Published online May 15, 2012. doi: 10.4239/wjd.v3.i5.94
Revised: April 19, 2012
Accepted: May 11, 2012
Published online: May 15, 2012
AIM: To investigate the signaling mechanism of anti-oxidative action by curcumin and its impact on glucose disposal.
METHODS: Male C57BL/6J mice were fed with either a normal diet (n = 10) or a high fat diet (HFD) (n = 20) to induce obesity and insulin resistance. After 16 wk, 10 HFD-fed mice were further treated with daily curcumin oral gavage at the dose of 50 mg/kg body weight (BW) (HFD + curcumin group). After 15 d of the curcumin supplementation, an intraperitoneal glucose tolerance test was performed. Fasting blood samples were also collected for insulin and glucose measurements. Insulin-sensitive tissues, including muscle, adipose tissue and the liver, were isolated for the assessments of malondialdehyde (MDA), reactive oxygen species (ROS) and nuclear factor erythroid-2-related factor-2 (Nrf2) signaling.
RESULTS: We show here that in a HFD mouse model, short-term curcumin gavage attenuated glucose intolerance without affecting HFD-induced BW gain. Curcumin also attenuated HFD-induced elevations of MDA and ROS in the skeletal muscle, particularly in its mitochondrial fraction, but it had no such an effect in either adipose tissue or the liver of HFD-fed mice. Correspondingly, in skeletal muscle, the levels of total or nuclear content of Nrf2, as well as its downstream target, heme oxygenase-1, were reduced by HFD-feeding. Curcumin intervention dramatically reversed these defects in Nrf2 signaling. Further analysis of the relationship of oxidative stress with glucose level by a regression analysis showed a positive and significant correlation between the area under the curve of a glucose tolerance test with MDA levels either in muscle or muscular mitochondria.
CONCLUSION: These findings suggest that the short-term treatment of curcumin in HFD-fed mice effectively ameliorates muscular oxidative stress by activating Nrf2 function that is a novel mechanism for its effect in improving glucose intolerance.
- Citation: He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes 2012; 3(5): 94-104
- URL: https://www.wjgnet.com/1948-9358/full/v3/i5/94.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i5.94
Obesity is associated with a high incidence of many metabolic disorders, including type 2 diabetes mellitus and cardiovascular diseases[1,2]. Insulin resistance is recognized as a core mechanism for these obesity-related diseases. Extensive recent studies have shown that chronic activation of inflammatory signaling, endoplasmic reticulum (ER) stress and mitochondrial dysfunction are among the etiological factors of insulin resistance[3,4]. In addition, the causal relationship between oxidative stress and the development of insulin resistance[5-7] has been suggested for a long time, while it has gained much more attention recently due to the recognition of the involvement of mitochondrial-derived oxidative stress in the etiology of insulin resistance[4,8]. Over nutrition supply or high fat diet (HFD) consumption lead to increased mitochondrial oxidative phosphorylation and inevitable reactive oxygen species (ROS) over-production[7,8]. Mammals however, have evolved endogenous anti-oxidative systems to prevent oxidative stress and maintain insulin sensitivity.
The transcription nuclear factor erythroid-2-related factor-2 (Nrf2) plays a major role in maintaining redox balance. In a resting cell, Nrf2 molecules mainly reside in cell cytosol and are anchored with its negative regulator Kelch-like ECH-associated protein 1 (Keap1). The coupling between Nrf2 and Keap1 leads to Nrf2 proteasomal degradation[9]. When oxidative stress occurs, Nrf2 and Keap1 will be separated, leading to the elevation of free Nrf2 level and the increase of Nrf2 nuclear translocation. Nuclear Nrf2 will then bind to the consensus nucleotide sequence, namely antioxidant response element (ARE), in the promoter regions of a battery of genes that encode antioxidant enzymes[9,10]. Since oxidative stress is an important etiological factor in aging, inflammation and tumor growth, it is not surprising that Nrf2 can modulate the progress of these diseases[11,12].
Interestingly, certain natural phyto-compounds, including curcumin, are Nrf2 activators and have a potential therapeutic effect for diseases, including inflammation, tumor, heart failure, neurodegenerative diseases and ischemia[13-16]. Curcumin has also been shown to sensitize insulin action in HFD-fed diabetic models[17]. The understanding of molecular mechanisms underlying the insulin sensitizing action by curcumin is essential for the generation of interventional approaches. Most in vivo investigations have been conducted to explore long-term effect of curcumin and its beneficial effect on insulin signaling mainly via reducing body weight (BW) gain and inhibiting inflammatory reactions[17,18]. However, in spite of the documented anti-oxidative function of curcumin[14], no attempt has been made to correlate the role of curcumin-mediated anti-oxidative function and its impact on insulin sensitivity. Since curcumin has been shown to activate Nrf2 in cultured cells[15], here we asked whether curcumin activates Nrf2 system in vivo and whether the activation leads to improved insulin signaling. To investigate whether the beneficial effect of curcumin can be achieved independent of its BW lowering effect, we delivered a low dose of curcumin by gavage short-term to avoid BW change.
Male C57BL/6J mice (8 wk of age) were purchased from the Laboratory Animal Center of Sun Yat-sen University. Curcumin (curcuminoid content ≥ 94%), common chemicals and protease inhibitors were obtained from Sigma Chemical Company (St Louis, MO). Normal diet (ND) (8% calories from fat) and HFD (60% calories from fat) were provided by Guangdong Animal Center, (Guangzhou, Guangdong, China). Nrf2 antibody was from Santa Cruze Biotechnology (Santa Cruz, CA). Heme oxygenase-1 (HO-1) and β-actin antibodies were from the Proteintech Group (Chicago, IL). Histone H3 antibody was obtained from Cell Signaling Technology (Denvers, MA, United States). Bicinchoninic acid (BCA) assay kit was purchased from Pierce Bio-technology, Inc. (Rockford, IL). Enhanced chemiluminescence (ECL) was purchased from Thermo Scientific (Rockford, IL). Blood glucose meter was obtained from ACON Laboratories, Inc. (San Diego, CA). ROS assay kit was purchased from GENMED Scientifics (Shanghai, China). Malondialdehyde (MDA) assay kit was purchased from ZeptoMetrix (Buffalo, NY).
The animal experiments were performed in accordance with the Guide for Care and Use of Experimental Animals (Sun Yat-sen University, SYSU). Thirty mice were housed in an environmentally controlled room at 22 ± 2.0 °C and 50% ± 5% humidity with a 12-h: 12-h light/dark cycle. The mice had access to food and water ad libitum. After a one week adaptive period, the mice were randomly divided into two weight-matched groups. Ten mice were fed with ND and the rest of them (20 mice) were fed with HFD. BW was measured weekly. After 16 wk of feeding, HFD-fed mice were further divided into two weight-matched groups, e.g., HFD group (n = 10) and HFD plus curcumin treated group (n = 10). Curcumin was given daily by oral gavage at the dose of 50 mg/kg BW in 1% carboxymethyl cellulose buffer solution for 15 d. Mice in the ND and HFD group were gavaged with vehicle only. For blood sample and tissue collection, all mice were euthanized after fasting for 6 h. Blood samples were taken and centrifuged at 4 °C at 3000 rpm for 10 min for collecting the serum. Meanwhile, skeletal muscle in quadriceps, livers and epididymal fat pads were rapidly isolated, followed by immediate freezing in liquid nitrogen and then stored at -80 °Cbefore further analyses.
Intraperitoneal glucose tolerance test was performed as previously described[19]. Briefly, mice were fasted overnight, followed by glucose (1 g/kg) injection intraperitoneally. Blood samples collected from a tail vein were used for glucose measurement.
Nuclear and mitochondrial fractionation were performed as previously described[20,21] with a slight modification. About 30 mg skeletal muscle was homogenized in 1 mL ice-cold buffer containing 20 mmol/L HEPES (pH 7.4), 250 mmol/L sucrose, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L dithiothreitol and the protease inhibitors [2 μg/mL aprotinin, 5 μg/mL leupeptin and 2 mmol/L phenylmethyl sulphonyl fluoride (PMSF)]. Following the homogenization procedure, samples were incubated on ice for 20 min. Centrifugation was then carried out at 720 ×g at 4 °C for 5 min. The nuclear pellet was dispersed by the buffer and passed through a 21G needle 20 times. The samples were then centrifuged again at 720 ×g at 4 °C for 10 min. After removing the supernatant, the nuclear pellet was re-suspended in 50 μL nuclear lysis buffer containing 20 mmol/L Tris (pH 7.5), 137 mmol/L NaCl, 2 mmol/L EDTA, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 1% Nonidet P-40 (NP-40), 2 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS) and the aforementioned protease inhibitors. The nuclear pellet was sonicated briefly for 3 s and saved for western blotting. For mitochondrial fractionation whole cell lysate after removing the nuclei was centrifuged at 10 000 ×g, 4 °C for 10 min and saved the pellet as mitochondrial fraction at -80 °C.
MDA level was determined by the thiobarbituric acid (TBA) method[22]. TBA reaction was performed according to manufactory guidance. To assess MDA level in serum, 100 μL serum was used. For tissue MDA assay about 30 mg of tissues (skeletal muscle, liver or adipose tissue) or mitochondria extracted from 30 mg skeletal muscle were homogenized in 300 μL ice-cold PBS. TBA reaction was performed according to manufactory guidance. Briefly, samples were incubated with TBA and SDS at 95 °C for 1 h, followed by a centrifugation at 800 ×g for 10 min. Supernatants were transferred to a 96-well plate and the absorbance was measured at 532 nm. The protein concentration in samples was determined by a BCA assay kit. Serum MDA level was calculated according to the formula provided by the company guidance, i.e., serum MDA (nmol/mL) = (sample OD value-background OD)/( Standard OD-blank OD) × standard concentration (10 nmol/mL) × sample dilution times, while tissue MDA level after the calculation was further corrected by sample protein concentration (mg protein/mL). MDA equivalents were expressed as nmol/mg tissue protein or nmol/mL serum.
Skeletal muscles were homogenized and ROS level analysis was performed using 2’,7’-dichlorofluorescein diacetate fluorescent dye as a probe and fluorescence density was measured at 490/520 nm according to manufactory guidance and the measured values of optical density [a relative fluorescence unit (RFU)] were corrected by the protein concentrations of samples and were expressed as RFU/μg protein.
After the mice were fasted for 6 h, blood samples were collected. To assess plasma insulin levels, an ELISA based method (Mercodia AB, Uppsala, Sweden) was used, the method was according to manufactory guidance and expressed as μg/L. Plasma glucose assay levels were determined using a glucose assay kit from Sigma-Aldrich (Saint Louis, United States), with the method provided by the manufacturer. The level of the original plasma glucose concentration was measured at 540 nm and expressed as mg/dL. Homeostasis model assessment- insulin resistance index (HOMA-IR) was calculated as [fasting plasma glucose (mmol/L) × fasting serum insulin (μIU/mL)] /22.5[23].
Methods for tissue protein preparation and western blotting were performed as described[19]. A fraction of liver, adipose tissue and skeletal muscle were homogenized in ice-cold lysis buffer containing 20 mmol/L Tris (pH 7.5), 137 mmol/L NaCl, 2 mmol/L EDTA, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 1% NP-40, 2 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 10% glycerol, 2 μg/mL aprotinin, 5 μg/mL leupeptin and 2 mmol/L PMSF. The homogenates were then sonicated on ice cold conditions three times for 20 s and centrifuged at 12 000 rpm for 10 min at 4 °C. The supernatant was collected. The protein concentration was determined by BCA assay kit. Equal amounts of proteins were used for western blot analysis. The proteins were heated at 95 °Cfor 5 min in SDS sample loading buffer and underwent SDS-PAGE analysis as described in the following. The cellular proteins were separated by SDS-PAGE (10%) and transferred to nitrocellulose membranes. Following the transfer, the membranes were incubated for 1.5 h at room temperature in the buffer containing 25 mmol/L Tris (pH 7.6), 154 mmol/L NaCl, 0.1% Tween-20 and 5% skimmed milk. The membranes were then probed with antibody overnight at 4 °C. The membranes were washed and incubated with secondary antibody conjugated to horseradish peroxidase. Immunoreactive proteins were visualized with ECL. The intensities of the bands were quantified by phosphorimager analysis using NIH image software.
All the values were expressed as mean ± SE. Statistical comparisons of means among three groups were carried out by using analysis of variance and the P value less than 0.05 was considered to be statistically significant. The correlation between area under the curve (AUC) and MDA or ROS was tested with the method of multiple linear regressions.
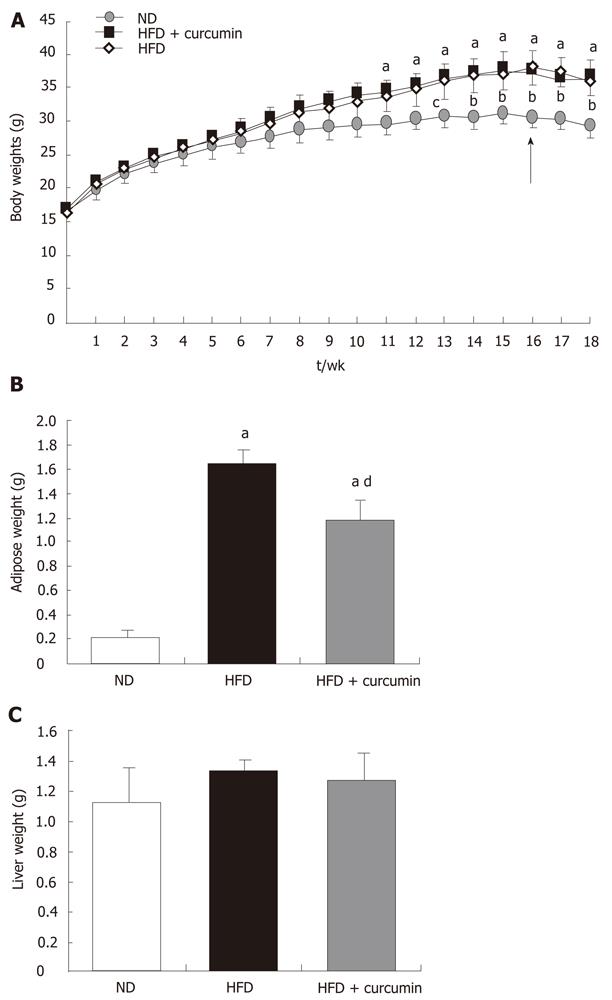
Firstly, we fed the mice with HFD to induce obesity and insulin resistance. As shown in Figure 1A, mice who received HFD feeding exhibited a more rigorous BW gain when compared with the mice of the ND group. The difference reached statistical significance at 11 wk after HFD feeding. Curcumin was supplemented after 16 wk feeding during HFD consumption, which was different from the previous studies when curcumin was administered at the beginning of HFD feeding and prevented HFD-induced obesity[17,18]. A short-term and low dose of curcumin administration was designed to avoid its BW lowering effect in order to detect the primary mechanism of its action. We gavaged a group of HFD-fed mice with 50 mg/kg BW of curcumin consecutively for 15 d. The control mice received the vehicle solution. This curcumin treatment did not alter BW gain in mice while fed with HFD (Figure 1A). To further detect its effect on obesity, the weight of epididymal fat pads (an indicator of visceral fat mass) was measured. We observed that HFD induced about an 8-fold increase in the weight of epididymal fat pads compared to ND mice (ND: 0.21 ± 0.07 g vs HFD: 1.65 ± 0.12 g, P < 0.001, Figure 1B) , while curcumin gavage only moderately reduced the weight of epididymal fat in mice who received HFD (HFD + curcumin: 1.19 ± 0.2 g vs HFD: 1.65 ± 0.12 g, P < 0.05, Figure 1B) and compared with mice in the ND group, the increase of the weight of epididymal fat in curcumin-treated mice was still about 5-fold (ND: 0.21 ± 0.07 g vs HFD + curcumin: 1.19 ± 0.2 g, P < 0.001, Figure 1B). We also measured the weight of the liver and there was no statistical difference among the three groups (Figure 1C).
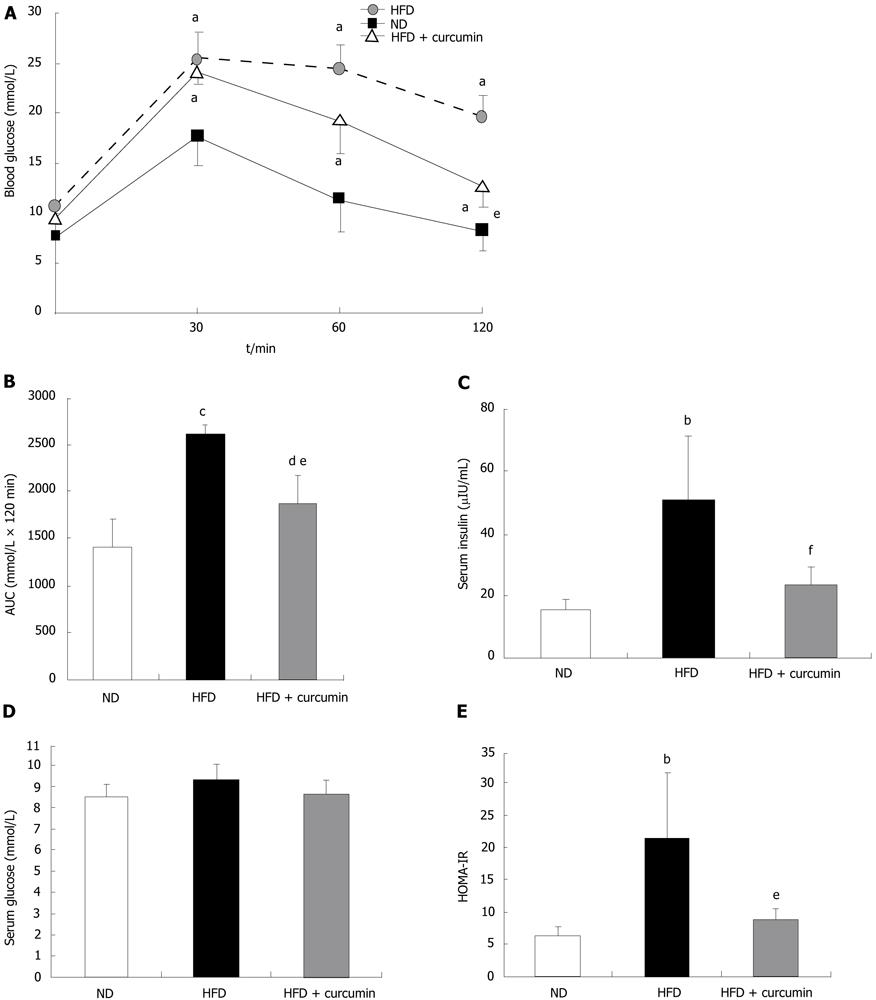
As indicated previously, curcumin administration significantly prevented HFD-induced insulin resistance by long-term supplementation[17]. To assess whether curcumin could reverse insulin insensitivity in an established obese model or to test its treatment efficacy, we performed an intraperitoneal glucose tolerance test (IPGTT). As shown in Figure 2A, after 18 wk feeding of HFD, glucose levels following the i.p. injection were significantly higher when compared with those in ND-fed mice. Curcumin treatment reduced blood glucose levels (Figure 2A). The analysis of AUC of the IPGTT during the tested 120 min also showed that HFD induced about a 2-fold increase in the AUC index compared to that in ND mice, while curcumin normalized this elevation by more than 50% (Figure 2B). Furthermore, in order to assess whether insulin sensitivity was enhanced by curcumin, we measured fasting glucose and insulin levels (Figure 2C and D) and calculated the HOMA-IR index (Figure 2E). We found that HFD induced almost a 3-fold increase in this index while curcumin significantly reversed this abnormality. These results indicate that the effect of curcumin on improving glucose disposal is via enhancing insulin action and this action of curcumin in HFD-fed mice is disposable with its effect on attenuating BW gain.
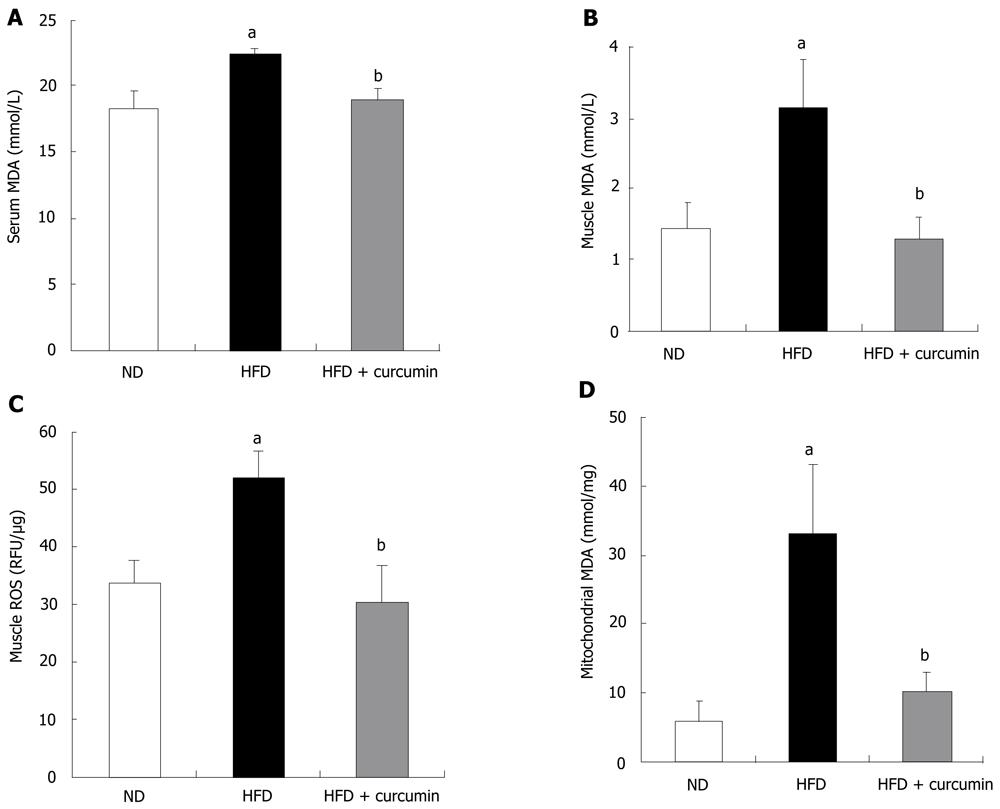
Increased evidence indicates that oxidative stress plays a causal role in the development of insulin resistance[7,24,25]. We tested whether HFD and curcumin alter redox balance by assessing MDA levels, a stable indicator of oxidative stress[22]. As shown in Figure 3A, HFD induced approximately 20% elevation of serum MDA level, while short-term curcumin gavage completely reversed this elevation (HFD: 22.24 ± 0.50 nmol/mL vs HFD + curcumin: 18.82 ± 0.91 nmol/mL, P < 0.01). We then assessed MDA levels in the insulin sensitive tissues. MDA level in the skeletal muscle of HFD-fed mice was increased approximately 2-fold when compared with that in the ND mice (Figure 3B). Curcumin administration decreased muscular MDA content to the level that was comparable with that in the ND mice (Figure 3B). Furthermore, muscular ROS content was increased about 40% by HFD feeding while curcumin treatment completely blocked this elevation (ND: 33.61 ± 4.03 RFU/μg vs HFD: 51.7 ± 4.92 RFU/μg vs P < 0.001; HFD: 51.7 ± 4.92 RFU/μg vs HFD + curcumin: 30.18 ± 6.66 RFU/μg, P < 0.001, Figure 3C).
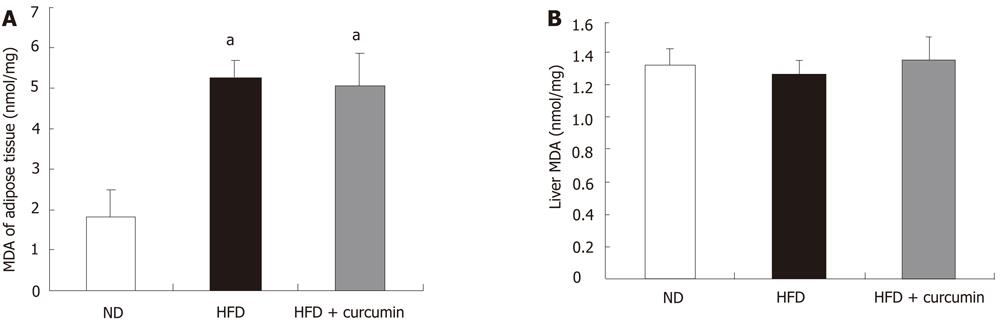
Since mitochondrial oxidative stress plays a major causative role in insulin resistance in the condition of nutrition over consumption[4,8], we measured the MDA level in the mitochondrial fraction of skeletal muscles. Indeed, the MDA level in the mitochondrial fraction was pronouncedly elevated in the HFD-fed mice (4-fold elevation compared to ND mice) and curcumin significantly attenuated the effect of HFD (HFD: 33.07 ± 10.16 nmol/mg vs HFD + curcumin: 10.05 ± 2.90 nmol/mg, P < 0.001, Figure 3D). We, however, did not detect a change of MDA level in liver or adipose tissue by curcumin gavage (Figure 4). By performing a regression analysis on the muscular MDA level and the AUC value in IPGTT, we found that there was a significantly positive correlation between them (P < 0.05). The regression analysis was also performed on the muscular mitochondrial MDA level and the AUC value in IPGTT. A more significantly positive correlation was observed (P < 0.001). These data suggest the existence of a tight link between muscular mitochondrial redox balance and the ability of the whole body on glucose disposal.
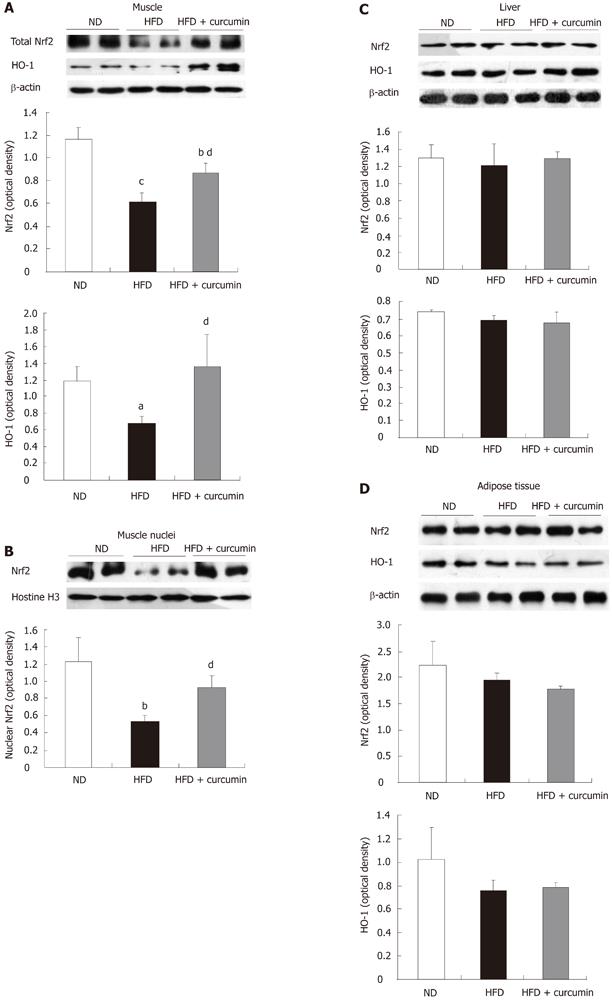
Curcumin has been shown to activate the Nrf2 system in cell culturing systems[15]. To further explore the potential mechanism of an anti-oxidative effect of curcumin, we determined whether curcumin stimulates Nrf2 signaling in vivo. We prepared whole lysates of tissues from the three groups of mice and examined the levels of Nrf2 as well as one of its target gene products, HO-1 by western blotting. As shown in Figure 5A, when compared with the control ND mice, HFD feeding reduced Nrf2 level approximately 50% in muscles, whereas curcumin administration significantly attenuated the repressive effect of HFD (Figure 5A). We however, did not see an apparent change in the protein levels of Nrf2 and HO-1 in the liver and the fat tissue by this short-term curcumin treatment (Figure 5C and D).
Consistently, Nrf2 content in the nuclei of skeletal muscle was reduced in HFD mice while curcumin treatment reversed this reduction (Figure 5B). Furthermore, HFD feeding led to a reduced level of HO-1 whereas curcumin significantly reversed this reduction. These results collectively suggest that HFD feeding impairs the function of Nrf2 systemwhile short-term treatment with curcumin significantly activates the Nrf2-ARE signaling pathway in skeletal muscles of HFD-fed mice.
Long-term curcumin administration in mice has been shown to improve insulin signaling and glucose disposal by attenuating inflammation and obesity[17,26]. However, it is not clear whether curcumin could exert its insulin-sensitizing action by anti-oxidative stress. Particularly, very few studies have been conducted to examine the acute effect of curcumin in an already insulin resistant model. We showed here that short-term curcumin gavage improved glucose disposal in a HFD-induced mouse model in the absence of an apparent change of BW. We then observed that short-term curcumin administration ameliorated oxidative stress in both serum and muscles of HFD mice, particularly in muscular mitochondria. Furthermore, we detected that these beneficial effects were accompanied by a reversal of impaired Nrf2 signaling, including increased Nrf2 expression, its nuclear location and the expression of the target antioxidant enzyme, HO-1. We hence suggest that the activation of the major anti-oxidative defense machinery Nrf2 in muscle is a novel mechanism for curcumin in the treatment of insulin resistance and associated metabolic disorders.
In a HFD-induced insulin resistant model, several mechanisms have been proposed to explain the causal role of HFD consumption in the development of insulin insensitivity. Inflammation, ER stress and mitochondrial dysfunction are all reported to be involved[3,6-8]. However, these abnormalities could be the chronic etiology factors of insulin resistance. It is still not known which factor initially induces insulin resistance. It is reasonable to believe that in the condition of nutrition over supply, mitochondrial oxidative phosphorylation metabolism would produce a larger amount ROS and if anti-oxidative machinery could not eliminate the mitochondrial derived oxidants, oxidative stress will occur, resulting in insulin resistance. In support, recent investigations have indicated that muscle mitochondrial derived ROS can acutely impair glucose disposal[27,28] and the administration of mitochondrial specific antioxidant corrects this defect[7,25]. Interestingly, curcumin has an anti-oxidant effect and in vitro studies have indicated its action on triggering Nrf2 signaling[14,15]. We therefore proposed the working hypothesis of this study that short-term curcumin supplementation could improve glucose homeostasis in HFD mice by its anti-oxidative action via Nrf2 activation. Firstly, we examined the redox status in the three groups of mice by MDA assay. We demonstrate here that HFD induces a severe oxidative stress in skeletal muscle especially in mitochondria, while curcumin administration reverses this deleterious effect. The regression analysis on the AUC data of IPGTT and MDA level has further ascertained the relationship of muscle mitochondrial redox status with insulin sensitivity. We hence suggest that muscular mitochondria are initial and important working sites for curcumin in attenuating oxidative stress and improving glucose tolerance. Detailed mechanisms underlying mitochondrial redox status in regulating insulin action are currently unclear. One possibility is that ROS impairs mitochondrial function, followed by reduced lipid oxidation and increased lipid accumulation in muscle. The elevation of muscle lipid content then activates protein kinase C, which blocks insulin receptor substrate-1 phosphorylation and downstream insulin signaling[29]. Further experiments are needed to assess whether curcumin is able to improve insulin signaling via blocking this pathological signaling pathway.
Redox balance is determined by both ROS production and anti-oxidative function (ROS elimination). Recent studies have indicated that an inability of the Nrf2 system in the elimination of ROS leads to the development of insulin resistance, whereas pharmacological or genetic activation of Nrf2 can improve insulin signaling, along with improved glucose metabolism[19,30]. To further investigate the underlying mechanism of curcumin on regulating redox balance, we examined Nrf2 signaling in insulin sensitive tissues. We found that HFD repressed Nrf2 function in muscles by reducing both total Nrf2 content and its nuclear portion, whereas curcumin markedly reversed these defects. Furthermore, curcumin also increased the expression of HO-1, a downstream target of Nrf2 and an essential anti-oxidative enzyme. These data are consistent with the reversal of oxidative stress in muscle but not in fat tissue or the liver. Therefore, these results suggest that curcumin-induced Nrf2 action is a novel mechanism against HFD-induced oxidative stress and to attenuate glucose intolerance. In line with the positive regulation of the Nrf2/HO-1 system on insulin sensitivity, HO-1 has been shown to repress oxidative stress and inflammation[31], and the administration of HO-1 inducer improves insulin sensitivity[32,33]. Due to the tight link of oxidative stress with inflammation[31], further studies should be conducted to address whether the activation of Nrf2 by curcumin is related to its anti-inflammatory effect. It should also be pointed out that although 15 d curcumin supplementation did not reduce the BW in HFD-fed animals, we began to see the effect of curcumin on reducing fat mass. We hence cannot rule out the possibility that curcumin-evoked Nrf2 signaling modulate lipid metabolism, adipogenesis, adipocyte differentiation, indirectly affecting glucose metabolism[17-19].
Curry containing curcumin, a popular natural spice in India, has been used as a traditional drug to treat inflammation, whereas modern studies further demonstrate the potential of curcumin to treat other major life-threatening diseases, such as heart failure, neurodegenerative diseases and diabetes[13,17,18,34]. The relative high safety and low cost of curcumin would also encourage its therapeutic applications in the future. In addition to the preventive effect of curcumin on insulin resistance, we demonstrated here that after the induction of obesity by HFD, short-term curcumin gavage still reversed glucose intolerance, clearly suggesting the therapeutic application of curcumin in the treatment of insulin resistance-related metabolic disorders. In light of its clinical usage, studies have been conducted and verified the effectiveness of curcumin in treating hyperglycemia-induced oxidative stress and related disorders, such as diabetic nephropathy[35,36]. In accordance, the present study provides a significant mechanism of Nrf2 activation by which curcumin can defend oxidative stress and mitochondrial redox imbalance and attenuate the abnormality of glucose metabolism in the condition of nutritional oversupply. Together these findings would place Nrf2 and curcumin as the new therapeutic targets and approaches for the treatment of hyperglycemia, as well as oxidative stress-related diseases.
Curcumin intervention attenuates insulin resistance and improves glucose disposal in various rodent models of insulin resistance and diabetes. The current mechanistic understanding on these beneficial effects is limited although curcumin has been shown to attenuate body weight (BW) gain and inflammatory response during high fat diet consumption. Whether curcumin improves insulin signaling via activating the endogenous nuclear factor erythroid-2-related factor-2 (Nrf2) anti-oxidative stress system and whether the improvement involves the attenuation of mitochondrial oxidative stress are unknown.
The use of a naturally occurring compound in intervention is an optimum approach for the treatment and prevention of diabetes and obesity. Mitochondria are a primary site of production of free radicals. Extensive recent studies have revealed the fundamental importance of mitochondrial oxidative stress in the etiology of insulin resistance. The activation of the major anti-oxidative stress system Nrf2 was shown to improve insulin signaling and glucose disposal in vivo. Curcumin is able to activate Nrf2 signaling in vitro. Here the authors addressed the question whether curcumin, a naturally-occurring compound, improves insulin signaling via attenuating oxidative stress in mitochondria, via activating the Nrf2 signaling pathway.
The study showed here for the first time the stimulatory effect of curcumin in activating endogenous Nrf2 system in vivo. This finding will initiate further investigations on mechanistic exploration of the stimulation of Nrf2 system by naturally-occurring plant compounds.
The authors demonstrated here that curcumin improved insulin signaling and glucose disposal, associated with the attenuation of muscle mitochondrial oxidative stress. This finding defined muscle mitochondria as novel targets for curcumin.
The improvement observed in this animal model is independent of the BW lowering effect of curcumin. The authors have hence not only deepened the mechanistic understanding of the therapeutic effect of curcumin, but also revealed that attenuation of endogenous anti-oxidative stress is an important pathological event of high fat diet consumption.
This study identified a new target for curcumin and other naturally-occurring compounds in improving insulin sensitivity. It further supports the hypothesis that curcumin can be utilized in the treatment and prevention of diabetes and other metabolic disorders that involve insulin resistance.
Insulin resistance is the pathophysiological condition that insulin can not exert normal function on its targeting tissues or cells, including muscle, liver and adipose tissue, or a higher concentration of insulin is required to evoke insulin signaling and glucose uptake. The abnormality is the basis for the development of insulin-resistant diseases, including type 2 diabetes mellitus, hypertension and certain cardiovascular diseases. Nrf2 anti-oxidative system: in the condition of oxidative stress, cytosol Nrf2 will be translocated into the nucleus. It will then bind to a battery of specific target genes in their promoter regions, stimulating the transcription of these genes. Nrf2 target genes encode a series of anti-oxidant enzymes, including HO-1.
The present study showed that short term treatment with curcumin activates Nrf2 signaling and lowers blood glucose levels and oxidative stress in C57BL/6J mice fed on high-fat diet. It has two strengths. Firstly, demonstration of Nrf2 activation and nuclear translocation following curcumin treatment in an animal model of high-fat diet induced obesity and insulin resistance. Secondly, demonstration of reversal of high fat diet-induced malondialdehyde elevations in serum, muscle and muscle mitochondria.
Peer reviewers: Suresh T Mathews, Professor, Department of Nutrition and Food Science, Auburn University, Auburn, AL 36849, United States; Dr. Serap Yalin, Pharmacy Faculty, Mersin University, Mersin 33169, Turkey
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 401] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1182] [Cited by in F6Publishing: 1075] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 4. | Martínez JA. Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J Physiol Biochem. 2006;62:303-306. [PubMed] [Cited in This Article: ] |
| 5. | Laight DW, Desai KM, Gopaul NK, Anggård EE, Carrier MJ. Pro-oxidant challenge in vivo provokes the onset of NIDDM in the insulin resistant obese Zucker rat. Br J Pharmacol. 1999;128:269-271. [PubMed] [Cited in This Article: ] |
| 6. | Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 867] [Cited by in F6Publishing: 882] [Article Influence: 67.8] [Reference Citation Analysis (1)] |
| 7. | Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1803] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 8. | Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809-14818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 323] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1199] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 10. | Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H: quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675-44682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27:999-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 354] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 13. | Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868-878. [PubMed] [Cited in This Article: ] |
| 14. | Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332-347. [PubMed] [Cited in This Article: ] |
| 15. | Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 789] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549-3558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 19. | Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, Fantus IG, Jin T. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia. 2011;54:922-934. [PubMed] [Cited in This Article: ] |
| 20. | Fernández-Vizarra E, López-Pérez MJ, Enriquez JA. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods. 2002;26:292-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Ackerman EJ, Iakoucheva LM. Nucleotide excision repair in oocyte nuclear extracts from Xenopus laevis. Methods. 2000;22:188-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1613] [Cited by in F6Publishing: 1689] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 23. | Turner RC, Holman RR, Matthews D, Hockaday TD, Peto J. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism. 1979;28:1086-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 247] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab. 2003;285:E295-E302. [PubMed] [Cited in This Article: ] |
| 25. | Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789-800. [PubMed] [Cited in This Article: ] |
| 26. | El-Moselhy MA, Taye A, Sharkawi SS, El-Sisi SF, Ahmed AF. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food Chem Toxicol. 2011;49:1129-1140. [PubMed] [Cited in This Article: ] |
| 27. | Bravard A, Bonnard C, Durand A, Chauvin MA, Favier R, Vidal H, Rieusset J. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab. 2011;300:E581-E591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab. 2010;299:E1096-E1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Roden M. Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes (Lond). 2005;29 Suppl 2:S111-S115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625-633. [PubMed] [Cited in This Article: ] |
| 31. | Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 578] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 32. | Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326:196-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365-370. [PubMed] [Cited in This Article: ] |
| 36. | Rema M, Pradeepa R. Diabetic retinopathy: an Indian perspective. Indian J Med Res. 2007;125:297-310. [PubMed] [Cited in This Article: ] |