Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.668
Peer-review started: March 30, 2022
First decision: April 25, 2022
Revised: May 3, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 15, 2022
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease in the world and represents a clinical-histopathologic entity where the steatosis component may vary in degree and may or may not have fibrotic progression. The key concept of NAFLD pathogenesis is excessive triglyceride hepatic accumulation because of an imbalance between free fatty acid influx and efflux. Strong epidemiological, biochemical, and therapeutic evidence supports the premise that the primary pathophysiological derangement in most patients with NAFLD is insulin resistance; thus the association between diabetes and NAFLD is widely recognized in the literature. Since NAFLD is the hepatic manifestation of a metabolic disease, it is also associated with a higher cardio-vascular risk. Conventional B-mode ultrasound is widely adopted as a first-line imaging modality for hepatic steatosis, although magnetic resonance imaging represents the gold standard noninvasive modality for quantifying the amount of fat in these patients. Treatment of NAFLD patients depends on the disease severity, ranging from a more benign condition of nonalcoholic fatty liver to nonalcoholic steatohepatitis. Abstinence from alcohol, a Mediterranean diet, and modification of risk factors are recommended for patients suffering from NAFLD to avoid major cardiovascular events, as per all diabetic patients. In addition, weight loss induced by bariatric surgery seems to also be effective in improving liver features, together with the benefits for diabetes control or resolution, dyslipidemia, and hypertension. Finally, liver transplantation represents the ultimate treatment for severe nonalcoholic fatty liver disease and is growing rapidly as a main indication in Western countries. This review offers a comprehensive multidisciplinary approach to NAFLD, highlighting its connection with diabetes.
Core Tip: Nonalcoholic fatty liver disease is the most common liver disease worldwide, characterized by fat accumulation in the hepatic parenchyma, with a range of different stages from mild inflammation to severe fibrosis. There is a biunivocal relationship with type 2 diabetes, with important consequences in terms of cardiovascular risk, which seems to also have occurred during the coronavirus disease 2019 pandemic. This review focuses on the pathogenesis, clinical aspects, and treatment, providing guidance for a non-invasive diagnosis and preferred therapy, medical and/or surgical.
- Citation: Bellini MI, Urciuoli I, Del Gaudio G, Polti G, Iannetti G, Gangitano E, Lori E, Lubrano C, Cantisani V, Sorrenti S, D’Andrea V. Nonalcoholic fatty liver disease and diabetes. World J Diabetes 2022; 13(9): 668-682
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/668.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.668
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease worldwide[1] and represents a clinico-histopathologic entity with features mimicking alcohol-induced liver injury, but occurring, by definition, in patients with little or no history of alcohol consumption. Its prevalence reaches up to 25%-30%[2,3] of the worldwide population, with approximately 2 billion of individuals being affected[4].
NAFLD includes a different variety of findings, ranging from hepatocyte fat accumulation without concomitant inflammation or fibrosis (simple hepatic steatosis), to hepatic steatosis with a necro-inflammatory component (steatohepatitis), which may or may not have associated fibrosis. Nonalcoholic steatohepatitis (NASH) may progress to cirrhosis in up to 20% of patients[5,6], and it is a leading cause of cryptogenic cirrhosis[7].
The cause of NAFLD has not been fully elucidated and is considered multifactorial. A two-hit model of NAFLD development was originally proposed. The first consists of hepatic steatosis, which then sensitizes the liver to a progressive injury and is mediated by “second hits” as inflammatory cytokines, adipokines, and oxidative stress. Together they lead to steatohepatitis and fibrosis[8]. Currently, the two-hit hypothesis has been replaced by the “multiple hit” theory, which recognizes the following components in NAFLD pathophysiology: insulin resistance, obesity, gut microbiota, and environmental and genetic factors[9].
The aim of this review is to report, from a comprehensive multidisciplinary perspective, the pathogenesis, diagnosis, and treatment of NAFLD, highlighting its relationship with diabetes.
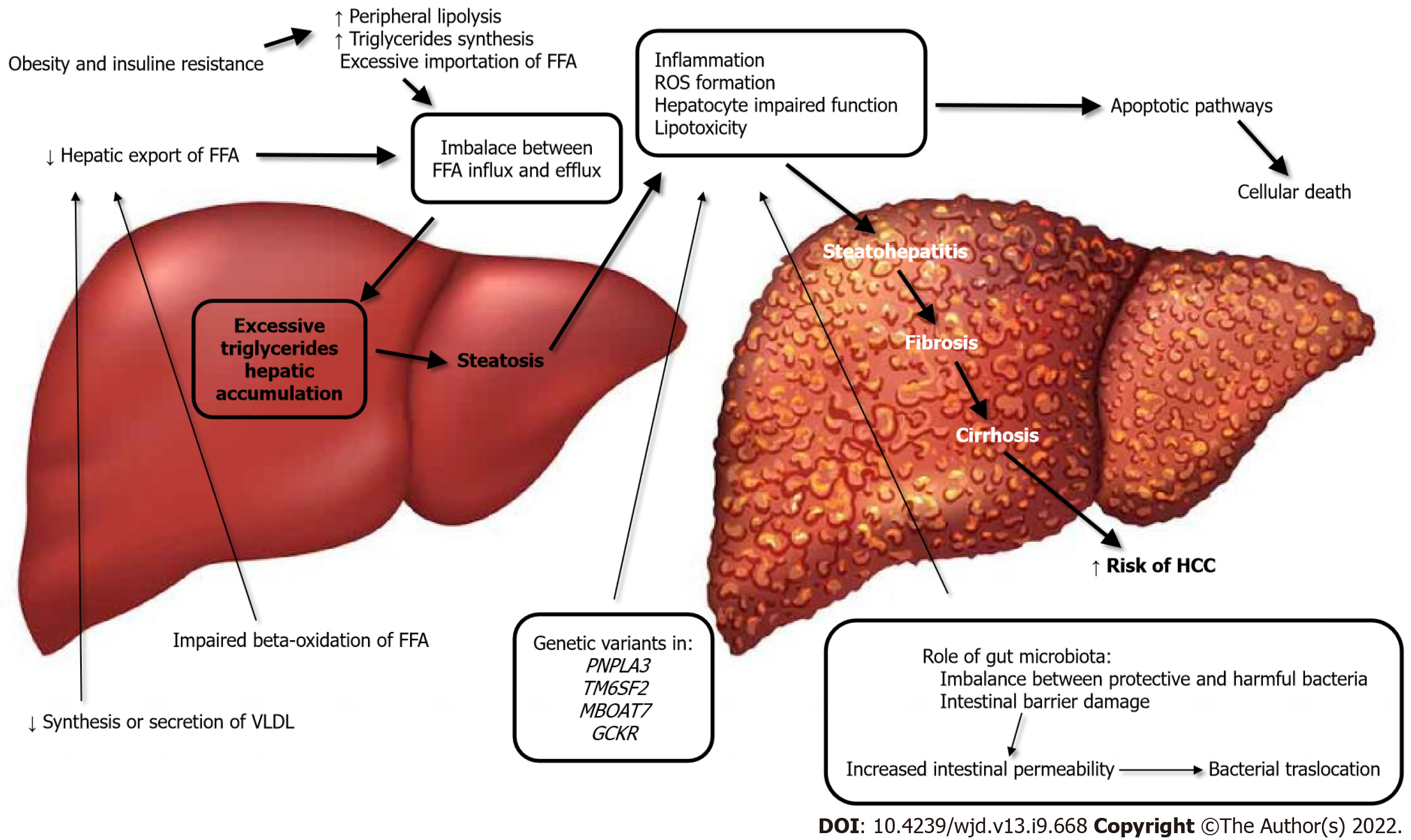
The key concept of NAFLD pathogenesis is excessive triglyceride hepatic accumulation as a result of an imbalance between free fatty acid (FFA) influx and efflux[10]. This can occur from the excessive importation of FFAs from the adipose tissue; diminished hepatic export of FFA, possibly secondary to reduced synthesis or secretion of very low-density lipoprotein; or the impaired beta-oxidation of FFA. The pathogenesis and evolution of NAFLD are depicted in Figure 1.
Strong epidemiological, biochemical, and therapeutic evidence supports the premise that the primary pathophysiological derangement in most patients with NAFLD is insulin resistance. Resistance to the action of insulin results in important changes in lipid metabolism. These include enhanced peripheral lipolysis, increased triglyceride synthesis, and increased hepatic uptake of fatty acids. Each of these may contribute to the accumulation of hepatocellular triglycerides, which in turn results in a preferential shift from carbohydrate to FFA beta-oxidation, an occurrence that has been demonstrated in patients with insulin resistance[11]. The association of liver steatosis and metabolic dysfunction is so strict that a new definition was recently proposed to define this entity, namely “metabolic (dysfunction)-associated fatty liver disease” (MAFLD)[12].
The excessive inflow of triglycerides to the liver leads to inflammation, reactive oxygen species (ROS) formation, hepatocyte impaired function, and lipotoxicity. Hepatocellular cells injury activates apoptotic pathways, ultimately causing cellular death. This results in the progression from noninflammatory isolated steatosis to the development of nonalcoholic steatohepatitis, with a risk of further evolution to fibrosis, cirrhosis and, worst-case scenario, to the development of hepatocellular carcinoma[9,13]. In this regard, the major role of mitochondrial dysfunction in the genesis of NAFLD has emerged in recent years; in fact mitochondria are responsible for the β-oxidation of FFAs and controlling the tricarboxylic acid cycle. Furthermore, mitochondria favor cell adaption to oxidative stress, mitigating the effects of ROS production[14].
Intestinal microbes have also been implicated as a potential source of hepatotoxic oxidative injury, and changes in the microbiome play a role in the lipotoxicity and pathogenesis of NAFLD[15,16].
The specific composition of gut microbiota may play a role in both the inflammatory and fibrosis responses in patients with NAFLD. The imbalance between protective and harmful bacteria, such as altered Firmicutes/Bacteroidetes ratio, relative abundance of alcohol-producing bacteria, growth of harmful genera, and lack of protective genera, together predispose[17] to damage of the intestinal barrier. The consequent epithelial disruption leads to an altered immune reaction and activation of inflammatory pathways, as a response to the bacterial products, namely short-chain fatty acids, trimethylamine N-oxide, and secondary bile acids[18]. Damage of the intestinal membrane finally results in impaired transport across the mucosa, increasing the filtration of bacterial lipopolysaccharides and thus further contributing to NAFLD development[17,19].
In terms of genetic risk factors, there is also a role in the development of NAFLD. Studies on twins have demonstrated a strong hereditary correlation, estimated to be approximately 50%, to both hepatic fat content and hepatic fibrosis[4]. It is recognized that at least four genetic variants in four different genes (PNPLA3, TM6SF2, MBOAT7, and GCKR) are responsible for the encoding of hepatic lipid metabolism regulatory proteins and are therefore involved in the development and progression of NAFLD[12,20].
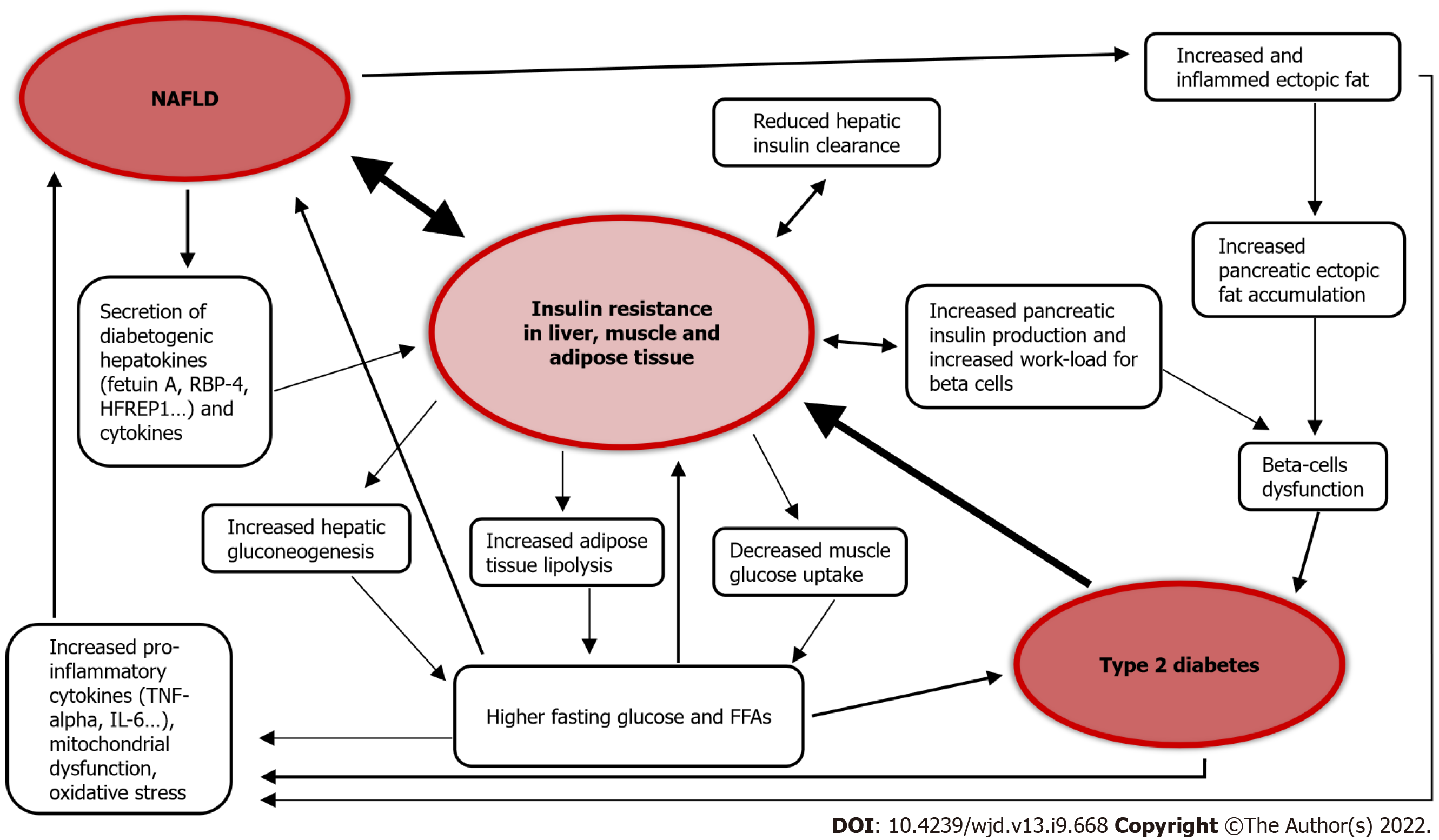
Among type 2 diabetes (T2D) patients, the prevalence of NAFLD is more than double compared to the general population, and is estimated to be over 55%. The global prevalence of NASH in T2D patients is 37%[1]. The prevalence of NAFLD in T1D is reportedly between 10% and 20%[21,22].
The association between T2D and NAFLD is widely recognized in the literature[23-26]. T2D is itself a risk factor for the development of NAFLD, and seems to accelerate the progression of liver disease[1,27]. On the other hand, NAFLD is a risk factor for the development of T2D and its complications[22,23,27-29]. In fact, NAFLD gives a two-fold increased risk of incident diabetes over a course of about 5 years[23,30], and the risk of patients affected by liver steatosis to develop diabetes increases in parallel to the extent of steatosis severity[30], becoming even higher when the fibrosis is advanced[23,30].
A study on 2020 participants, with a 10-year follow-up, observed that the fatty liver index (FLI), an indirect assessment used to quantify the amount of hepatic fat with a mathematical formula, predicts incident risk of developing T2D and glycemic alterations preceding diabetes. Individuals with a high FLI had an increased risk of developing diabetes, and among these high FLI patients, overweight and obese people had a risk that increased by more than 10- and 15-fold compared to similar body mass index-matched people but lower FLI[31]. Similarly, another study on 28991 pre-diabetic patients with a 3-year follow-up found that high FLI is a risk factor for developing diabetes, even in nonobese patients[32]. Of note, NAFLD predicts the development of metabolic syndrome over a period of less than 5 years[33], and metabolic syndrome is considered a risk factor for T2D.
NAFLD is associated with the development of macrovascular and microvascular complications in T2D patients, including chronic kidney disease (CKD)[29], retinopathy and autonomic neuropathy, although the results across studies are not completely concordant[34,35]. Liver fibrosis is also independently associated with macrovascular and microvascular complications in diabetic patients[36], and although T2D is a well-known risk factor to CKD, NAFLD predicts deterioration of renal function even in healthy subjects.
As per dietary advice, adherence to a Mediterranean diet is inversely associated with NAFLD and prevents the development of T2D and cardiovascular disease (CVD) in patients with NAFLD over a 10-year span[37], whereas the low adherence to these food habits is associated with diabetes and CVD onset in NAFLD patients[38]. Virtually, most studies assessing liver fat content have reported positive results after very low-calorie diets and ketogenic diets. While it is acknowledged that weight loss is associated with amelioration of NAFLD, less is known about the effect of macronutrient distribution on such outcomes. Carbohydrate restriction, with its well-established role in modulating insulin levels, and the newly proposed pathway involving the microbiome shift with increased folate production, likely plays a primary role in the reported effectiveness of ketogenic diets towards NAFLD[39].
Figure 2 summarizes the pathophysiological link between NAFLD and T2D.
CVD is among the leading causes of death worldwide[40], and the prevention of cardiovascular events is crucial from a global health perspective.
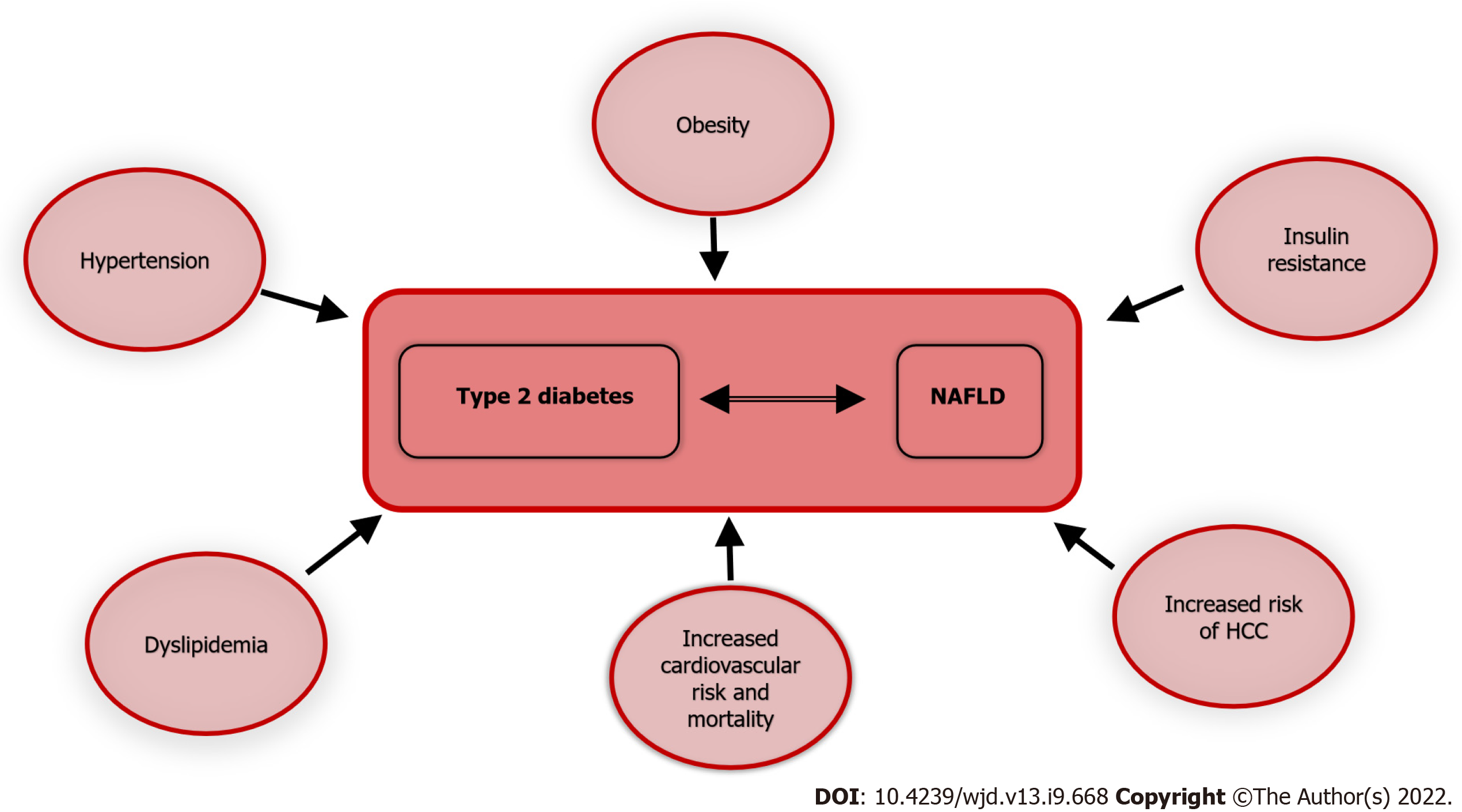
Atherosclerotic CVD is the major cause of morbidity and mortality in diabetic patients[41]. CVD comorbidities often present in diabetic patients as hypertension and dyslipidemia, are additive risk factors for cardiovascular events. T2D is a recognized cardiovascular risk factor as well, and NAFLD contributes independently to CVD[42].
Since NAFLD is the hepatic manifestation of a metabolic disease, it is also associated with a higher cardiovascular risk[43]. A recent meta-analysis assessed the long-term higher risk of fatal and nonfatal CVD events, observing an increase across steatosis stages, reaching the maximum when fibrosis was present[44]. NAFLD is also significantly associated with hypertension[45] and heart failure[46], thus significantly increasing the overall mortality risk[46]. In a retrospective study comparing more than 900 subjects affected either by NAFLD or AFLD or with normal liver appearance on computed tomography, fatty liver independently from the cause of the steatosis was associated with a higher cardiovascular risk[47]. Since NAFLD is a dynamic entity, it is, by definition, subject to variation over time. In the same study, Lee et al[47] evaluated 3 million subjects for NAFLD with FLI for a minimum of four times, between 2009 and 2013, concluding that higher persistent FLI led to a higher mortality rate for all causes, myocardial infarction, and stroke. These results were confirmed after correcting for many possible confounders such as age, sex, smoking, alcohol consumption, income, dyslipidemia, body mass index, diabetes, hypertension, and physical activity[47].
As already discussed, diabetes and NAFLD are often associated; thus they may act synergistically to maximally increase cardiovascular risk[48]; the higher incidence of CVD in diabetic patients with steatosis compared to diabetic patients without steatosis[48] seems to confirm this detrimental association.
A study on > 130000 T2D patients with a hospital record of NAFLD or AFLD, and no record of any other liver disease, showed an increased risk for recurrent CVD, cancer, and mortality for all causes[49]. Patients with a history of hospital admission and fatty liver were younger than those without liver disease[50]. Of note, similar to what happens in healthy subjects and T2D patients, even in T1D patients, NAFLD increases the cardiovascular risk[51].
Figure 3 illustrates the association of T2D and NAFLD with multiple morbid conditions; thus the coexistence and interaction of the two, further exacerbates the prognosis of each.
From the very beginning of the severe acute respiratory syndrome coronavirus 2 pandemic, diabetes has shown an association to this virus infection. In fact, a study on 5700 patients admitted to 12 hospitals in the New York City area demonstrated that the most common comorbidities in admitted coronavirus disease 2019 (COVID-19) patients were hypertension (56.6%), obesity (41.7%), and diabetes (33.8%)[52]. Diabetes prevalence in COVID-19 patients is high, varying from 15%, in a pool of more than 23000 patients[53], up to almost 40% in another study on 200 hospitalized patients[54].
Diabetic patients have a higher risk of contracting COVID-19[55], a higher risk of hospitalization[54] and mortality[56].
NAFLD is also associated to COVID-19[57], to its severity progression, risk of intubation, dialysis and use of vasopressors[58], although in contrast, some authors[59-61] did not observe a higher risk of severe COVID-19 and intensive care unit access for NAFLD patients.
A longer viral shedding time[62] and a higher mortality for COVID-19 in NASH patients with advanced fibrosis[63] have also been reported.
NAFLD diagnosis is based on three criteria: (1) Absence of significant alcohol intake; (2) presence of hepatic steatosis; and (3) exclusion of other causes of liver disease.
Some clinical biomarkers are used to screen for or diagnose NAFLD, used in complex algorithms for risk stratification. They aim to combine various conditions, such as arterial hypertension with laboratory exams, like transaminases, to predict outcomes of the liver disease, but as single markers, they only provide poor sensitivity and specificity. Yet, their overall performance is limited, with further studies needed to transfer the initial thought cut-off values into the real clinical scenario[64].
It can therefore be asserted that due to the lack of available noninvasive methods to confirm the diagnosis of NAFLD, liver biopsy remains the gold standard to classify steatosis, and NASH. However, biopsy has limitations[65]; namely it is invasive, subject to sampling variability and observer-dependence, and most importantly, carries risks. Therefore, it is not offered to routinely assess the amount of fatty liver in NAFLD patients who may have simple steatosis, as reported in the majority of cases[6].
As previously mentioned, since NAFLD is a dynamic entity[47], varying through lifetime, imaging methods remain the most widely utilized tools to assess NAFLD patients and quantify the relative hepatic steatosis.
To date, various imaging methods have been utilized: ultrasonography, CT, magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS). More recently, other diagnostic tools measuring liver stiffness have entered clinical practice, in view of their practical utility, as reported in Table 1.
| Modality | Pros | Cons |
| US B-Mode | Lack of ionizing radiation | No panoramic view |
| Less expensive | Operator dependency | |
| Repeatable | Limited accuracy diagnosing mild hepatic steatosis | |
| Fast | Rather qualitative nature | |
| Can be performed at the bedside (no need to transport the patient) | Non simple steatosis/NASH differentiation | |
| Useful also for identification of other pathology such as liver lesions | ||
| QUS | Same as US B-Mode | Not always available |
| Quantitative and semiquantitative fat evaluation (less operator sensitive) | Need to buy newer machines and software | |
| Fibroscan | Quantitative evaluation (less operator sensitive) | Expensive equipment that doesn’t supply imaging evaluation |
| Lack of ionizing radiation | ||
| Fast | ||
| Can be performed at the bedside (no need to transport the patient) | ||
| CT | Fast | Ionizing radiation |
| Panoramic view | Limited accuracy diagnosing mild hepatic steatosis | |
| Volumetric rendering | Non simple steatosis/NASH differentiation | |
| High spatial resolution | ||
| Quantitative density evaluation | ||
| MRI | Highly accurate and reproducible for measuring hepatic fat | Expensive |
| Panoramic view | Examination time | |
| Lack of ionizing radiation | Software not always available | |
| Quantitative fat evaluation | ||
| MRS | Highly accurate and reproducible for measuring hepatic fat | Expensive |
| Panoramic view | Examination time | |
| Lack of ionizing radiation | Software not always available | |
| Quantitative fat evaluation | Evaluation of small portion of the liver | |
| Expertise required for data acquisition and analysis |
Conventional B-mode ultrasound (US) is the most widely used imaging modality for the noninvasive evaluation of hepatic steatosis, as first-line diagnostic imaging procedure, according to clinical practice guidelines[66]. Fatty liver infiltration is characterized by hyperechogenicity of the parenchyma and increasing attenuation of US waves in deeper parts, specifically where there is increasing steatosis[67]. However, US evaluation of fatty livers is based on the operator’s experience; in comparison to histology as reference standard, the overall sensitivity and specificity of B-mode US are, respectively, 84.8% and 93.6%, with 0.93 accuracy[68].
US elastography quantitatively evaluates liver stiffness. Two broad categories of imaging-based sonoelastography are currently in clinical use: strain elastography, which is influenced by the operator or physiologic forces that produce tissue deformation; and shear wave elastography (SWE), which instead results from the acoustic radiation force of the tissue displacement[69,70].
Fibroscan uses transient US elastography (TE) to measure hepatic elasticity by quantifying the shear wave velocity with ultrasonic echo pulses from low-frequency vibrations that are transmitted into the liver[71,72]. Since patients with > 66% steatosis at liver biopsy have a false-positive higher rate, via the Fibroscan XL probe it is also possible to investigate obese patients, given that during TE the transmission of a mechanical wave through the skin and subcutis could cause technical failure and unreliable measurements[73].
Controlled attenuation parameter (CAP) is another technique implemented on the Fibroscan device. The principle of CAP is to measure the acoustic attenuation in liver of shear waves generated by the probe. The amount of fat deposited in the liver can be inferred from the degree of attenuation[74]. In a multimodality study in patients with biopsy-proven NAFLD, it was shown that using a threshold of 261 dB/m CAP the methodic accuracy was 0.85 (95% confidence interval of 0.75–0.96) for steatosis diagnosis[75].
Two-dimensional SWE is an US technique providing visualization of viscoelastic properties of soft tissues in real time[76]. These techniques employ acoustic radiation force impulses that induce tissue motion at a microscopic level, which in turn produces tissue shear waves. The shear waves are related to tissue stiffness under simple assumptions, expressed as Young's module[77].
In the last several years, quantitative US measures, such as the ultrasonic attenuation coefficient and backscatter coefficient, derived from the raw radiofrequency echo data, have been considered a noninvasive tool for the objective assessment of hepatic steatosis[78].
A general limitation of all US-based methods evaluating liver fat content, including CAP, is that sonography exploits the attenuation of the propagated and reflected waves. While liver fat attenuates sound waves, many other liver pathologies such as hepatitis, hemochromatosis or fibrosis can also affect sound waves in the same manner[79].
CT evaluation of hepatic steatosis is based on the attenuation values of the liver parenchyma, assessed as Hounsfield units (HU), in association with tissue composition. The attenuation value of fat (approximately -100 HU) is much lower than that of soft tissue, so hepatic steatosis lowers the attenuation of liver parenchyma. Some studies have reported that contrast-enhanced venous CT and nonenhanced CT have comparable diagnostic accuracy for hepatic steatosis[80]; however, nonenhanced CT is usually preferred to avoid the potential errors of contrast-enhanced CT caused by variations in hepatic attenuation related to contrast injection methods and scan times. The two CT indexes most frequently used to assess steatosis are the absolute liver attenuation value (i.e. HU-liver) and the attenuation difference between the liver and spleen.
CT is accurate for the diagnosis of moderate-to-severe steatosis but is not as accurate for detecting mild steatosis. The threshold values of CT indices for the diagnosis of hepatic steatosis are quite variable, depending on the methods and populations used[81-83]. Furthermore, some factors may affect hepatic attenuation on CT, such as the presence of excess iron in the liver and ingestion of certain drugs such as amiodarone[84].
While CT and US assess hepatic steatosis through proxy parameters (echogenicity and attenuation, respectively), MRI can more directly measure the amount of hepatic fat, in fact it is an imaging modality with a rich range of contrast mechanisms detecting and quantifying hepatic fat content through the measurement of proton signals present in water and fat[85].
There are conventional MRI methods providing qualitative estimates of hepatic steatosis and fully quantitative MRS and MRI methods that allow for an accurate and precise measurement of hepatic fat content[86-88].
MRS and chemical shift-encoded MRI, when performed in expert hands, can serve as confounder-corrected methods able to discern the number of fat-bound protons divided by the amount of all protons in the liver, including fat- and water-bound protons[89].
To date, MRI especially with the techniques reported above, represent the noninvasive gold standard evaluation of these patients; however, US is broadly gaining popularity.
NAFLD treatment depends on the severity of the disease, ranging from a more benign condition of nonalcoholic fatty liver to nonalcoholic steatohepatitis, which is at the more severe end of the spectrum. However, there are some measures that can be applied to all patients. These include the following. (1) Abstinence from alcohol: evidence shows that in NAFLD patients, there is no liver-safe limit of alcohol intake[90]. Heavy alcohol use is well-known to be associated with hepatic steatosis, hepatic injury, and progression of parenchymal fibrosis[91], but even low alcohol consumption in individuals with metabolic abnormalities could be harmful, thus abstinence from alcohol for patients with NAFLD is always recommended. (2) Immunizations: for patients without serologic evidence of immunity, vaccination for hepatitis A virus and hepatitis B virus is recommended, and, in general, standard, age-appropriate immunizations for all patients[7]. (3) Modification of risk factors for CVD: For patients with hyperlipidemia, lipid-lowering therapy; for patients with diabetes, optimizing blood glucose control[9].
For patients with NASH and T2D, the presence of the liver disease can inform the choice of glucose lowering therapy, and although this is typically with metformin, the beneficial impact on liver histology with certain other insulin-sensitizing agents could be of note when choosing a second-line agent in NASH patients, if metformin is contraindicated or in need of additional glucose-lowering therapy[33,35]. In this setting, pioglitazone and GLP-1 receptor agonists (e.g., liraglutide, semaglutide) are reasonable options[92] and the apparent benefit of certain insulin-sensitizing agents for NAFLD is likely related to the role that insulin resistance plays in the development of NAFLD[9].
For patients with biopsy-proven NASH and fibrosis stage 2 but without diabetes, the use of vitamin E (800 international units per day) is suggested. The antioxidant, anti-inflammatory, and anti-apoptotic properties of vitamin E accompanied by the ease-of-use and exceptional tolerability have made vitamin E a pragmatic therapeutic choice in nondiabetic patients with histologic evidence of NASH[93].
In every case, weight loss is the primary therapy for most patients with NAFLD. It can lead to improvement in liver biochemical tests, liver histology, serum insulin levels, and quality of life[94-96].
Several studies have suggested that weight loss of at least 5% of body weight is necessary to improve hepatic steatosis, although the long-term benefits of such weight loss are unknown. In a meta-analysis of eight trials including 373 patients, losing 5% of body weight resulted in improvement in hepatic steatosis, while losing of 7% of body weight was associated with improvement in NALFD activity score, which is used to grade disease activity[97].
Unfortunately, only less than 10% of patients that try to lose weight with lifestyle modifications, including diet and physical activity, achieve this target at 1-year, and fewer maintain the weight loss at 5 years[98]. Bariatric surgery is an option that may be considered in those who fail to lose weight by lifestyle changes.
Although weight loss seems to be the main mechanism, bariatric surgery has been shown to improve also liver histology and fibrosis secondary to NASH, in addition to other benefits including an improvement or resolution of T2D mellitus, dyslipidemia, and hypertension, and a reduction of cardiovascular morbidity or mortality[99-101].
A meta-analysis of 10 studies showed that the bariatric surgery group had significantly lower odds of major adverse cardiovascular events as compared to no surgery (odds ratio = 0.49; 95% confidence interval: 0.40-0.60; P < 0.00001; I2 = 93%) suggesting the benefit of bariatric surgery in reducing the occurrence of serious events in patients with obesity and CVDs[102].
In the SPLENDOR study of 1158 patients with histologically confirmed NASH and obesity, bariatric surgery (gastric bypass or sleeve gastrectomy) was associated with a much lower 10-year cumulative incidence of major adverse liver outcomes (2.3% vs 9.6%) and major cardiovascular events (8.5% vs 15.7%) compared with nonsurgical management[103].
Weight reduction due to bariatric surgery causes inflammatory changes in patients with obesity. After gastric bypass there is a proven reduction of hepatic expression of factors involved in the progression of liver inflammation (macrophage chemoattractant protein 1, and interleukin-8) and fibrogenesis [transforming growth factor-β1, tissue inhibitor of metalloproteinase 1, α-smooth muscle actin, and collagen-α1(I)][104], a significant decrease in mean NAFLD fibrosis score after Roux-en-Y gastric bypass (RYGB) and resolution rate of 55% of severe fibrosis in 12-mo observation[105], and, moreover, RYGB contributes to significant reduction in NAFLD activity score, steatosis, inflammation and liver ballooning during 1-year observation[106].
In a long-term follow-up of patients with NASH who underwent bariatric surgery, Lassailly et al[107] observed resolution of NASH in liver biopsies from 84% of patients 5 years later. The reduction of fibrosis is progressive, beginning during the 1st year and continuing through 5 years[107].
Among recently available surgical methods, RYGB and laparoscopic sleeve gastrectomy (LSG) are the most performed worldwide. The remaining question is whether RYGB or LSG is more effective[108].
A systematic review and meta-analysis performed by Baldwin et al[109] compared RYGB and LSG using separate criteria: transaminases concentration, NAFLD activity score and NAFLD fibrosis score. Overall, both RYGB and LSG significantly improved liver enzymes, NAFLD activity score, and NAFLD fibrosis score postoperatively. Direct comparisons of RYGB to LSG in any of the criteria failed to demonstrate superiority[109]. These findings, without any significant difference between the two groups, are confirmed in other studies[110,111].
Even if the role of bariatric surgery in the treatment of NAFLD is significant, there are some patients that will develop new or worsened features of NAFLD after a bariatric procedure[112]. A 5-year prospective study performed by Mathurin et al[113] showed that 19.8% of patients experienced fibrosis progression at 5 years follow up for unknown reason.
Aggravation of NAFLD after surgery should be kept in mind when qualifying patients for a bariatric procedure. At the extreme consequences, and when the progression of liver fibrosis is irreversible, also liver transplantation becomes an option, and indeed NASH is nowadays representing the fastest growing indication in Western countries to this kind of surgery. Yet, lifestyle modifications, as well as pharmacological strategies and tailored immunosuppression via a strategic multidisciplinary approach are still key to control diabetes and CVD risk in this setting, too[114].
NAFLD is intimately related to T2D and both diseases are highly prevalent worldwide, representing a public health alarm. The diagnosis and management of NAFLD in T2D is challenging, given the inherent cardiovascular risk and the underlying liver parenchymal degeneration. As well as to insulin resistance, NAFLD may be related to other hormonal alterations, quite common in patients with obesity, and potentially contributing to the onset and the worsening of steatohepatitis. A complete hormonal workout, in patients with severe NAFLD, and conversely investigation of NAFLD in patients with T2D, severe obesity or other metabolic disorders is recommended to prevent and monitor NAFLD risk.
Current medical treatments aim to mitigate insulin resistance, optimizing metabolic control and halting hepatic disease progression; yet they are still under debate for their efficacy, and new classes of drugs targeting different pathways need experimentation in the forms of randomized controlled trials, to pursue a tailor-made approach, for example assessing gut permeability and modification of individual human microbiota.
Identification of simple, inexpensive biomarkers would be also of help as an additional diagnostic tool, or to predict disease progression and response to treatment.
Surgery is considered a more advanced therapeutic option, either to improve obesity and control of the associated metabolic conditions, via bariatric interventions, either by substituting the cirrhotic liver via organ transplantation.
Future research should focus on the treatment of NAFLD, as a risk factor for developing T2D and in how to prevent and detect NAFLD progression in patients with T2D, obesity or other severe metabolic conditions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hua J, China; Luo LY, China; Yang M, United States; Zhang XL, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 773] [Cited by in F6Publishing: 1099] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6281] [Article Influence: 785.1] [Reference Citation Analysis (0)] |
| 3. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 215] [Article Influence: 107.5] [Reference Citation Analysis (2)] |
| 4. | Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, Nguyen P, Hernandez C, Richards L, Salotti J, Lin S, Seki E, Nelson KE, Sirlin CB, Brenner D; Genetics of NAFLD in Twins Consortium. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 5. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 781] [Cited by in F6Publishing: 727] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 680] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 7. | Li B, Zhang C, Zhan YT. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1706] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 9. | Głuszyńska P, Lemancewicz D, Dzięcioł JB, Razak Hady H. Non-Alcoholic Fatty Liver Disease (NAFLD) and Bariatric/Metabolic Surgery as Its Treatment Option: A Review. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49:471-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1163] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 12. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1188] [Cited by in F6Publishing: 1576] [Article Influence: 394.0] [Reference Citation Analysis (1)] |
| 13. | Hirsova P, Ibrabim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Di Ciaula A, Passarella S, Shanmugam H, Noviello M, Bonfrate L, Wang DQ, Portincasa P. Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies? Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 58] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 15. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 16. | Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 603] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 17. | Di Ciaula A, Bonfrate L, Portincasa P. The role of microbiota in nonalcoholic fatty liver disease. Eur J Clin Invest. 2022;52:e13768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Portincasa P, Bonfrate L, Khalil M, Angelis M, Calabrese FM, D'Amato M, Wang DQ, Di Ciaula A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, Wu P, Liu X, Yu Y, Farris AB, Nusrat A, Parkos CA, Anania FA. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151:733-746.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Dongiovanni P, Romeo S, Valenti L. Genetic Factors in the Pathogenesis of Nonalcoholic Fatty Liver and Steatohepatitis. Biomed Res Int. 2015;2015:460190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue-Valleskey JM, Hoogwerf BJ, Haupt A. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017;19:1630-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | de Vries M, Westerink J, Kaasjager KHAH, de Valk HW. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients With Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 364] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 24. | Gangitano E, Ginanni Corradini S, Lubrano C, Gnessi L. La Non-Alcoholic Fatty Liver Disease, una patologia epatica di interesse endocrinologico. L'Endocrinologo. 2021;22:436-440. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Dharmalingam M, Yamasandhi PG. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Indian J Endocrinol Metab. 2018;22:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 303] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 27. | Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 28. | Franch-Nadal J, Caballeria L, Mata-Cases M, Mauricio D, Giraldez-García C, Mancera J, Goday A, Mundet-Tudurí X, Regidor E; PREDAPS Study Group. Fatty liver index is a predictor of incident diabetes in patients with prediabetes: The PREDAPS study. PLoS One. 2018;13:e0198327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Bellini MI, Paoletti F, Herbert PE. Obesity and bariatric intervention in patients with chronic renal disease. J Int Med Res. 2019;47:2326-2341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 205] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 31. | Cuthbertson DJ, Koskinen J, Brown E, Magnussen CG, Hutri-Kähönen N, Sabin M, Tossavainen P, Jokinen E, Laitinen T, Viikari J, Raitakari OT, Juonala M. Fatty liver index predicts incident risk of prediabetes, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Ann Med. 2021;53:1256-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kitazawa A, Maeda S, Fukuda Y. Fatty liver index as a predictive marker for the development of diabetes: A retrospective cohort study using Japanese health check-up data. PLoS One. 2021;16:e0257352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 34. | Song D, Li C, Wang Z, Zhao Y, Shen B, Zhao W. Association of non-alcoholic fatty liver disease with diabetic retinopathy in type 2 diabetic patients: A meta-analysis of observational studies. J Diabetes Investig. 2021;12:1471-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Mantovani A, Dalbeni A, Beatrice G, Cappelli D, Gomez-Peralta F. Non-Alcoholic Fatty Liver Disease and Risk of Macro- and Microvascular Complications in Patients with Type 2 Diabetes. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, Maffeis C, Colecchia A, Villani R, Rinaldi L, Orsi E, Pisano G, Adinolfi LE, Fargion S, Fracanzani AL. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020;40:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Takahashi S, Tanaka M, Furuhashi M, Moniwa N, Koyama M, Higashiura Y, Osanami A, Gocho Y, Ohnishi H, Numata K, Hisasue T, Hanawa N, Miura T. Fatty liver index is independently associated with deterioration of renal function during a 10-year period in healthy subjects. Sci Rep. 2021;11:8606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Kouvari M, Boutari C, Chrysohoou C, Fragkopoulou E, Antonopoulou S, Tousoulis D, Pitsavos C, Panagiotakos DB, Mantzoros CS; ATTICA study Investigators. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: Results from the ATTICA prospective cohort study. Clin Nutr. 2021;40:3314-3324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 40. | GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4187] [Cited by in F6Publishing: 3317] [Article Influence: 552.8] [Reference Citation Analysis (0)] |
| 41. | American Diabetes Association. . 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S111-S134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 348] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 42. | Han AL. Association of Cardiovascular Risk Factors and Metabolic Syndrome with non-alcoholic and alcoholic fatty liver disease: a retrospective analysis. BMC Endocr Disord. 2021;21:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 44. | Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:903-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 45. | Higashiura Y, Furuhashi M, Tanaka M, Takahashi S, Mori K, Miyamori D, Koyama M, Ohnishi H, Moniwa N, Numata K, Hisasue T, Hanawa N, Miura T. Elevated Fatty Liver Index Is Independently Associated With New Onset of Hypertension During a 10-Year Period in Both Male and Female Subjects. J Am Heart Assoc. 2021;10:e021430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Park J, Kim G, Kim H, Lee J, Lee YB, Jin SM, Hur KY, Kim JH. The association of hepatic steatosis and fibrosis with heart failure and mortality. Cardiovasc Diabetol. 2021;20:197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Lee CH, Han KD, Kim DH, Kwak MS. The Repeatedly Elevated Fatty Liver Index Is Associated With Increased Mortality: A Population-Based Cohort Study. Front Endocrinol (Lausanne). 2021;12:638615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Caussy C, Aubin A, Loomba R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr Diab Rep. 2021;21:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 49. | Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, McGurnaghan S, McCrimmon R, Read SH, Sattar N, Byrne CD; Scottish Diabetes Research Network Epidemiology Group. Cardiovascular Disease, Cancer, and Mortality Among People With Type 2 Diabetes and Alcoholic or Nonalcoholic Fatty Liver Disease Hospital Admission. Diabetes Care. 2018;41:341-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 50. | Cavender MA, Steg PG, Smith SC Jr, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL; REACH Registry Investigators. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 51. | Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of chronic kidney disease in patients with Type 1 diabetes and non-alcoholic fatty liver. Diabet Med. 2012;29:220-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6024] [Cited by in F6Publishing: 6161] [Article Influence: 1540.3] [Reference Citation Analysis (0)] |
| 53. | Hussain S, Baxi H, Chand Jamali M, Nisar N, Hussain MS. Burden of diabetes mellitus and its impact on COVID-19 patients: A meta-analysis of real-world evidence. Diabetes Metab Syndr. 2020;14:1595-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, Chaudhry Z, Vahia A, Abreu Lanfranco O, Ramesh M, Zervos MJ, Alangaden G, Miller J, Brar I. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 410] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 55. | Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 56. | Miller LE, Bhattacharyya R, Miller AL. Diabetes mellitus increases the risk of hospital mortality in patients with Covid-19: Systematic review with meta-analysis. Medicine (Baltimore). 2020;99:e22439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Dongiovanni P, Meroni M, Longo M, Fracanzani AL. MAFLD in COVID-19 patients: an insidious enemy. Expert Rev Gastroenterol Hepatol. 2020;14:867-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 59. | Trivedi HD, Wilechansky R, Goyes D, Vieira Barbosa J, Canakis A, Lai M, Long MT, Fricker Z. Radiographic Hepatic Steatosis Is Not Associated With Key Clinical Outcomes Among Patients Hospitalized With COVID-19. Gastroenterology Res. 2021;14:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, Chen P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553-1560.e78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 61. | Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 62. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 377] [Article Influence: 94.3] [Reference Citation Analysis (2)] |
| 63. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 64. | Buckley AJ, Thomas EL, Lessan N, Trovato FM, Trovato GM, Taylor-Robinson SD. Non-alcoholic fatty liver disease: Relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res Clin Pract. 2018;144:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 66. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2694] [Article Influence: 336.8] [Reference Citation Analysis (2)] |
| 67. | Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053-6062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 180] [Cited by in F6Publishing: 195] [Article Influence: 39.0] [Reference Citation Analysis (4)] |
| 68. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 974] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 69. | Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, Yin M, Miller FH, Low RN, Hassanein T, Godfrey EM, Asbach P, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 70. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 876] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 71. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 72. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 73. | Dyson JK, McPherson S, Anstee QM. Republished: Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. Postgrad Med J. 2014;90:254-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Abdelmalek M, Doo E, Tonascia JA, Kleiner DE, Sanyal AJ, Chalasani N; NASH Clinical Research Network. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 75. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 76. | Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1835] [Cited by in F6Publishing: 1418] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 77. | Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Saito H, Ebinuma H, Lupsor M, Badea R, Fierbinteanu-Braticevici C, Petrisor A, Friedrich-Rust M, Sarrazin C, Takahashi H, Ono N, Piscaglia F, Marinelli S, D'Onofrio M, Gallotti A, Salzl P, Popescu A, Danila M. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis. 2013;45:762-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Jeon SK, Lee JM, Joo I. Clinical Feasibility of Quantitative Ultrasound Imaging for Suspected Hepatic Steatosis: Intra- and Inter-examiner Reliability and Correlation with Controlled Attenuation Parameter. Ultrasound Med Biol. 2021;47:438-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Siegelman ES. MR imaging of diffuse liver disease. Hepatic fat and iron. Magn Reson Imaging Clin N Am. 1997;5:347-365. [PubMed] [Cited in This Article: ] |
| 80. | Kim DY, Park SH, Lee SS, Kim HJ, Kim SY, Kim MY, Lee Y, Kim TK, Khalili K, Bae MH, Lee JY, Lee SG, Yu ES. Contrast-enhanced computed tomography for the diagnosis of fatty liver: prospective study with same-day biopsy used as the reference standard. Eur Radiol. 2010;20:359-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, Suh DJ, Kim KM, Bae MH, Lee JY, Lee SG, Yu ES. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 82. | van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, Stoker J. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 83. | Hepburn MJ, Vos JA, Fillman EP, Lawitz EJ. The accuracy of the report of hepatic steatosis on ultrasonography in patients infected with hepatitis C in a clinical setting: a retrospective observational study. BMC Gastroenterol. 2005;5:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Lv HJ, Zhao HW. Amiodarone-induced hepatotoxicity - quantitative measurement of iodine density in the liver using dual-energy computed tomography: Three case reports. World J Clin Cases. 2020;8:4958-4965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, Reeder SB. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 86. | Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9:715-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 446] [Article Influence: 63.7] [Reference Citation Analysis (13)] |
| 87. | Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology. 2018;68:763-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 88. | Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, Hu HH, Hetterich H, Kühn JP, Kukuk GM, Loomba R, Middleton MS, Obuchowski NA, Song JS, Tang A, Wu X, Reeder SB, Sirlin CB; RSNA-QIBA PDFF Biomarker Committee. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology. 2018;286:486-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 89. | Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 90. | Di Ciaula A, Bonfrate L, Krawczyk M, Frühbeck G, Portincasa P. Synergistic and Detrimental Effects of Alcohol Intake on Progression of Liver Steatosis. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, Kechagias S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 92. | Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 93. | Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, Cholankeril G, Kim D, Ahmed A. The Role of Vitamin E in the Treatment of NAFLD. Diseases. 2018;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 94. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1181] [Cited by in F6Publishing: 1302] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 95. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 973] [Cited by in F6Publishing: 884] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 96. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 97. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 98. | Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S-225S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1171] [Cited by in F6Publishing: 1093] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 99. | Ha J, Jang M, Kwon Y, Park YS, Park DJ, Lee JH, Lee HJ, Ha TK, Kim YJ, Han SM, Han SU, Heo Y, Park S. Metabolomic Profiles Predict Diabetes Remission after Bariatric Surgery. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Diemieszczyk I, Woźniewska P, Gołaszewski P, Drygalski K, Nadolny K, Ładny JR, Razak Hady H. Does weight loss after laparoscopic sleeve gastrectomy contribute to reduction in blood pressure? Pol Arch Intern Med. 2021;131:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, Thabane L, Hong D, Yusuf S, Anvari M. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease:: A Population-Based Retrospective Cohort Study. Circulation. 2021;143:1468-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 102. | Sutanto A, Wungu CDK, Susilo H, Sutanto H. Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 103. | Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, Albaugh VL, Shariff FU, Rodriguez NA, Jin J, Brethauer SA, Dasarathy S, Alkhouri N, Schauer PR, McCullough AJ, Nissen SE. Association of Bariatric Surgery With Major Adverse Liver and Cardiovascular Outcomes in Patients With Biopsy-Proven Nonalcoholic Steatohepatitis. JAMA. 2021;326:2031-2042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 104. | Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: a prospective study. Obes Surg. 2015;25:982-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 106. | Pedersen JS, Rygg MO, Serizawa RR, Kristiansen VB, Albrechtsen NJW, Gluud LL, Madsbad S, Bendtsen F. Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Non-Alcoholic Fatty Liver Disease: A 12-Month Follow-Up Study with Paired Liver Biopsies. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 108. | Cerci M, Bellini MI, Russo F, Benavoli D, Capperucci M, Gaspari AL, Gentileschi P. Bariatric surgery in moderately obese patients: a prospective study. Gastroenterol Res Pract. 2013;2013:276183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 109. | Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis. 2019;15:2123-2130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 110. | de Brito E Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-analysis. Obes Surg. 2021;31:2762-2772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 111. | Cherla DV, Rodriguez NA, Vangoitsenhoven R, Singh T, Mehta N, McCullough AJ, Brethauer SA, Schauer PR, Aminian A. Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg Endosc. 2020;34:2266-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1040-1060.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 113. | Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, Romon M, Pattou F. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 114. | Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |