Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.683
Peer-review started: April 25, 2022
First decision: May 30, 2022
Revised: June 13, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 15, 2022
Recently added to the therapeutic arsenal against chronic heart failure as a first intention drug, the antidiabetic drug-class sodium-glucose cotransporter-2 inhibitors (SGLT2i) showed efficacy in decreasing overall mortality, hospitalization, and sudden death in patients of this very population, in whom chronic or acute ischemia count among the first cause. Remarkably, this benefit was observed independently from diabetic status, and benefited both preserved and altered ventricular ejection fraction. This feature, observed in several large randomized controlled trials, suggests additional effects from SGLT2i beyond isolated glycemia control. Indeed, both in-vitro and animal models suggest that inhibiting the Na+/H+ exchanger (NHE) may be key to preventing ischemia/ reperfusion injuries, and by extension may hold a similar role in ischemic damage control and ischemic preconditioning. Yet, several other mechanisms may be explored which may help better target those who may benefit most from SGLT2i molecules. Because of a large therapeutic margin with few adverse events, ease of prescription and potential pharmacological efficacity, SGLT2i could be candidate for wider indications. In this review, we aim to summarize all evidence which link SGLT2i and ischemia/reperfusion injuries modulation, by first listing known mechanisms, including metabolic switch, prevention of lethal arrythmias and others, which portend the latter, and second, hypothesize how the former may interact with these mechanisms.
Core Tip: The antidiabetic drug-class sodium-glucose cotransporter-2 inhibitors (SGLT2i) showed efficacy in decreasing mortality in patients with chronic heart failure, in whom ischemia counts among the first cause. Remarkably, this benefit was observed independently from diabetic status. This feature, yielded from several randomized controlled trials, suggests additional effects from SGLT2i beyond isolated glycemia control. Indeed, previous in-vitro and animal models analyzed altogether suggests the role of the inhibition of the Na+/H+ exchanger, which holds a pivotal role in ischemia/reperfusion injuries. In this review, we aim to summarize evidence which associate SGLT2i and ischemia/reperfusion injuries, by first listing known mechanisms which portend the latter, and second, hypothesize how the former may interact with these mechanisms.
- Citation: Quentin V, Singh M, Nguyen LS. A review of potential mechanisms and uses of SGLT2 inhibitors in ischemia-reperfusion phenomena. World J Diabetes 2022; 13(9): 683-695
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/683.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.683
Although sodium-glucose cotransporter-2 inhibitors (SGLT2i) represent a decade-old drug class, the range of their indications has expanded since the first Food and Drug Administration label in 2013 in patients with type 2 diabetes[1,2]. Indeed, SGLT2i which include empagliflozin, dapagliflozin and canagliflozin are now indicated in patients with heart failure, independently from their status towards diabetes[3].
To understand how SGLT2i went from an antidiabetic to a cardioprotective treatment, one must recall how in patients with type 2 diabetes treated by SGLT2i, there were numerous observations of a decrease in heart failure events, all-cause mortality, cardiovascular mortality[4]. Furthermore, subgroup analyses confirmed that this risk decrease was consistent across a wide range of cardiovascular risk[5,6].
Hence, specific randomized controlled trials were launched to assess the hypothesis of a benefit to be treated by SGLT2i for patients with heart failure, regardless of the presence or absence of diabetes. Preliminary reports were then confirmed, and SGLT2i improved clinical outcomes in patients presenting with heart failure, be they with preserved and reduced ejection fraction[2,7-9].
Nevertheless, while the main pharmacological effect of SGLT2i is to decrease renal glucose reabsorption, thereby increasing urinary glucose excretion, the benefits observed even in non-diabetic patients question off-target mechanisms. As an illustration, in the EMPA-REG OUTCOME trial which compared empagliflozin to placebo in patients with type 2 diabetes at high risk for cardiovascular events, the proportion of acute myocardial or cerebral ischemic event was similar in both groups, however, patients in the treatment group were more likely to surviving a cardiovascular event. This element may be supportive of a cellular protective association in ischemic injury[10]. In the dapagliflozin and prevention of adverse-outcomes in heart failure trial (DAPA-HF), administration of dapagliflozin reduced risk of serious ventricular arrythmia, cardiac arrest or sudden death[11].
In the following review, we aimed to suggest several mechanisms which may explain how SGLT2i act as immunomodulators, and how they may act beyond the sole increase in urinary loss of glucose. We first described the ischemia-reperfusion injury phenomenon and then expanded on the interactions between SGLT2i and ischemia-reperfusion mechanisms. Our main assumption lied on a protective role against ischemia-reperfusion lesions, which involve an increase in functional ketones, associated with a metabolic change, an impact on sodium/hydrogen exchanger, endothelial dysfunction, inflammation biomarkers, and platelet function.
While mortality of acute myocardial infarction, has been decreasing over time[12], subsequent morbidity manifested by heart failure has grown. Mitigating infarct size is a therapeutic goal which may be attained by decreasing the delay between first signs of ischemia and revascularization[13], and by managing secondary lesions.
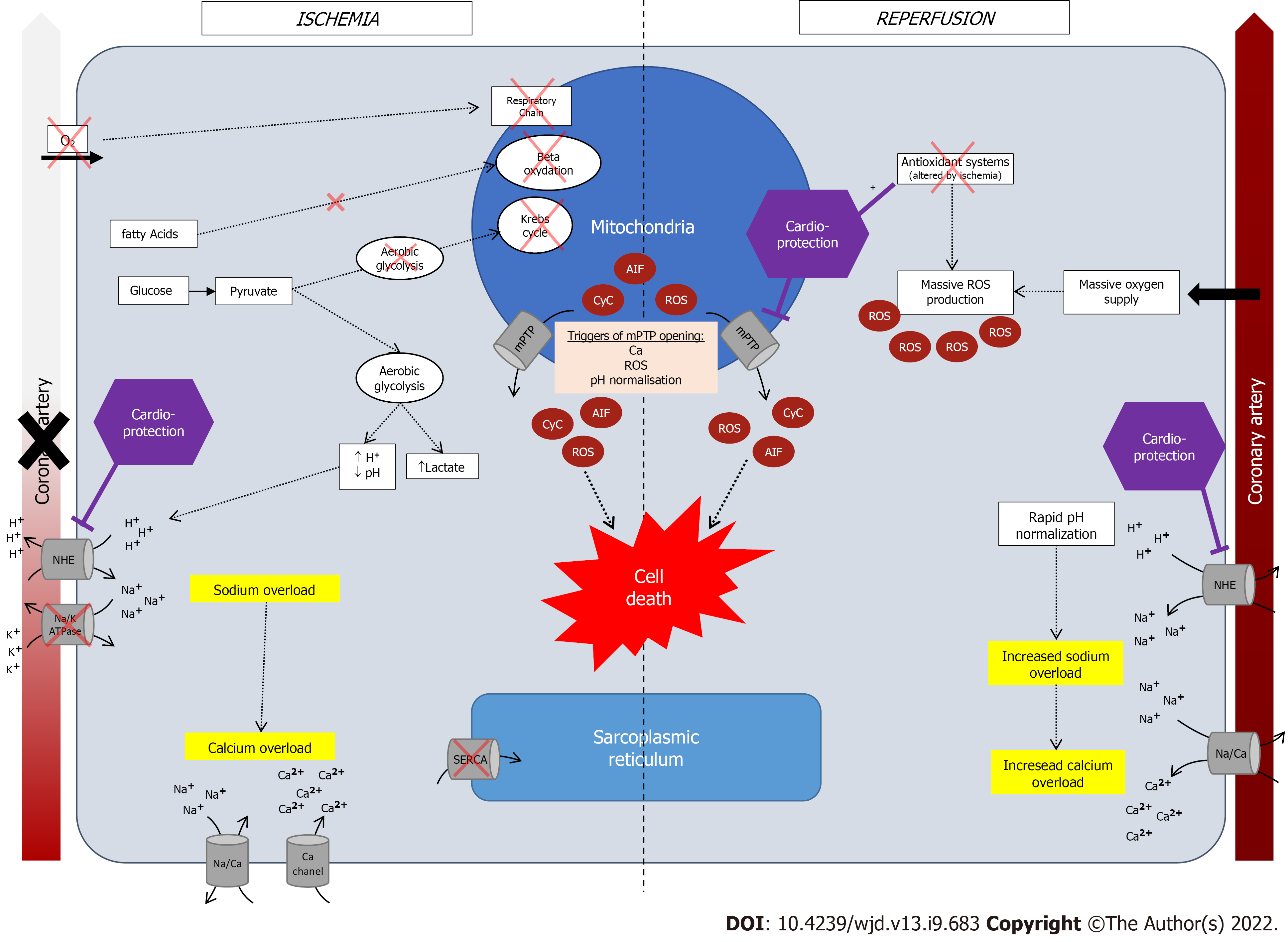
Myocardial ischemia is often caused by the occlusion of epicardial artery resulting in the ischemia of the coronary vascular territory which it depends upon. If prolonged, it may lead to myocardial infarction, an irreversible condition[14,15]. Therefore, quickly restoring blood flow in the occluded artery is the only way to limit the extent of infarction and subsequent complications including mortality. The reperfusion phenomenon however has been associated with secondary lesions[16], responsible for additional cardiomyocyte injuries[17,18]. These additional lesions may be partly responsible of final infarction size and therefore associated with adverse outcomes as there is a link between infraction size and long-term mortality or heart failure[19].
In cardiac surgery, these lesions are detected in 25% to 45% of patients[20]. They may be assessed by CK-MB and/or troponin levels, associated with postoperative adverse events[21]: arrythmias, myocardial stunning, low cardiac output syndrome and perioperative infarction[22]. Although, situations leading to these myocardial injuries are either unpredictable (i.e., acute myocardial infarction) or unavoidable (i.e., cardiac surgery), cardioprotective strategies aiming at reducing ischemia/ reperfusion injury are critical[23].
Defined by a mismatch between supply and need in oxygen and nutrients, its consequences depend on its severity, duration and the existence of collateral circulation[24]. In normal blood flow situation, oxygen is used by mitochondrial respiratory chain to produce ATP by using fatty acids (65%), glucose (15%), lactate (15%) and amino-acids and ketones (5%). Ninety percent of produced ATP are used by cardiomyocytes for contraction and the rest for homeostasis[25]. Following arterial occlusion and oxygen supply arrest, oxidative phosphorylation by mitochondrial respiratory chain stops and metabolism becomes anaerobic with the use of anaerobic glycogenolysis, leading to formation of H+ and lactates[26]. Hence, during ischemia, ATP is mainly produced from glucose instead of fatty acids, due to a higher energy-consumption rate of fatty acids catabolism[27]. This metabolic shift leads to the accumulation of AcylCoA and AcylCarnitine, both considered toxic for cardiomyocytes (enzymatic inhibition, alters cell membrane etc.). The small amount of produced ATP is used to maintain cellular homeostasis by using ATP-dependent ion pumps, until all ATP are depleted. Owing to ATP deficiency some cellular functions are not further ensured such as myocardial contraction, protein synthesis[28].
Then, an intracellular sodium accumulation creates a cellular oedema due to the activation of Na+/H+ exchanger (NHE) and inhibition of NA/K ATPase, which in turn, leads to a cytosolic calcium overload by activation of Na+/Ca2+ exchanger[29,30], inhibition of SERCA[30], and increased calcium entry via other channels[30].
The subsequent activation of protease, lipase, nuclease[27], and mitochondrial ultrastructural damage, are associated with myocardial stunning. In normal conditions, mitochondria’s membrane is impermeable to ions and proteins[22], with a channel on the inner membrane called the mitochondrial permeability transition pore (mPTP)[25]. During ischemia, this permeability transitions, opening mPTP[22], leading to mitochondrial oedema and death and release of its contents: Cytochrome c, apoptosis-inducing factor AIF, reactive oxygen species (ROS)[31,32].
ROS are highly reactive elements responsible for cellular injury because of reactions with lipids, proteins, and nucleic acids. The accumulation of xanthine and hypoxantine during ischemia[33], allows for their use by xanthine oxidase, activated during reperfusion and leading to the formation of ROS[34]. One of the many sources of hypoxanthine during ischemia, is ATP degradation by adenine nucleotide translocase which synthesize ADP, then degraded into hypoxanthine. This phenomenon increases energetic deficiency.
After myocardial ischemia, restoring blood flow is an emergency, and clinical guidelines all advocate for the shortest delay possible[13,25]. However, reperfusion is also associated with secondary injuries[35], due to the sudden oxygen supply which allows for the formation of superoxide anions. The mechanisms which are hypothesized include: (1) The activation of oxidative phosphorylation; (2) the activation of xanthine oxidase; and (3) local neutrophil accumulation and NADPH oxidase activation, also leading to ROS accumulation[25]. In normal conditions, superoxide anions are antagonized by antioxidant elements (catalase, superoxide dismutase, glutathione peroxidase, vitamins, etc.). However, in case of massive ROS production and altered defense mechanisms by ischemia, the balance is tipped off towards ROS accumulation. A graphic summary of these mechanisms is available in Figure 1.
Another mechanism of reperfusion injury is the pH paradox[25,36]. Reperfusion restores pH by quickly extracting accumulated H+, by activating of NHE; yet, pH restoration has been associated with deleterious outcomes[37]. Indeed, an abrupt accumulation of Na+ may lead to cellular oedema and calcium overload (due to a Na+/Ca2+ exchanger), and since cytoplasmic acidosis inhibits the mPTP opening, rapid normalization of intracellular pH leads to mitochondrial permeability transition with mPTP reopening[27]. Hence, phenomena similar to that of ischemia may occur even though reperfusion was achieved[29].
Cardioprotective strategies aim to reduce cardiomyocytes injuries, secondary to ischemia-reperfusion phenomena, and include 4 methods: preconditioning, postconditioning, remote conditioning and pharmacological treatment.
Preconditioning consists in applying cycles of brief coronary occlusion immediately before sustained occlusion. Clinical benefit has been observed in dog models, where repetitive short coronary occlusions preceding sustained occlusion resulted with an infarction smaller more delayed than that of a sustained occlusion without preconditioning[38]. While the benefit was initially observed shortly after ischemia, more lasting effects have been recently highlighted suggesting the role of protein synthesis [inducible nitric oxide (NO) synthase, cyclooxygenase, aldose reductase, superoxide dismutase][18]. Elements which are thought to mediate preconditioning benefit include but are not limited to adenosine, bradykinin or mechanical stretch activating various intracellular signaling pathways including RISK-pathway (increasing AKT and ERK1/2) and SAFE-pathway (increasing JAK and STAT) whose end targets are inhibition of mPTP opening, inhibition of Na/H exchanger or upregulation of antioxidant systems (superoxide dismutase, aldose reductase, etc.)[18,38].
Although promising, preconditioning is not reliable in clinical practice since it could not be used before acute coronary syndrome because of the brief effects of such procedure or the unpredictability of ACS. Hence, preconditioning could only be used in patient before CABG, by cross-clamping the aorta and then releasing for several minutes. Studies showed that it decreased post-operative ventricular arrhythmias, inotrope use and limited ICU stay[39].
On the other end, ischemic postconditioning consists in the same procedure, performed after the ischemic event, during reperfusion procedures. Similarly, it was associated with smaller infarct size[40,41], a more progressive pH restoration, decreasing ROS production and calcium-induced mPTP opening, resulting in anti-apoptotic, anti-autophagic et anti-arrhythmic benefit[25].
While pre- and postconditioning aim at stimulating local anti-inflammatory pathways, remote conditioning consists in applying cycles of brief occlusion in other territories than that which is affected by ischemia (i.e., neighboring coronary artery, limb). Theoretical advantages of this method lie in the fact that it may be applied at any time, is non-invasive and easily feasible. On top of the abovementioned mechanisms, additional systemic signal pathways may be involved with neuronal (peripheral sensory nerves, spinal cord, brainstems and vagal nerves) and humoral inducing a renal production of adenosine[42]. While this approach also aims at diminishing infarct size, mortality, and hospitalization for heart failure, phase III clinical trials failed to yield significant benefit, excepts in the most severe patients (cardiogenic shock or cardiac arrest)[18].
Yet, while multiple drugs have been tested, none showed clinical significance in human patients. Na/H exchangers inhibitors showed improvement in cardiovascular outcomes but increased stroke incidence[25,43,44]. Cyclosporine A, a nonspecific inhibitor of mPTP[45], promising initial results infarct, which were not translated in clinical studies[18]. Adenosine, acting as a vasodilator, was associated with pre- and postconditioning-like effects[46], through inhibition of mPTP opening[47]. Similarly, results were not conclusive in clinical trials[48]. Finally, NO was associated with potentially benefit in ischemia-reperfusion injuries by acting on oxygen consumption[49], platelet aggregation[50], leucocyte adhesion[51], and free radical scavenging[52].
These discrepancies between theoretical promises and disappointing clinical results require further research in the field, investigating novel pathways.
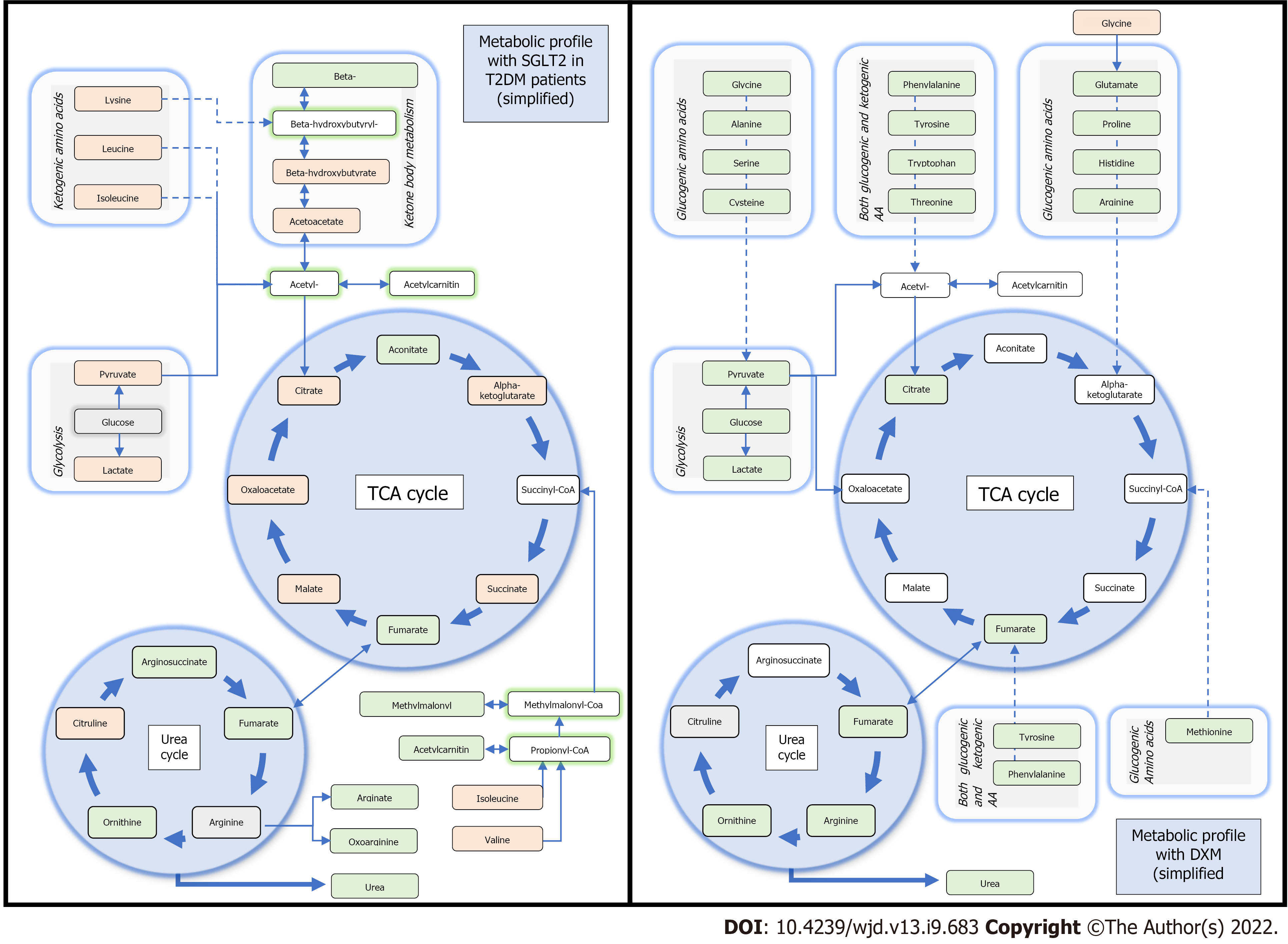
In normal oxygenation conditions, myocardial mitochondrial oxidative metabolism exploits fatty acids (60%), glucose (30%), lactate and to a lesser degree ketones and amino acids, with a capacity to rapidly change substrates depending on workload or conditions. Under hypoxic conditions, myocardial substrate oxidation switches from free fatty acids to glucose and carbohydrate oxidation, because transformation of glucose to lactate is independent of oxygen supply[53]. During prolonged anaerobia, ketone becomes predominant as a resource. For instance, in animal models increasing the uptakes of 3-hydroxybutyrate (3HB) is associated with an improvement in cardiac function, pathologic cardiac remodeling, and oxygen consumption, whereas the capacity to oxidate substrate such as fatty acid is reduced[54]. Of note, 3HB is generated in the liver and may be used as a substrate for generating acetyl-CoA leading to increased production of NADH to drive energy transfer and ATP production.
Remarkably, in patient treated by SGLT2i, an uprising of ketone circulation was observed[55,56].
One of the hypotheses is that SGLT2i improves myocardial fuel metabolism, contractility, and cardiac efficiency by shifting catabolism away from lipids and glucose to that of ketone bodies[57]. Improved oxygen consumption and work efficiency at a mitochondrial level have been hypothesized[58]. Similarly as fasting, with the expected glucose depletion under SGLT2i, insulin-glucagon ratio is modulated, delivery of free fatty acids is increased to the liver which then stimulates ketogenesis[59]. Metabolomic profiles of patients with type 2 diabetes further support this hypothesis[55]. In addition to an expected reduction in glucose, SGLT2i increased 3HB levels suggesting an accrued utilization of ketone bodies. Moreover, increased intermediate metabolites of the urea cycle may indicate its use as well as amino-acids[55]. Remarkably, the same metabolic changes were observed in non-diabetic patients: Ferrannini et al[54] showed that SGLT2i reduced end-tissular glucose catabolism, accelerated lipolysis and fat oxidation. While these changes were more prominent after long-term exposition, an effect was observed as early as the first administration[53]. When compared to serum profiles of patients under corticoids treatment (widely tested in ischemia-injury model), SGLT2 might represent a different therapeutic candidate because of alternative energy income pathways involved[60]. A comparison between the metabolomic changes due to SGLT2i molecules as compared to glucocorticoids is available in Figure 2.
Because use of ketone bodies depends on the targeted organ, heart as well as kidneys may be those which benefit the most from an increase in 3HB[57]. Furthermore, similarly to an ischemia-hypoxia setting, during incremental atrial pacing, fractional extraction of 3HB persist, with improved energy efficiency; and a lower use of free fatty acids in low oxygenation conditions prevents the formation of ROS[59].
Of note, even if data from animal studies are promising and suggest benefit regarding infarct size and recovery, opposite signals appear when focusing on ketone bodies[58,61]. A recent work reported a suppression in ketone body utilization by myocardial during ischemia, based on levels of ß-hydroxybutyrate in patient presenting chest pain in a retrospective population[62]. Animal models with low-carbohydrate diet inducing mild nutritional ketosis showed a worse recovery and survival, more arrythmias after induced ischemia[63,64]. However, these contradictory results, well summarized in Kolwicz and al. review[65], only raise the need for additional studies at the metabolic level.
SGLT2i were also associated with the inhibition of the NHE in myocardial cells[66]. We previously described the role of NHE in the homeostasis of ischemic cells, which induce oxidative stress with elevated cytosolic Na+ and increased mitochondrial formation of ROS through a final intracellular calcium overload. The counterbalance of such mechanism requires the regeneration of antioxidative enzymes by mitochondria, relying on NADPH, indirectly produced by the Krebs cycle, in turn activated by intramitochondrial calcium[67]. NHE inhibitors were associated with cardioprotective features in animal models of acute myocardial infarction[68]. Moreover, a chronic inhibition of NHE was associated with improvement against cardiomyocyte injury, remodeling, and systolic dysfunction[69].
Remarkably, SGLT2i indirectly interacts with NHE. In mice, empagliflozin reversed the effects of ouabain (an agent increasing intracellular sodium)[70]. Moreover, this effect was independent from SGLT2 and indirectly caused a decreased activation of the Na+/Ca2+ exchanger. The same results were observed with other SGLT2i (dapagliflozin, canagliflozin)[66].
This inhibition with empagliflozin was associated with lower rates of tumor necrosis factor alpha (TNF-α), attesting of a cell preservation and lowered inflammation through NHE inhibition.
Additional mechanisms which were hypothesized include: improved AMPK activation in myocytes[71], and cardio-fibroblasts[72]. In contrast, another study showed that concrete benefit on AMPK-pathway with SGLT2 in human cells and mouse cells in vitro seems unlikely because activation appeared with concentrations corresponding to the peak plasma concentrations of therapeutic doses[73].
In human cells, NHE inhibition showed similar results in atrial and ventricular myocytes, as compared to that of mice ventricular myocytes. Heart failure and atrial fibrillation were associated with increased NHE expression[74]. Finally, in human coronary endothelial cells, empagliflozin was associated with a similar reduction of oxidative stress supporting the previous hypothesis[75].
Positive effects of inhibition of NHE are not limited to better myocardial function, ionic homeostasis, or reduction of myocyte ischemic inflammation. Empagliflozin and canagliflozin in short-term treatment enhanced coronary vasodilation through NHE inhibition[66], whereas dapagliflozin needed a more prolonged treatment to reach comparable effect[76]. However, in cases of acute inflammation, a non-specific vasodilatation may occur, making it difficult to interpret supposed effect of inhibition of SGLT2[77].
Sudden deaths and ventricular arrythmias may occur after acute ischemia and reperfusion events, and SGLT2i were associated with fewer such events. Yet, because SGLT2i do not inherently feature anti-arrhythmic properties, several mechanisms have been hypothesized[78]. An improved ionic homeo-stasis through NHE inhibition has been suggested in the DAPA-HF trial, where 5.9% of the subjects assigned to the dapagliflozin group experimented serious rhythmic event (sudden death, cardiac arrest, ventricular arrythmias), with 7.4% in the placebo group[11]. In animal models, pre-treatment with empagliflozin reduced the incidence of reperfusion-induced ventricular arrythmia after an ischemia/reperfusion event, with the participation of the ERK1/2 pathway, involved in the RISK reperfusion-signaling pathway[79].
Role of the autonomous nervous system has also been investigated. In 2020, effects of empagliflozin vs placebo on cardiac sympathetic activity in acute myocardial infarction patients with T2DM (EMBODY Trial) compared empagliflozin with placebo for various electrocardiographic parameters. Heart rate variability, heart rate turbulence and electrocardiographic variations were recorded after acute myocardial infarction. Authors aimed to assess the variables associated with lethal ventricular arrhythmias. With a 6-mo-follow-up, a difference was observed between the two groups regarding sympathetic and parasympathetic stimulation[80]. Of note, to date, no study described these elements in the first few hours after an ischemic event index.
Finally, in a recent meta-analysis which analyzed the effects of SGLT2i on atrial arrythmia, sudden death and ventricular arrythmia which included 34 trials in patients with diabetes, use of SGLT2i were protective towards atrial arrythmia and sudden cardiac death, albeit several limitations existed[81].
Even if ionic homeostasis is the main hypothesis for the observed data, a plausible mechanism concurring to these results may lie on inhibition of platelet function, and antithrombin generation observed with SGLT2i. Unbalanced platelet activation and coagulation disturbance have been described during ischemic stress and associated with arrhythmia. SGLT2i have recently been associated with antiplatelet and antithrombotic features. Empagliflozin and dapagliflozin partially reduced the effects of stearic acid, an inflammatory agent inducing oxidative stress and impaired endothelial repair processes. As a result, platelets were less activated, in addition to that of ADP inhibition[82]. In male mice with T2DM model, administration of dapagliflozin showed a decreased activation and recruitment with an improved thrombin-platelet-mediated inflammation profile in vivo and less activated platelet with thrombin stimulation or CRP. Prolonged treatment did not affect hemostasis suggesting safety of utilization[83]. Gliflozin via NHE inhibition participate to maintain endothelial function[84] and endothelial production of NO. In a recent study, pharmacological analysis in vitro suggested that the gliflozin’s antiplatelet activity synergize with NO and prostacyclin[85]. Substantial evidence sustaining an intricated mechanism.
Taken altogether, these elements encourage to explore concrete platelet and hemostasis parameters with SGLT2i in ischemic situation, to sustain a potential benefit in ischemic-reperfusion context.
Beyond the theoretical data and focused exploratory clinical investigations, many animal models have been developed to assess the benefits of SGLT2 inhibitors in ischemia-reperfusion.
Acute administration of canagliflozin in male rat models of myocardial infarction showed decrease in infarct size, improved left ventricular systolic and diastolic function during and after ischemia, and decreased ROS[86]. Similar results were obtained with dapagliflozin[61], and the delay before the first ventricular arrythmia was lower when treated by SGLT2i. An improved communication between cardiac cells with preserved phosphorylation of gap junction protein connexin-43 was suggested[87,88]. Empagliflozin also showed similar results: reduced infarction size, better ventricular parameters, reduced systemic inflammation and ROS production, in acute or chronic administration[89,90]. The role of STAT3 phosphorylation was observed in several models[89-91]. Even if the beneficial mechanism is not yet fully determined, acute lowering of the blood glucose might be one of the potential hypothesis[92]. Interestingly, dipeptidyl peptidase 4 inhibitors were also compared to SGLT2i in murine models: SGLT2i showed greater efficacy than dipeptidyl peptidase 4 inhibitors to improve metabolic impairments and left ventricular function[93].
Recently, 16 independent animal models experiments which compared SGLT2i to control, and included 224 subjects overall, were summarized in a recent meta-analysis[94]. Regardless of diabetes, SGLT2is were significantly associated with fewer myocardial ischemia-reperfusion injuries and infarct size. Additionally, systemic treatment performed better than local administration, and longer-term treatment was associated with better results.
On top of myocardial protection, other organs have been tested.
In a model of lung injury due to ischemia-reperfusion, empagliflozin was tested on respiratory function, tissular and cellular analyses. Similarly, as in cardiac usage, SGLT2i was associated with lower levels of circulating cytokines in bronchoalveolar liquids, those were dependent on improved phosphorylation of pulmonary ERK1/2[95].
In models of ischemia-reperfusion-induced kidney injury, dapagliflozin was associated decreased biomarkers of renal failure (blood urea and creatinine) and fewer tubular injuries. Furthermore, under hypoxic condition, dapagliflozin reversed cellular death. Similarly, as in heart and lung, phosphorylation of AMPK and ERK1/2 was improved[96]. Remarkably, similar observations were made in non-diabetic rats[97].
Finally, in neurons, SGLT2i may interact with SGLT2 and SGLT1, expressed in human center nervous system[98]. Similar ischemia-reperfusion injuries may be performed in neurons, and empagliflozin in was associated with smaller infarct size and improved neuronal functions than in control rats. The main pathway studied was the HIF-1α/VEGF cascade, on which suppression of neuronal expression of Caspase-3 by empagliflozin had positive neuronal effects[99]. Moreover, role of Caspase-3 repression in hyperglycemic rats suggested an association between empagliflozin use and a decrease in TNF-α[100].
Beyond the cardiovascular benefits observed in patients with chronic heart failure treated by SGLT2i, data from large clinical trials including EMPA-REG or DAPA-HF may suggest a benefit through ischemia-reperfusion events. The inhibition of the NHE may play a pivotal role in such cardioprotective feature and further investigations towards the immunomodulatory properties of SGLT2i drug-class are warranted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Athyros VG, Greece; Ved J, India S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374:1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | McMurray JJV, Docherty KF, Jhund PS. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. Reply. N Engl J Med. 2020;382:973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 711] [Article Influence: 355.5] [Reference Citation Analysis (0)] |
| 4. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7124] [Cited by in F6Publishing: 7286] [Article Influence: 809.6] [Reference Citation Analysis (0)] |
| 5. | Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139:1384-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 6. | Verma S, Mazer CD, Fitchett D, Inzucchi SE, Pfarr E, George JT, Zinman B. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia. 2018;61:1712-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Correction to: Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021;143:e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1192] [Cited by in F6Publishing: 1821] [Article Influence: 607.0] [Reference Citation Analysis (0)] |
| 9. | Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M, Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 264] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 10. | Bell RM, Yellon DM. SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol. 2018;6:435-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, Solomon SD, McMurray JJV. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42:3727-3738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5364] [Cited by in F6Publishing: 5911] [Article Influence: 985.2] [Reference Citation Analysis (0)] |
| 13. | Park J, Choi KH, Lee JM, Kim HK, Hwang D, Rhee TM, Kim J, Park TK, Yang JH, Song YB, Choi JH, Hahn JY, Choi SH, Koo BK, Chae SC, Cho MC, Kim CJ, Kim JH, Jeong MH, Gwon HC, Kim HS; KAMIR‐NIH (Korea Acute Myocardial Infarction Registry–National Institutes of Health) Investigators. Prognostic Implications of Door-to-Balloon Time and Onset-to-Door Time on Mortality in Patients With ST -Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Heart Assoc. 2019;8:e012188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Heusch G. Myocardial Ischemia: Lack of Coronary Blood Flow or Myocardial Oxygen Supply/Demand Imbalance? Circ Res. 2016;119:194-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Maroko PR, Libby P, Ginks WR, Bloor CM, Shell WE, Sobel BE, Ross J Jr. Coronary artery reperfusion. I. Early effects on local myocardial function and the extent of myocardial necrosis. J Clin Invest. 1972;51:2710-2716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 279] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 527] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 17. | Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 984] [Cited by in F6Publishing: 961] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17:773-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 498] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 19. | Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 399] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 20. | Weman SM, Karhunen PJ, Penttilä A, Järvinen AA, Salminen US. Reperfusion injury associated with one-fourth of deaths after coronary artery bypass grafting. Ann Thorac Surg. 2000;70:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Costa MA, Carere RG, Lichtenstein SV, Foley DP, de Valk V, Lindenboom W, Roose PC, van Geldorp TR, Macaya C, Castanon JL, Fernandez-Avilèz F, Gonzáles JH, Heyer G, Unger F, Serruys PW. Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS). Circulation. 2001;104:2689-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 440] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 23. | Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 24. | Jennings RB, Reimer KA. Factors involved in salvaging ischemic myocardium: effect of reperfusion of arterial blood. Circulation. 1983;68:I25-I36. [PubMed] [Cited in This Article: ] |
| 25. | Benhabbouche S, Crola da Silva C, Abrial M, Ferrera R. [The basis of ischemia-reperfusion and myocardial protection]. Ann Fr Anesth Reanim. 2011;30 Suppl 1:S2-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Jennings RB, Murry CE, Steenbergen C Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation. 1990;82:II2-I12. [PubMed] [Cited in This Article: ] |
| 27. | Chassot PG. Precis d’anesthesie cardiaque 5. [cited 1 April 2022]. Available from: https://www.pac5.ch/fr/node/1076/take. [Cited in This Article: ] |
| 28. | Herzig JW, Peterson JW, Solaro RJ, Rüegg JC. Phosphate and vanadate reduce the efficiency of the chemo-mechanical energy transformation in cardiac muscle. Adv Exp Med Biol. 1982;151:267-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Berdeaux A, Tissier R, Couvreur N, Salouage I, Ghaleh B. [Heart rate reduction: beneficial effects in heart failure and post-infarcted myocardium]. Therapie. 2009;64:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Tani M, Neely JR. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989;65:1045-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 539] [Cited by in F6Publishing: 506] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+. FEBS J. 2005;272:3593-3601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880-9884. [PubMed] [Cited in This Article: ] |
| 34. | Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;265:6656-6663. [PubMed] [Cited in This Article: ] |
| 35. | Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 284] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, Cascio WE. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Bond JM, Herman B, Lemasters JJ. Protection by acidotic pH against anoxia/reoxygenation injury to rat neonatal cardiac myocytes. Biochem Biophys Res Commun. 1991;179:798-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5406] [Cited by in F6Publishing: 5434] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 39. | Walsh SR, Tang TY, Kullar P, Jenkins DP, Dutka DP, Gaunt ME. Ischaemic preconditioning during cardiac surgery: systematic review and meta-analysis of perioperative outcomes in randomised clinical trials. Eur J Cardiothorac Surg. 2008;34:985-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1396] [Cited by in F6Publishing: 1439] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 41. | Lie RH, Hasenkam JM, Nielsen TT, Poulsen R, Sloth E. Post-conditioning reduces infarct size in an open-chest porcine acute ischemia-reperfusion model. Acta Anaesthesiol Scand. 2008;52:1188-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Chakrabarti S, Hoque AN, Karmazyn M. A rapid ischemia-induced apoptosis in isolated rat hearts and its attenuation by the sodium-hydrogen exchange inhibitor HOE 642 (cariporide). J Mol Cell Cardiol. 1997;29:3169-3174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Mentzer RM Jr, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasché P, Myers ML, Nicolau J, Simoons M, Thulin L, Weisel RD; EXPEDITION Study Investigators. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85:1261-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 45. | Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1001] [Cited by in F6Publishing: 956] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 46. | Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 927] [Cited by in F6Publishing: 826] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 47. | Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47:684-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 369] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 49. | Loke KE, McConnell PI, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze TH. Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res. 1999;84:840-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1034] [Cited by in F6Publishing: 1002] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 51. | Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 321] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5053] [Cited by in F6Publishing: 4855] [Article Influence: 142.8] [Reference Citation Analysis (1)] |
| 53. | Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes. 2016;65:1190-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 54. | Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 55. | Kappel BA, Lehrke M, Schütt K, Artati A, Adamski J, Lebherz C, Marx N. Effect of Empagliflozin on the Metabolic Signature of Patients With Type 2 Diabetes Mellitus and Cardiovascular Disease. Circulation. 2017;136:969-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 56. | Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 408] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 57. | Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? Diabetes Care. 2016;39:1115-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 422] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 58. | Yurista SR, Silljé HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21:862-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 59. | Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes Care. 2016;39:1108-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 647] [Cited by in F6Publishing: 679] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 60. | Bordag N, Klie S, Jürchott K, Vierheller J, Schiewe H, Albrecht V, Tonn JC, Schwartz C, Schichor C, Selbig J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci Rep. 2015;5:15954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 62. | Arima Y, Izumiya Y, Ishida T, Takashio S, Ishii M, Sueta D, Fujisue K, Sakamoto K, Kaikita K, Tsujita K. Myocardial Ischemia Suppresses Ketone Body Utilization. J Am Coll Cardiol. 2019;73:246-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Liu J, Wang P, Douglas SL, Tate JM, Sham S, Lloyd SG. Impact of high-fat, low-carbohydrate diet on myocardial substrate oxidation, insulin sensitivity, and cardiac function after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2016;311:H1-H10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Wang P, Tate JM, Lloyd SG. Low carbohydrate diet decreases myocardial insulin signaling and increases susceptibility to myocardial ischemia. Life Sci. 2008;83:836-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Kolwicz SC Jr. Ketone Body Metabolism in the Ischemic Heart. Front Cardiovasc Med. 2021;8:789458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61:722-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 374] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 67. | Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Böhm M, O'Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 68. | Avkiran M, Marber MS. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 69. | Jun S, Aon MA, Paolocci N. Empagliflozin and HFrEF: Known and Possible Benefits of NHE1 Inhibition. JACC Basic Transl Sci. 2019;4:841-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Peng X, Li L, Lin R, Wang X, Liu X, Li Y, Ma C, Ruan Y, Liu N. Empagliflozin Ameliorates Ouabain-Induced Na+ and Ca2+ Dysregulations in Ventricular Myocytes in an Na+-Dependent Manner. Cardiovasc Drugs Ther. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Li X, Lu Q, Qiu Y, do Carmo JM, Wang Z, da Silva AA, Mouton A, Omoto ACM, Hall ME, Li J, Hall JE. Direct Cardiac Actions of the Sodium Glucose Co-Transporter 2 Inhibitor Empagliflozin Improve Myocardial Oxidative Phosphorylation and Attenuate Pressure-Overload Heart Failure. J Am Heart Assoc. 2021;10:e018298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Ye Y, Jia X, Bajaj M, Birnbaum Y. Dapagliflozin Attenuates Na+/H+ Exchanger-1 in Cardiofibroblasts via AMPK Activation. Cardiovasc Drugs Ther. 2018;32:553-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 73. | Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes. 2016;65:2784-2794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 74. | Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S, Arzt M, Maier LS, Wagner S. Empagliflozin inhibits Na+ /H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Uthman L, Li X, Baartscheer A, Schumacher CA, Baumgart P, Hermanides J, Preckel B, Hollmann MW, Coronel R, Zuurbier CJ, Weber NC. Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na+]c/ROS-pathway in human endothelial cells. Biomed Pharmacother. 2022;146:112515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D'Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res. 2020;157:104781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 77. | Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18:385-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 78. | Kolesnik E, Scherr D, Rohrer U, Benedikt M, Manninger M, Sourij H, von Lewinski D. SGLT2 Inhibitors and Their Antiarrhythmic Properties. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Hu Z, Ju F, Du L, Abbott GW. Empagliflozin protects the heart against ischemia/reperfusion-induced sudden cardiac death. Cardiovasc Diabetol. 2021;20:199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 80. | Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Ishikawa M, Maruyama M, Ogano M, Tanabe J; EMBODY trial investigators. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 81. | Fernandes GC, Fernandes A, Cardoso R, Penalver J, Knijnik L, Mitrani RD, Myerburg RJ, Goldberger JJ. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18:1098-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 82. | Spigoni V, Fantuzzi F, Carubbi C, Pozzi G, Masselli E, Gobbi G, Solini A, Bonadonna RC, Dei Cas A. Sodium-glucose cotransporter 2 inhibitors antagonize lipotoxicity in human myeloid angiogenic cells and ADP-dependent activation in human platelets: potential relevance to prevention of cardiovascular events. Cardiovasc Diabetol. 2020;19:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Kohlmorgen C, Gerfer S, Feldmann K, Twarock S, Hartwig S, Lehr S, Klier M, Krüger I, Helten C, Keul P, Kahl S, Polzin A, Elvers M, Flögel U, Kelm M, Levkau B, Roden M, Fischer JW, Grandoch M. Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia. 2021;64:1834-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Park SH, Belcastro E, Hasan H, Matsushita K, Marchandot B, Abbas M, Toti F, Auger C, Jesel L, Ohlmann P, Morel O, Schini-Kerth VB. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 85. | Lescano CH, Leonardi G, Torres PHP, Amaral TN, de Freitas Filho LH, Antunes E, Vicente CP, Anhê GF, Mónica FZ. The sodium-glucose cotransporter-2 (SGLT2) inhibitors synergize with nitric oxide and prostacyclin to reduce human platelet activation. Biochem Pharmacol. 2020;182:114276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Sayour AA, Korkmaz-Icöz S, Loganathan S, Ruppert M, Sayour VN, Oláh A, Benke K, Brune M, Benkő R, Horváth EM, Karck M, Merkely B, Radovits T, Szabó G. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med. 2019;17:127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 87. | Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 88. | Richards TS, Dunn CA, Carter WG, Usui ML, Olerud JE, Lampe PD. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J Cell Biol. 2004;167:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Andreadou I, Efentakis P, Balafas E, Togliatto G, Davos CH, Varela A, Dimitriou CA, Nikolaou PE, Maratou E, Lambadiari V, Ikonomidis I, Kostomitsopoulos N, Brizzi MF, Dimitriadis G, Iliodromitis EK. Empagliflozin Limits Myocardial Infarction in Vivo and Cell Death in Vitro: Role of STAT3, Mitochondria, and Redox Aspects. Front Physiol. 2017;8:1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 90. | Nikolaou PE, Efentakis P, Abu Qourah F, Femminò S, Makridakis M, Kanaki Z, Varela A, Tsoumani M, Davos CH, Dimitriou CA, Tasouli A, Dimitriadis G, Kostomitsopoulos N, Zuurbier CJ, Vlahou A, Klinakis A, Brizzi MF, Iliodromitis EK, Andreadou I. Chronic Empagliflozin Treatment Reduces Myocardial Infarct Size in Nondiabetic Mice Through STAT-3-Mediated Protection on Microvascular Endothelial Cells and Reduction of Oxidative Stress. Antioxid Redox Signal. 2021;34:551-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 91. | Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 92. | Zuurbier CJ. Does acute treatment of dapagliflozin reduce cardiac infarct size through direct cardiac effects or reductions in blood glucose levels? Cardiovasc Diabetol. 2020;19:141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, Thummasorn S, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J Endocrinol. 2018;236:69-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 94. | Sayour AA, Celeng C, Oláh A, Ruppert M, Merkely B, Radovits T. Sodium-glucose cotransporter 2 inhibitors reduce myocardial infarct size in preclinical animal models of myocardial ischaemia-reperfusion injury: a meta-analysis. Diabetologia. 2021;64:737-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Huang D, Ju F, Du L, Liu T, Zuo Y, Abbott GW, Hu Z. Empagliflozin Protects against Pulmonary Ischemia/Reperfusion Injury via an Extracellular Signal-Regulated Kinases 1 and 2-Dependent Mechanism. J Pharmacol Exp Ther. 2022;380:230-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 96. | Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, Choi DE. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS One. 2016;11:e0158810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 97. | Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin Enhances Autophagy, Mitochondrial Biogenesis, and Antioxidant Defense and Ameliorates Renal Ischemia/Reperfusion in Nondiabetic Rats. Oxid Med Cell Longev. 2022;2022:1197061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 98. | Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci. 2016;130:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 99. | Abdel-Latif RG, Rifaai RA, Amin EF. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch Pharm Res. 2020;43:514-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 100. | Amin EF, Rifaai RA, Abdel-Latif RG. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam Clin Pharmacol. 2020;34:548-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |