Published online Sep 25, 2015. doi: 10.4239/wjd.v6.i12.1207
Peer-review started: December 26, 2014
First decision: February 10, 2015
Revised: August 15, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: September 25, 2015
Progression of normal glucose tolerance (NGT) to overt diabetes is mediated by a transition state called impaired glucose tolerance (IGT). Beta cell dysfunction and insulin resistance are the main defects in type 2 diabetes mellitus (type 2 DM) and even normoglycemic IGT patients manifest these defects. Beta cell dysfunction and insulin resistance also contribute to the progression of IGT to type 2 DM. Improving insulin sensitivity and/or preserving functions of beta-cells can be a rational way to normalize the GT and to control transition of IGT to type 2 DM. Loosing weight, for example, improves whole body insulin sensitivity and preserves beta-cell function and its inhibitory effect on progression of IGT to type 2 DM had been proven. But interventions aiming weight loss usually not applicable in real life. Pharmacotherapy is another option to gain better insulin sensitivity and to maintain beta-cell function. In this review, two potential treatment options (lifestyle modification and pharmacologic agents) that limits the IGT-type 2 DM conversion in prediabetic subjects are discussed.
Core tip: Behavioral changes (dieting plus exercising) are effective in preventing impaired glucose tolerance (GT)-type 2 diabetes mellitus (type 2 DM) conversion as well as impaired fasting glucose (FG) - type 2 DM conversion but loosing weight is hard and also difficult to maintain. Pharmacological interventions (plus dieting and exercising) improving and preserving beta-cell function and enhancing insulin sensitivity may be suitable choices for high-risk IGT patients. Troglitazone in Prevention of Diabetes Study, Pioglitazone in Prevention of Diabetes Study, Diabetes Reduction Assessment with ramipril and rosiglitazone Medication Trial, Actos Now for the prevention of diabetes study and Diabetes Prevention Program have proven that thiazolidinediones obviously prevent the development of type 2 DM in IGT subjects as well as IFG subjects. In Diabetes Prevention Program and Indian Diabetes Prevention Program, metformin slowed down the progression of IGT to type 2 DM, and eventually American Diabetes Association Consensus Conference Statement proposed metformin usage in high-risk IGT individuals. However, the efficacy of pioglitazone and rosiglitazone efficacy in preventing IGT progression to type 2 DM nearly doubles metformin’s efficacy (31% vs 72% and 62%, respectively). Rosiglitazone (low dose = 2 mg/d) together with metformin (850 mg/d) was proven to slow down IGT progression to type 2 DM as well as being more tolerable.
- Citation: Kanat M, DeFronzo RA, Abdul-Ghani MA. Treatment of prediabetes. World J Diabetes 2015; 6(12): 1207-1222
- URL: https://www.wjgnet.com/1948-9358/full/v6/i12/1207.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i12.1207
Impaired glucose tolerance (IGT) (second hour plasma glucose level 140-199 mg/dL) was first described in 1979 as “an intermediate stage in the transition from normal glucose tolerance (NGT) to overt type 2 diabetes mellitus (type 2 DM)”[1]. Individuals with IGT possess higher risk for type 2 DM later in life[2]. ADA-revised type 2 DM diagnostic criteria declared a new term called impaired fasting glucose (IFG) (glucose level 100-125 mg/dL) in 1997[3]. IFG is an intermediate stage that GT changes from NGT to type 2 DM gradually and defined by fasting plasma glucose level. Subjects who have IFG are also candidates for developing type 2 DM later. But clinical and epidemiologic studies showed that IFG and IGT are different sorts of glucose intolerance[4]. Both IGT and IFG are called “prediabetes” because of gradual progression to type 2 DM. Nearly 70 million prediabetics (IGT and/or IFG) live in America. Since prediabetes is so prevalent[5], increase mortality, morbidity and healthcare costs (annually $245 billion in 2012) it is accepted as an important public health problem. Thus, alleviating the progression of IGT and/or IFG to type 2 DM is a reasonable way to combat with diabetes epidemic and to lessen healthcare costs.
The Diabetes Control and Complications Trial[6], the United Kingdom Prospective Diabetes Study (UKPDS)[7,8] and the Kumamoto Study[9] showed hyperglycemia is a risk factor for macrovascular and especially for microvascular complications[10,11]. Latest evidence illuminated that strict glycemic control is more effective in controlling diabetic vascular complications in new-onset diabetes patients than in long-standing, poorly-controlled type 2 DM patients[12,13]. Therefore, in new-onset type 2 DM, main target must be to achieve normoglycemic control[14]. Early detection and effective intervention of type 2 DM diminishes long-term complications leading morbidity and mortality and eventually expected to provide social, medical, and economic benefits. Treatment should be initiated in IGT period in order to reverse the main pathophysiological defects in prediabetes[4,15-18] because this is a hopeful way of intervention to prevent hyperglycemia-related vascular complication development[15-18].
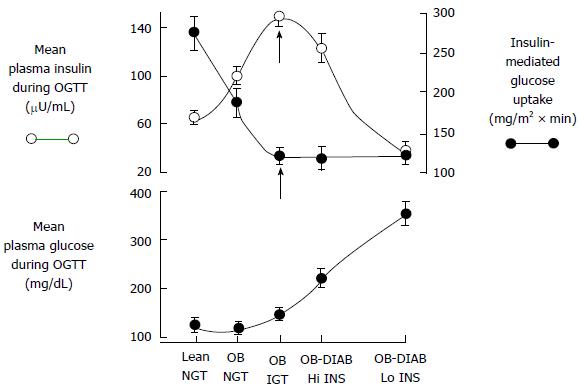
Recent proof favors dual-level emergence of type 2 DM[19-24] (Figure 1). In individuals tended to progress type 2 DM, earliest metabolic abnormality is the insulin resistance. When insulin resistance appears, beta-cells increase their insulin secretion to maintain normoglycemia. Thus, hyperinsulinemia is the main sign of insulin resistance. If beta-cells can not overcome insulin resistance, GT aggrevates. Eventually, IGT appears and followed by overt type 2 DM[22-25].
Thus, IGT individuals’ plasma insulin levels are high but their beta-cell function are extremely diminished[22,23,25]. Therefore, noticing the difference between insulin secretion and beta-cell function is important.
The common defect in prediabetes and type 2 DM is insulin resistance[26-29] and involves liver[22,23,30], muscle[22,23,28,31,32], and adipose tissue[23]. Insulin resistance antecedents the glucose intolerance and type 2 DM[22,23,33]. NGT offspring of two diabetic parents[34,35] and people with IGT[36] are markedly insulin resistant and develop hyperinsulinemia in order to compensate the pathologic state[14,34,35]. Evience supports that insulin resistance may have a genetic component that worsens by environmental factors such as sedantary lifestyle and gaining weight. Hence, interventions that ameliorate insulin resistance and limits the insulin secretory demand on beta-cells shown to stop or postpone IGT conversion to type 2 DM[37-40].
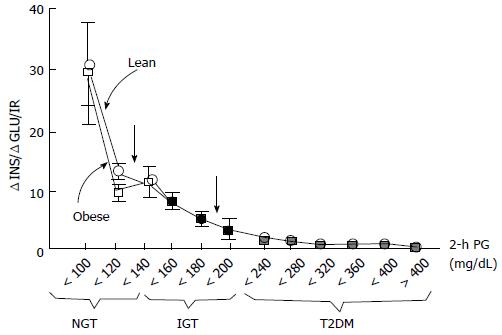
Insulin resistance is the basic characteristics of IGT while deficiency of beta-cell function is the reason of IGT and its conversion to type 2 DM[22,23,41]. Thus, interventions preserving beta-cell function may be a good idea to prevent the generation of type 2 DM. In order to estimate IGT progression to type 2 DM oral glucose tolerance test (OGTT) can be used and a low plasma insulin response is a clue for progression. Especially, reduction of insulin secretion in the first phase (0-10 min later following intravenous glucose challenge) is a good indicator for conversion to diabetes[33,36,42,43]. The first phase insulin secretion deteriorates gradually when the fasting plasma glucose (PG) exceeds 90 mg/dL and is almost completely lost when the fasting PG reaches over 110 mg/dL[22,23,44,45]. As previously described, it is crucial to discriminate insulin secretion from beta-cell function. Beta-cells respond unit glucose increase (ΔG) with unit insulin increase (ΔI), and this response is modulated by severity of insulin resistance[46]. Pure plasma insulin response measurement can lead to confusing about the health of beta-cells. The gold standard for the estimation of beta-cell function is to calculate insulin secretion/insulin resistance (disposition) index (ΔI/ΔG/IR). Both genetic and acquired factors (glucotoxicity[47] lipotoxicity[48], incretin deficiency/resistance[49-51]) effect loss of beta-cell function. Compared to normal glucose tolerant individuals, impaired glucose tolerant individuals have a 4-6 fold increment in type 2 DM risk[52]. Prospective epidemiologic studies reveal that nearly 40% of subjects developing type 2 DM at follow-up had normal glucose tolerant initially. Beta-cell dysfunction is an optimal predictor for 2-h plasma glucose during OGTT in normal glucose tolerant individuals[43,52]. Beta-cell dysfunction is also an optimal predictor for NGT conversion to IGT and thereby to type 2 DM[23,24,52]. Individuals in the upper tertile of NGT have lost 50% of their beta-cell function, wheras subjects in the upper tertile of IGT 70%-80% (Figure 2). Individuals in the upper tertile of IGT are maximally insulin resistant and decline in beta-cell function is about 70%-80%. At this point, minimal extra reduction in insulin secretion causes a prominent increase in fasting and postprandial blood glucose levels. Once overt type 2 DM emerges, beta-cell function diminishes progressively[53] despite therapies with metformin, sulfonylureas, and insulin to control glycemia. Genetics, insulin resistance leading insulin secretory demand increment, glucotoxicity, lipotoxicity, impaired incretin release/action, amylin accumulation, and decreased beta-cell mass are causitive factors in the progression of beta-cell dysfunction. Interventions in order to postpone or preclude beta-cell failure are valuable tools in combatting with the conversion of IGT to type 2 DM.
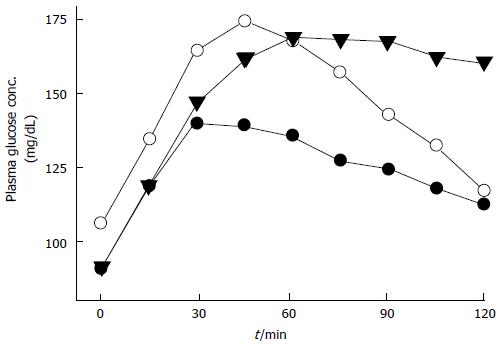
IGT or IFG patients, and particularly people possessing both IGT and IFG[54,55] carry high risk for type 2 DM[56-58]. IGT and IFG are eventually end up with type 2 DM but they exhibit different physiological and pathological processes and have distinct reflections on atherosclerotic cardiovascular disease emergence. In people with IFG hepatic insulin resistance is moderate and OGTT-early insulin response (0-30 min) is diminished[59]. When hyperglycemic clamp and IVGTT techniques were used in OGTT, first phase insulin secretion is found to be blunted in IFG[60,61] (Figure 3). But, late (60-120 min) plasma insulin response is unspoilt and muscle insulin sensitivity is near-normal in IFG patients; therefore two-hour plasma glucose levels returns to its initial fasting PG levels[62-64]. Adversely, people with IGT have moderate to severe muscle insulin resistance and impaired plasma insulin responses (both early and late responses) during oral GT test[63,64]. Even if fasting PG is relatively stable, it rises progressively during OGTT and not come back to normal levels for a long time while two-hour plasma glucose remains well above the fasting plasma glucose level. On the other hand, IGT and IFG share a characteristic impaired insulin secretion pattern in the first phase. However, insulin secretion in second-phase is intact in IFG states. Whereas, muscle insulin resistance is the dominant factor in IGT, in IFG tissue responsible for insulin resistance is that of liver. Also, IGT and IFG exhibit distinct characteristics for atherosclerotic cardiovascular disease. IGT seems to be related with metabolic syndrome and a good indicator of cardiovascular disease, while IFG predicts these events to a lesser extent[65].
ADA recommends considering HbA1c = 5.7%-6.4% level as an instrument to detect future diabetes risk. However, no previous study has adopted HbA1c level as a screening tool to identify subjects at high risk (HbA1c = 5.7%-6.5%) and has examined the efficacy of interventions to reduce the risk of transition to type 2 DM. Kanat et al[66] and Færch et al[67] previously have demonstrated the concordance of HbA1c vs OGTT in high risk individuals and found only little overlap between them. Moreover, Kanat et al[66] have shown that HbA1c was a poor predictor of impaired beta cell function which is the principle factor mediating the process in which high risk individuals become overt diabetes. Discussion below is about how we should prevent diabetes among high risk individuals, namely individuals with IFG/IGT identified by OGTT results.
First step in the progression of NGT to type 2 DM is IGT and IFG[22-24,33]. The IGT and IFG shares 2 features in common: Beta-cell function impairment and insulin resistance. Thereby, it seems logical to assume that efforts to preserve or increase functions of beta-cells and/or decrease insulin resistance may be a potent way to delay the conversion of IGT to DM.
The basic risk factor in the progression of IGT to diabetes is obesity[34,68]. The main reason of type 2 DM epidemic confronted during the last two decades may be the obesity epidemic itself. Sedantary lifestyle and eventually gaining weight triggers insulin resistance and force the capacity of beta-cell insulin secretion. On the other hand, loosing weight by means of lifestyle interventions, pharmacologic therapies or bariatric surgery augments insulin sensitivity, decreases beta-cell work overload, and gets GT better in IGT states[69-71]. Four studies have shown that loosing weight through dieting and/or exercising improves insulin sensitivity and ameliorates beta-cell function, thus is a good way to limit IGT progression to type 2 DM[72-74]. When individuals loose the 5% of their body weight, total body insulin sensitivity improves by 30%[73] and decrease in their IGT to type 2 DM progression nearly by 58%[37].
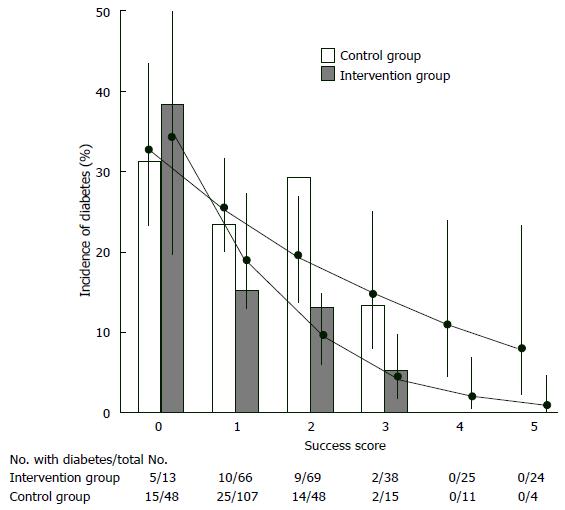
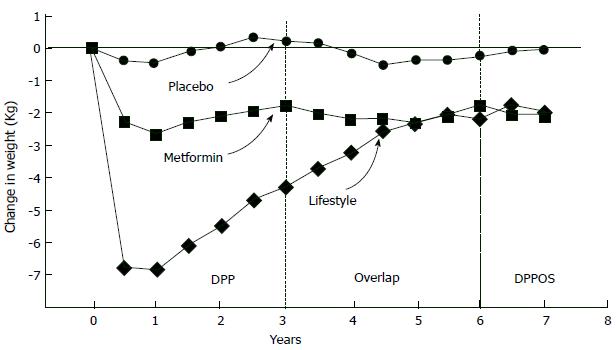
Finnish Diabetes Prevention Study, intervention individuals were given special advice to loose weight (> 5% of total body weight), to decrease total fat consumption (< 30% of total calories) as well as saturated fat consumption (< 10% of total fat), to increase fiber consumption (15 g for each 1000 kilocalories) and to increase physical activity (30 min/d). These individuals were followed up 3.2 years. Cumulative diabetes incidence was 58% lower in the intervention individuals compared to controls (HR = 0.4, P < 0.001). Individuals in the study were categorized considering whether they succeeded their initial targets at one year of assessment (Figure 4). Reciprocal relationship was determined between achievement score and new diabetes cases. If an individual succeeded 4-5 goals, diabetes did not develop[72]. Another landmark clinical trial [Dipeptidyl peptidase (DPP)] assigned 3234 prediabetic patients (IFG + IGT) to placebo, metfomin (2 × 850 mg per day), or a lifestyle modification program. In this program targets are loosing 7% of body weight, taking 150 min-physical exercise every week and reducing (25% of total calories) total intake of fat. Individuals were followed up to 2.8 years. Lifestyle modifications (compared to placebo) decreased the new diabetes cases by 58%. Hovewer, in subjects who lost weight and who met physical exercise/dieting targets, risk of diabetes decreased > 90%. These results are consistent with the Finnish Diabetes Prevention Study in which participants met four or five of their goals. In post-hoc analyses of both studies, weight loss was the most important contributor to type 2 DM prevention. In the DPP trial, a 5-kg weight loss over time could account for the 55% reduction in the risk of diabetes over the mean of 3.2 years of follow-up in this high-risk population[37].
Isolated IFG and isolated IGT individuals carry nearly the same risk about the progression of IFG to type 2 DM, but there is no major clinical trial assessing the lifestyle intervention efficacy on preventing IFG - type 2 DM conversion. A small study[75] in Japanese subjects with IFG has reported that an intensive weight loss program is more effective in reducing the conversion rate from IFG to type 2 DM compared to less intensive intervention (HR = 0.56, 95%CI: 0.36-0.87). Subgroup analysis revealed that subjects who had IFG + IGT at baseline manifested greater reduction in the conversion to type 2 DM (HR = 0.41, 95%CI: 0.24-0.69) while it was not statistically significant in subjects with isolated IFG (HR = 1.17, 95%CI: 0.50-2.74). A significant difference achieved by lifestyle intervention on diabetes conversion between two groups (P = 0.03).
Lifestyle intervention is the most effective approach to combat with progression of IGT to type 2 DM, but preserving the final weight and exercising is unsustainable[76]; for example, when DPP trial ended, people gained weight again[77] (Figure 5). Weight loss achieved by drugs is also a good way to diminish conversion of IGT to type 2 DM. Orlistat brings 5.8 kg loss while lifestyle changes brings 3.0 kg loss, while IGT - type 2 DM conversion limited by orlistat was about a 37% in XENDOS study[78]. But, when placebo was given instead of the drug, individuals gained weight again although they continued their diets so weight loss provided by pharmacologic interventions is also unsustainable[79]. Typically, most weight loss programs resulted in weight regain no matter what intervention type (lifestyle or pharmacologic) was used and when loosing weight programme stopped, IGT - type 2 DM progression rate mimics control individuals[80]. Thus, we can conclude that “legacy” effect via weight loss is not much in terms of slowing down the IGT - type 2 DM progression. In real-life, even maintaining 5% weight loss is unrealistic. In a study performed in Finland community[81] a diabetes prevention program aiming 5%-7% weight loss applied 10149 registered subjects and 1/3 of these subjects lost more than 2.5% of their body weight. Moreover, in case of achievement of sustainable weight loss, diabetes incidence decrease was about 50%-60%. In other words, IGT - type 2 DM progression continued in 40% to 50% of subjects although they lost weight successfully. Therefore, changes in lifestyle are insufficient in preventing diabetes in prediabetic people. But opposite to behavioral interventions such as dieting and exercising, pharmacological interventions always limits IGT or IFG progression to type 2 DM.
Lifestyle intervention is impractical and not satisfactory for insulin sensitivity improvement, pharmacologic agents used as an alternative way of enhancing insulin impact and limiting IGT - type 2 DM progression. In some clinical studies, pharmacotherapy getting insulin sensitivity better in adipocytes, in muscle-cells or liver-cells have found to diminish conversion of IGT - type 2 DM.
Metformin: Fasting PG concentration and hemoglobin A1c can be decreased by metformin in type 2 DM through inhibition of liver glucose production[82-84] or through preserving beta-cell function[85]. However, in some studies including UKPDS and ADOPT, it is shown that hemoglobin A1c decreases first and then rises again gradually[7,8,85,86]. In DPP study, IGT conversion to type 2 DM by 31% when metformin was given at the dose of 1700 mg/d; also this therapy corrected insulin sensitivity and diminishes new metabolic syndrome cases. Again, metformin in Indian Diabetes Prevention Program limits the IGT - type 2 DM progression[87]. Other minor studies[88-90] show that metformin lowers the plasma glucose concentration in obese adolescents. However, there is no study investigating the efficacy of metformin on diminishing the conversion rate of IFG to type 2 DM. It is proven that metformin and weight loss has similar effectiveness on decreasing the progression of IGT to type 2 DM in younger than 65-year-old subjects, subjects with body mass index over 35 and subjects whose fasting plasma glucose exceeding 110 mg/dL[37]. Thus, it is not unusual to claim that metformin would significantly lower the conversion rate from IFG to type 2 DM. A prospective randomized clinical trial illuminated the answer. Eventually, American Diabetes Association advices metformin useage in high-risk individuals (younger than 60-year-old, body mass index over 30 kg/m2 and HbA1c over 6.0%) with IGT or IFG, taking into account that metformin has been known as a safe generic drug[91]. However, similar to sulfonylureas, metformin cannot stop beta-cell failure which is crucial for type 2 DM. While metformin response initially seems good, HbA1c begins to rise eventually.
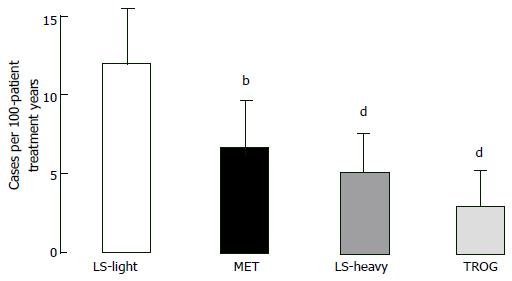
Thiazolidinediones: Thiazolidinediones act on “peroxisome proliferator activator receptor gamma” (PPAR-γ) and eventually improve two main defects generated by IGT. Thiazolidinediones bring adipocytes as well as liver and muscle cells sensitivity to insulin[92-94] and also support and protect beta-cells function[95]. Hypothesis that defends “thiazolidinediones improve muscle insulin sensitivity by reducing plasma free fatty acid levels and intramyocellular lipid content, and redistributing fat from visceral to subcutaneous adipose depots” finds lots of evidence. Moreover, muscle and fat cell PPAR-γ receptors mediates insulin-sensitizing effect directly[92-94]. There is no significant difference between troglitazone[96], pioglitazone[97], and rosiglitazone[98] in controlling glycemia and increasing insulin sensitivity in type 2 DM. Troglitazone increase GT and insulin sensitivity as well as limits type 2 DM conversion in IGT individuals[38,99,100] and in women developing diabetes during their pregnancies[101]. In Diabetes Prevention Program, IGT - type 2 DM progression reduced by 23% by troglitazone within three years, even if the drug was stopped after 10 mo[38]. After 1.5 years of follow-up diabetes incidence was markedly reduced for every 100 person-treatment years in IGT subjects taking troglitazone compared with placebo (3.0 vs 12.0 cases; P < 0.001), compared with metformin (3.0 vs 6.7 cases; P = 0.02) and compared with lifestyle changing activities (3.0 vs 5.1, P = 0.18) (Figure 6). IGT - type 2 DM conversion decrease attributed to rosiglitazone was 62% in DREAM trial[39] and best indicator of diabetes prevention was recovery in insulin secretion/insulin resistance index. Pioglitazone and troglitazone slows down IGT progression to type 2 DM in women with gestational diabetes history[101-103]. In Actos Now for the prevention of diabetes study, IGT - type 2 DM conversion rate fall attributed to pioglitazone was 72% (P < 0.00001)[40].
Because IGT - type 2 DM conversion and appearance of hyperglycemia led by gradual beta-cell failure, improving beta-cell function in IGT individuals are expected to be useful in lowering the new cases of type 2 DM. Although thiazolidinediones strikingly increase insulin sensitivity in IGT individuals, the best indicator of type 2 DM prevention is reinforcing beta-cell function. In diabetic human trials[101,103] and animal studies[104] troglitazone[99-101], pioglitazone[95,97,102], and rosiglitazone[95,98] increased the function of beta-cells by: (1) unloading beta-cells via advancing insulin sensitivity; (2) decreasing plasma free fatty acid levels; (3) correcting lipotoxicity; in other words sending toxic lipid metabolites (diacylglycerol, ceramides and fatty acyl CoAs) away from beta-cells; and (4) exerting direct PPAR-γ receptor-mediated beta-cell effect[48,94,95]. Thiazolidinediones both advance insulin sensitivity and protect beta-cell function so that they blocks IGT - type 2 DM conversion and create a longstanding HbA1c decrement in type 2 DM[23]. Nevertheless, thiazolidinediones induce fluid retention plus fat weight gain and they have the disadvantage of being expensive[39,105]. For that reason, American Diabetes Association decleared metformin instead of thiazolidinediones for treatment of IGT or IFG[91] even if thiazolidinediones doubles the effect of metformin in preventing IGT - type 2 DM conversion[105,106] (Figure 6). In Actos Now for the prevention of diabetes study titrated pioglitazone dose was 45 mg per day. But, even 15 to 30 mg daily pioglitazone dose increased insulin secretion and sensitivity in type 2 DM[107] while causing lesser fluid retention and lesser fat gain[108]. Also, Canadian individuals with IGT were given 2 mg per day rosiglitazone plus 1000 mg per day metformin, and IGT - type 2 DM conversion reduction with this regimen was about 71% with no significant fluid retention and weight gain[109].
In all of the 8 studies continued over 1.5 years, thiazolidinediones reduced HbA1c levels and maintained this decrement in type 2 DM subjects. In ADOPT, 5-year rosiglitazone-associated HbA1c decrease was obtained[86]. Sustained reduction in HbA1c implicates that thiazolidinediones are long-acting drugs on beta-cell functionality. Parallely, in another study, insulin secretion/insulin resistance index which is the gold standart in the measurement of beta-cell function is calculated in 61 type 2 DM subjects and functions of beta-cells improved by rosiglitazone and pioglitazone in a similar way[95]. Consequently, thiazolidinediones protect and augment beta-cell function, sensitize insulin as well as preserve long standing HbA1c reduction and delay IGT- type 2 DM progression.
Glucagon-like peptide-1 analogues: Oral glucose consumption provides 2-3-fold greater plasma insulin response compared to same level of hyperglycemia enhanced by intravenous glucose and this is called “incretin effect”[110-112]. Ninety percent of incretin effect derived from L cell-associated glucagon-like peptide-1 (GLP-1) release and K cell-associted GIP release. GIP and GLP-1 are strong stimuli for insulin secretion. GLP-1 also blocks secretion of glucagon, postpones emptying of stomach, diminishes appetite, limits food consumption and potentiates loosing weight. Dipeptidyl peptidase-IV cleaves GLP-1 and GIP rapidly within one or two minutes, those peptides are not suitable for therapy of type 2 DM and/or IGT individuals. GLP-1 receptor agonists (namely liraglutide and exenatide) mimicing GLP-1 actions are resistant to degenarating effect of dipeptidyl peptidase-IV[113,114]. Like endogenous GLP-1, liraglutide and exenatide are powerful insulin secretagogues, and they decrease secretion of glucagon, potentiate loosing weight and effectively decrease plasma glucose levels in type 2 DM. A three-year prospective study showed exenatide reduced HbA1c for a long time, augmented functions of beta-cells and provided gradual weight loss[115]. One favorable aspect of GLP-1 analogues is that hypoglycemia is uncommon during therapy because GLP-1 analogues merely increase secretion of insulin whenever there is hyperglycemia. Glucose physiologically triggers release of insulin. Glucose increases the ATP generation, eventually generated ATPs close the potassium channels. Consequently, membrane of beta-cells are depolarized, calcium influx occurs and exocytosis begins in insulin-containing vesicles[116]. Eventually, glucose mediates insulin secretion. But effect of GIP and GLP-1 on beta-cells are totally independent from hyperglycemia. After they bind self receptors, adenylate cyclase is activated, ATP is converted to cAMP so they “amplifies” insulin secretion by means of hyperglycemia. If hyperglycemia does not exist, GLP-1 or GIP can not augment secretion of insulin[117].
The typical signs in subjects with IGT and type 2 DM are severe decrease in functions of beta-cells and obvious decrease in incretin effect after meal or after glucose consumption[110-112]. Studies have pointed out that in IGT and type 2 DM cases the main defect is the incapability of beta-cells to respond glucose. Incretin hormones partially overcome beta-cell “blindness” to glucose[118]. In IGT cases GLP-1 response after meal usually is not changed or slight impairment is observed[119-121] while GLP-1 response in the first 10 min is usually lessened (this implicates phasic defect in GLP-1 secretion) but GIP secretion is mildly elevated[122]. On the contrary, in type 2 DM beta-cells are resistant to GLP-1-mediated insulin secretion[123]. Also, beta-cells are resistant to GIP-mediated stimulation of insulin secretion. If insulin is given and glycemia reverted to normal, susceptibility of beta-cells to GIP can be improved, but this is not true for GLP-1[50].
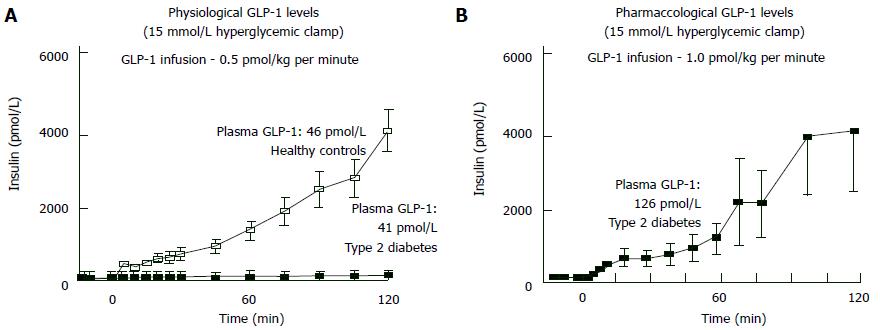
If hyperglycemia exists, NGT individuals give powerful insulin secretion response against the GLP-1 increase. Inversely, in type 2 DM the same GLP-1 amount cannot increase insulin secretion even hyperglycemia exists[50,51]. But whenever plasma GLP-1 levels increased pharmacologically, insulin response becomes normal in hyperglycemic states (Figure 7). Hence, pharmacological plasma GLP-1 levels may restore “beta-cell glucose blindness” in IGT and type 2 DM. Although GLP-1-analogue-mediated beta cell stimulation is only sustainable during wash-out period, a novel trial declared that 3-year exenatide therapy partially recovered responsiveness of beta-cell to glucose[124].
Conversion of NGT to IGT and eventually to type 2 DM is mediated by nonstop failure of beta-cells (Figures 1 and 2). Exenatide: (1) increases responsiveness of beta cells to glucose and augments functions of beta-cells in type 2 DM; (2) facilitates loosing weight; (3) does not induce hypoglycemia; and (4) is applied once a week (Bydureon). For that reason, exenatide could be a good choice to decrease the conversion of IFG/IGT to type 2 DM and to a guarantee for NGT. There is no study investigating GLP-1 analogue effect on IGT - type 2 DM conversion. On the other hand liraglutide was investigated in obese but nondiabetic individuals (31% had IGT)[125]. In these IGT individuals, 84%-96% decrement was observed in type 2 DM progression. Five percent weight loss was achieved in 61% of individuals while ten percent weight loss achieved in 19% of individuals. New metabolic syndrome cases was decreased up to 60%. Therefore, long-acting GLP-1 analogues could be preferable drugs in order to prevent conversion of IGT to type 2 DM, because they carry additional effects such as weekly administration, beta cell function augmentation, and facilitation of loosing weight[126].
DPP-IV inhibitors: DPP-IV is the enzyme that cleaves GLP-1; DPP-IV inhibitors block this enzyme and therefore rise plasma GLP-1 concentrations. But, DPP-IV inhibitor-related increase in GLP-1 concentrations is uniquely dependent on endogenous GLP-1 secretion. Thus, DPP-IV inhibitor-related plasma GLP-1 rise usually is lower than GLP-1 analogue-related rise. DPP-IV inhibitors accomplish moderate increase in insulin secretion and have moderate inhibition on glucagon[110]. Vildagliptin administration in IGT individuals reveals little augmentation on functionality of beta-cells. However, vildagliptin effect totally disappeared after washout[116]. There is no study calculating DPP-IV inhibitor-mediated conversion rate of impiared GT - type 2 DM switch. In contrary to GLP-1 analogues, DPP-IV inhibitors cannot help loosing weight and they exert insufficient effect on beta-cells. Accordingly, GLP-1 analogues may be superior to DPP-IV inhibitors in IGT treatment.
Alpha-glucosidase inhibitors: IGT-type 2 DM conversion rate decreased about 25% by acarbose[127] and voglibose[128]. This effect was attributed to inhibition of carbohydrate absorption but increment in incretin secretion induced by alpha-glucosidase inhibitors may be the real reason of positive impact on glucose homeostasis[129]. Alpha-glucosidase inhibitors changes microbial flora of gut, thus they may help to heal glucose intolerance[130].
Pharmacotherapy cessation and emergence of diabetes: Pharmacological therapy applied to increase insulin sensitivity and beta-cell function have potent impact on prediabetes-diabetes conversion. But, we are not sure whether this effect is transient or sustained when the intervention is discontinued. Pharmacologic interventions prevents or delays diabetes onset by: (1) masking diabetes appearance by suppressing glucose; (2) preventing or delaying diabetes development only while it is being used; or (3) retaining their effects even after withdrawal.
Reassessing glycemic status after washing out the pharmacotherapy could clarify which possibility is relevant for the intervention[131]. Several studies investigating wash out effect are conducted in order to answer these questions. After 2.8 years of intervention in DPP trial, the incidence of diabetes in individuals with IGT was reduced by 58% with lifestyle modifications while the reduction is only 31% with metformin therapy compared with placebo. At the end of the trial 11-d washout period applied, participants who were taking metformin or placebo and had not developed diabetes were tested with a repeat OGTT in order to assess whether the observed metformin effect was sustained after cessation of the drug. Washout control reveals metformin participants had a significant increase in fasting glucose levels. It is concluded that one-quarter of the beneficial effect of metformin to prevent type 2 DM was attributable to a pharmacological effect and this effect did not persist when the drug was withdrawn. However, the overall effect of metformin in preventing diabetes remained substantial at 25% after withdrawal of the intervention[132].
In DREAM trial rosiglitazone slows down the new-onset diabetes in people with IGT ± IFG significantly (HR = 0.40, P < 0.0001). After a median 71-d medication washout period, the incidence of diabetes is similar both in intervention and placebo groups. This evidence suggests rosiglitazone does not have a sustained effect on the underlying disease pathophysiology and effective as long as the therapy is being given[131].
In STOP-NIDDM trial acarbose given to IGT patients delayed progression to Type 2 DM. The risk of progression to diabetes over 3.3 years was reduced by 25%. In the last 3 mo of the study placebo was given to all subjects. During this placebo treatment period, the incidence of diabetes was higher in the group originally assigned to acarbose than in the group first randomized to placebo (HR = 0.45, P < 0.005). On the other hand, STOP-NIDDM trial demonstrated that beneficial effect of acarbose preventing type 2 DM was partially attributable to its pharmacological effect and similar to metformin, the effect is not sustainable when drug use is stopped.
Prediabetics prone to develop type 2 DM plus atherosclerosis-induced cardiovascular complications are usually sub-maximally insulin resistant. In addition, these individuals have lost two thirds of their beta cell functions, their HbA1c levels usually are around 6% and at least 10% have diabetic retinopathy[133,134], nearly the same percentage of individuals have peripheral neuropathy[135]. Characteristic primers of diabetes are beta-cell dysfunction and insulin resistance. Gold standard measurement method for insulin sensitivity is euglycemic insulin clamp technique while the gold standart measurement method for insulin secretion is hyperglycemic clamp technique. These techniques are not much applicable for screening in clinical practice. Other predictive models studied IGT - type 2 DM conversion[136] and it is concluded that neither anthropometric criteria (waist-to-hip ratio or body mass index) nor metabolic syndrome components are superior to two-hour plasma glucose of OGTT. Another study illuminated two subgroups carrying high type 2 DM risk: First group consisted of IGT individuals whose total plasma glucose is in the upper fifth percentile during OGTT while the second group consisted of fasting plasma glucose over 95 mg/dL[137]. Best predictive criterion for future type 2 DM in IGT subjects is one-hour plasma glucose over 155 mg/dL, independent of their GT status in the Botnia[54] and San Antonio Heart[138] studies. Some biomarkers such as fasting PG, ferritin, insulin, adiponectin, HbA1c, IL-2 receptor A, high-sensitivity C-reactive protein predict diabetes development in later life[139]. Actos Now for the prevention of diabetes study and Diabetes Prevention Program gives inspiration to select IGT subjects carrying extra risks for type 2 DM, in order to discriminate people that take advantage from pharmacotherapy.
The optimal strategy is to prevent development of hyperglycemia intervening at the stage of IGT and also to revert GT back to normal. Individuals with IGT are insulin resistant and lost 50%-80% of their beta-cell function. Also, in order to prevent vascular complications resumption of normoglycemia is crucial in type 2 DM. This algorithm is also cheaper in long run. Diabetes Prevention Program Research Group wrote “Over 3 years, metformin was clinically effective (in preventing diabetes in IGT subjects) and cost-effective from the perspective of a health system and society, especially if implemented with generic medication pricing”[140,141]. When model simulations performed, similar results were reached[142,143]. IGT - type 2 DM conversion blockage by pioglitazone[40] is two fold that of metformin[37], so it is logical to assume that pioglitazone also could be cost-effective. But, monitoring and side effect treatment costs of those drugs should be remembered. Two aspects should be taken into account while performing cost analysis of pioglitazone. First one is edema management (if occurs) and the second is monitoring and treating osteoporosis. Possible long bone fracture in postmenopausal women should also be evaluated in cost analysis. Some studies implies bladder cancer risk in individuals who are given 45 mg pioglitazone over two-year time. But FDA mandated a prospective study in order to clear the pioglitazone safety (Kaiser Permanente study) as after eight-year observation, in comparison to those who never used pioglitazone, hazard risk ratio of bladder cancer was 0.98 in diabetics receiving pioglitazone.
GLP-1 analogues are expensive and they may not be put on market in near future. For that reason, cost analysis of GLP-1 analogue use in prediabetes states should be done cautiously. From community perspective, different criteria are considered in drug usage. But from patient perspective any solution to postpone or avert hyperglycemia probably decreases new onset microvascular complications such as nephropathy, neuropathy and/or retinopathy. When the main argument is reducing new cases of blindness, amputations and/or end-stage renal disease, “cost” cannot be top criterion for the individual for ethical reasons.
Another option is to prefer waiting till diabetes emerges and initiate therapy at this stage rather than treating individuals with prediabetes. But there is several limitations for this option. First, it brings handicaps on detecting exact timing of diabetes onset, namely, prediabetic individuals should be regularly controlled during this period. Secondly, UKPDS results make us to realize that in initial stages of diabetes tight glucose control cannot prevent microvascular complications. Besides, progression of euglycemia to dysglycemia is a silent but secular process. Thus, defining diabetes initiation in the basis of plasma glucose (namely fasting plasma glucose or two-hour plasma glucose) levels or in the basis of HbA1c is controversial. In reality, one tenth of prediabetics already have evidence of diabetic microvascular complications. Thirdly, upper tertile of IGT group is insulin resistant, their beta cell function loss is nearly 70%-80% whereas volume loss is about 30%-40%. Fourthly, a major diminution in beta-cell mass in prediabetes accelerates the conversion process to type 2 DM[144]. There is no remedy to increase human beta cell mass, today.
All pathophysiological events observed in type 2 DM also appears in prediabetic individuals and nearly 10% of prediabetics exhibit microvascular complications. Consequently, initiating lifestyle changes and pharmacotherapy in high-risk prediabetics instead of waiting till diabetes emerges seems reasonable. However there is no study comparing prediabetic stage therapy vs the diabetic stage therapy. Because these studies necessitate large sample sizes and very long study periods in order to demonstrate incidence differences in terms of microvascular complications. Therefore, response to the question “when should we institute pharmacological therapy?” is unclear, yet.
Lastly, prediabetics carry high risk for cardiovascular complications (myocardial infarction, stroke, cardiovascular death) besides their type 2 DM risk. IGT individuals are highly insulin resistant and thereby, exhibit some typical metabolic abnormalities observed in insulin resistance. For example they become dysglycemic, dyslipidemic, hypertensive, obese, insulin resistant, prone to coagulation, vulnerable to inflammation and endothelial dysfunction. Those abnormalities are also the main risk factors for cardiovascular disease. Moreover, insulin resistance is an independent atherosclerotic risk factor irrespective of other associated risk factors[94]. Thus cardiovascular disease risk of prediabetics is much more compared to normal individuals. Some measures diminishing diabetes risk also reduce cardiovascular risk. For instance, pioglitazone decreases triglyceride concentrations and increases HDL levels while loosing weight decreases blood pressure and heals lipid profile[37]. Eventually, in order to decrease cardiovascular disease risk of these individuals one should apply measures diminishing type 2 DM risk on one hand, while giving special attention on treating CVD risk factors (blood pressure and dyslipidemia) on the other hand.
Restoration of normoglycemia in prediabetics obviously lessens diabetes risk. Diabetes Prevention Program Outcome Study (DPPOS) compared the 894 people who had at least one normal OGTT with the 1096 people who never regressed to normoglycemia in Diabetes Prevention Program. In follow-up period of the study relative risk of diabetes emergence was 56% lower in the first group (OR = 0.44)[145]. Regression from prediabetes to normoglycemia not only reduces the risk of diabetes, but also the risk of cardiovascular disease. DPPOS has proven that if prediabetes can regress to normal glucose state, cardiovascular complications decrease[146]. Because, nearly one tenth of prediabetics possess microvascular complications, it is likely that restoration of normoglycemia improves microvascular complications[147].
Behavioral changes (dieting plus exercising) are effective in preventing IGT-type 2 DM conversion as well as IFG - type 2 DM conversion but loosing weight is hard and also difficult to maintain. Pharmacological interventions (plus dieting and exercising) improving and preserving beta-cell function and enhancing insulin sensitivity may be suitable choices for high-risk IGT patients. Troglitazone in Prevention of Diabetes Study, Pioglitazone in Prevention of Diabetes Study, Diabetes Reduction Assessment with ramipril and rosiglitazone Medication Trial, Actos Now for the prevention of diabetes study and Diabetes Prevention Program proven that thiazolidinediones obviously prevent the development of type 2 DM in IGT subjects as well as IFG subjects (Table 1). In Diabetes Prevention Program and Indian Diabetes Prevention Program, metformin slowed down the progression of IGT to type 2 DM, eventually ADA Consensus Conference Statement proposed metformin usage in high-risk IGT individuals. However, pioglitazone and rosiglitazone efficacy in preventing IGT progression to type 2 DM nearly doubles metformin’s efficacy (31% vs 72% and 62%, respectively). Rosiglitazone (low dose = 2 mg/d) together with metformin (850 mg/d) was proven to be slows down IGT progression to type 2 DM as well as to be more tolerable. GLP-1 analogues: (1) effectively treats type 2 DM; (2) blocks IGT - type 2 DM progression; (3) preserves and augments functions of beta-cells; (4) facilitates loosing weight; (5) combat with cardiovascular risks; (6) do not cause hypoglycemia; and (7) can be used once a day (liraglutide) or once a week (Bydureon). For these reasons we speculate that this drug group, especially long-acting preperations[127], be ideal for obese patients with IGT.
| Study | n | Duration (yr) | Incidence of DM in control (%) | Relative risk reduction (%) |
| IDPP | 269 | 2.5 | 18.3 | 26 |
| USDPP | 2151 | 2.8 | 11 | 31 |
| USDPP | 1172 | 0.9 | 11 | 75 |
| TRIPOD | 236 | 2.5 | 13.1 | 55 |
| PIPOD | 89 | 3 | 13.1 | 55 |
| DREAM | 5269 | 3 | 6.5 | 60 |
| ACT NOW | 602 | 2.8 | 6 | 72 |
| CANOE | 207 | 3.9 | 10.1 | 66 |
| STOP NIDDM | 1368 | 3.2 | 8.1 | 36 |
| XENDOS | 3305 | 4 | 2.2 | 37 |
The benefits and disadvantages of pharmacotherapy must be evaluated simultaneously. Although rare, metformin can induce lactic acidosis. If serum creatinine levels exceeds 1.4 mg/dL in females and 1.5 mg/dL in males, metformin is contraindicated. Gastrointestinal side effects are often and one tenth of patients are metformin intolerable. On the other hand, pioglitazone users experience fluid retention, fat weight gain and congestive heart failure. Paradoxically, while fat weight gain increses, reduction in HbA1c becomes more prevalent and much more insulin sensitivity/beta-cell function improvement is achieved. Easily detected clinical sign of fluid retention is peripheral edema and can be controlled easily with distally acting diuretics such as amiloride or spironolactone. Because these side effects are dose-related, restricting pioglitazone to 30 mg daily dose may decrease side effects. Trauma-related fracture cases were increased in postmenopausal women treated with pioglitazone. For that reason pioglitazone should be used carefully in postmenopausal women. Nausea/vomiting are main handicaps of GLP-1 receptor agonist usage; nearly one third of subjects experience nausea/vomitting. Though adverse effects are generally mild or temporary, liraglutide/exenatide intolerance is about 5%. Pancreatitis is also pronounced, but when large national databases were analysed retrospectively, there was no such increment in pancreatitis in GLP-1 receptor agonist users.
In conclusion, we recommend strict lifestyle modification for patients with IGT ± IFG. Another option is to initiate pharmacotherapy with metformin plus low-dose pioglitazone. In high risk IGT individuals long-acting GLP-1 analogue use as well as diet plus exercise may be another option. Each component of this approach is effective in type 2 DM prevention and turning IGT back to normal. Depending on evidence described earlier, we believe “combination therapy” would especially be preventive for microvascular complications and is associated with lower adverse effects. Also, pharmacotherapy with generic drugs may be cost effective.
Special thanks to Meagan Pate, MD for her editing assistance.
P- Reviewer: Faerch K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. [PubMed] [Cited in This Article: ] |
| 2. | Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708-723. [PubMed] [Cited in This Article: ] |
| 3. | Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. [PubMed] [Cited in This Article: ] |
| 4. | Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9:193-199. [PubMed] [Cited in This Article: ] |
| 5. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [PubMed] [Cited in This Article: ] |
| 6. | The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17510] [Cited by in F6Publishing: 15819] [Article Influence: 510.3] [Reference Citation Analysis (3)] |
| 7. | UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [PubMed] [Cited in This Article: ] |
| 8. | UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865. [PubMed] [Cited in This Article: ] |
| 9. | Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117. [PubMed] [Cited in This Article: ] |
| 10. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [PubMed] [Cited in This Article: ] |
| 11. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5314] [Cited by in F6Publishing: 4992] [Article Influence: 312.0] [Reference Citation Analysis (0)] |
| 12. | Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 821] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 13. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3445] [Cited by in F6Publishing: 3204] [Article Influence: 213.6] [Reference Citation Analysis (0)] |
| 14. | American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011;34 Suppl 1:S11-S61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 1858] [Article Influence: 142.9] [Reference Citation Analysis (0)] |
| 15. | Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430-1435. [PubMed] [Cited in This Article: ] |
| 18. | Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89-94. [PubMed] [Cited in This Article: ] |
| 19. | Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Järvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 405] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191-197. [PubMed] [Cited in This Article: ] |
| 21. | Lee ET, Howard BV, Savage PJ, Cowan LD, Fabsitz RR, Oopik AJ, Yeh J, Go O, Robbins DC, Welty TK. Diabetes and impaired glucose tolerance in three American Indian populations aged 45-74 years. The Strong Heart Study. Diabetes Care. 1995;18:599-610. [PubMed] [Cited in This Article: ] |
| 22. | DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667-687. [PubMed] [Cited in This Article: ] |
| 23. | Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1971] [Cited by in F6Publishing: 1793] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 24. | Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH. A two-step model for development of non-insulin-dependent diabetes. Am J Med. 1991;90:229-235. [PubMed] [Cited in This Article: ] |
| 25. | Jallut D, Golay A, Munger R, Frascarolo P, Schutz Y, Jéquier E, Felber JP. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism. 1990;39:1068-1075. [PubMed] [Cited in This Article: ] |
| 26. | DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173-194. [PubMed] [Cited in This Article: ] |
| 27. | Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607. [PubMed] [Cited in This Article: ] |
| 28. | Bajaj M, Defronzo RA. Metabolic and molecular basis of insulin resistance. J Nucl Cardiol. 2003;10:311-323. [PubMed] [Cited in This Article: ] |
| 29. | Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 30. | DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387-395. [PubMed] [Cited in This Article: ] |
| 31. | Abdul-Ghani MA, Matsuda M, DeFronzo RA. Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med. 2008;25:1289-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 551] [Cited by in F6Publishing: 591] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319:1500-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 314] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes. 1990;39:283-288. [PubMed] [Cited in This Article: ] |
| 35. | Pratipanawatr W, Pratipanawatr T, Cusi K, Berria R, Adams JM, Jenkinson CP, Maezono K, DeFronzo RA, Mandarino LJ. Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation. Diabetes. 2001;50:2572-2578. [PubMed] [Cited in This Article: ] |
| 36. | Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, Dailey G, Gerich J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA. 1995;273:1855-1861. [PubMed] [Cited in This Article: ] |
| 37. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13206] [Cited by in F6Publishing: 11846] [Article Influence: 538.5] [Reference Citation Analysis (0)] |
| 38. | Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150-1156. [PubMed] [Cited in This Article: ] |
| 39. | Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1076] [Cited by in F6Publishing: 1036] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 40. | DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 41. | Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 417] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 42. | Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1372] [Cited by in F6Publishing: 1231] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 43. | Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 44. | Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, DeFronzo RA. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2008;295:E401-E406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Godsland IF, Jeffs JA, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia. 2004;47:1157-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Diamond MP, Thornton K, Connolly-Diamond M, Sherwin RS, DeFronzo RA. Reciprocal variations in insulin-stimulated glucose uptake and pancreatic insulin secretion in women with normal glucose tolerance. J Soc Gynecol Investig. 1995;2:708-715. [PubMed] [Cited in This Article: ] |
| 47. | Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610-630. [PubMed] [Cited in This Article: ] |
| 48. | Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 493] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 49. | Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 530] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 50. | Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 51. | Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 386] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 52. | Abdul-Ghani MA, Williams K, DeFronzo R, Stern M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care. 2006;29:1613-1618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337-442. [PubMed] [Cited in This Article: ] |
| 54. | Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA. Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care. 2010;33:557-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Cowie CC, Harris MI, Silverman RE, Johnson EW, Rust KF. Effect of multiple risk factors on differences between blacks and whites in the prevalence of non-insulin-dependent diabetes mellitus in the United States. Am J Epidemiol. 1993;137:719-732. [PubMed] [Cited in This Article: ] |
| 57. | Jarrett RJ, Keen H, McCartney P. The Whitehall Study: ten year follow-up report on men with impaired glucose tolerance with reference to worsening to diabetes and predictors of death. Diabet Med. 1984;1:279-283. [PubMed] [Cited in This Article: ] |
| 58. | Eriksson KF, Lindgärde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study. Diabetologia. 1991;34:891-898. [PubMed] [Cited in This Article: ] |
| 59. | Jani R, Molina M, Matsuda M, Balas B, Chavez A, DeFronzo RA, Abdul-Ghani M. Decreased non-insulin-dependent glucose clearance contributes to the rise in fasting plasma glucose in the nondiabetic range. Diabetes Care. 2008;31:311-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Faerch K, Vaag A, Holst JJ, Glümer C, Pedersen O, Borch-Johnsen K. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia. 2008;51:853-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Kanat M, Mari A, Norton L, Winnier D, DeFronzo RA, Jenkinson C, Abdul-Ghani MA. Distinct β-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012;61:447-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev. 2010;26:280-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo RA, Abdul-Ghani MA. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. 2011;48:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 65. | DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397-405. [PubMed] [Cited in This Article: ] |
| 66. | Kanat M, Winnier D, Norton L, Arar N, Jenkinson C, Defronzo RA, Abdul-Ghani MA. The relationship between {beta}-cell function and glycated hemoglobin: results from the veterans administration genetic epidemiology study. Diabetes Care. 2011;34:1006-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Færch K, Johansen NB, Witte DR, Lauritzen T, Jørgensen ME, Vistisen D. Relationship between insulin resistance and β-cell dysfunction in subphenotypes of prediabetes and type 2 diabetes. J Clin Endocrinol Metab. 2015;100:707-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Keen H, Jarrett RJ, McCartney P. The ten-year follow-up of the Bedford survey (1962-1972): glucose tolerance and diabetes. Diabetologia. 1982;22:73-78. [PubMed] [Cited in This Article: ] |
| 69. | Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506-2514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 701] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 70. | Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrannini E. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med. 2005;118:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Tiikkainen M, Bergholm R, Rissanen A, Aro A, Salminen I, Tamminen M, Teramo K, Yki-Järvinen H. Effects of equal weight loss with orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. Am J Clin Nutr. 2004;79:22-30. [PubMed] [Cited in This Article: ] |
| 72. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7148] [Cited by in F6Publishing: 6390] [Article Influence: 277.8] [Reference Citation Analysis (0)] |
| 73. | Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404-2414. [PubMed] [Cited in This Article: ] |
| 74. | Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1074] [Cited by in F6Publishing: 1004] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 75. | Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, Fukunaga R, Bandai Y, Tajima N, Nakamura Y. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011;171:1352-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 76. | Wing RR, Goldstein MG, Acton KJ, Birch LL, Jakicic JM, Sallis JF, Smith-West D, Jeffery RW, Surwit RS. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care. 2001;24:117-123. [PubMed] [Cited in This Article: ] |
| 77. | Venditti EM, Bray GA, Carrion-Petersen ML, Delahanty LM, Edelstein SL, Hamman RF, Hoskin MA, Knowler WC, Ma Y. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond). 2008;32:1537-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161. [PubMed] [Cited in This Article: ] |
| 79. | Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 844] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 80. | Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1999] [Cited by in F6Publishing: 1922] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 81. | Saaristo T, Moilanen L, Korpi-Hyövälti E, Vanhala M, Saltevo J, Niskanen L, Jokelainen J, Peltonen M, Oksa H, Tuomilehto J. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care. 2010;33:2146-2151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 82. | DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 937] [Cited by in F6Publishing: 841] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 83. | DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 284] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059-4067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005-2012. [PubMed] [Cited in This Article: ] |
| 86. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2205] [Cited by in F6Publishing: 2041] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 87. | Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1219] [Cited by in F6Publishing: 1141] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 88. | Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 89. | Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55. [PubMed] [Cited in This Article: ] |
| 90. | Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339-348. [PubMed] [Cited in This Article: ] |
| 91. | Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 917] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 92. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1492] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 93. | Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507-514. [PubMed] [Cited in This Article: ] |
| 94. | DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 95. | Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E871-E883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 96. | Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 575] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 97. | Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001;24:710-719. [PubMed] [Cited in This Article: ] |
| 98. | Miyazaki Y, He H, Mandarino LJ, DeFronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes. 2003;52:1943-1950. [PubMed] [Cited in This Article: ] |
| 99. | Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 682] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 100. | Cavaghan MK, Ehrmann DA, Byrne MM, Polonsky KS. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. J Clin Invest. 1997;100:530-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Berkowitz K, Peters R, Kjos SL, Goico J, Marroquin A, Dunn ME, Xiang A, Azen S, Buchanan TA. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes. 1996;45:1572-1579. [PubMed] [Cited in This Article: ] |
| 102. | Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517-522. [PubMed] [Cited in This Article: ] |
| 103. | Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796-2803. [PubMed] [Cited in This Article: ] |
| 104. | Higa M, Zhou YT, Ravazzola M, Baetens D, Orci L, Unger RH. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1999;96:11513-11518. [PubMed] [Cited in This Article: ] |
| 105. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3109] [Cited by in F6Publishing: 2873] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 106. | Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab. 2014;16:719-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 107. | Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25:517-523. [PubMed] [Cited in This Article: ] |
| 108. | Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605-1611. [PubMed] [Cited in This Article: ] |
| 109. | Zinman B, Harris SB, Neuman J, Gerstein HC, Retnakaran RR, Raboud J, Qi Y, Hanley AJ. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. 2010;376:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 110. | Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 111. | Wajcberg E, Tavaria A. Exenatide: clinical aspects of the first incretin-mimetic for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2009;10:135-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 113. | DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092-1100. [PubMed] [Cited in This Article: ] |
| 114. | Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 805] [Cited by in F6Publishing: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 115. | Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 116. | Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 117. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2461] [Cited by in F6Publishing: 2465] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 118. | Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 918] [Cited by in F6Publishing: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 119. | Rask E, Olsson T, Söderberg S, Holst Jj Jj, Tura A, Pacini G, Ahrén B. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism. 2004;53:624-631. [PubMed] [Cited in This Article: ] |
| 120. | Byrne MM, Gliem K, Wank U, Arnold R, Katschinski M, Polonsky KS, Göke B. Glucagon-like peptide 1 improves the ability of the beta-cell to sense and respond to glucose in subjects with impaired glucose tolerance. Diabetes. 1998;47:1259-1265. [PubMed] [Cited in This Article: ] |
| 121. | Ahrén B, Larsson H, Holst JJ. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137:127-131. [PubMed] [Cited in This Article: ] |
| 122. | Jones IR, Owens DR, Luzio S, Williams S, Hayes TM. The glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:668-677. [PubMed] [Cited in This Article: ] |
| 123. | Knop FK, Vilsbøll T, Højberg PV, Larsen S, Madsbad S, Vølund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56:1951-1959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 124. | Bunck MC, Cornér A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, Smith U, Yki-Järvinen H, Diamant M. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 125. | Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 743] [Cited by in F6Publishing: 714] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 126. | Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 790] [Cited by in F6Publishing: 751] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 127. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1820] [Cited by in F6Publishing: 1584] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 128. | Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 129. | Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358-E1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 130. | Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 131. | DREAM Trial Investigators. Incidence of diabetes following ramipril or rosiglitazone withdrawal. Diabetes Care. 2011;34:1265-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 132. | Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care. 2003;26:977-980. [PubMed] [Cited in This Article: ] |
| 133. | Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 134. | Massin P, Lange C, Tichet J, Vol S, Erginay A, Cailleau M, Eschwège E, Balkau B. Hemoglobin A1c and fasting plasma glucose levels as predictors of retinopathy at 10 years: the French DESIR study. Arch Ophthalmol. 2011;129:188-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 136. | Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S194-S198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 137. | Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701-710. [PubMed] [Cited in This Article: ] |
| 138. | Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31:1650-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 139. | Kolberg JA, Jørgensen T, Gerwien RW, Hamren S, McKenna MP, Moler E, Rowe MW, Urdea MS, Xu XM, Hansen T. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care. 2009;32:1207-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 140. | Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518-2523. [PubMed] [Cited in This Article: ] |
| 141. | Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323-332. [PubMed] [Cited in This Article: ] |
| 142. | Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, Davies MJ, Khunti K. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ. 2008;336:1180-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 143. | Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, Gregg E, Holman RR, Kirkman MS, Stern M. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010;375:1365-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 144. | Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102-110. [PubMed] [Cited in This Article: ] |
| 145. | Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243-2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 146. | Perreault L, Temprosa M, Mather KJ, Horton E, Kitabchi A, Larkin M, Montez MG, Thayer D, Orchard TJ, Hamman RF. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program outcomes study. Diabetes Care. 2014;37:2622-2631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 147. | Yakubovich N, Gerstein HC. Is regression to normoglycaemia clinically important? Lancet. 2012;379:2216-2218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |