Revised: December 9, 2011
Accepted: January 9, 2012
Published online: January 15, 2012
Diabetes mellitus and its complications are becoming one of the most important health problems in the world. Diabetic nephropathy is now the main cause of end-stage renal disease. The mechanisms leading to the development and progression of renal injury are not well known. Therefore, it is very important to find new pathogenic pathways to provide opportunities for early diagnosis and targets for novel treatments. At the present time, we know that activation of innate immunity with development of a chronic low grade inflammatory response is a recognized factor in the pathogenesis of diabetic nephropathy. Numerous experimental and clinical studies have shown the participation of different inflammatory molecules and pathways in the pathophysiology of this complication.
- Citation: Luis-Rodríguez D, Martínez-Castelao A, Górriz JL, Álvaro F, Navarro-González JF. Pathophysiological role and therapeutic implications of inflammation in diabetic nephropathy. World J Diabetes 2012; 3(1): 7-18
- URL: https://www.wjgnet.com/1948-9358/full/v3/i1/7.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i1.7
Diabetes mellitus (DM), especially type 2, represents one of the most important health problems worldwide and, according to recent estimations, it is likely to worsen to critical levels in the next decades, with the great concern that this disease is rising rapidly in younger population groups, including children and adolescents[1,2]. According to data from the International Diabetes Federation, the number of diabetics older than twenty will rise from 285 million in 2010 to 439 million in 2030. Therefore, target organ complications secondary to diabetes, especially micro and macro vascular complications will be one of the most important medical concerns in the near future. Because of this, a growing number of researches have focused on diabetes and its complications, with the aim to expand our knowledge about pathogenic and pathophysiological mechanisms, preventive strategies and potential novel therapies.
Diabetic nephropathy (DN) is one of the most relevant diabetic complications. In the last decade, DN has become the main cause of end-stage renal disease (ESRD) in the Western world, with estimations indicating that type 2 diabetes contributes to a great proportion of patients in renal replacement therapy programs[3,4]. However, this situation is starting to change. While in the general population the incidence of ESRD rises continuously due to the increased prevalence of diabetes mellitus, a recent study found that diabetes-related ESRD incidence in the population with diabetes has shown a declining trend, suggesting that current efforts in the prevention of ESRD may be successful[5].
Insulin resistance and relative insulin deficiency play key roles in the development of type 2 diabetes[6,7]. Hyperglycemia occurring as result of these factors is critical in the genesis of diabetic complications. Poor glycemic control has been demonstrated as an independent predictor of the development and progression of DN[8], although the intimate mechanisms by which hyperglycemia leads to renal injury are not completely known.
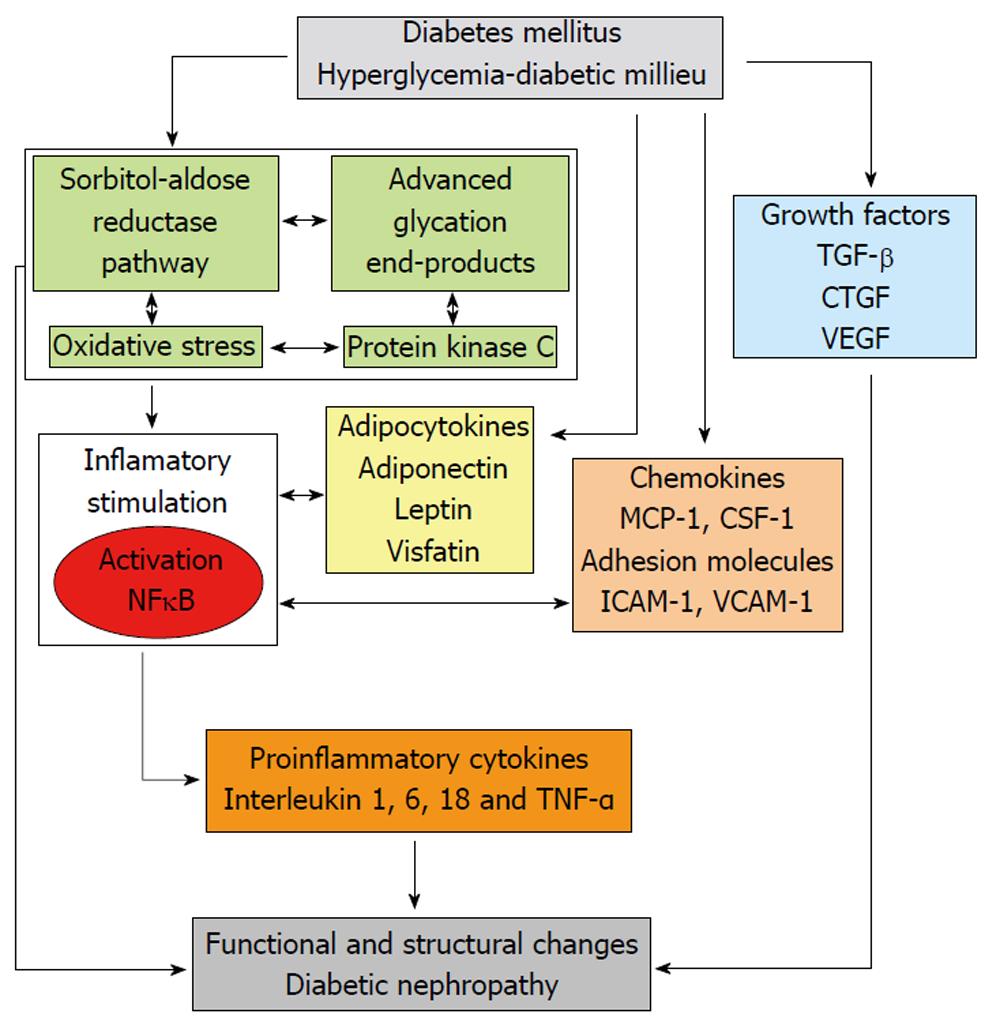
Over the last years, growing evidence supports the role of different enzymes and metabolic pathways in the activation of inflammatory mechanisms involved in the pathophysiology of DN (Figure 1).
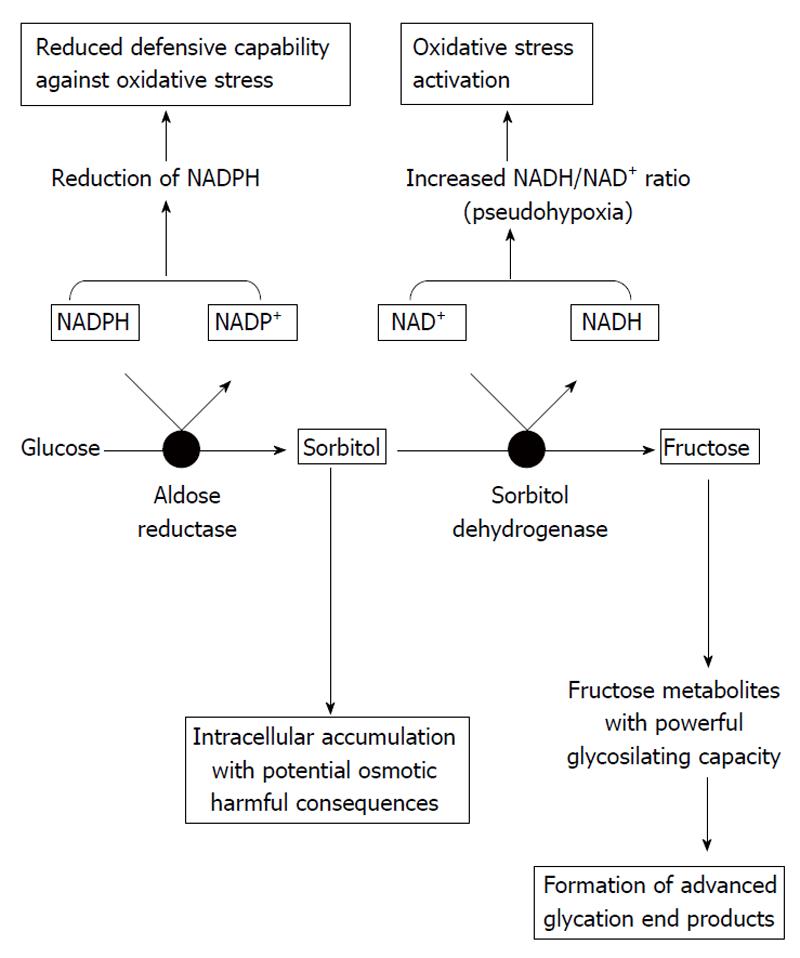
When intracellular glucose levels are increased, the polyol pathway of glucose metabolism becomes active. The first and rate-limiting enzyme in this pathway is aldose reductase, which reduces glucose to sorbitol using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor; sorbitol is then metabolized to fructose by sorbitol dehydrogenase that uses nicotinamide adenine dinucleotide (NAD+) as a cofactor. The affinity of aldose reductase for glucose rises in the hyperglycemic state, causing sorbitol to accumulate and using much more NADPH.
Activation of aldose reductase enzyme itself is able to cause damage, as well as through other mechanisms such as activation of protein kinase C (PKC) and protein glycosylation. In addition, as referred to above, excessive activation of the polyol pathway increases sorbitol and fructose levels. Sorbitol, a strongly hydrophilic alcohol, does not diffuse readily through cell membranes and accumulates intracellularly with potential osmotic harmful consequences. Regarding fructose, this molecule can be phosphorylated to fructose-3-phosphate, which is broken down to 3-deoxyglucosone; both compounds are powerful glycosylating agents that participate in the formation of advanced glycation end products (AGEs).
The excessive usage of NADPH by the overactivated aldose reductase may result in less cofactor available for other processes of cellular metabolism and enzymes; for instance, glutathione reductase, which is critical for the maintenance of the intracellular pool of reduced glutathione. This would lessen the capability of cells to respond to oxidative stress, resulting in enhanced activity of compensatory mechanisms, such as the activity of the glucose monophosphate shunt, the principal supplier of cellular NADPH. On the other hand, the usage of NAD+ by sorbitol dehydrogenase leads to an increased ratio of NADH/NAD+, which has been termed “pseudohypoxia” and linked to a multitude of metabolic and signaling changes known to alter cell function. It has been proposed that the excess of NADH may be a substrate for NADH oxidase, with the subsequent generation of intracellular oxidant species. Thus, activation of the polyol pathway can initiate and multiply cellular damage through different mechanisms, including alteration of intracellular tonicity, generation of AGEs precursors, and exposition to oxidative stress as a result of both reduction of antioxidant defences and generation of oxidant species (Figure 2)[9,10].
Some studies have shown that the inhibition of aldose reductase may have a beneficial effect on proteinuria and glomerular filtration rate. However, some of these drugs have been associated with significant toxicities[11]. Epalrestat, one of these drugs, was evaluated in patients with type 2 diabetes and microalbuminuria. After 5 years of follow-up, the rate of urinary albumin excretion did not change in patients under this therapy, while it increased in control patients who did not receive this treatment[12].
This is an enzyme with different isoforms that phosphorylates various target proteins responsible for intracellular signal transduction involved in regulating vascular function and contractility, flow, cell proliferation and vascular permeability. Activation of PKC results in a myriad of potential harmful effects related to diabetic complications (Table 1)[13].
| Reduction of nitric oxide production |
| Increased endothelin-1, prostaglandin E2 and thromboxane A2 |
| Induction of growth factor expression: Transforming growth factor-β and vascular endothelial growth factor |
| Accumulation of microvascular matrix, fibronectin and type IV collagen |
| Overexpression of fibrinolytic inhibitor plasminogen activator inhibitor-1 |
| Activation of the transcription factor nuclear factor kappa B |
| Increased nicotinamide adenine dinucleotide phosphate oxidase activity |
| Blood-flow abnormalities |
| Alteration of vascular permeability |
| Induction of angiogenesis |
| Organ fibrosis |
| Capillary occlusion |
| Induction of inflammatory mediators |
| Stimulation of oxidative stress |
In cultured vascular cells, elevated glucose concentrations primarily activates the β and δ isoforms of PKC. In the diabetic retina, hyperglycemia persistently activates PKC and p38α mitogen-activated protein kinase (MAPK), resulting in increased expression of SHP-1 (Src homology-2 domain-containing phosphatase-1), a protein tyrosine phosphatase which is a previously unknown target of PKC signaling. This signaling cascade finally results in pericyte apoptosis. The same pathway, activated by increased fatty acid oxidation in insulin-resistant arterial endothelial and cardiac cells, has been suggested to play an important role in diabetic atherosclerosis and cardiomyopathy. In addition, overactivity of PKC has been implicated in the decreased nitric oxide (NO) production in smooth muscle cells and has been shown to inhibit insulin-stimulated expression of endothelial NO synthase in cultured endothelial cells. Activation of PKC by high glucose also induces expression of vascular endothelial growth factor (VEGF) in vascular smooth muscle cells.
Regarding diabetic kidney disease (DKD), a hyperglycemic environment induces increased PKC-β2 activity in renal endothelial cells to produce prostaglandin E2 and thromboxane A2, substances that alter the permeability and the response to angiotensin II of vascular cells. In addition to alterations of blood flow and permeability, activation of PKC contributes to the accumulation of micro vascular matrix protein by inducing expression of transforming growth factor (TGF)-β, fibronectin and type IV collagen in both cultured mesangial cells and in experimental animal models. This effect appears to be mediated by inhibition of NO production. Finally, hyperglycemia-induced activation of PKC has been also implicated in the overexpression of the fibrinolytic inhibitor plasminogen activator inhibitor (PAI)-1 and in the activation of the transcription factor nuclear factor kappa B (NF-κB) in cultured endothelial and vascular smooth muscle cells[14].
Ruboxistaurin is a specific inhibitor of the β isoform of PKC. In a variety of experimental models of DKD, ruboxistaurin normalized glomerular hyperfiltration, decreased urinary albumin excretion, preserved kidney function and was associated with structural benefits, including reduction of mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis. Regarding clinical studies, initial works of PKC-β inhibition in type 2 diabetic patients with DN under treatment with angiotensin converting enzyme inhibitors (ACEi) and/or angiotensin receptor blockers (ARB) showed a reduction of urinary albumin excretion and stabilization of the glomerular filtration rate. In addition, secondary analyses of clinical trials in patients with diabetic retinopathy or neuropathy have suggested that ruboxistaurin appears safe and may also prevent the onset of DKD[15].
Nonenzymatic protein glycation by glucose is a complex cascade of reactions yielding a heterogeneous class of compounds, collectively called AGEs. This process begins with the Maillard reaction, by which the carbonyl group (aldehyde or ketone) of the reducing sugar reacts with the amino group of the biomolecule to form a reversible Schiff base. After this initial process, the Schiff bases can undergo an intramolecular rearrangement to form the Amadori products, which finally form irreversible AGEs. When AGEs are formed at critical sites in enzymes or proteins, they may alter the structure and function of these molecules in plasma, as well as in the arterial wall, mesangium and glomerular basement membranes. AGEs can elicit their effects via ligation to specific receptors (RAGEs) on different cells, including podocytes, endothelial and smooth muscle cells, as well as mesangial and tubular epithelial cells. The AGE-RAGE interaction determines the activation of intracellular signaling pathways leading to diverse consequences, including generation of reactive oxygen species (ROS), release of inflammatory cytokines such as tumor necrosis factor alpha (TNF α) and interleukin (IL)-1 and 6, activation of transcription factors such as NF-κB, and expression of adhesion molecules and growth factors like connective tissue growth factor (CTGF) or TGF-β[16-19].
AGEs represent a potential target in the treatment of DN. This therapeutic strategy includes different possibilities, from inhibition of AGE formation to blockade of their receptors[20,21]. Aminoguanidine, one of the earliest identified inhibitors of AGE-formation, reduces AGE accumulation by scavenging free reactive carbonyl groups. In addition, different agents widely used in diabetic patients, including aspirin, metformin, ACEi and ARB, have also demonstrated the capacity to decrease AGE accumulation. Another therapeutic approach is based on cross-links cleavage within and between tissue proteins and other organic compounds. Thus, new agents such as N-phenacylthiazolium bromide and alagebrium chloride allow the clearance of glycated proteins via scavenger receptors and subsequent renal excretion. Finally, experimental studies have shown that it is possible to block the binding of AGEs to their specific receptor with soluble forms of RAGE (produced either from alternative gene splicing or by proteolysis) or by using neutralizing RAGE antibodies. These therapeutic approaches based on anti-AGE actions are associated with beneficial effects, such as reduction of cellular oxidative stress or modulation of inflammation.
Oxidative stress is caused by an imbalance between the production of oxidants or ROS and the capacity of a biological system to readily detoxify the reactive intermediates or repair the resulting damage. The final result is the oxidation of important macromolecules, including proteins, lipids, carbohydrates and DNA. Growing evidence indicates that oxidative stress plays a pivotal role in the development of both micro and macro vascular diabetic complications[22].
ROS include free radicals such as superoxide, hydroxyl and peroxyl, and nonradical species such as hydrogen peroxide. It is important to note that there is also a reactive nitrogen species produced from similar pathways, which include the radicals nitric oxide and nitrogen dioxide, as well as the nonradical peroxynitrite. There are a number of enzymatic and nonenzymatic sources of ROS in the diabetic kidney, including autoxidation of glucose, advanced glycation, polyol pathway flux, mitochondrial respiratory chain deficiencies, xanthine oxidase activity, peroxidases and NAD(P)H oxidase. Importantly, a direct relationship has been demonstrated between the severity of renal injury and the degree of oxidative stress in DN. Moreover, histological studies have shown the presence of glyco- and lipo-oxidation products in the mesangial matrix and nodular lesions of DN[23,24].
Growing evidence indicates that metabolic abnormalities in diabetes lead to mitochondrial superoxide production, which results in the activation of major biochemical harmful pathways, including increased AGE formation, activation of protein kinase C, increased flux through the polyol pathway, and overactivity of the hexosamine pathway, each of which, in addition, can initiate and/or perpetuate cellular ROS generation[23,24].
The ability of individual cell types to process glucose is the most important factor in the excessive intracellular generation of ROS induced by hyperglycemia. Thus, the control of glucose influx into the cytosol in presence of elevated glucose concentrations is critical in order to maintain an adequate intracellular glucose homeostasis. However, certain renal cell populations, such as endothelial, mesangial, epithelial and tubular cells, are particularly susceptible since they are unable to decrease glucose transport rates adequately. Therefore, intensive glycemic control and interventions that decrease cellular glucose uptake may limit ROS generation in the diabetic kidney[25].
In addition to approaches aimed to reduce ROS production, a critical factor to avoid oxidative damage is the adequate function of endogenous antioxidant systems, including free radical scavengers and enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase. A reduction in expression and activity of these antioxidant enzymes have been reported in diabetic micro vascular disease. Importantly, overexpression of SOD or catalase protects against end organ damage in models of DN[24]. Finally, the therapeutic use of antioxidants might be a useful approach. However, conventional antioxidants are unlikely to be effective because these compounds neutralize reactive oxygen molecules on a one-for-one basis, whereas hyperglycemia-induced overproduction of superoxide is a continuous process. On the contrary, based on the beneficial effects of antioxidant enzymes overexpression, the catalytic antioxidant SOD/catalase mimetic has demonstrated beneficial effects in experimental models[22,23,24].
Extracellular matrix (ECM) comprises an insoluble network of different molecules (glycoproteins, elastins, collagens) that provides mechanical support for renal cells and participates in cell-cell interactions as well as between cells and other elements[25]. ECM abnormalities are a structural hallmark in DN and fibrosis, characterized by ECM accumulation and angiogenesis, and is a critical pathophysiological process directly related to renal function alterations and renal disease progression. Growth factors are molecules participating in the regulation of ECM formation and turn-over, with evidence of their important role in the pathogenesis of diabetic long-term complications.
Transforming growth factor-β: TGF-β is considered the main pro-fibrotic factor in DN, playing a critical role in the hypertrophic process and fibrotic/sclerotic manifestations, including glomerulosclerosis and interstitial fibrosis. Multiple mediators in the diabetic environment converge to up-regulate TGF-β in the diabetic kidney[26,27]. TGF-β is crucial in the induction and maintenance of interstitial fibrosis due to its regulatory effect on cell proliferation and the synthesis and degradation of the extracellular matrix. In addition, TGF-β promotes the increased production of extracellular matrix components, as well as mesenchymal cell proliferation, migration and accumulation following inflammatory responses[28].
Specific inhibition of TGF-β with neutralizing antibodies or TGF-β1 antisense oligodeoxynucleotides in experimental models of DN have resulted in the prevention of glomerular enlargement and the attenuation of the excess matrix expression[28,29].
Connective tissue growth factor: CTGF is a mediator of TGF-β effects, promoting glomerular damage through increased production of extracellular matrix proteins and induction of changes in cytoskeletal structure. However, CTGF is able to mediate profibrotic activity directly and also participates in processes related to cell proliferation, migration and differentiation. CTGF also has the potential to modulate other factors such as VEGF and bone morphogenic proteins, which are involved in the repair process inherent to renal fibrogenesis[30,31].
Down-regulation of CTFG expression in murine models of diabetes is associated with a reduction in mesangial matrix expansion and the components involved in glomerulosclerosis and interstitial fibrosis, with a lesser degree of kidney disease[32].
Vascular endothelial growth factor: The primary function of the VEGF is to maintain the integrity and viability of the endothelium throughout diverse actions, including promotion of endothelial cell proliferation, differentiation and survival, participation in interstitial matrix remodeling, and mediation of endothelium-dependent vasodilatation.
In the kidney, VEGF expression is most prominent in podocytes and tubular epithelial cells, while VEGF receptors are mainly found on endothelial cells. It has recently been proved that VEGF participates in processes of neovascularization and glomerulosclerosis[33,34] and growing evidence highlights the relevance of VEGF in the pathogenesis of DN. Different studies suggest that podocyte-derived VEGF is directly involved in the glomerular capillary hyperpermeability of macromolecules[35]. VEGF expression is significantly increased in the diabetic state and stimulation of VEGF secretion by podocytes can affect blood flow, glomerular endothelial cell function and also have an autocrine effect, altering podocyte synthesis of glomerular basement membranes constituents and foot processes[36,37].
From a therapeutical perspective, animal studies using inhibition of VEGF activity by neutralizing antibodies or small molecule inhibitors of VEGF receptor kinase signaling have demonstrated a marked amelioration of albuminuria in the diabetes setting[38-40].
Adipocytokines are cell-to-cell bioactive peptides secreted by the adipose tissue that act locally and distally through autocrine, paracrine and endocrine effects. These molecules have been related to multiple functions as well as to pathophysiological processes.
Adiponectin: The most abundant adipocytokine in the human plasma is adiponectin, which has been inversely correlated with body mass index and insulin resistance[41]. The role of this molecule has been highlighted in the pathogenesis of obesity-related illnesses, including Type 2 DM, because it is an important factor in the regulation of insulin sensitivity as well as vascular endothelial function[42]. Adiponectin has been implicated in the functions of endothelial and inflammatory cells, including preservation of endothelial NO by directly shifting the balance between this molecule and ROS generation in a direction favorable to NO availability. Adiponectin acts through its receptors Adipo-R1 and Adipo-R2, with participation of diverse downstream signaling mechanisms including the AMP-activated protein kinase (AMPK) and the cAMP-dependent protein kinase (cAMP/PKA) pathways.
Experimental studies have shown that adiponectin improves insulin sensitivity through the stimulation of glucose utilization and fatty acid oxidation in skeletal muscles and liver, facilitating glucose uptake and deleting hepatic gluconeogenesis[43,44]. A negative relationship between systemic oxidative stress and circulating adiponectin levels has been shown in rodent models of obesity and the metabolic syndrome[45]. Additionally, adiponectin suppresses inflammatory changes in endothelial cells induced by TNF-α. Finally, the accumulation of this adipocytokine in the injured kidney has been described, which is able to prevent glomerular and tubulointerstitial injury through modulating inflammation and oxidative stress[46].
In the clinical setting, the interrelationships between adiponectin, inflammation and renal damage are controversial. Thus, reduced levels of adiponectin have been associated with elevated concentrations of inflammatory parameters, such as C-reactive protein (CRP) and IL-6, and correlated with metabolic syndrome[47]. However, in type 1 diabetic patients, plasma adiponectin concentrations are found to be significantly elevated compared to healthy controls[48,49] and, moreover, DN and progression to ESRD are found to be associated with higher serum adiponectin levels in these patients[50,51]. On the contrary, reduced adiponectin levels have been reported in patients with type 2 diabetes in the basal state with impaired utilization of adiponectin in the coronary artery and/or the heart, which has been suggested as a promoting factor for the development of atherosclerosis[52,53]. In addition, genetic variability in the adiponectin gene has been related to plasma adiponectin isoforms and the risk of DN[54,55].
From a therapeutic perspective, both non-pharmacological strategies, such as weight reduction and lifestyle modifications[56-59], as well as pharmacological interventions, treatment with thiazolidinediones[60-65], blockade of the renin-angiotensin system with ACEI or ARB[66-71], clonidine-like sympatho-inhibitory antihypertensive agents[72], fenofibrate[73] and the cannabinoid-1 receptor blocker rimonabant[74], have been demonstrated to be able to enhance adiponectin levels, which might provide a scientific rationale for the use of these drugs in order to increase plasma adiponectin concentrations in high-risk populations.
Leptin: Leptin plays a key role in regulating energy intake and expenditure. In addition, this molecule has been related to diverse potentially pro-inflammatory effects, such as impairment of endothelial function, stimulation of inflammatory signaling pathways, increase in oxidative stress, stimulation of platelet aggregation and migration, and stimulation of hypertrophy and proliferation of vascular smooth muscle cells.
Leptin levels have been reported to be increased in both type 1 and type 2 diabetic patients with microalbuminuria or macroalbuminuria[75,76]. In this context, direct and indirect renal effects of leptin could be relevant for the development and progression of nephropathy. Endothelial cells express leptin receptors, showing an increased ROS production in response to leptin stimulation. In addition, leptin stimulates the proliferation of glomerular endothelial cells, increases TGF-β1 synthesis and collagen type IV production. It also stimulates hypertrophy in cultured rat mesangial cells and, moreover, infusion of leptin into normal rats promotes the development of glomerulosclerosis and proteinuria[77].
Visfatin: Visfatin is preferentially produced by visceral fat and promotes B cell maturation and inhibits neutrophil apoptosis.
Recent investigations suggest the participation of visfatin in the pathogenesis of DN. Experimental in vivo and in vitro studies have shown that visfatin is produced by renal cells (podocytes, mesangial and proximal tubular cells) and diabetic animals produce higher levels of this adipocytokine. In these models, plasma visfatin levels are elevated in the early stages of diabetes, which are positively correlated with body weight, fasting plasma glucose and microalbuminuria[78,79]. In vitro studies have demonstrated that visfatin treatment of cultured cells resulted in the activation of downstream signaling pathways (including Erk-1, Akt and p38 MAPK) and increased NF-κB transcriptional activity, leading to a marked increment in the synthesis of profibrotic factors such as TGF-β1, plasminogen activator inhibitor-1 and type I collagen[78-80].
From a clinical perspective, plasma visfatin levels are significantly increased in diabetic patients compared to control subjects, showing a positive correlation with systolic blood pressure, body weight, fasting glucose, plasma level of monocyte chemoattractant protein-1 (MCP-1) and urine albumin excretion (UAE)[80].
Monocyte-specific chemokines attract macrophages to tissues, migrating from vessels following an endothelial gradient[81]. Experimental studies have demonstrated that kidney macrophage accumulation is associated with renal damage in DN.
The most potent chemokine factor for monocytes is the MCP-1, an essential molecule involved in monocyte traffic across endo and epithelial barriers[82]. It has been demonstrated that MCP-1-mediated macrophage accumulation and activation is a critical mechanism in the development of early DN in animal models[83]. In addition, MCP-1 binding to the MCP-1 receptor 2 (CCR2) is able to induce a significant reduction in nephrin (both mRNA and protein expression) via a Rho-dependent mechanism in podocytes[84]. In streptozotocin-treated mice, MCP-1 was overexpressed within the glomeruli and the absence of MCP-1 reduced both albuminuria and downregulation of nephrin[84]. In clinical studies, upregulation of kidney MCP-1 is associated with macrophage recruitment, albuminuria, tubulointerstitial damage and disease progression[85-88], indicating that blockade of MCP-1 activity should be considered as a therapeutic target in the treatment of DN.
Blockade of the CCR2 pathway ameliorated glomerulosclerosis in experimental studies[89]. Others works in type 2 diabetic patients with DN have shown a reduction of urinary MCP-1 levels as well as an improvement in renal function after blockade of the renin-angiotensin-aldosterone system by ACEi or aldosterone[90-92]. More recent studies have shown that vitamin D analogues can inhibit the synthesis and activity of MCP-1 and ameliorate glomerular injury in diabetes[93].
Another molecule involved in chemoattraction is the colony-stimulating factor (CSF)-1, which is produced in diabetic kidneys and promotes macrophage accumulation, activation and survival. CSF-1 acts exclusively through the c-fms receptor, which is only expressed on cells of the monocyte-macrophage lineage. One experimental study has demonstrated that blockade of c-fms can suppress the progression of established DN in db/db mice[94].
Cell adhesion molecules are proteins located on the cell surface involved in the binding with other cells or with the extracellular matrix. These proteins are typically transmembrane receptors composed of three domains: an intracellular domain that interacts with the cytoskeleton, a transmembrane domain, and an extracellular domain that interacts either with other adhesion molecules of the same kind (homophilic binding) or different kind or the extracellular matrix (heterophilic binding). Four protein groups are the most important families of cell adhesion molecules: the immunoglobulin superfamily, the integrins, the cadherins and the selectins.
Intercellular adhesion molecule-1: Intercellular adhesion molecule-1 (ICAM-1), also known as CD54, is an endothelial and leukocyte associated transmembrane protein with relevance in stabilizing cell-cell interactions and facilitating leukocyte endothelial transmigration. It is constitutively present in the membranes of leukocytes and endothelial cells; upon cytokine stimulation, the concentrations greatly increase. ICAM-1 ligation produces proinflammatory effects such as inflammatory leukocyte recruitment by signaling through cascades involving a number of kinases[95].
This adhesion molecule is involved in the pathogenesis of diabetic kidney disease[96,97]. Renal expression of ICAM-1 is elevated in DN and has been associated with progression of renal damage[98,99]. Regarding clinical studies, several studies have shown that plasma concentrations of ICAM-1 are increased in both type 1 and type 2 diabetic patients with DN[100,101].
It has been suggested that modulation of ICAM-1 activity (blockade of receptor activation or reduction of expression) may be a therapeutic approach in DN. In a recent experimental study, colchicine administration to streptozotocin-induced diabetic rats significantly reduced UAE, inflammatory cell infiltration and extracellular matrix accumulation. These beneficial effects were associated with inhibition of inflammatory molecules expression in the renal tissue, including ICAM-1[102].
Vascular cell adhesion molecule-1: Vascular cell adhesion molecule-1 (VCAM-1) mediates the adhesion of lymphocytes, monocytes, eosinophils and basophils to vascular endothelium. It also functions in leukocyte-endothelial cell signal transduction and has been suggested to play a role in the development of several pathological processes, including atherosclerosis, rheumatoid arthritis and DN.
Experimental works in diabetic mice demonstrated an increased expression of VCAM-1 by endothelial as well as by infiltrating cells in the renal interstitium[103]. Studies in subjects with DN have shown elevated circulating concentrations of VCAM-1[104,105]. Prospective studies in type 2 diabetic subjects have shown that markers of endothelial dysfunction and inflammatory activity were related to elevated UAE during follow-up and, furthermore, elevated plasma levels of soluble VCAM-1 and CRP were associated with an increased risk of death[105].
Inflammatory cytokines are key molecules involved in the inflammatory process, playing a significant role as regulators as well as final effectors. Importantly, they have been involved in the pathogenesis of several diseases, including DN (Table 2).
| Increase expression and synthesis of chemokines, adhesion molecules, transcription factors, cytokines, growth factors and mediators of inflammation |
| Alteration of synthesis of prostaglandins and hyaluronan |
| Stimulation of oxidative stress |
| Induction of intraglomerular hemodynamic abnormalities |
| Increase of vascular endothelial cell permeability |
| Induction of cell proliferation and contraction, and inhibition of endothelium relaxation |
| Increase fibronectin expression |
| Induction of cell apoptosis and necrosis |
| Induction of glomerular hypertrophy |
| Stimulation of plasminogen activator inhibitor-1 production |
| Reduction of tissue factor inhibitor and thrombomodulin expression |
| Stimulation of inflammatory cells recruitment and activation |
| Induction of major histocompatibility complex antigen expression |
Interleukins: Macrophages incubated with glomerular basement membranes from diabetic rats produced significantly greater concentrations of IL-1 and TNF-α than macrophages incubated with membranes from normal animals, suggesting the potential role of inflammatory cytokines as pathogenic factors in DN for the first time[106]. Later studies have demonstrated that renal cells (endothelial, mesangial, glomerular and tubular epithelial cells) are able to synthesize inflammatory cytokines[107-109].
IL- 1 and IL-6 are up-regulated in the diabetic kidney[110,111]. IL-1 has been related to increased endothelial permeability, proliferation of mesangial cells and matrix synthesis, and intraglomerular hemodynamic abnormalities secondary to alteration of prostaglandin production[112,113]. IL-6 stimulates proliferation of mesangial cells, increases fibronectin expression, affects extracellular matrix dynamics, and enhances endothelial permeability[114,115]. Elevated concentrations of IL-6 have been reported in type 2 diabetic patients and overt nephropathy, with a direct association between membrane thickening and IL-6. In addition, IL-6 has been related to the progression of renal disease[116].
IL-18 is an inflammatory cytokine recently involved in the pathogenesis of DN. Type 2 diabetic patients show elevated levels of IL-18, which were related to the development of increased urinary albumin excretion[117]. Later studies confirmed the independent and significant association between albuminuria and both serum and urinary IL-18 concentration and, moreover, these levels correlated positively with changes in urinary albumin excretion[118]. Finally, high levels of IL-18 have been suggested as a significant predictor of early renal dysfunction in type 2 diabetes[119].
Tumor necrosis factor-α
Diverse cells, including monocytes, macrophages and intrinsic renal cells, are also able to synthesize this inflammatory cytokine[108,109,112,120]. Experimental studies have demonstrated that mRNA expression levels for TNF-α are increased by approximately 2.5-fold in the renal cortex of diabetic rats compared with the expression observed in normal rats[121]. This is a relevant finding since this cytokine has a variety of bioactivities that may promote renal injury[122,123], including intraglomerular blood flow dysregulation[124] and abnormalities of the glomerular barrier function due to the induction of local generation of ROS[125].
Urinary albumin excretion significantly correlates with renal cortical expression levels and urinary TNF-α excretion[121] and, moreover, elevated urinary TNF-α concentrations and increased TNF-α levels in the renal interstitial fluid preceded the increase in urinary albumin excretion[126]. Clinical studies show similar results, with a direct and significant association between serum TNF-α and urinary protein excretion in diabetic patients with normal renal function and microalbuminuria, as well as in subjects with overt nephropathy and renal failure[127,128].
Importantly, modulation of TNF-α activity may have an important therapeutic strategy for DN. Experimental studies have shown that administration of infliximab (a chimeric anti-TNF-α antibody), etanercept (a soluble TNF-α receptor) or pentoxifylline (a phosphodiesterase inhibitor able to inhibit TNF-α mRNA accumulation and the transcription of the TNF-α gene) to diabetic rats were associated with reduction of albuminuria and urinary TNF-α excretion[127,129-133]. Moreover, clinical trials have shown that pentoxifylline reduces urinary protein excretion in diabetic patients with normal renal function as well as in those with renal insufficiency[128,134,135]. In addition, combination of pentoxifylline and renin-angiotensin system blockers shows an additive antiproteinuric effect[136,137].
Significant advances have been made in recent years in relation to the pathogenesis of diabetic nephropathy, which have recognized the involvement of inflammation in the development and progression of renal damage in diabetic patients. This has expanded the state of knowledge very significantly of one of the most important complications of diabetes, allowing identification of molecules and signaling pathways involved in the genesis and evolution of renal damage in this context. Understanding these processes is a key factor to enable the identification of new therapeutic targets for the treatment of this complication. In fact, there are clinical studies (so far based on the inhibition of TNF-α) demonstrating that inhibition of inflammatory factors is a strategy that can realistically be applied to patients. However, we are still unable to understand globally how these inflammatory pathways interact with each other or how they interact with other pathogenic factors. Moreover, once new potential therapeutic targets of interest are identified, it is necessary to develop molecules that inhibit these inflammatory factors or pathways and conduct studies from the bench to the bedside that provide new strategies for the treatment of diabetic nephropathy.
Recent studies in the last decade have shown that inflammation is a key process in the development of diabetes mellitus and diabetic complications. Different inflammatory molecules and pathways are involved in the pathogenesis and progression of DN. The growing knowledge and better understanding of the role of the inflammatory processes in the context of DN will represent an important therapeutic opportunity for the development of new strategies that can be translated successfully into clinical applications for the treatment of this complication.
Peer reviewers: Chi-Feng Liu, Associate Professor, National Taipei University of Nursing and Sciences, NO. 365, ming Te Road, Peitou, Taipei 11211, Taiwan, China; Nikolaos Papanas, Dr., Second Department of Internal Medicine, Democritus University of Thrace, Alexandroupolis 68100, Greece; Armin Rashidi, Dr., Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23507, United States
S- Editor Wu X L- Editor Roemmele A E- Editor Zhang DN
| 1. | Vivian EM. Type 2 diabetes in children and adolescents--the next epidemic? Curr Med Res Opin. 2006;22:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23:381-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 824] [Cited by in F6Publishing: 728] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 3. | Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 527] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 4. | Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005;S14-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care. 2010;33:73-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1582] [Cited by in F6Publishing: 1490] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 7. | Beck-Nielsen H, Groop LC. Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:1714-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 217] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Iseki K, Ikemiya Y, Kinjo K, Iseki C, Takishita S. Prevalence of high fasting plasma glucose and risk of developing end-stage renal disease in screened subjects in Okinawa, Japan. Clin Exp Nephrol. 2004;8:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233-S236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Passariello N, Sepe J, Marrazzo G, De Cicco A, Peluso A, Pisano MC, Sgambato S, Tesauro P, D'Onofrio F. Effect of aldose reductase inhibitor (tolrestat) on urinary albumin excretion rate and glomerular filtration rate in IDDM subjects with nephropathy. Diabetes Care. 1993;16:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001;15:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 14. | Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med. 2001;18:945-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Tuttle KR. Protein kinase C-beta inhibition for diabetic kidney disease. Diabetes Res Clin Pract. 2008;82 Suppl 1:S70-S74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Shimoike T, Inoguchi T, Umeda F, Nawata H, Kawano K, Ochi H. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metabolism. 2000;49:1030-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Sourris KC, Harcourt BE, Forbes JM. A new perspective on therapeutic inhibition of advanced glycation in diabetic microvascular complications: common downstream endpoints achieved through disparate therapeutic approaches? Am J Nephrol. 2009;30:323-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2969] [Cited by in F6Publishing: 3273] [Article Influence: 233.8] [Reference Citation Analysis (0)] |
| 23. | Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:146-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 815] [Cited by in F6Publishing: 812] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 25. | Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol. 2005;4:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575-596. [PubMed] [Cited in This Article: ] |
| 27. | Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 Suppl 1:S38-S41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746-756. [PubMed] [Cited in This Article: ] |
| 29. | Wang A, Ziyadeh FN, Lee EY, Pyagay PE, Sung SH, Sheardown SA, Laping NJ, Chen S. Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol. 2007;293:F1657-F1665. [PubMed] [Cited in This Article: ] |
| 30. | Schmidt-Ott KM. Unraveling the role of connective tissue growth factor in diabetic nephropathy. Kidney Int. 2008;73:375-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 589] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 32. | Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355-3368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol. 2007;292:F1665-F1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 432] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Chen S, Kasama Y, Lee JS, Jim B, Marin M, Ziyadeh FN. Podocyte-derived vascular endothelial growth factor mediates the stimulation of alpha3(IV) collagen production by transforming growth factor-beta1 in mouse podocytes. Diabetes. 2004;53:2939-2949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993-1000. [PubMed] [Cited in This Article: ] |
| 39. | Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090-3094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093-3104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3510] [Cited by in F6Publishing: 3418] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 42. | Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784-1792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1982] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 43. | Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 450] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 45. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [PubMed] [Cited in This Article: ] |
| 46. | Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:1910-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Valle M, Martos R, Gascón F, Cañete R, Zafra MA, Morales R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005;31:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De Cobelli F, Battezzati A, Secchi A, Del Maschio A, Luzi L. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1174-E1181. [PubMed] [Cited in This Article: ] |
| 49. | Imagawa A, Funahashi T, Nakamura T, Moriwaki M, Tanaka S, Nishizawa H, Sayama K, Uno S, Iwahashi H, Yamagata K. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25:1665-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Lindström T, Frystyk J, Hedman CA, Flyvbjerg A, Arnqvist HJ. Elevated circulating adiponectin in type 1 diabetes is associated with long diabetes duration. Clin Endocrinol (Oxf). 2006;65:776-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Saraheimo M, Forsblom C, Fagerudd J, Teppo AM, Pettersson-Fernholm K, Frystyk J, Flyvbjerg A, Groop PH. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005;28:1410-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Furuhashi M, Ura N, Moniwa N, Shinshi Y, Kouzu H, Nishihara M, Kokubu N, Takahashi T, Sakamoto K, Hayashi M. Possible impairment of transcardiac utilization of adiponectin in patients with type 2 diabetes. Diabetes Care. 2004;27:2217-2221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Takano H, Kodama Y, Kitta Y, Nakamura T, Obata JE, Mende A, Kawabata K, Saitoh Y, Fujioka D, Kobayashi T. Transcardiac adiponectin gradient is independently related to endothelial vasomotor function in large and resistance coronary arteries in humans. Am J Physiol Heart Circ Physiol. 2006;291:H2641-H2646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Jaziri R, Aubert R, Roussel R, Emery N, Maimaitiming S, Bellili N, Miot A, Saulnier PJ, Travert F, Hadjadj S. Association of ADIPOQ genetic variants and plasma adiponectin isoforms with the risk of incident renal events in type 2 diabetes. Nephrol Dial Transplant. 2010;25:2231-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Zhang D, Ma J, Brismar K, Efendic S, Gu HF. A single nucleotide polymorphism alters the sequence of SP1 binding site in the adiponectin promoter region and is associated with diabetic nephropathy among type 1 diabetic patients in the Genetics of Kidneys in Diabetes Study. J Diabetes Complications. 2009;23:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815-3819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 57. | Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1081] [Cited by in F6Publishing: 1010] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 58. | Valsamakis G, McTernan PG, Chetty R, Al Daghri N, Field A, Hanif W, Barnett AH, Kumar S. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism. 2004;53:430-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, Möhlig M, Pfeiffer AF, Spranger J. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712-2719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 60. | Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968-2974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 553] [Cited by in F6Publishing: 540] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 61. | Hirose H, Kawai T, Yamamoto Y, Taniyama M, Tomita M, Matsubara K, Okazaki Y, Ishii T, Oguma Y, Takei I. Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism. 2002;51:314-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, Baxi S, Mudaliar SR, Henry RR. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, Wang JP, Chen CL, Tai TY, Chuang LM. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 64. | Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152-12162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 840] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 66. | Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 67. | Koh KK, Quon MJ, Han SH, Ahn JY, Jin DK, Kim HS, Kim DS, Shin EK. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005;45:1088-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687-3692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 69. | Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108:96-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Shmieder R, Schlaich M, Mimran A, Fauvel JP, Ruilope LM. The cytokine adiponectin is disparately influenced by telmisartan and ramipril in type 2 diabetes. J Hypertens. 2006;24 Suppl 6:225. [Cited in This Article: ] |
| 71. | Fogari R, Derosa G, Mugellini A. Effect of valsartan and eprosartan on adiponectin, leptin and insulin sensitivity in hypertensive obese patients. J Hypertens. 2006;24 Suppl 6:258. [Cited in This Article: ] |
| 72. | Nowak Ł, Adamczak M, Wiecek A. Blockade of sympathetic nervous system activity by rilmenidine increases plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2005;18:1470-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Choi IS, Shin EK. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 74. | Després JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1062] [Cited by in F6Publishing: 946] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 75. | Rudberg S, Persson B. Serum leptin levels in young females with insulin-dependent diabetes and the relationship to hyperandrogenicity and microalbuminuria. Horm Res. 1998;50:297-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Fruehwald-Schultes B, Kern W, Beyer J, Forst T, Pfützner A, Peters A. Elevated serum leptin concentrations in type 2 diabetic patients with microalbuminuria and macroalbuminuria. Metabolism. 1999;48:1290-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS, Han JY, Han SY, Han KH, Kim HK. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1485-F1494. [PubMed] [Cited in This Article: ] |
| 79. | Kang YS, Song HK, Lee MH, Ko GJ, Han JY, Han SY, Han KH, Kim HK, Cha DR. Visfatin is upregulated in type-2 diabetic rats and targets renal cells. Kidney Int. 2010;78:170-181. [PubMed] [Cited in This Article: ] |
| 80. | Kang YS, Song HK, Lee MH, Ko GJ, Cha DR. Plasma concentration of visfatin is a new surrogate marker of systemic inflammation in type 2 diabetic patients. Diabetes Res Clin Pract. 2010;89:141-149. [PubMed] [Cited in This Article: ] |
| 81. | Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 405] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 82. | Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 83. | Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 84. | Tarabra E, Giunti S, Barutta F, Salvidio G, Burt D, Deferrari G, Gambino R, Vergola D, Pinach S, Perin PC. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes. 2009;58:2109-2118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, Koizumi M, Funabiki K, Horikoshi S, Shirato I. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Chiarelli F, Cipollone F, Mohn A, Marini M, Iezzi A, Fazia M, Tumini S, De Cesare D, Pomilio M, Pierdomenico SD. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care. 2002;25:1829-1834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 88. | Takebayashi K, Matsumoto S, Aso Y, Inukai T. Association between circulating monocyte chemoattractant protein-1 and urinary albumin excretion in nonobese Type 2 diabetic patients. J Diabetes Complications. 2006;20:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, Abe H, Iehara N, Fukatsu A, Okamoto H. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 90. | Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26:2421-2425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 91. | Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17:1362-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. J Clin Endocrinol Metab. 2006;91:2214-2217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Lim AK, Ma FY, Nikolic-Paterson DJ, Thomas MC, Hurst LA, Tesch GH. Antibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db mice. Diabetologia. 2009;52:1669-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52:925-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 695] [Cited by in F6Publishing: 715] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 96. | Okada S, Shikata K, Matsuda M, Ogawa D, Usui H, Kido Y, Nagase R, Wada J, Shikata Y, Makino H. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes. 2003;52:2586-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 97. | Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 98. | Matsui H, Suzuki M, Tsukuda R, Iida K, Miyasaka M, Ikeda H. Expression of ICAM-1 on glomeruli is associated with progression of diabetic nephropathy in a genetically obese diabetic rat, Wistar fatty. Diabetes Res Clin Pract. 1996;32:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 100. | Clausen P, Jacobsen P, Rossing K, Jensen JS, Parving HH, Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with Type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17:644-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Güler S, Cakir B, Demirbas B, Yönem A, Odabasi E, Onde U, Aykut O, Gürsoy G. Plasma soluble intercellular adhesion molecule 1 levels are increased in type 2 diabetic patients with nephropathy. Horm Res. 2002;58:67-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, Kim SH, Han SH, Lee JE, Moon SJ. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F200-F209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Ina K, Kitamura H, Okeda T, Nagai K, Liu ZY, Matsuda M, Fujikura Y. Vascular cell adhesion molecule-1 expression in the renal interstitium of diabetic KKAy mice. Diabetes Res Clin Pract. 1999;44:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Lim SC, Caballero AE, Smakowski P, LoGerfo FW, Horton ES, Veves A. Soluble intercellular adhesion molecule, vascular cell adhesion molecule, and impaired microvascular reactivity are early markers of vasculopathy in type 2 diabetic individuals without microalbuminuria. Diabetes Care. 1999;22:1865-1870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 480] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 106. | Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Hasegawa G, Nakano K, Kondo M. Role of TNF and IL-1 in the development of diabetic nephropathy. Nefrologia. 1995;15:1-4. [Cited in This Article: ] |
| 108. | Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450-456. [PubMed] [Cited in This Article: ] |
| 109. | Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia. 1999;42:878-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Sassy-Prigent C, Heudes D, Mandet C, Bélair MF, Michel O, Perdereau B, Bariéty J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 254] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 111. | Navarro JF, Milena FJ, Mora C, León C, García J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 112. | Royall JA, Berkow RL, Beckman JS, Cunningham MK, Matalon S, Freeman BA. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am J Physiol. 1989;257:L399-L410. [PubMed] [Cited in This Article: ] |
| 113. | Pfeilschifter J, Pignat W, Vosbeck K, Märki F. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochem Biophys Res Commun. 1989;159:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 164] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 114. | Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16 Suppl 1:S78-S82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 115. | Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 763] [Cited by in F6Publishing: 825] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 116. | Buraczynska M, Jozwiak L, Ksiazek P, Borowicz E, Mierzicki P. Interleukin-6 gene polymorphism and faster progression to end-stage renal failure in chronic glomerulonephritis. Transl Res. 2007;150:101-105. [PubMed] [Cited in This Article: ] |
| 117. | Kersting F, Follath F, Moulds R, Mucklow J, McCloy R, Sheares J, Dollery C. A comparison of cardiovascular effects of dobutamine and isoprenaline after open heart surgery. Br Heart J. 1976;38:622-626. [PubMed] [Cited in This Article: ] |
| 118. | Nakamura A, Shikata K, Hiramatsu M, Nakatou T, Kitamura T, Wada J, Itoshima T, Makino H. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care. 2005;28:2890-2895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 119. | Araki S, Haneda M, Koya D, Sugimoto T, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A. Predictive impact of elevated serum level of IL-18 for early renal dysfunction in type 2 diabetes: an observational follow-up study. Diabetologia. 2007;50:867-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Hasegawa G, Nakano K, Kondo M. Role of TNF and IL-1 in the development of diabetic nephropathy. Nefrologia. 1995;15:1-4. [Cited in This Article: ] |
| 121. | Navarro JF, Milena FJ, Mora C, León C, Claverie F, Flores C, García J. Tumor necrosis factor-alpha gene expression in diabetic nephropathy: relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int Suppl. 2005;S98-S102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Baud L, Ardaillou R. Tumor necrosis factor in renal injury. Miner Electrolyte Metab. 1995;21:336-341. [PubMed] [Cited in This Article: ] |
| 123. | Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134:419-430. [PubMed] [Cited in This Article: ] |
| 124. | Baud L, Perez J, Friedlander G, Ardaillou R. Tumor necrosis factor stimulates prostaglandin production and cyclic AMP levels in rat cultured mesangial cells. FEBS Lett. 1988;239:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol. 1998;9:433-438. [PubMed] [Cited in This Article: ] |
| 126. | Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 128. | Navarro JF, Mora C, Rivero A, Gallego E, Chahin J, Macía M, Méndez ML, García J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am J Kidney Dis. 1999;33:458-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Han J, Thompson P, Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990;172:391-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 389] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 130. | Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192-198. [PubMed] [Cited in This Article: ] |
| 131. | Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, Yamamoto T. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 132. | DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113-F121. [PubMed] [Cited in This Article: ] |
| 133. | DiPetrillo K, Gesek FA. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am J Nephrol. 2004;24:352-359. [PubMed] [Cited in This Article: ] |
| 134. | Guerrero-Romero F, Rodríguez-Morán M, Paniagua-Sierra JR, García-Bulnes G, Salas-Ramírez M, Amato D. Pentoxifylline reduces proteinuria in insulin-dependent and non insulin-dependent diabetic patients. Clin Nephrol. 1995;43:116-121. [PubMed] [Cited in This Article: ] |
| 135. | Navarro JF, Mora C, Muros M, Maca M, Garca J. Effects of pentoxifylline administration on urinary N-acetyl-beta-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am J Kidney Dis. 2003;42:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Harmankaya O, Seber S, Yilmaz M. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail. 2003;25:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Navarro JF, Mora C, Muros M, García J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16:2119-2126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |