmonary complications in diabetes mellitus. Mayo Clin Proc. 1989;64:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 222. | Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 36-1987. A 14-year-old girl with diabetic ketoacidosis and pneumonitis with cavitation. N Engl J Med. 1987;317:614-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 223. | Chadli-Chaieb M, Bchir A, Fathallah-Mili A, Ach K, Maaroufi A, Garrouche A, Chaieb L. [Mucormycosis in the diabetic patient]. Presse Med. 2005;34:218-222. [PubMed] [Cited in This Article: ] |
| 224. | Passi GR, Menon PS, Gupta DK, Lodha R. Recovery from pulmonary mucormycosis and candidiasis in diabetic ketoacidosis. Indian Pediatr. 1996;33:1047-1050. [PubMed] [Cited in This Article: ] |
| 225. | Bhansali A, Suresh V, Chaudhry D, Vaiphei K, Dash RJ, Kotwal N. Diabetes and rapidly advancing pneumonia. Postgrad Med J. 2001;77:734-735, 740-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 226. | Westphal SA, Sarosi GA. Diabetic ketoacidosis associated with pulmonary coccidioidomycosis. Clin Infect Dis. 1994;18:974-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 227. | Chen HF, Wang CY, Lee HY, See TT, Chen MH, Jiang JY, Lee MT, Li CY. Short-term case fatality rate and associated factors among inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: a hospital-based analysis over a 15-year period. Intern Med. 2010;49:729-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 228. | Fujiwara S, Oshika H, Motoki K, Kubo K, Ryujin Y, Shinozaki M, Hano T, Nishio I. Diabetic ketoacidosis associated with Guillain-Barré syndrome with autonomic dysfunction. Intern Med. 2000;39:495-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 229. | Noviello TB, Noviello TC, Purisch S, Lamounier RN, Reis JS, Menezes PA, Calsolari MR. [Diabetes ketoacidosis associated with Guillan-Barré syndrome]. Arq Bras Endocrinol Metabol. 2008;52:562-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 230. | Bachynski BN, Flynn JT, Rodrigues MM, Rosenthal S, Cullen R, Curless RG. Hyperglycemic acidotic coma and death in Kearns-Sayre syndrome. Ophthalmology. 1986;93:391-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 231. | Harvey JN, Barnett D. Endocrine dysfunction in Kearns-Sayre syndrome. Clin Endocrinol (Oxf). 1992;37:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 232. | Nagappan R, Parkin G, Wright CA, Walker CS, Vallance N, Buchanan D, Nazaretian S. Adult long-segment tracheal stenosis attributable to complete tracheal rings masquerading as asthma. Crit Care Med. 2002;30:238-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 233. | Batsis JA, Craici IM, Froehling DA. Central venous catheter thrombosis complicated by paradoxical embolism in a patient with diabetic ketoacidosis and respiratory failure. Neurocrit Care. 2005;2:185-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 234. | Murdoch IA, Pryor D, Haycock GB, Cameron SJ. Acute renal failure complicating diabetic ketoacidosis. Acta Paediatr. 1993;82:498-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 235. | Al-Matrafi J, Vethamuthu J, Feber J. Severe acute renal failure in a patient with diabetic ketoacidosis. Saudi J Kidney Dis Transpl. 2009;20:831-834. [PubMed] [Cited in This Article: ] |