Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.673
Peer-review started: December 18, 2020
First decision: March 16, 2021
Revised: March 17, 2021
Accepted: April 12, 2021
Article in press: April 12, 2021
Published online: May 15, 2021
Type 1 diabetes originates from gene-environment interactions, with increasing incidence over time.
To identify correlates of childhood type 1 diabetes in European countries using an ecological approach. Several environmental variables potentially influencing the onset of type 1 diabetes have been previously evaluated. However, the relationships between epidemiologic data and exposure to toxic airborne mole
We employed an ecological model to explore, in a wide time period (1990-2018), associations between type 1 diabetes incidence in 19 European countries (systematic literature review) and the nationwide production of five widely diffused air pollutants: particulate matter < 10 μm (PM10), nitrogen oxides (NO), non-methane volatile organic compounds (VOCs), sulphur oxide (SO2), and ammonia.
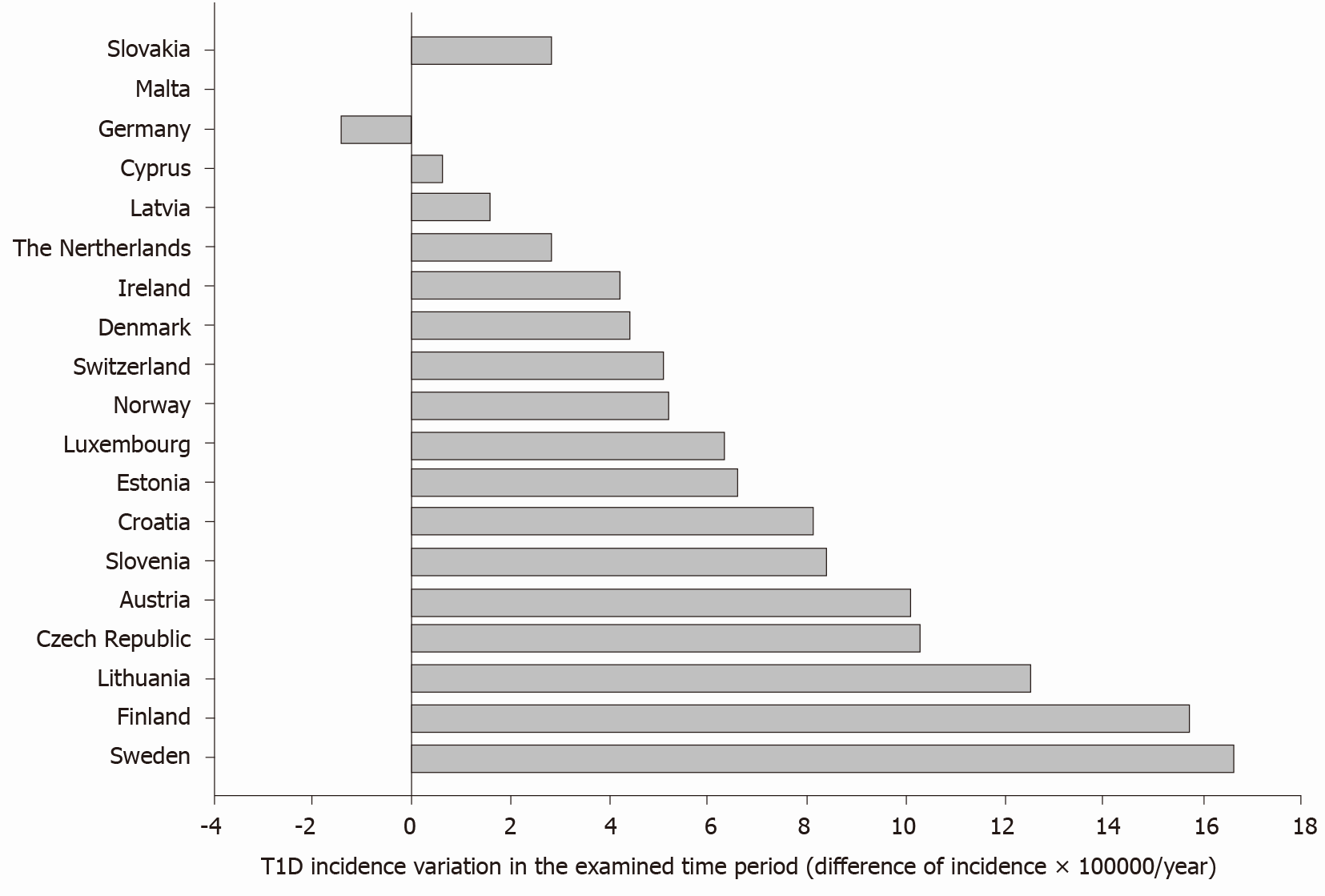
Data confirm a raising incidence of type 1 diabetes in 18 out of 19 explored countries. The average difference (last vs first report, all countries) was +6.9 × 100000/year, with values ranging from -1.4 (Germany) to +16.6 (Sweden) per 100000/year. Although the overall production of pollutants decreased progressively from 1990 to 2018, type 1 diabetes incidence was positively associ
Evidence justify further studies to explore better links between long-term air quality and type 1 diabetes onset at the individual level, which should include exposures during pregnancy. In this respect, type 1 diabetes could be, at least in part, a preventable condition. Thus, primary prevention policies acting through a marked abatement of pollutant emissions might attenuate future type 1 diabetes incidence throughout Europe.
Core Tip: The environment has a role in the onset of type 1 diabetes. Possible associa
- Citation: Di Ciaula A, Portincasa P. Relationships between emissions of toxic airborne molecules and type 1 diabetes incidence in children: An ecologic study. World J Diabetes 2021; 12(5): 673-684
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/673.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.673
The onset of type 1 diabetes is linked with pathogenic processes involving formation of autoantibodies, islet specific T-cells, and progressive inflammatory destruction of the beta-cells[1]. These pathways result from complex interactions between genetic[2,3] and environmental factors[4], which also involve epigenetic mechanisms [5].
Epidemiologic studies reveal a progressive increase in the incidence of type 1 diabetes in children, which doubled over the last 20 years, with a 3.4% increase per annum[6]. In European countries, the incidence of type 1 diabetes in pediatric age shows an increment rate of about 3% per year[7], but different trends occur in different geographical areas[7].
According to preliminary observations, the epidemiologic increment of type 1 diabetes could be due, at least in part, to an unhealthy environment. In particular, type 1 diabetes onset has been linked with the concentration of airborne molecules (introduced with breath), and with chemicals introduced by oral ingestion or direct cutaneous contact (endocrine disrupting chemicals)[8].
These molecules can also operate during the in utero life[9,10], increasing the risk of developing type 1 diabetes mainly through immune alterations[11-13] and damage to pancreatic beta-cells[14].
Preliminary evidence, in particular, suggest a role for airborne pollutants as ozone[10], nitrogen oxides (NO)[9], particulate matter[8,15], sulphate, nitrates, nitrites, N-nitroso compounds, persistent organic pollutants, heavy metals, and volatile organic compounds (VOCs)[8].
However, so far, the possibility that long-term exposure to a large number of diffused air pollutants may affect the incidence of type 1 diabetes in children has not been fully confirmed.
We aimed to identify correlates of childhood type 1 diabetes in European countries using an ecological approach. The methodology links temporal trends of the global nationwide production of toxic airborne molecules with type 1 diabetes incidence in children living in the same European areas.
We performed a systematic review of literature (PubMed) to collect and examine available data about the incidence of type 1 diabetes in pediatric age (0-15 years) in 19 European countries (Supplementary Tables 1 and 2). All publications until December 4, 2020 were considered. Papers were selected by the following criteria: (1) the study period was ≥ 2 years; (2) the study considered the overall age-standardized incidence of type 1 diabetes (per 100000 per year) in at least one European country and in the age range 0-15 years; (3) the study was based on a nationwide dataset; (4) study periods starting from the year 1990. This time limit depended on the lack of information about countrywide pollutant production before 1990 (see below); and (5) studies published in English language.
In the selected studies, the incidence of type 1 diabetes was assessed, on average, on a time period of 17.8 years (range 4-28 years), from 1990 to 2018 (Supplementary Table 2). In the case of different studies examining similar time periods in the same country, data from the study with the shortest period were excluded from the analysis, in order to avoid time overlapping and correlation bias.
Data about pollutant emissions were derived from the European Environmental Agency and Eurostat database (https://ec.europa.eu/eurostat/databrowser/view/ENV_AIR_EMIS__custom_210633/default/table?lang=en, last update 25 November 2020), and measured as tonnes of emissions per year, considering total sectors of emissions for each pollutant and for the national territory, in the period 1990-2018. Average emissions in selected periods (corresponding to type 1 diabetes incidence time periods in each country) were thereafter calculated for the following pollutants: Particulate matter < 10 μm (PM10), NO, non-methane organic VOCs, sulphur oxide (SO2), and ammonia. Correlations between the global amount of pollutant emissions and type 1 diabetes incidence were checked for each country/time period by Spearman’s rank correlation coefficient.
We categorized each pollutant according to tertiles of emissions in the entire study period (low, medium, and high pollutant emissions). To calculate odds ratios and confidence intervals for type 1 diabetes incidence associated with emissions of specific pollutants, we fit separate logistic regression models with type 1 diabetes incidence as the dependent variable, and tertiles of each pollutant as the independent variable. Kruskal-Wallis analysis of variance by ranks followed by multiple-comparison Z-value test or Mann-Whitney U-test were employed to compare differences among groups, as appropriate. P < 0.05 were considered statistically significant for all analysis. Graphic representation of data is provided by SigmaPlot software (https://systatsoftware.com/products/sigmaplot/). Statistical analyses were performed with NCSS10 Statistical Software (NCSS, LLC, Kaysville, UT, United States).
Altogether, in 19 European countries, 18 studies considering the incidence of type 1 diabetes met the inclusion criteria and were considered in the analysis (Supplementary Table 1).
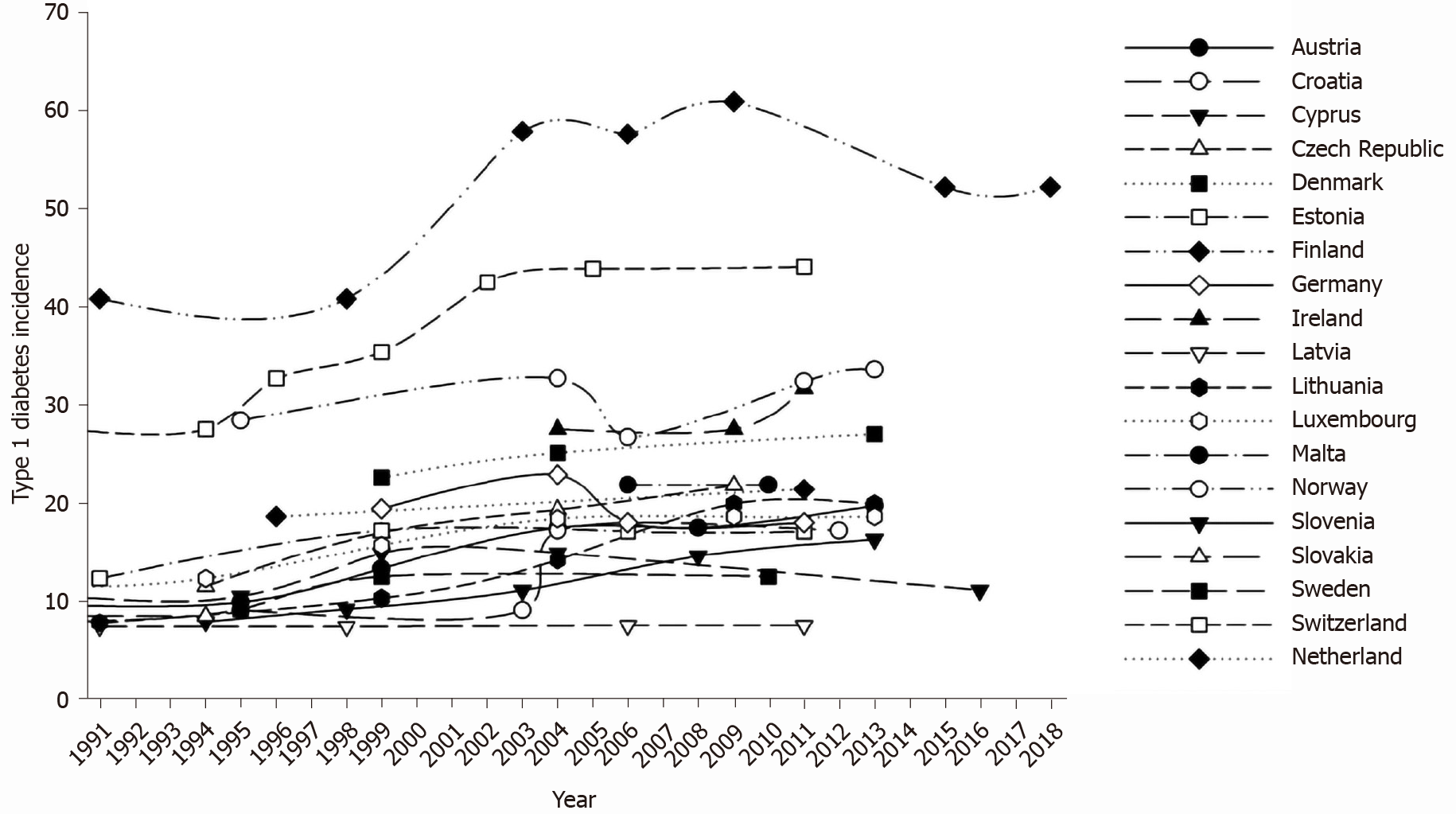
Figure 1 shows time variations in type 1 diabetes incidence across Europe in each of the explored countries, in the whole observation period. During the explored time interval, type 1 diabetes incidence increased in all explored countries, with the exception of Germany.
The average difference (last vs first report, all countries) was +6.9 × 100000/year, with values ranging from -1.4 (Germany) to +16.6 (Sweden) per 100000/year (Figure 2).
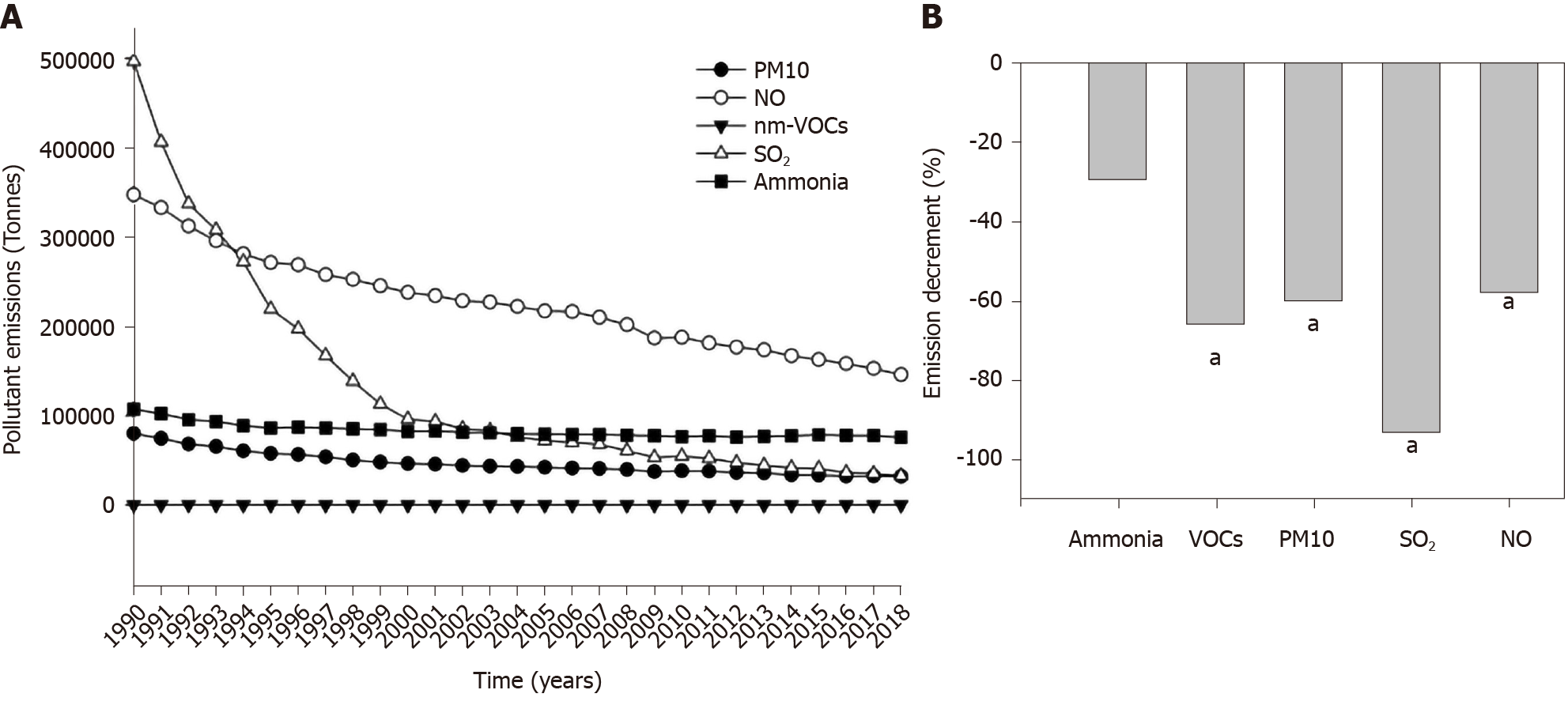
Considering the whole group of explored countries, the global, nationwide production of pollutants decreased progressively from 1990 to 2018 (Figure 3A), with significant reductions recorded in the case of VOCs, PM10, SO2, and NO but not for ammonia (Figure 3B).
The incidence of type 1 diabetes in European countries was significantly positively correlated with national PM10 emissions (ρ = 0.32, P = 0.004), VOCs (ρ = 0.35, P = 0.001), and NO (ρ = 0.44, P = 0.0001) but not with those of SO2 and ammonia (P = NS, data not shown).
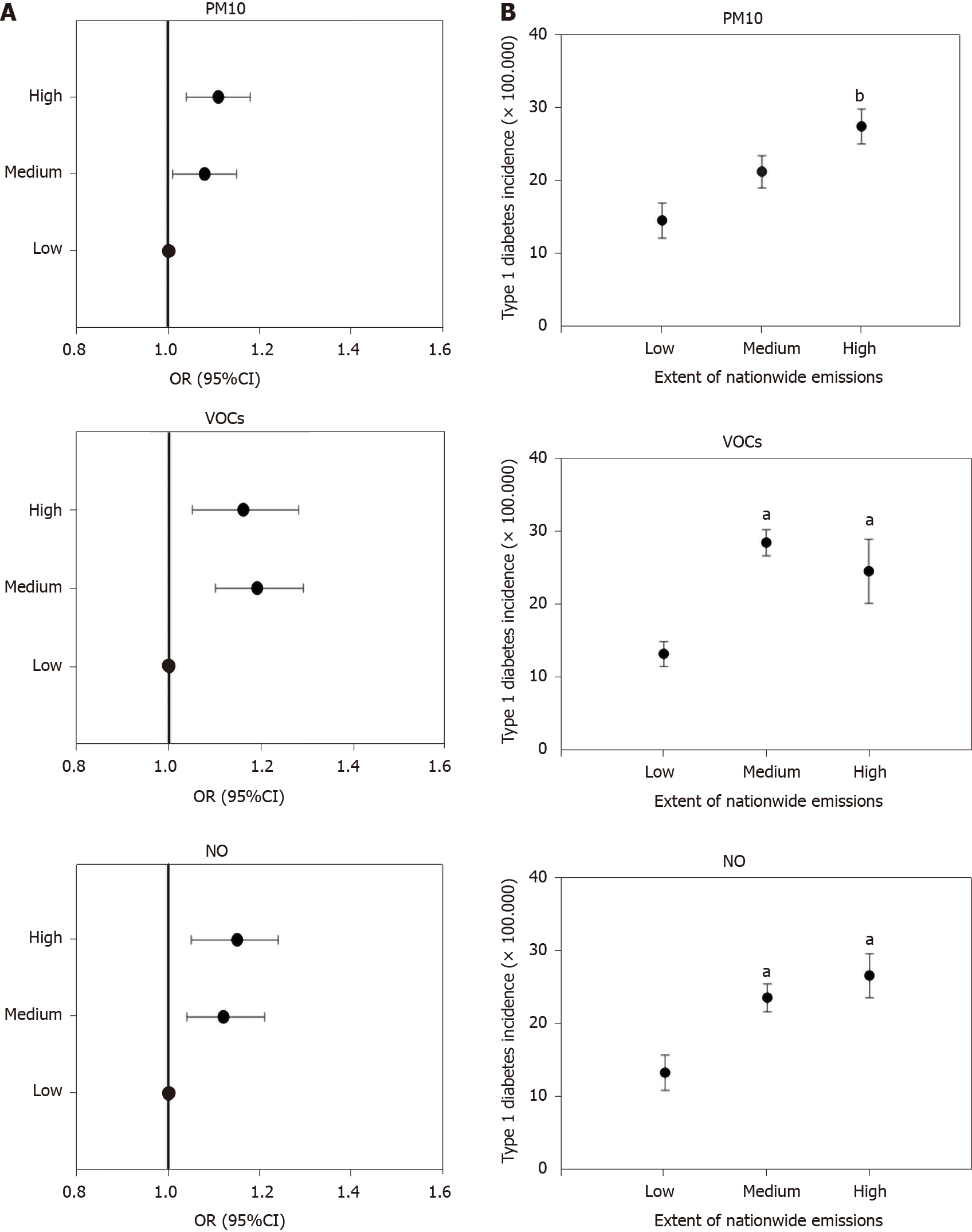
When countries were stratified according to tertiles of pollutants emitted in the entire period (Supplementary Table 3), the odds of elevated type 1 diabetes incidence were significantly higher in countries in the medium and high tertiles of PM10, VOCs, and NO production (but not SO2 or ammonia, data not shown) than in those in the low production group (Table 1 and Figure 4A). Mean incidence of type 1 diabetes was significantly higher in countries with high production than in countries with low production of PM10, VOCs, and NO (Supplementary Table 3, Table 2 and Figure 4B). The incidence of type 1 diabetes was similar when countries were compared in terms of SO2 and ammonia production (data not shown).
| Tertiles of emission | PM10 | VOCs | NO |
| Reference, low | 1 | 1 | 1 |
| II tertile, medium | 1.08 (1.01-1.15) | 1.19 (1.1-1.29) | 1.12 (1.04-1.21) |
| III tertile, high | 1.10 (1.04-1.18) | 1.16 (1.05-1.28) | 1.15 (1.05-1.24) |
The present study examined type 1 diabetes incidence during about three decades (1990-2018) and reports a progressive increase in the majority of the 19 explored European countries. We employed an ecologic approach and found an association between type 1 diabetes incidence and the global burden of anthropogenic emissions of three widely diffused air pollutants (namely PM10, non-methane VOCs, and NO), with increased odds of high incidence in countries with the highest pollutant emissions.
We confirm previous observations about the worldwide rise in type 1 diabetes incidence with time in pediatric age[7,16-19].
The selected papers cover a time window of about 18 years. Although genetic susceptibility is a well-known risk factor for the onset of type 1 diabetes[2,3], the relatively short time interval does not explain the increase in pediatric type 1 diabetes incidence simply by shifts in individual genetic susceptibility. A larger time window is usually needed to establish genetic changes at a population level. Indeed, prior studies found a rise in type 1 diabetes incidence associated with unchanged[3] or even decreased[20] frequency of major genetic risk factors for type 1 diabetes. Furthermore, only a minority of genetically susceptible children progress to clinical disease[21-23], and the concordance rate among monozygotic twins ranges from 13% to 68%, and is approximately 6% in siblings[21-23].
These elements therefore point to a critical role for environmental factors that also operate during pregnancy[4,23,24], as well as for mechanisms acting through the interplay gene-environment[25].
The relationships between the onset of type 1 diabetes and the environment have investigated a number of factors, including deprivation[26]. None among these factors, however, is convincingly implicated in the etiology of type 1 diabetes as a chronic autoimmune disease[23,24,26,27]. Environmental pollution is still scarcely explored, despite the link with several autoimmune diseases[28,29], namely rheumatic diseases[30], thyroid diseases[31], and systemic lupus erythematosus[32].
The pathogenic relationship environment-autoimmune diseases might also exist in pediatric age[33-36]. In particular, toxicants strongly affect the development of the immune system also during in utero life. In newborns, living in a highly polluted urban area is associated with lower percentage of CD4+ T-lymphocytes and a lower CD4+ / CD8+ ratio, but higher percentage of natural killer cells[37]. In the same population, prenatal maternal exposure to polycyclic aromatic hydrocarbons and fine particulate matter was associated with a lower percentage of CD3+, CD4+, and CD8+ T-lymphocytes and with a higher percentage of CD19+ B-lymphocytes[38].
Among environmental toxics, bisphenol A[39-42], higher intake of nitrates, nitrites, N-nitroso compounds, and persistent organochlorine pollutants[43-45] are potentially associated with the onset of type 1 diabetes. Limited evidence[43,44] exist for heavy metals like chromium[46], cadmium[47], and lead[48].
Air pollution is a heterogeneous mixture of molecules present in gases and solid particles, each having its own potential pathogenic effect. This study found a possible association between country emissions of PM10, NO, VOCs, and type 1 diabetes incidence. Of note, maternal exposure before and during pregnancy to PM10, NO[49], and VOCs[49,50] affects blood lymphocyte immunophenotype distribution in newborns, even at low air concentrations.
Our results about PM10 and type 1 diabetes incidence are in line with previous epidemiological observations. A large cohort study in Bavarian children found that exposure to high levels of PM10 and nitrogen dioxide can accelerate the onset of type 1 diabetes and the risk of developing type 1 diabetes in children with less than 5 years of age. Mechanisms involve a more severe inflammatory state[51]. Overall, results indicate that the exposure to PM10 is contributing to the increased incidence of type 1 diabetes in pediatric age[8], in particular in children with less than 5 years of age[52].
Non-methane VOCs are widely diffused indoor and outdoor pollutants. Although systematic studies are lacking, there are evidence showing that in utero exposure to VOCs affects the immune system in animal models[53] and in newborns[49,50]. More studies should explore the role of VOCs on the pathogenesis and on the epidemio
The relationships between chronic exposure to NO and the risk of type 1 diabetes is also scarcely explored. A previous report from a large cohort study in Swedish children found a significant association between NO exposure during the third trimester of pregnancy and offspring type 1 diabetes[9]. Of note, the amount of NO emission is among the main factors causing ozone pollution[54]. Studies found that cumulative exposure to ozone and sulfate in ambient air increased the risk of developing type 1 diabetes in pediatric age[52,55]. The role of ozone exposure as a risk factor for type 1 diabetes has been also confirmed by a recent retrospective-population based cohort study, suggesting an increased incidence of type 1 diabetes in children aged 0-5 years exposed to high ozone levels during the prenatal period (first trimester of pregnancy)[10].
An additional aspect emerging from this study was the apparent contradiction between the increased incidence of type 1 diabetes over time and the progressive nationwide decrement of pollutants. It should be underlined that, in the whole group of explored countries, the statistical association between the type 1 diabetes incidence and the global burden of pollutants (i.e. tonnes of emissions per year) does not express a temporal trend. The analysis correlates the type 1 incidence recorded in a specific period, with the burden of pollutants emitted in the same explored period. From this point of view, the countries with the highest emissions of PM10, VOCs, and NO (i.e. nationwide emissions in the II and III tertiles) have the highest incidence of type 1 diabetes in corresponding time periods. This finding is confirmed by logistic regression models exploring the odds of elevated type 1 diabetes incidence in countries divided according to tertiles of emissions and by an analysis of variance exploring differences between the average incidence of type 1 diabetes in countries with low, medium, and high production of pollutants.
The decreasing trend in the emission of pollutants observed over time was significant for all explored pollutants, with the exclusion of ammonia. Of note, we found no significant association between type 1 diabetes incidence and SO2 (the pollutant with the highest percent decrement in the examined period, i.e. -93%). The possibility exists that the decreased production of VOCs, PM10, and NO (about -60% in 2018 vs 1990) might be still insufficient to generate beneficial effects in terms of global type 1 diabetes incidence. In addition, previous epidemiologic findings based on other diseases could not identify a definitive threshold linking air concentration of particulate matter[56] and NO[57] with health effects. Biological effects might also occur during moderate exposure to multiple pollutants (cumulative effect).
This study has some limitations. Firstly, the analysis included children with age comprised between 0 and 15 years, as a whole group. Type 1 diabetes is a heterogeneous disease in terms of epidemiologic findings, and different trends exist in children with early onset of disease, as compared to children diagnosed type 1 diabetes in older ages[8]. However, the wide time period in the present study (about 18 years) might partly limit the bias from different epidemiologic trends in different pediatric age groups. Additional surveys should better explore the link between air pollution and type 1 diabetes incidence in different age classes, employing a specific study design (case-control or cohort studies). Secondly, the present study used an ecological approach. This methodology can only indicate the existence of ecological associations, which not necessarily point to pathogenic associations between explored pollutants and type 1 diabetes onset at an individual level. Further studies are needed to examine in details pathophysiological and epidemiological links between individual exposure to PM10, NO, and VOCs and the onset of type 1 diabetes. Future analyses, in particular, should comprehensively consider in utero exposures, epigenetic mechanisms, and individual variables exploring other known risk factors of type 1 diabetes as genetic factors, viral infection history, family history, individual diet, and lifestyle. Finally, the present study was not designed to explore time variations in type 1 diabetes incidence according to temporal trends of emission in each country. Studies conducted at a national level could correlate local epidemiologic and environmental data on a wide time window, possibly in different age classes[8]. Although genetic factors seem to play a limited role in the epidemiological variations of type 1 diabetes incidence[21-23], nation-based cohort or case-control studies should also allow comparisons between subgroups with similar genetic background but living in different countries.
In conclusion, the present study confirms the increasing epidemiologic trend of type 1 diabetes in pediatric age in European countries. Results point, in particular, to the association with the global burden of emissions of specific environmental pollutants (PM10, NO, VOCs). We advocate further studies exploring additional links between long-term air quality and type 1 diabetes onset. Such surveys should employ specific models and should consider exposures during pregnancy. We speculate that type 1 diabetes is, at least in part, a preventable condition and that primary prevention policies might attenuate future type 1 diabetes incidence throughout Europe, by marked abatement of pollutant emissions.
Type 1 diabetes onset depends on gene-environment interactions, and several reports show, worldwide, an increased incidence of type 1 diabetes over time.
The effect of environmental factors on type 1 diabetes incidence in pediatric age is still incompletely unexplored.
To correlate the incidence of childhood type 1 diabetes in European countries with the global, nationwide production of toxic airborne molecules.
We employed a systematic literature review to explore type 1 diabetes incidence in pediatric age in 19 European countries (time period: 1990-2018). We therefore applied an ecological study design to explore possible associations with the nationwide production of five widely diffused air pollutants: Particulate matter < 10 μm (PM10), nitrogen oxides (NO), non-methane volatile organic compounds (VOCs), sulphur oxide (SO2), and ammonia.
A raising incidence of type 1 diabetes was evident in 18 out of 19 countries. Considering the whole group of countries, type 1 diabetes incidence was associated with the nationwide emissions of PM10, NO, non-methane VOCs, but not with those of SO2 and ammonia.
The global burden of emission of specific air pollutants is associated with type 1 diabetes incidence. The study design employed in the present study can only indicate the existence of ecological associations and does not necessarily point to specific pathogenic links. However, results suggest the possibility that type 1 diabetes could be, at least in part, a preventable condition.
Further studies conducted with specific models are needed to explore better the pathogenic links between type 1 diabetes, air pollutants and other known risk factors of disease as genetic factors, viral infection history, individual diet, and lifestyle.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: International Society of Doctors for Environment.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Novita BD, Xia MF S-Editor: Fan JR L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 861] [Cited by in F6Publishing: 784] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 2. | Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12:781-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS; Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1270] [Cited by in F6Publishing: 1289] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 4. | Hasham A, Tomer Y. The recent rise in the frequency of type 1 diabetes: who pulled the trigger? J Autoimmun. 2011;37:1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Dang MN, Buzzetti R, Pozzilli P. Epigenetics in autoimmune diseases with focus on type 1 diabetes. Diabetes Metab Res Rev. 2013;29:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle EJ, Bingley PJ, Dahlquist G, Jarosz-Chobot PK, Marčiulionytė D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Rojnic Putarek N, deBeaufort CE, Samardzic M, Green A. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 7. | Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C, Bingley PJ, Schoenle E, Jarosz-Chobot P, Urbonaité B, Rothe U, Krzisnik C, Ionescu-Tirgoviste C, Weets I, Kocova M, Stipancic G, Samardzic M, de Beaufort CE, Green A, Dahlquist GG, Soltész G. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142-2147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Di Ciaula A. Type I diabetes in paediatric age in Apulia (Italy): Incidence and associations with outdoor air pollutants. Diabetes Res Clin Pract. 2016;111:36-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Malmqvist E, Larsson HE, Jönsson I, Rignell-Hydbom A, Ivarsson SA, Tinnerberg H, Stroh E, Rittner R, Jakobsson K, Swietlicki E, Rylander L. Maternal exposure to air pollution and type 1 diabetes--Accounting for genetic factors. Environ Res. 2015;140:268-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Elten M, Donelle J, Lima I, Burnett RT, Weichenthal S, Stieb DM, Hystad P, van Donkelaar A, Chen H, Paul LA, Crighton E, Martin RV, Decou ML, Luo W, Lavigne É. Ambient air pollution and incidence of early-onset paediatric type 1 diabetes: A retrospective population-based cohort study. Environ Res. 2020;184:109291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. 2019;125:350-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Howard SG, Lee DH. What is the role of human contamination by environmental chemicals in the development of type 1 diabetes? J Epidemiol Community Health. 2012;66:479-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Bodin J, Stene LC, Nygaard UC. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? Biomed Res Int. 2015;2015:208947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Di Ciaula A, Portincasa P. Fat, epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med. 2014;25:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Michalska M, Zorena K, Wąż P, Bartoszewicz M, Brandt-Varma A, Ślęzak D, Robakowska M. Gaseous Pollutants and Particulate Matter (PM) in Ambient Air and the Number of New Cases of Type 1 Diabetes in Children and Adolescents in the Pomeranian Voivodeship, Poland. Biomed Res Int. 2020;2020:1648264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G; Swedish Childhood Diabetes Study Group. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60:577-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | van Wouwe JP, Mattiazzo GF, el Mokadem N, Reeser HM, Hirasing RA. [The incidence and initial symptoms of diabetes mellitus type 1 in 0-14-year-olds in the Netherlands, 1996-1999]. Ned Tijdschr Geneeskd. 2004;148:1824-1829. [PubMed] [Cited in This Article: ] |
| 18. | Chae HW, Seo GH, Song K, Choi HS, Suh J, Kwon A, Ha S, Kim HS. Incidence and Prevalence of Type 1 Diabetes Mellitus among Korean Children and Adolescents between 2007 and 2017: An Epidemiologic Study Based on a National Database. Diabetes Metab J. 2020;44:866-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 875] [Cited by in F6Publishing: 925] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 20. | Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, Harrison LC. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, Leslie RD. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. J Autoimmun. 2009;33:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Nisticò L, Iafusco D, Galderisi A, Fagnani C, Cotichini R, Toccaceli V, Stazi MA; Study Group on Diabetes of the Italian Society of Pediatric Endocrinology and Diabetology. Emerging effects of early environmental factors over genetic background for type 1 diabetes susceptibility: evidence from a Nationwide Italian Twin Study. J Clin Endocrinol Metab. 2012;97:E1483-E1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Stene LC, Gale EA. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56:1888-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | MacFarlane AJ, Strom A, Scott FW. Epigenetics: deciphering how environmental factors may modify autoimmune type 1 diabetes. Mamm Genome. 2009;20:624-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Sheehan A, Freni Sterrantino A, Fecht D, Elliott P, Hodgson S. Childhood type 1 diabetes: an environment-wide association study across England. Diabetologia. 2020;63:964-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Howard SG. Exposure to environmental chemicals and type 1 diabetes: an update. J Epidemiol Community Health. 2019;73:483-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Ritz SA. Air pollution as a potential contributor to the 'epidemic' of autoimmune disease. Med Hypotheses. 2010;74:110-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Qian-Wu, Dan YL, Tao SS, Zhang Q, Sam NB, Fan YG, Zou YF, Ye DQ, Pan HF. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. 2019;18:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 30. | Farhat SC, Silva CA, Orione MA, Campos LM, Sallum AM, Braga AL. Air pollution in autoimmune rheumatic diseases: a review. Autoimmun Rev. 2011;11:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Duntas LH. Environmental factors and thyroid autoimmunity. Ann Endocrinol (Paris). 2011;72:108-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE). Environ Health Perspect. 2011;119:45-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Wang CM, Jung CR, Chen WT, Hwang BF. Exposure to fine particulate matter (PM2.5) and pediatric rheumatic diseases. Environ Int. 2020;138:105602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Goulart MFG, Alves AGF, Farhat J, Braga ALF, Pereira LAA, de Faria Coimbra Lichtenfels AJ, de Arruda Campos LM, Silva CAAD, Elias AM, Farhat SCL. Influence of air pollution on renal activity in patients with childhood-onset systemic lupus erythematosus. Pediatr Nephrol. 2020;35:1247-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Alves AGF, de Azevedo Giacomin MF, Braga ALF, Sallum AME, Pereira LAA, Farhat LC, Strufaldi FL, de Faria Coimbra Lichtenfels AJ, de Santana Carvalho T, Nakagawa NK, Silva CA, Farhat SCL. Influence of air pollution on airway inflammation and disease activity in childhood-systemic lupus erythematosus. Clin Rheumatol. 2018;37:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Zeft AS, Prahalad S, Schneider R, Dedeoglu F, Weiss PF, Grom AA, Mix C, Pope Rd CA. Systemic onset juvenile idiopathic arthritis and exposure to fine particulate air pollution. Clin Exp Rheumatol. 2016;34:946-952. [PubMed] [Cited in This Article: ] |
| 37. | Hertz-Picciotto I, Dostál M, Dejmek J, Selevan SG, Wegienka G, Gomez-Caminero A, Srám RJ. Air pollution and distributions of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology. 2002;13:172-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Hertz-Picciotto I, Herr CE, Yap PS, Dostál M, Shumway RH, Ashwood P, Lipsett M, Joad JP, Pinkerton KE, Srám RJ. Air pollution and lymphocyte phenotype proportions in cord blood. Environ Health Perspect. 2005;113:1391-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Xu J, Huang G, Nagy T, Guo TL. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch Toxicol. 2019;93:1083-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Ahn C, Kang HS, Lee JH, Hong EJ, Jung EM, Yoo YM, Jeung EB. Bisphenol A and octylphenol exacerbate type 1 diabetes mellitus by disrupting calcium homeostasis in mouse pancreas. Toxicol Lett. 2018;295:162-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Cetkovic-Cvrlje M, Thinamany S, Bruner KA. Bisphenol A (BPA) aggravates multiple low-dose streptozotocin-induced Type 1 diabetes in C57BL/6 mice. J Immunotoxicol. 2017;14:160-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Bodin J, Kocbach Bølling A, Wendt A, Eliasson L, Becher R, Kuper F, Løvik M, Nygaard UC. Exposure to bisphenol A, but not phthalates, increases spontaneous diabetes type 1 development in NOD mice. Toxicol Rep. 2015;2:99-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Longnecker MP, Daniels JL. Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect. 2001;109 Suppl 6:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Rignell-Hydbom A, Elfving M, Ivarsson SA, Lindh C, Jönsson BA, Olofsson P, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants in relation to risk of type 1 diabetes. PLoS One. 2010;5:e11281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Cetkovic-Cvrlje M, Olson M, Schindler B, Gong HK. Exposure to DDT metabolite p,p'-DDE increases autoimmune type 1 diabetes incidence in NOD mouse model. J Immunotoxicol. 2016;13:108-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Karagun BS, Temiz F, Ozer G, Yuksel B, Topaloglu AK, Mungan NO, Mazman M, Karagun GM. Chromium levels in healthy and newly diagnosed type 1 diabetic children. Pediatr Int. 2012;54:780-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Singh PK, Baxi D, Diwedi R, Ramachandran AV. Prior cadmium exposure improves glucoregulation in diabetic rats but exacerbates effects on metabolic dysregulation, oxidative stress, and hepatic and renal toxicity. Drug Chem Toxicol. 2012;35:167-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Forte G, Bocca B, Peruzzu A, Tolu F, Asara Y, Farace C, Oggiano R, Madeddu R. Blood metals concentration in type 1 and type 2 diabetics. Biol Trace Elem Res. 2013;156:79-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Baïz N, Slama R, Béné MC, Charles MA, Kolopp-Sarda MN, Magnan A, Thiebaugeorges O, Faure G, Annesi-Maesano I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn's cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Lehmann I, Thoelke A, Rehwagen M, Rolle-Kampczyk U, Schlink U, Schulz R, Borte M, Diez U, Herbarth O. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environ Toxicol. 2002;17:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Beyerlein A, Krasmann M, Thiering E, Kusian D, Markevych I, D'Orlando O, Warncke K, Jochner S, Heinrich J, Ziegler AG. Ambient air pollution and early manifestation of type 1 diabetes. Epidemiology. 2015;26:e31-e32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW. Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes. 2002;3:184-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Wang F, Liu F, Liu H, Chen W, Si X, Ma X. Effects of immunological and hematological parameter in mice exposed to mixture of volatile organic compounds. Inhal Toxicol. 2016;28:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Toro MV, Cremades LV, Calbó J. Relationship between VOC and NOx emissions and chemical production of tropospheric ozone in the Aburrá Valley (Colombia). Chemosphere. 2006;65:881-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Hathout EH, Beeson WL, Ischander M, Rao R, Mace JW. Air pollution and type 1 diabetes in children. Pediatr Diabetes. 2006;7:81-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Ware JH. Particulate air pollution and mortality--clearing the air. N Engl J Med. 2000;343:1798-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Kraft M, Eikmann T, Kappos A, Künzli N, Rapp R, Schneider K, Seitz H, Voss JU, Wichmann HE. The German view: effects of nitrogen dioxide on human health--derivation of health-related short-term and long-term values. Int J Hyg Environ Health. 2005;208:305-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |