Published online May 15, 2022. doi: 10.4239/wjd.v13.i5.408
Peer-review started: December 13, 2021
First decision: January 12, 2022
Revised: January 28, 2022
Accepted: April 28, 2022
Article in press: April 28, 2022
Published online: May 15, 2022
Diabetic retinopathy (DR) is a diabetic complication that can severely affect the patients’ vision, eventually leading to blindness. DR is the most important manifestation of diabetic micro-vasculopathy and is mainly related to the course of diabetes and the degree of blood glucose control, while the age of diabetes onset, sex, and type of diabetes have little influence on it.
To explore the changes in blood oxygen saturation and oxidative stress indices of retinal vessels in patients with DR.
In total, 94 patients (94 eyes) with DR (DR group) diagnosed at Jianyang people’s Hospital between March 2019 and June 2020, and 100 volunteers (100 eyes) (control group) without eye diseases, were included in this study. Arterial and venous blood oxygen saturation, retinal arteriovenous vessel diameter, and serum oxidative stress indicators in the two groups were compared. Based on the stage of the disease, the DR group was divided into the simple DR and proliferative DR groups for stratified analysis.
The oxygen saturation of the retinal vessels in the DR group was significantly higher than that in the control group (P < 0.05). The retinal vessel diameters between the DR and control groups were not significantly different. The serum malondialdehyde (MDA) and 8-hydroxydehydroguanosine (8-OHdG) levels in the DR group were significantly higher than those in the control group (P < 0.05). The serum superoxide dismutase (SOD) and reduced glutathione (GSH) levels in the DR group were significantly lower than those in the control group (P < 0.05). The oxygen saturation of the retinal vessels in the patients with proliferative DR was significantly higher than that in the patients with simple DR (P < 0.05). The retinal vessel diameter in patients with proliferative DR was not significantly different from that of patients with simple DR (P > 0.05). Serum MDA and 8-OHdG levels in patients with proliferative DR were significantly higher than those in patients with simple DR (P < 0.05). Serum SOD and GSH levels in patients with proliferative DR were significantly lower than those in patients with simple DR (P < 0.05).
Increased blood oxygen saturation of retinal arteries and veins and increased oxidative stress damage in patients with DR may be associated with decreased retinal capillary permeability and arterial oxygen dispersion, possibly reflecting the patient’s condition.
Core Tip: Diabetic retinopathy (DR) is a complication of diabetes. Studies have shown that retinal blood oxygen saturation and oxidative stress are closely associated with hypoxic-ischemic injury of retinal tissues caused by DR. Although DR treatment has improved in recent years, the long-term prognosis for late DR is not optimistic, and effective methods are needed to prevent DR from deteriorating in the later stages. Therefore, determining the incidence of DR and establishing its early diagnosis are considered clinically important. Our study monitored and analyzed retinal blood vessels, reflecting changes in serum biological indicators of blood oxygen saturation and oxidative stress levels in patients with DR, to determine the patient’s condition, thus improving the existing diagnosis and treatment methods and developing new methods for the treatment of serious complications of diabetes and subsequently ameliorating the cure rate of patients.
- Citation: Wang XL, Cai FR, Gao YX, Zhang J, Zhang M. Changes and significance of retinal blood oxygen saturation and oxidative stress indexes in patients with diabetic retinopathy. World J Diabetes 2022; 13(5): 408-416
- URL: https://www.wjgnet.com/1948-9358/full/v13/i5/408.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i5.408
Diabetic retinopathy (DR) is one of the main complications of diabetes and includes ophthalmic and microvascular lesions caused by abnormal insulin metabolism in patients with diabetes. This can lead to impaired ocular nutrition and visual function, which, in turn, can lead to adult blindness[1,2], making DR one of the main causes of vision impairment and blindness globally. The incidence of retinal complications can increase with disease duration. Over time, up to 50% of people with type 1 diabetes and 30% of people with type 2 diabetes may potentially develop visually threatening retinal changes, with symptoms that are not evident during the early stage[3,4]. High blood glucose level, hypertension, renal disease, and hyperlipidemia are all typical diseases associated with diabetes, which are related to the pathogenesis of DR. Moreover, in diabetes, the pathological cascade of blood vessel injury can be caused by high blood glucose level and the related oxidative stress[5]. Due to the subsequent vascular wall interference, the permeability of the blood-retinal barrier is disrupted, which leads to hypoxia and eventually, retinal nutrition degeneration and photoreceptor cell death. The subsequent progression of retinopathy leads to retinal neovascularization, vitreous hemorrhage, and fibrous tissue formation in the preretinal hemorrhage focus[6]. Although the treatment for DR has improved in recent years, the long-term prognosis for late DR remains poor, and it is necessary to determine effective ways to prevent progression of DR in the later stages. Further, despite the transparency regarding the pathogenesis and treatment of DR in recent years, several questions remain unanswered. Currently, most DR treatments are mainly focused on the later stage, failing to address the early, potentially reversible stage of the disease; moreover, patients in the late stage have more complications. Therefore, determining the incidence of DR and establishing its early diagnosis have important prognostic and scientific values, which may help to improve the existing methods and provide new methods for the diagnosis and treatment of diabetes with severe complications.
According to recent studies[7-9], hypoxic-ischemic injury of retinal tissues caused by diabetic microangiopathy is closely associated with retinal blood oxygen saturation. Retinal blood oxygen saturation is associated with disease severity and is significantly higher in patients with DR than in normal individuals. The human retina, an energy-demanding organ that is particularly sensitive to reactive oxygen species and lipid peroxides, is rich in polyunsaturated fatty acids and free radicals/reactive oxygen species. Studies have suggested that oxidative stress is an important pathogenic mechanism of diabetic complications caused by hyperglycemia, and the incidence of DR is associated with high oxidative stress in the body and the accompanying oxidative damage[10,11]. Biomarkers are biochemical indicators that can be used to mark changes in the structure or function of systems, organs, tissues, and cells. Effective biomarkers have been used for the diagnosis and classification of various diseases, including diabetes and diabetic microangiopathy. Serum malondialdehyde (MDA), 8-hydroxydehydroguanosine (8-OHdG), superoxide dismutase (SOD), and reduced glutathione (GSH) are common indicators of clinical oxidative stress. Currently, studies assessing the correlation between changes in blood oxygen saturation, oxidative stress markers, and risk of DR are limited. Therefore, our study mainly aimed to explore and discuss the changes in blood oxygen saturation and oxidative stress markers in patients with DR to reflect their condition to a certain extent, and for the early detection and treatment of patients, thus, improving the cure rate of patients.
A total of 94 patients (94 eyes) with DR (DR group) diagnosed at Jianyang people’s Hospital between March 2019 and June 2020, and 100 volunteers (100 eyes) (control group) without eye diseases, were included in this study. The inclusion criteria comprised: patients (1) With DR aged 48–79 years; and (2) who met the diagnostic criteria of DR, as established by Practical Ophthalmology (3rd Edition)[12]. The exclusion criteria comprised: patients (1) With eye infection; (2) cataract; (3) glaucoma; (4) high myopia or hyperopia; (5) acute myocardial infarction; and (6) diseases of the blood system and autoimmune diseases. Based on the formation of retinal neovascularization, DR was divided into proliferative and non-proliferative DR.
Fundus vessel findings were confirmed by fundus fluorescein angiography (FFA). The control group consisted of volunteers with normal fundus and FFA findings.
The study bidding document and related materials were used after the medical ethics committee approved this study and decided the disease release. Informed consent was signed by patients and their families before the implementation of the study.
The OXYMAP T1 non-invasive retinal oxygen saturation analyzer was used to measure the retinal dynamic venous oxygen saturation of all participants before and after treatment. All the test operations were performed by the same technician; the test was repeated three times, and the average value was recorded.
We administered tropicamide and waited for 5 min for pupil dilation, following which, an APS-B fundus color camera (Chongqing Kanghua Technology Co., Ltd.) was used to obtain 300-field fundus photos of the posterior pole of each eye. A 2.5-cm diameter circle was drawn with the center of the optic disc as the center. Two concentric circles (0.5- and 1.0-cm diameters, respectively) were then drawn from the edge of optic disc. The superior temporal and inferior retinal arteriovenous diameters intersecting the two concentric circles were measured twice at each measuring point.
Serum MDA, 8-OHdG, SOD, and GSH levels were evaluated as follows: After maintaining 1 wk of glucose stability, a 5-mL fasting venous blood sample was collected from the patients with type 2 diabetes mellitus and from the control group on the next morning. Serum separation was performed at 3000 r/min. SOD level was measured using the xanthine oxidase method, MDA level was measured using the thiobarbital method, and GSH level was measured using the dithiobarbital colorimetric method. A competitive inhibition enzyme-linked immunosorbent assay was used to measure the 8-OHdG level. The kit was purchased from Trevigen (USA), and the procedure was performed strictly in accordance with the kit instructions.
The measurement indices of the patients in this study, such as retinal artery and retinal vein oxygen saturation, age, body mass index, and serum MDA and 8-OHdG levels, were in line with approximate normal distribution or normal distribution, based on the P-P plot and Q-Q plot, and expressed as mean ± SD. Enumeration data are expressed as percentage. The
Baseline data, including age, sex, affected side distribution, hypertension, and diabetes in the DR and control groups were collected and analyzed; the two groups had good equilibrium and comparability (P > 0.05), and all patients in the two groups had good equilibrium (Table 1).
| Group | DR group (n = 94) | Control group (n = 100) | t/χ2 value | P value |
| Age (yr) | 65.8 ± 7.0 | 64.4 ± 8.1 | 1.284 | 0.201 |
| BMI (kg/m2) | 24.6 ± 1.9 | 24.4 ± 2.0 | 0.713 | 0.477 |
| Sex | 1.195 | 0.274 | ||
| Male | 51 (54.26) | 62 (62.00) | ||
| Female | 43 (45.74) | 38 (38.00) | ||
| Affected side distribution | 1.959 | 0.162 | ||
| Left side | 47 (50.00) | 60 (60.00) | ||
| Right | 47 (50.00) | 40 (40.00) | ||
| Hypertension | 2.561 | 0.110 | ||
| Yes | 18 (19.15) | 29 (29.00) | ||
| No | 76 (80.85) | 71 (71.00) | ||
| Dyslipidemia | 1.909 | 0.176 | ||
| Yes | 32 (34.04) | 25 (25.00) | ||
| No | 62 (65.96) | 75 (75.00) |
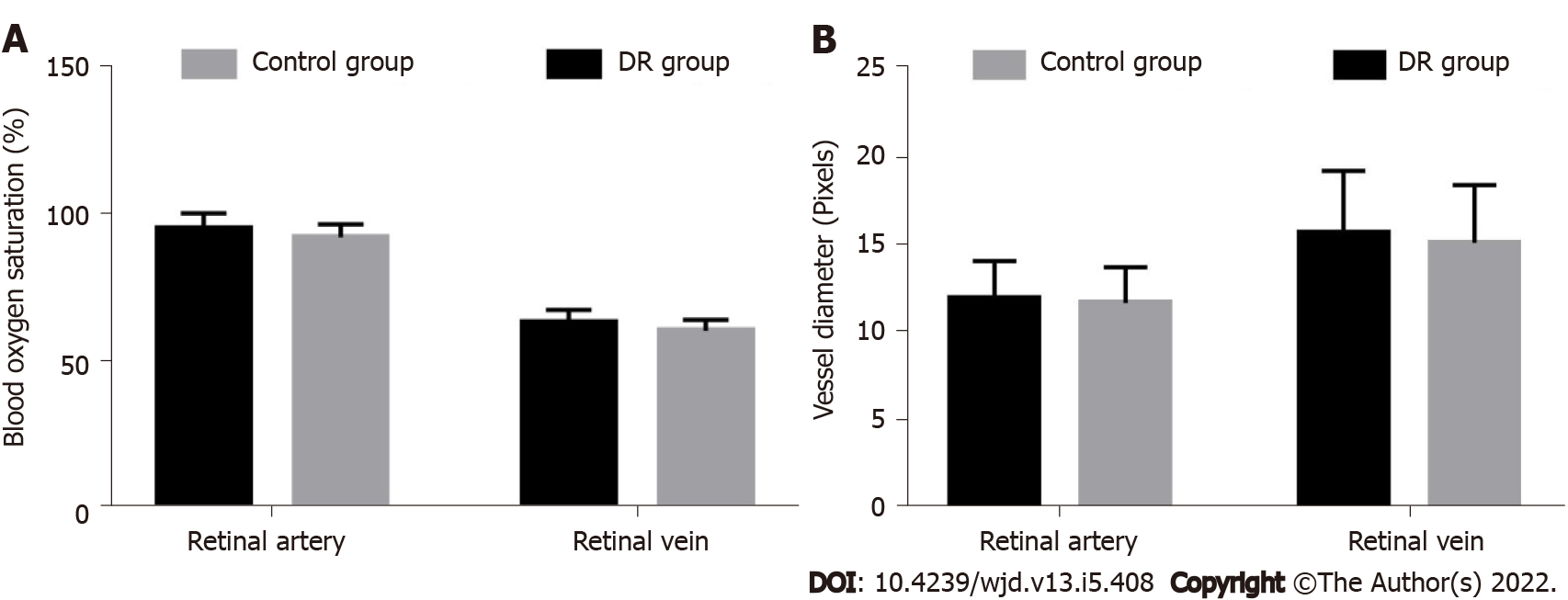
The retinal artery and venous oxygen saturation in the DR group were 95.70 ± 5.20% and 63.50 ± 4.41%, respectively, which were significantly higher than those in the control group (92.63 ± 4.50% and 60.83 ± 3.72%, respectively) (t-values, 4.405 and 4.568, respectively; P < 0.05) (Figure 1A).
The diameters of the retinal arteries and veins were 12.06 ± 2.15 pixels and 15.83 ± 3.56 pixels in the DR group and 11.80 ± 2.07 pixels and 15.27 ± 3.30 pixels in the control group, respectively. The difference was not statistically significant (t-values, 0.858 and 1.137, respectively; P > 0.05) (Figure 1B).
Serum MDA and 8-OHdG levels in the DR group were significantly higher than those in the control group (P < 0.05). Compared with the control group, patients in the DR group had a significantly more aggravated oxidative stress injury (Table 2).
| Group | DR group (n = 94) | Control group (n = 100) | t value | P value |
| MDA (μmol/L) | 7.50 ± 1.50 | 3.82 ± 0.84 | 21.246 | < 0.05 |
| 8-HdG (ng/mL) | 107.50 ± 22.51 | 49.63 ± 8.11 | 24.102 | < 0.05 |
| SOD (U/L) | 71.33 ± 14.80 | 93.64 ± 17.26 | -9.637 | < 0.05 |
| GSH (mg/L) | 163.81 ± 38.51 | 211.07 ± 25.46 | -10.14 | < 0.05 |
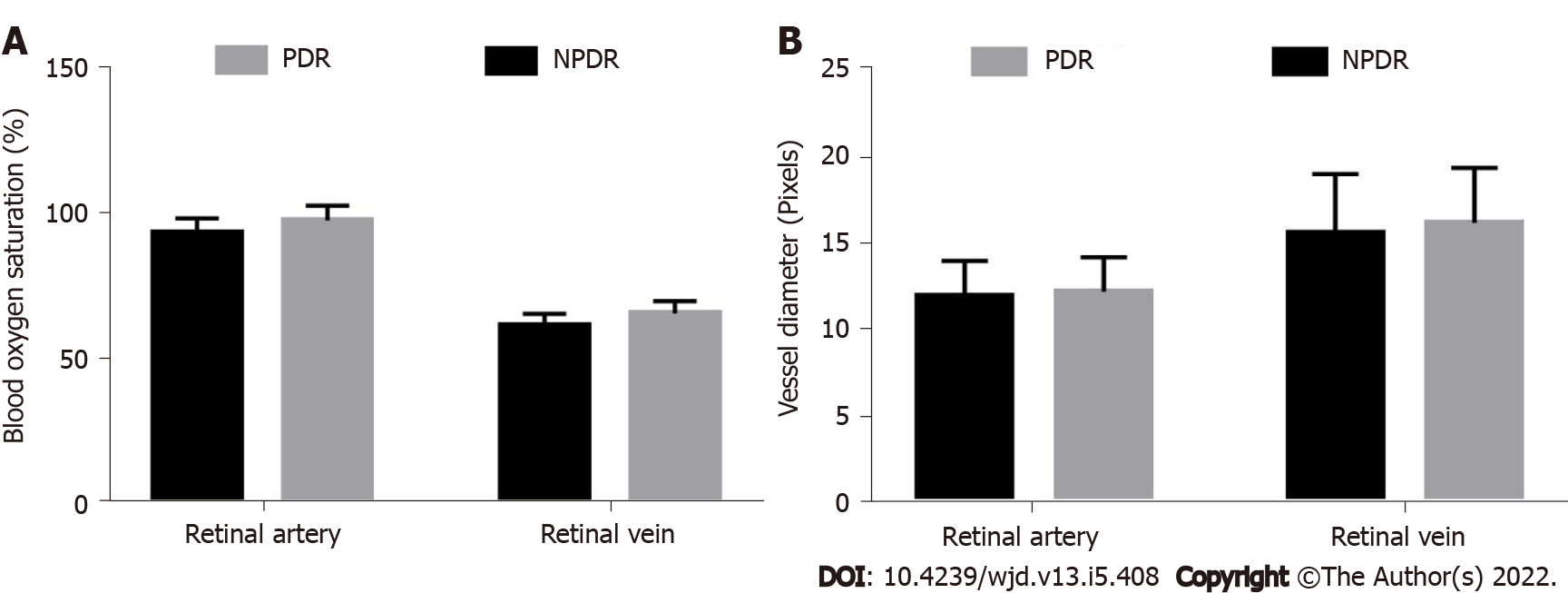
In patients with proliferative DR, the retinal artery and venous oxygen saturation were 94.00 ± 4.95% and 61.80 ± 4.17%, respectively, which were significantly lower than those in patients with simple DR (98.21 ± 5.13% and 66.01 ± 4.28%, respectively) (t-values, −3.988 and −4.753, respectively; P < 0.05) (Figure 2A).
The retinal artery and vein diameters were 12.22 ± 1.98 pixels and 16.17 ± 3.18 pixels, respectively, in the proliferative DR group, and 11.95 ± 2.04 pixels and 15.60 ± 3.38 pixels, respectively, in the simple DR group. The difference was not statistically significant (t-values, −0.637 and −0.822, respectively; P > 0.05) (Figure 2B).
Serum MDA and 8-OHdG levels in patients with proliferative DR were significantly higher than those in patients with simple DR (P < 0.05). Serum SOD and GSH levels in patients with proliferative DR were significantly lower than those in patients with simple DR (P < 0.05) (Table 3).
| Disease classification | Simple (n = 56) | Proliferative (n = 38) | t value | P value |
| MDA (μmol/L) | 6.11 ± 1.38 | 9.55 ± 1.43 | -11.688 | < 0.05 |
| 8-HdG (ng/mL) | 90.53 ± 20.54 | 132.51 ± 19.82 | -9.862 | < 0.05 |
| SOD (U/L) | 83.02 ± 11.68 | 54.10 ± 13.01 | 11.249 | < 0.05 |
| GSH (mg/L) | 187.46 ± 32.74 | 128.96 ± 26.74 | 9.135 | < 0.05 |
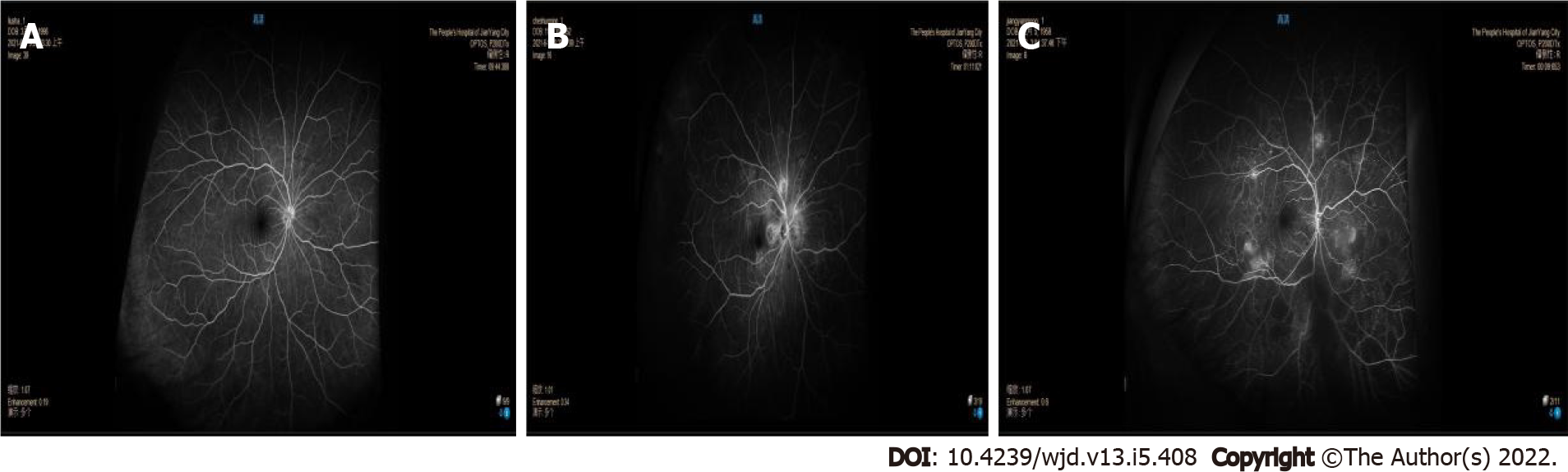
The results of retinal color images of different subjects were shown in the Figure 3.
DR is the most common and severe blood vessel complication in diabetes, and its incidence has been increasing annually. Progression of DR from the non-proliferative stage to the proliferative stage can lead to irreversible visual damage, which is an important cause of blindness in the patient. The pathogenesis of DR is complex, and the main pathological changes include changes in the intraocular environment caused by abnormal blood vessel proliferation, resulting in retinal tissue hypoxic-ischemic injury[13,14].
Our study showed that the oxygen saturation of the retinal artery and retinal vein in the DR group was significantly higher than that in the control group. This can be explained by the reduced retinal oxygen consumption in DR. Considering these results, more in-depth and reliable studies on retinal oxygen consumption in DR should be conducted in the future. Furthermore, in this study, the retinal artery and retinal vein oxygen saturation of patients with proliferative DR was significantly higher than that of patients with simple DR. Some researchers[15-17] used a device similar to the one used in this study to evaluate the main arteries and veins around the retinal optic disc and showed that blood oxygen saturation increased with the severity of DR, which was consistent with our results. Due to retinal hypoxic-ischemic injury, oxygen accumulates in retinal arteries and veins, and blood oxygen saturation increases, leading to insufficient retinal blood perfusion and oxygen supply. Simultaneously, oxygen free radicals and related cytokines in the body infiltrate the blood-retinal barrier, acting on retinal blood vessels, which also induces retinal tissue damage[18].
Some researchers[19-21] have used a variety of methods to measure the retinal vessel diameter in DR. In this study, there was no statistically significant difference in the diameter of the retinal artery and vein between the DR and control groups, or in the diameter of the retinal artery and vein between patients with proliferative DR and patients with simple DR. However, although the difference was not significant, it can be observed that the diameter of the retinal arteriovenous vessels in the DR group showed an upward trend compared with those in the control group, which is consistent with the results of most previous studies. However, the exact mechanism associated with the increase in retinal vessel diameter in DR remains unclear, which may be related to the obstruction of blood flow in small vessels caused by high blood glucose level and hypoxia. Moreover, retinal inflammation may also affect the diameter of the retinal arteries and veins.
Oxidative stress is not only an important factor in diabetes but also a key risk factor for DR. 8-OHdG is a biomarker for oxidative stress[22]. MDA is one of the final products of the peroxidation reaction between oxygen free radicals and unsaturated fatty acids in the cell membrane, which can indirectly reflect the content of oxygen free radicals and the degree of oxidative damage[23]. 8-OHdG and MDA are currently recognized as sensitive indicators for assessing oxidative stress. Due to their function and structure, nerve cells have higher oxygen supply requirements and are more sensitive to peroxide damage[24]. SOD and GSH are strong reducing agents in the body and can directly reduce oxidants, thus, reducing oxidative stress damage[25]. Therefore, the content of SOD and GSH can reflect the ability of the body to resist oxidative stress. The results of this study showed that the serum MDA and 8-OHdG levels in the DR group were significantly higher than those in the control group, and the serum SOD and GSH levels were significantly lower in the DR group than those in the control group, suggesting that the oxidative stress level of patients with DR increased with the increase in the degree of retinopathy. Therefore, oxidative stress may be involved in the incidence of DR, and high oxidative stress level may be a risk factor for the incidence of DR. Furthermore, the serum MDA and 8-OHdG levels in patients with proliferative DR were higher than those of patients with simple DR, and serum SOD and GSH levels were lower in patients with proliferative DR than those in patients with simple DR, indicating that oxidative stress increased significantly with the progression of the disease and retinopathy. Therefore, early simultaneous use of antioxidant stress and anti-inflammatory therapy may be more effective in delaying and treating DR.
Based on the findings of existing studies[26-30], this study compared serum oxidative stress and blood oxygen saturation indices of patients at different DR stages and simultaneously measured the retinal vessel diameter to explore the exact mechanism of oxidative stress-induced DR and to provide a valuable basis for the clinical diagnosis and treatment of patients with DR. However, it should be noted that the average age of the patients with DR in this study was relatively old, and our sample size was small. However, age, sex, and other factors have little impact on the retinal vascular oxygen saturation and retinal vessel diameter; therefore, we do not expect a large experimental deviation associated with these factors in our study.
In conclusion, increased blood oxygen saturation of retinal arteries and veins and increased oxidative stress damage in patients with DR may be associated with decreased retinal capillary permeability and arterial oxygen dispersion, and may reflect the patient’s condition to a certain extent.
Diabetic retinopathy (DR) is the most important manifestation of diabetic micro-vasculopathy and is mainly related to the course of diabetes and the degree of blood glucose control, while the age of diabetes onset, sex, and type of diabetes have little influence on it.
This study explored the relationship between the changes of retinal arterial and venous oxygen saturation and oxidative stress injury and retinal capillary permeability and arterial oxygen diffusion, and whether it can reflect the patient’s condition.
The study aimed to explore the effective monitoring index to effectively reflect the condition of patients with diabetic retinopathy.
Total 94 patients (94 eyes) with DR (DR group) and 100 volunteers (100 eyes) (control group) without eye diseases, were included in this study. Arterial and venous blood oxygen saturation, retinal arteriovenous vessel diameter, and serum oxidative stress indicators in the two groups were compared. Based on the stage of the disease, the DR group was divided into the simple DR and proliferative DR groups for stratified analysis.
The oxygen saturation of the retinal vessels in the DR group was significantly higher than that in the control group. The retinal vessel diameters between the DR and control groups were not significantly different. The oxygen saturation of the retinal vessels in the patients with proliferative DR was significantly higher than that in the patients with simple DR. The retinal vessel diameter in patients with proliferative DR was not significantly different from that of patients with simple DR.
Increased blood oxygen saturation of retinal arteries and veins and increased oxidative stress damage in patients with DR may be associated with decreased retinal capillary permeability and arterial oxygen dispersion, possibly reflecting the patient’s condition.
Large sample studies should be performed in the further.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lemmen KD, Germany; Ortega AL, Spain; Sasongko MB, Indonesia S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2576] [Cited by in F6Publishing: 2727] [Article Influence: 227.3] [Reference Citation Analysis (3)] |
| 2. | Safi H, Safi S, Hafezi-Moghadam A, Ahmadieh H. Early detection of diabetic retinopathy. Surv Ophthalmol. 2018;63:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Lechner J, O'Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Qiu X, Wang X, Hong P, Liu M, Wen Q, Chen Q. Retinal blood oxygen saturation and vascular endothelial growth factor-A in early diabetic retinopathy: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e20562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Bek T, Stefánsson E, Hardarson SH. Retinal oxygen saturation is an independent risk factor for the severity of diabetic retinopathy. Br J Ophthalmol. 2019;103:1167-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Mandal LK, Choudhuri S, Dutta D, Mitra B, Kundu S, Chowdhury IH, Sen A, Chatterjee M, Bhattacharya B. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can J Diabetes. 2013;37:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lv X, Ran X, Chen X, Luo T, Hu J, Wang Y, Liu Z, Zhen Q, Liu X, Zheng L, Tang Y, Zhao Q, Han S, Zhou Y, Luo W, Yang L, Li Q, Wang Z. Early-onset type 2 diabetes: A high-risk factor for proliferative diabetic retinopathy (PDR) in patients with microalbuminuria. Medicine (Baltimore). 2020;99:e20189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Hardarson SH, Stefánsson E. Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol. 2012;96:560-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Shikama M, Sonoda N, Morimoto A, Suga S, Tajima T, Kozawa J, Maeda N, Otsuki M, Matsuoka TA, Shimomura I, Ohno Y. Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig. 2021;12:1007-1014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Keilani C, Halalchi A, Wakpi Djeugue D, Regis A, Abada S. Retinal oximetry during treatment of retinal vein occlusion by ranibizumab in patients with high blood pressure and dyslipidemia. J Fr Ophtalmol. 2016;39:816-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hao H, Sasongko MB, Wong TY, Che Azemin MZ, Aliahmad B, Hodgson L, Kawasaki R, Cheung CY, Wang JJ, Kumar DK. Does retinal vascular geometry vary with cardiac cycle? Invest Ophthalmol Vis Sci. 2012;53:5799-5805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Prum BE Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, Herndon LW Jr, Lim MC, Williams RD. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016;123:P41-P111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 13. | Bek T. Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr Diab Rep. 2017;17:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Unoki N, Nishijima K, Sakamoto A, Kita M, Watanabe D, Hangai M, Kimura T, Kawagoe N, Ohta M, Yoshimura N. Retinal sensitivity loss and structural disturbance in areas of capillary nonperfusion of eyes with diabetic retinopathy. Am J Ophthalmol. 2007;144:755-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Orlando G, Sacchetti M, D'Errico V, Haxhi J, Rapisarda G, Pugliese G, Balducci S. Muscle fatigability in patients with type 2 diabetes: relation with long-term complications. Diabetes Metab Res Rev. 2020;36:e3231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Simó-Servat O, Hernández C, Simó R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019;62:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Browning DJ, Stewart MW, Lee C. Diabetic macular edema: Evidence-based management. Indian J Ophthalmol. 2018;66:1736-1750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Saw M, Wong VW, Ho IV, Liew G. New anti-hyperglycaemic agents for type 2 diabetes and their effects on diabetic retinopathy. Eye (Lond). 2019;33:1842-1851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Voigt M, Schmidt S, Lehmann T, Köhler B, Kloos C, Voigt UA, Meller D, Wolf G, Müller UA, Müller N. Prevalence and Progression Rate of Diabetic Retinopathy in Type 2 Diabetes Patients in Correlation with the Duration of Diabetes. Exp Clin Endocrinol Diabetes. 2018;126:570-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, Mitchell P, Klein R, Cheung CMG, Wong TY. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 21. | van der Heijden AA, Abramoff MD, Verbraak F, van Hecke MV, Liem A, Nijpels G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018;96:63-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Medhat D, El-Mezayen HA, El-Naggar ME, Farrag AR, Abdelgawad ME, Hussein J, Kamal MH. Evaluation of urinary 8-hydroxy-2-deoxyguanosine level in experimental Alzheimer's disease: Impact of carvacrol nanoparticles. Mol Biol Rep. 2019;46:4517-4527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Kumawat M, Kharb S, Singh V, Singh N, Singh SK, Nada M. Plasma malondialdehyde (MDA) and anti-oxidant status in diabetic retinopathy. J Indian Med Assoc. 2014;112:29-32. [PubMed] [Cited in This Article: ] |
| 24. | Wei LF, Zhang HM, Wang SS, Jing JJ, Zheng ZC, Gao JX, Liu Z, Tian J. Changes of MDA and SOD in Brain Tissue after Secondary Brain Injury with Seawater Immersion in Rats. Turk Neurosurg. 2016;26:384-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front Physiol. 2018;9:1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 26. | Ghamdi AHA. Clinical Predictors of Diabetic Retinopathy Progression; A Systematic Review. Curr Diabetes Rev. 2020;16:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Jansson RW, Hufthammer KO, Krohn J. Diabetic retinopathy in type 1 diabetes patients in Western Norway. Acta Ophthalmol. 2018;96:465-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ren D, Kang W, Xu G. Meta-Analysis of Diagnostic Accuracy of Retinopathy for the Detection of Diabetic Kidney Disease in Adults With Type 2 Diabetes. Can J Diabetes. 2019;43:530-537.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Matuszewski W, Baranowska-Jurkun A, Stefanowicz-Rutkowska MM, Modzelewski R, Pieczyński J, Bandurska-Stankiewicz E. Prevalence of Diabetic Retinopathy in Type 1 and Type 2 Diabetes Mellitus Patients in North-East Poland. Medicina (Kaunas). 2020;56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Matuszewski W, Stefanowicz-Rutkowska MM, Szychlińska M, Bandurska-Stankiewicz E. Differences in Risk Factors for Diabetic Retinopathy in Type 1 and Type 2 Diabetes Mellitus Patients in North-East Poland. Medicina (Kaunas). 2020;56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |