Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1894
Peer-review started: July 22, 2021
First decision: August 16, 2021
Revised: August 29, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 15, 2021
Gestational diabetes mellitus (GDM) is associated with a heightened level of oxidative stress, which is characterized by the overproduction of reactive oxygen species (ROS) from mitochondria. Previous studies showed that mitochondrial dysfunction is regulated by dynamin-related protein 1 (Drp1) and p66Shc in GDM.
The aim was to investigate the expression of Drp1 and p66Shc and their possible mechanisms in the pathogenesis of GDM.
A total of 30 pregnant women, 15 with GDM and 15 without GDM, were enrolled. Peripheral blood mononuclear cells and placental tissue were collected. The human JEG3 trophoblast cell line was cultivated in 5.5 mmol/L or 30 mmol/L glucose and transfected with wild-type (wt)-p66Shc and p66Shc siRNA. P66Shc and Drp1 mRNA levels were detected by quantitative real-time polymerase chain reaction. The expression of p66Shc and Drp1 was assayed by immunohistochemistry and western blotting. ROS was assayed by dihydroethidium staining.
The p66Shc mRNA level was increased in the serum (P < 0.01) and placentas (P < 0.01) of women with GDM, and the expression of Drp1 mRNA and protein were also increased in placentas (P < 0.05). In JEG3 cells treated with 30 mmol/L glucose, the mRNA and protein expression of p66Shc and Drp1 were increased at 24 h (both P < 0.05), 48 h (both P < 0.01) and 72 h (both P < 0.001). ROS expression was also increased. High levels of Drp1 and ROS expression were detected in JEG3 cells transfected with wt-p66Shc (P < 0.01), and low levels were detected in JEG3 cells transfected with p66Shc siRNA (P < 0.05).
The upregulated expression of Drp1 and p66shc may contribute to the occurrence and development of GDM. Regulation of the mitochondrial fusion-fission balance could be a novel strategy for GDM treatment.
Core Tip: The role of placental mitochondria in the etiology of gestational diabetes mellitus (GDM) is an emerging area of research. In this study, we report the expression levels of dynamin-related protein 1 (Drp1) and p66Shc in GDM and in the JEG3 human trophoblast cell line. Increased expression of p66Shc was induced by high glucose-activated Drp1 and promoted overproduction of reactive oxygen species, which may be the primary cause of cell damage and apoptosis during the occurrence and development of GDM.
- Citation: Huang TT, Sun WJ, Liu HY, Ma HL, Cui BX. p66Shc-mediated oxidative stress is involved in gestational diabetes mellitus. World J Diabetes 2021; 12(11): 1894-1907
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1894.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1894
Gestational diabetes mellitus (GDM) is a glucose metabolism disorder that is first discovered or first occurs after pregnancy. It is one of the most common complications of pregnancy, and its incidence is increasing worldwide. Women with a history of GDM have an increased risk of type 2 (T2DM) later in life[1,2]. One study showed that an increased blood glucose level in pregnant women with GDM can lead to an increase in fetal glucose levels, fetal insulin secretion, fetal macrosomia, and a risk of cesarean section and birth trauma, such as vaginal tears, shoulder dystocia, and asphyxia neonatorum[3]. Substantial evidence has shown that the fetuses of women with GDM have an increased risk of developing chronic metabolic diseases, such as obesity, hypertension, cardiovascular disease and T2DM[4].
Currently, the pathogenesis of GDM is still unclear. It is the result of interactions between environmental factors and genetic factors. The pathogenesis of GDM has been proven to be similar to that of DM, which is closely related to inflammatory factors, fat factors, lipids, and so on[1]. The oxidative stress hypothesis is an important advance in the study of placental pathophysiology in recent years. Studies have found that oxidative stress and mitochondrial dysfunction in the placenta are closely related to the onset of GDM[5,6]. Mitochondrial dynamics are necessary for reactive oxygen species (ROS) production[7]. Mitochondria continuously undergo dynamic fusion and fission. Damaged mitochondria are digested by mitochondrial phagocytosis to maintain a healthy mitochondrial pool. Hyperglycemia can lead to fragmentation of mitochondria and the accumulation of damaged mitochondria results in excessive oxidative stress[8]. During the process of metabolism, aerobic cells produce a series of ROS, including O2−, H2O2, HO2−, −OH, etc. ROS can activate a series of signal trans
p66Shc is a member of the Shc protein family, and an important regulatory protein involved in oxidative stress. It has oxidoreductase activity and regulates mitochondrial oxidative stress, apoptosis, and age-related diseases in mammals. As significant oxidative stress occurs in DM, the relationship between p66Shc and oxidative stress in DM has become a research focus in recent years. Researchers have shown that the expression of p66Shc in peripheral blood mononuclear cells in T2DM patients is significantly higher than that in nondiabetic patients[10], and that high glucose levels enhance the transcription of p66Shc mRNA in cultured human umbilical vein endothelial cells in vitro[11]. Whether p66Shc is involved in oxidative stress in the placenta, and the mechanism of mitochondrial dysfunction in GDM have not yet been elucidated. Dynamin-related protein 1 (Drp1) is a dynamic protein that is necessary for mitosis of mitochondria and is primarily located in the cytosol[12]. The equilibrium between fusion and fission is essential for cell integrity and survival. Excessive activation of Drp1 activates complicated mechanisms, disrupting this equilibrium, impairing the function of mitochondria and increasing cell apoptosis[13]. Drp1 has also been proven to contribute to the pathogenesis of obesity, diabetes and cancer[14]. However, the role of Drp1 in the pathogenesis of GDM is still unclear. Here, we investigated the expression of Drp1 and p66Shc in GDM patients and preliminarily discussed the possible mechanism of the pathogenesis of GDM.
Fifteen patients diagnosed with GDM and 15 healthy pregnant women were recruited from the Department of Obstetrics in Taian City Central Hospital between May 2016 and February 2017. All women had singleton pregnancies with no other pregnancy complications. The patients with GDM had not received any prenatal treatment and had poor blood glucose control during pregnancy. Women were excluded if they had a history of diabetes mellitus, anemia, or hypertension, a family history of diabetes mellitus, or any other medical complications. The women in the two groups were matched for age, gestational age, number of pregnancies, parity, nutritional status, and educational level. All pregnant women were tested with a standardized oral glucose tolerance test. GDM was diagnosed by one or more of the following criteria: (1) A fasting blood sugar level ≥ 5.1 mmol/L; and (2) A blood sugar level ≥ 10.0 mmol/L 1 h after consuming glucose and ≥ 8.5 mmol/L 2 h after consuming glucose. The study was approved by the Ethics Committee of Taian City Central Hospital and was in accord with the code of ethics of the World Medical Association. All participants were fully informed about the study and provided written informed consent.
Blood was collected from elbow veins of fasted pregnant women into anticoagulant tubes with ethylenediaminetetraacetic acid (EDTA, TaKaRa, China) before delivery. Mononuclear cells were extracted over 30 min in 1 mL Total RNA Extraction Reagent (TRIzol, TaKaRa, China), and the cells were homogenized, mixed, and stored at −80 °C. Placental specimens were cut from the maternal surface near the umbilical cord, avoiding obvious fibrosis and calcified areas. One piece was quickly stored in liquid nitrogen, and the other was fully rinsed, fixed in 10% formaldehyde solution for at least 24 h, placed in an embedding box, dehydrated, and embedded in paraffin. Placental tissues (50–100 mg) were homogenized adequately, 1 mL TRIzol was added, and the tissues were incubated for 5 min at room temperature to ensure complete dissociation of the nucleic acid protein complex.
The human trophoblast cell line JEG-3 was purchased from Shanghai Cell Bank (China) and cultured in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/mL penicillin and 100 ng/mL streptomycin (Sangon Biotech, Shanghai) at 37 ℃ in a 5% CO2 incubator. The cells were treated with 5.5 mmol/L or 30 mmol/L glucose and then cultured in a high-glucose environment for 24 h, 48 h or 72 h and collected for further analysis.
JEG3 cells were incubated in a 6-well plate until the cell density reached 90%–95% on the day of transfection. Plasmid DNA expressing p66Shc was generated by Biosune Biotechnology (Shanghai Co., Ltd., China) using a green fluorescent protein granule-N1 (pEGFP-N1) plasmid, and p66Shc-siRNA (5’-GCAAACAGAUCAUCGCCAATT-3’ sense and 5’-UUGGCGAUGAUCUGUUUGCTT-3’ antisense) were generated by Shanghai GenePharma (Shanghai, China). Following the manufacturer's instructions, 240 μL serum-free MEM and 10 μL Lipofectamine 2000 were added to each well, mixed, and incubated for 5 min. Then, 4 μg plasmid was diluted in 246 μL serum-free MEM and added to the solution in each well and incubated for 20 min at room temperature before adding 500 μL of the plasmid-Lipofectamine 2000 mixture with gentle mixing and incubation at 37 °C in a CO2 incubator. After 4–6 h, the medium was replaced with complete medium containing FBS. The transfection efficiency was determined 48 h later.
Homogenates of blood mononuclear cells and of placenta tissue were treated with 0.2 mL chloroform, mixed for 15 s and incubated for 5 min at room temperature. Then, the specimens were centrifuged at 12000 × g for 15 min. The supernatant was transferred to a new tube, treated with 0.5 mL 100% isopropanol, mixed, incubated for 10 min, and then centrifuged at 12000 × g for 10 min. The RNA precipitate at the bottom of the tube was then washed with a mixture of TRIzol and absolute ethanol, dried, and resuspended in 0.1% diethyl pyrocarbonate solution. The RNA concentration was then measured with an ultraviolet spectrophotometer (Amersham Biosciences, United States).
cDNA was isolated from all of the samples by reverse transcription in a 10 µL system according to the instructions of the PrimeScriptTM RT Reagent Kit (Takara, China). The primer sequences are listed in Table 1. qRT-PCR was carried out in a MicroAmp® Optical 96-well reaction plate using a qRT-PCR instrument (ABI, United States). The reaction volume was 10 µL, and the reaction system included 0.8 μL primers, 1 µL diluted cDNA template, 3.2 µL enzyme-free water, and 5 μL SYBR Green (Takara, China). Amplification was performed in quadruplicate using the following cycling parameters: 95 °C for 30 s; 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 45 s; and melting curve analysis (95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s). β-Actin was used as an internal standard. The relative levels of target mRNAs were calculated by the 2−ΔΔCt method.
| Primers | Forward | Reverse |
| p66Shc | 5’-GCCAAAGACCCTGTGAATCAG-3’ | 5’-GTATTGTTTGAAGCGCAACTCG-3’ |
| Drp1 | 5’-TGCCGTGAACCTGCTAGATG-3’ | 5’-GCCTTTGGCACACTGTCT TG-3 |
| β-actin | 5’-CTCACCATGGATGATGATATCGC-3’ | 5’-AGG AATCCTTCTGACCCATGC-3’ |
Placental tissue was sectioned at 6 μm on a tissue microtome, heated for 30 min at 65 °C, dewaxed and hydrated. The sections were soaked in hematoxylin-eosin solution for 5 min, washed and then placed in 1% hydrochloric acid alcohol for 2 s and ammonia for 10 s. After that, the sections were stained with 1% eosin solution for 10 min, washed, dehydrated step by step, sealed, and imaged.
For immunohistochemistry, the sections underwent heat-induced antigen retrieval for 15 min and cooled to room temperature. Endogenous peroxides were inactivated by incubation with 0.3% H2O2 for 15 min, the sections were washed three times with 1× PBS, and incubated with sheep serum for 30 min at 37 °C. After that, the sections were incubated with primary antibodies overnight at 4 °C. The next morning, the sections were rewarmed for 30 min at 37 °C, washed, and incubated with biotin-labeled goat anti-rabbit IgG secondary antibody solution for 20 min at 37 °C. Then, the sections were incubated with horseradish peroxidase (HRP) enzyme-labeled streptomyces ovalbumin working solution for 15 min and washed three times with 1× PBS. Diaminobenzidine chromogen solution (ZSGB-BIO, China) and Harris’ hematoxylin (Solarbio, China) were then added to the sections in sequence for a few minutes. Finally, the sections were dehydrated, mounted and sealed. Photographs were taken with a microscope (Olympus, Japan).
Placental sections were dewaxed, hydrated, and soaked for 10 min in 1× PBS; cell culture plates were washed two times with 1× PBS. The sections and cell plates were then incubated with diluted dihydroethidium (DHE) solution (Beyotime, China) for 30 min at 37 °C in the dark, washed, and sealed with glycerin. Images were taken using a fluorescence microscope (Olympus, Japan).
After washing JEG3 cells two times with 1× PBS, total protein was extracted using a protein extraction reagent (Beyotime, China) containing protease and phosphatase inhibitors. A bichinchonic acid (BCA) protein assay it (Beyotime, China) was used to measure the protein concentration with a NanoDrop 2000c ultramicro spectrophotometer (Thermo Scientific, USA). After 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels were prepared, the extracted proteins and ladders were added to the wells, electrophoresed, and transferred onto a nitrocellulose filter membrane (Pall, United States). The membrane was then incubated in 5% skim milk overnight at 4 °C and washed three times with 1× PBST. Immunoblotting was performed using anti-p66Shc (1:500, Abcam, UK), anti-Drp1(1:100, Abcam, UK), rabbit polyclonal anti-GAPDH IgG (1:500, CST, USA) and mouse anti-β-actin IgG (1:1000, CST, United States) antibodies at room temperature for 2 h. After washing three times with 1× PBS, the polyvinylidene difluoride (PVDF) membrane was incubated with an HRP-conjugated rabbit anti-mouse IgG secondary antibody (1:5000, Zsbio, China) for 1 h and then washed. The specific bands were detected using Pierce™ enhanced chemoluminescence (ECL) western blotting substrate (Millipore, USA) with a Bio–Rad electrophoresis image analyzer (Bio–Rad, United States). The gray values were analyzed with image analysis software.
SPSS Statistics 24.0 (IBM, United States) and GraphPad Prism 5.0 (GraphPad Software, United States) were used for statistical analysis. The clinical characteristics and biochemical indices were compared by t-tests or chi-squared tests. A P value < 0.05 was considered statistically significant.
Fifteen GDM patients and 15 women with healthy pregnancies were included in this study (Table 2). The mean maternal age and gestational age were not significantly different between the two groups (P > 0.05). The prepregnancy body mass index (BMI) and late pregnancy BMI of the GDM group were significantly higher than those of the control group (both P < 0.001). The fasting blood glucose level was significantly higher in the GDM group than in the control group (P < 0.001).
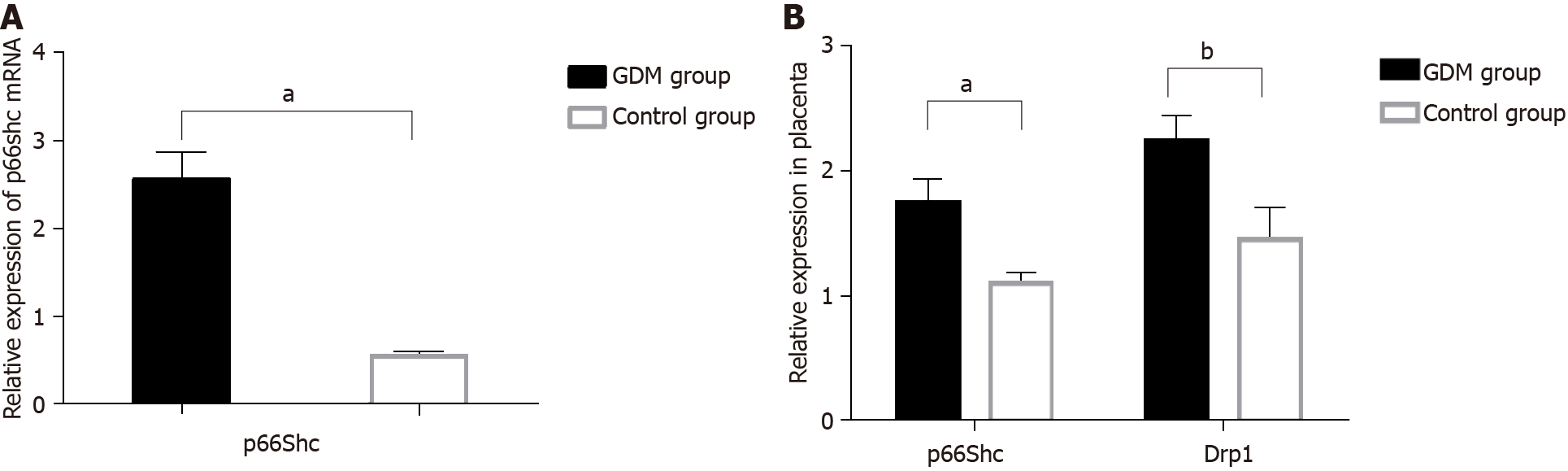
To investigate the roles of p66Shc and Drp1 in GDM, we measured the p66Shc mRNA level in the maternal serum and the expression of p66Shc and Drp1 mRNA in the placentas of GDM patients and in patients with healthy pregnancies by qRT-PCR. The protein expression of p66Shc and Drp1 was determined by immunohistochemical staining, and the level of ROS in the placentas was determined by DHE staining.
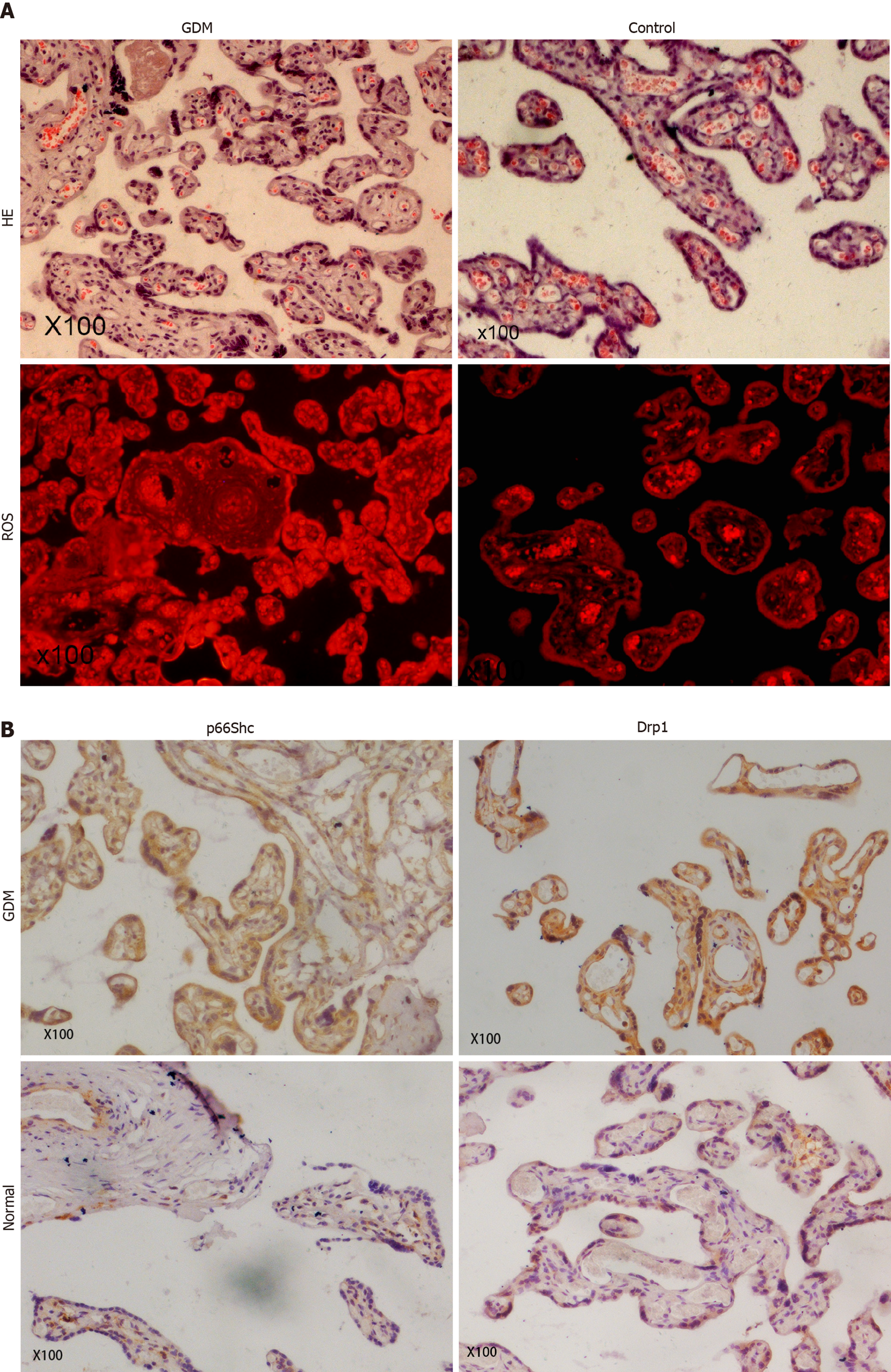
The results revealed significantly higher p66Shc mRNA levels in the maternal serum of GDM patients than in control group patients (P < 0.01) (Figure 1A). The mRNA expression levels of p66Shc (P < 0.01) and Drp1 (P < 0.05) were also significantly increased in the placentas of GDM patients compared with those of patients with normal pregnancies (Figure 1B). HE staining showed thickened arterioles of placental villi, narrowed lumens, poorly matured terminal capillaries and fewer terminal capillaries in the placentas of GDM patients. The levels of ROS were also significantly increased in the placentas of GDM patients (Figure 2A). Immunohistochemical staining showed that the expression levels of p66Shc and Drp1 were significantly higher in the placentas of the GDM group than in the placentas of the control group (Figure 2B).
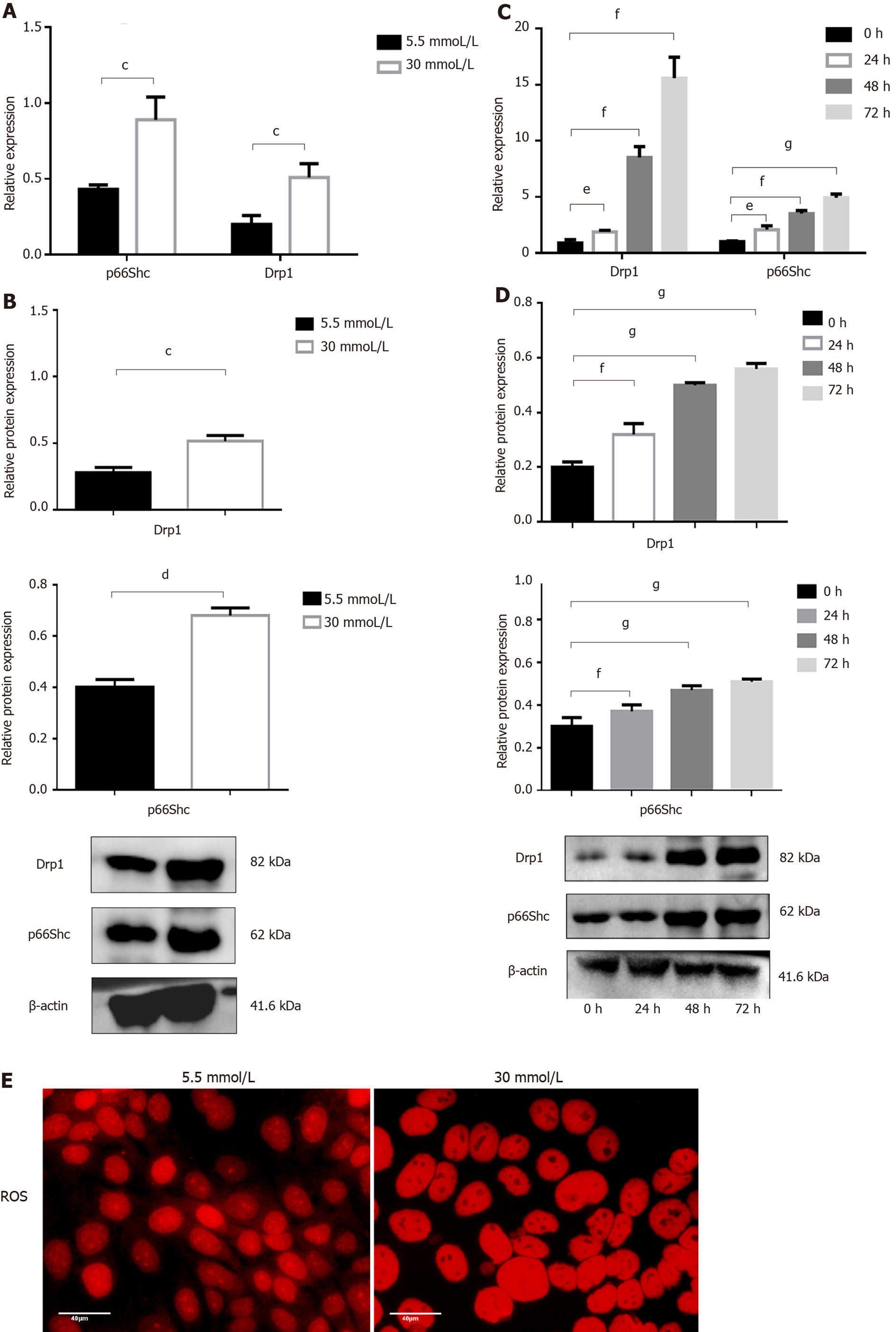
To further explore the roles of p66Shc and Drp1 in GDM, we measured the expression of p66Shc and Drp1 in JEG3 cells treated with different concentrations of glucose by qRT-PCR and Western blotting. The results showed that the mRNA level and protein expression of Drp1 and p66Shc were significantly increased (both P < 0.05) in the 30 mmol/L group compared with the 5.5 mmol/L group at 48 h (Figure 3A and B) and were also significantly increased at 0 h and 24 h (both P < 0.05), at 48 h (both P < 0.01), and at 72 h (P < 0.01 and P < 0.001) in the 30 mmol/L group. The longer the treatment time, the more significant the increase was (Figure 3C and D). Moreover, ROS levels were significantly elevated in the 30 mmol/L group compared with the 5.5 mmol/L group at 24 h (Figure 3E).
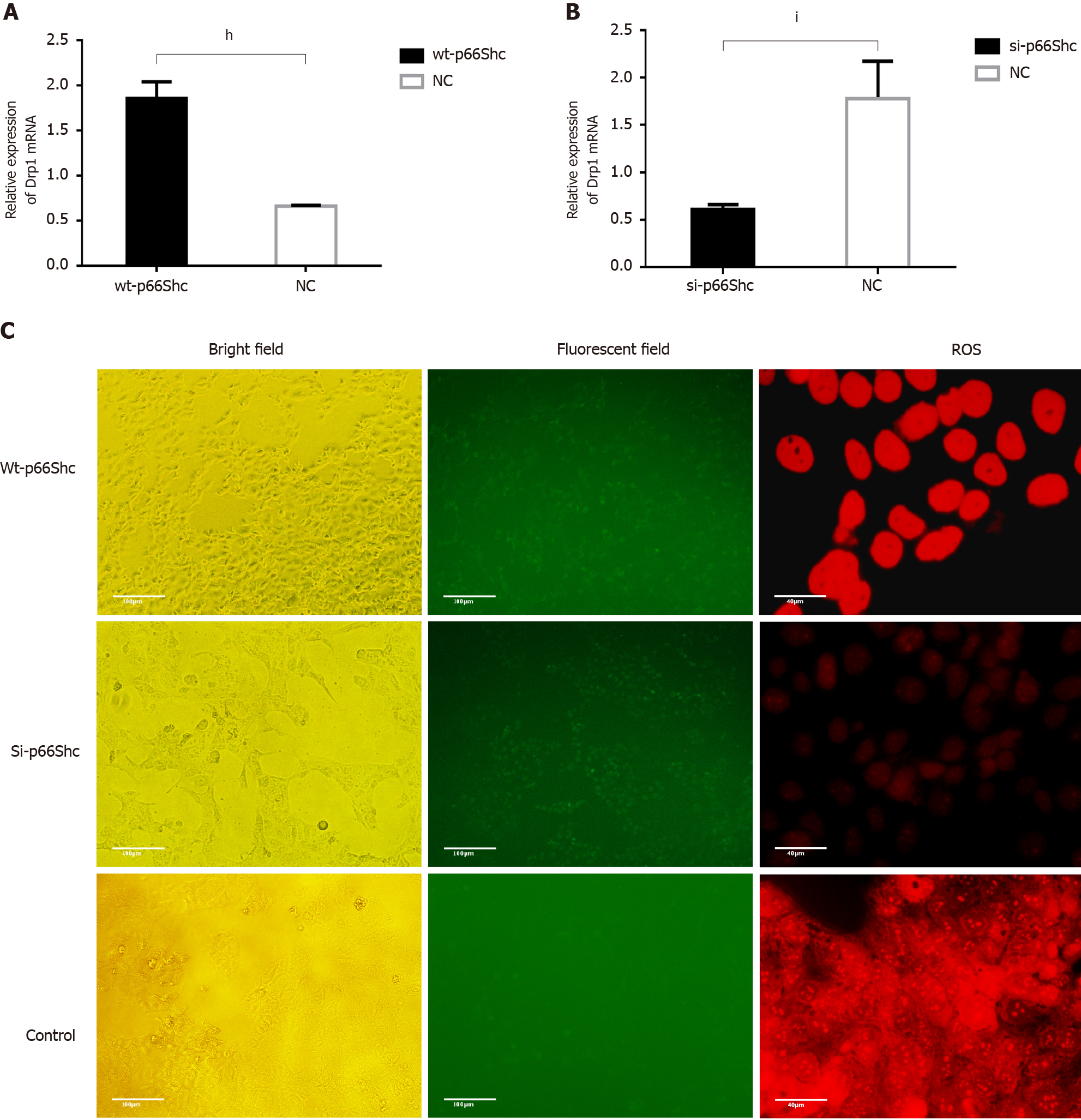
To determine the possible relationship among p66Shc, Drp1, and ROS, we altered the expression of Drp1 by transfecting cells with wt-p66Shc and p66Shc siRNA. The results showed that the expression of Drp1 was significantly upregulated in JEG3 cells after transfection with wt-p66Shc (P < 0.01) but significantly downregulated after transfection with p66Shc siRNA (P < 0.05, Figure 4A and B). The levels of ROS were also significantly increased in JEG3 cells after transfection with wt-p66Shc and significantly decreased after transfection with p66Shc siRNA (Figure 4C).
Oxidative stress refers to an imbalance between oxidation and antioxidation
Physiological oxidative stress is indeed a protective and adaptive mechanism of the body during normal pregnancies. However, excessive active oxygen can disrupt the balance between oxidation and antioxidation, cause pathological oxidative stress, and ultimately lead to tissue damage in GDM[6,19]. A previous study showed that placental cell biology and mitochondrial dysfunction are central to the pathophy
In a pathological pregnancy, the morphology and content of the mitochondria in the placenta change adaptively with changes in ROS levels, and the changes are accompanied by simultaneous damage and adaptive regulation[25]. Mitochondrial morphology and density are regulated by a reduction in mitochondrial fusion and an increase in mitosis[26]. In obese women with GDM, there is no significant change in the biogenesis of placental mitochondria, but a dynamic change has been found in placental mitochondrial morphology[27]. The mitochondrial changes contribute to ROS overproduction under hyperglycemic conditions and may become a target to control the production of ROS in hyperglycemia-associated disorders[28].
Mitochondrial dynamics are regulated by mitotic/fusion proteins, such as Drp1. Overexpression of Drp1 accelerates mitochondrial fission and promote the production of mitochondrial fragmentation and ROS[10]. Drp1 is activated in high glucose-treated cardiovascular cells, participates in fission-mediated fragmentation of mitochondrial tubules, enhances the production of mitochondrial ROS, opens the membrane permeability transition pore, and ultimately leads to cell injury and apoptosis[7]. In this study, high expression of Drp1 was found in the placentas of GDM patients. In addition, Drp1 was more highly expressed in a high-glucose environment than in a low-glucose environment, and its expression level increased with time under high-glucose conditions. The result shows that abnormal mitochondrial function is dominated by Drp1 at the onset of GDM. Notably, ROS levels were increased in the placentas of GDM patients and JEG3 cells treated with high glucose. Therefore, we speculated that Drp1 overexpression resulted in abnormal mitochondrial morphology and function and excessive production of ROS, promoting IR and human trophoblast cell apoptosis and contributing to the pathogenesis of GDM. Abbade et al[29] showed that placental mitochondrial dynamics are skewed toward fusion in GDM, as demonstrated by transmission electron microscopy and changes in the expression of key mechanochemical enzymes such as OPA1 and active phosphorylated Drp1. They found decreased Drp1 levels in placental tissue from women with GDM, which is contrary to our results. However, he also mentioned that proper glycemic control an important factor in GDM patients. In our study, we selected untreated GDM patients with poor glycemic control during pregnancy. However, the limited sample size hindered the capacity to assess the impact of Drp1 on the placentas of women with GDM.
p66Shc is an important regulatory protein of oxidative stress in the mitochondrial pathway. After phosphorylation at Ser36, p66Shc is transported to the mitochondrial membrane and generates mitochondrial ROS through the oxidation of cytochrome c[30]. In a DM mouse model, the expression of p66Shc was found to be significantly increased. However, knockout of the p66Shc gene reduced the generation of oxidative stress in renal tissues and delayed the progression of disease, suggesting that p66Shc is involved in mediating oxidative stress induced by high glucose[31]. In T2DM, the expression of p66Shc in peripheral blood monocytes was also found to be significantly increased[32], and the transcription of p66Shc mRNA was enhanced in human umbilical vein endothelial cells cultured with high glucose in vitro[33]. Activated p66Shc can translocate from the cytoplasm to mitochondria, oxidize cytochrome c, induce ROS production, and ultimately cause oxidative damage and cell apoptosis. It further promotes the phosphorylation of insulin receptor inhibitor-1, activates mammalian target of rapamycin receptor ribosome S6 protein kinase, leads to IR and exacerbates DM progression[34]. Thus, p66Shc plays an important role in the pathogenesis of DM.
However, the role of p66Shc in GDM remains unclear. In this study, we found that the expression of p66Shc was increased in the peripheral blood and placentas of patients with GDM and was significantly higher in JEG3 cells treated with high glucose than in those treated with low glucose in vitro. Compared with healthy pregnant women, our data showed that the expression of Drp1 and the level of ROS in GDM patients increased when the expression of p66Shc was increased, but decreased when p66Shc was decreased. Therefore, the activity of Drp1 and ROS may be regulated by p66Shc in the placentas of patients with GDM. This reveals that the increased expression of p66Shc was involved in high-glucose induced oxidative stress in human trophoblast cells and placentas. In addition, the expression of p66Shc and Drp1 in JEG3 cells under high glucose conditions was time dependent, showing that mitochondrial damage increased over time. Therefore, mitochondrial damage and ROS in patients with poor glycemic control may be increased during the development of GDM, and the possibility of pregnancy complications will also increase.
To date, the relationship among p66Shc, Drp1 and ROS in GDM is still unclear. Some studies have reported that p66Shc inhibited mitochondrial division in several types of cells, such as neuronal cells and fat-derived stem cells, thereby promoting cell damage and apoptosis[35,36]. In addition, the ability of p66Shc to alter the mitochondrial crista morphology of immune T cells has also been reported[37]. Drp1, a gene that promotes mitochondrial division, is a dynein necessary for mitochondrial division. The concept that mitochondrial fragments are prerequisites for the production of ROS has recently been proposed[38]. In DM, mitochondrial debris and ROS interact, creating a vicious cycle. Based on previous research, we found that GDM involved mitochondrial oxidant stress generation, perturbation of mitochondrial dynamics and mitochondrial fragmentation. In this study, we observed a significant increase in the expression of Drp1 and the levels of ROS in JEG3 cells overexpressing activated p66Shc. In contrast, p66Shc knockdown reduced the expression of Drp1 and the level of ROS. Therefore, several lines of evidence have demonstrated that p66Shc is a crucial mediator of mitochondrial dysfunction. This result shows that high glucose promoted mitochondrial fragmentation following p66Shc activation and excessive ROS, which may further cause mitochondrial damage and cell apoptosis, contributing to the occurrence and development of GDM. Our study investigated the potential mechanism of p66Shc in GDM in vitro, finding that p66Shc may be an important molecule in the development of GDM, which needs to be confirmed in large clinical studies. This study may provide a new molecular mechanism and experimental basis for the role of mitochondrial damage in the pathogenesis of gestational diabetes mellitus and provide a new approach for the treatment of GDM and its complications.
The expression of p66shc in peripheral blood and the levels of p66shc and Drp1 in placental tissues were significantly increased in patients with GDM. The increased expression of p66Shc induced by high glucose-activated Drp1 and promoted ROS overproduction, which may be the primary cause of cell damage and apoptosis during the occurrence and development of GDM. This study preliminarily explored the relationships of p66Shc, mitochondria, and oxidative stress in GDM, providing new ideas and evidence regarding the etiology and treatment of GDM.
Oxidative stress and mitochondrial dysfunction in the placenta are closely related to the onset of gestational diabetes mellitus (GDM). p66Shc plays a role in regulating mitochondrial oxidative stress, and dynamin-related protein 1 (Drp1) is a necessary dynamic protein for mitosis of primarily localized mitochondria.
The motivation was to add to what is known of the role of placental mitochondria in the etiology of GDM.
The study aimed to investigate the potential mechanism of p66Shc in GDM.
We detected the expression of Drp1 and p66Shc in patients with GDM and investigated the possible pathogenesis of GDM through in vitro culture of the JEG3 human trophoblast line.
P66Shc, Drp1, and reactive oxygen species (ROS) were highly expressed in the placentas and peripheral blood during GDM and in JEG3 cells under high glucose conditions. A significant increase in the expression of Drp1 and the level of ROS was detected in JEG3 cells overexpressing activated p66Shc. In contrast, p66Shc knockdown reduced the expression of Drp1 and the level of ROS.
The increased expression of p66Shc induced by high glucose-activated Drp1 and promoted ROS overproduction, which may contribute to the occurrence and development of GDM.
This study may provide a new understanding of molecular mechanism and experimental basis for the role of mitochondrial damage in the pathogenesis of gestational diabetes mellitus and provide a new approach for the treatment of the condition and its complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Obstetrics and Gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ajjan RA, Kim M, Rodrigues GR S-Editor: Wang JL L-Editor: Filipodia P-Editor: Guo X
| 1. | Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2141] [Cited by in F6Publishing: 2163] [Article Influence: 144.2] [Reference Citation Analysis (1)] |
| 2. | Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59:1396-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 351] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 3. | Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics. 2019;14:215-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Simmons R. Developmental origins of adult metabolic disease. Endocrinol Metab Clin North Am. 2006;35:193-204, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 494] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 6. | Holland O, Dekker Nitert M, Gallo LA, Vejzovic M, Fisher JJ, Perkins AV. Review: Placental mitochondrial function and structure in gestational disorders. Placenta. 2017;54:2-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 7. | Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 354] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol. 2015;26:1304-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Sun Y, Kopp S, Strutz J, Gali CC, Zandl-Lang M, Fanaee-Danesh E, Kirsch A, Cvitic S, Frank S, Saffery R, Björkhem I, Desoye G, Wadsack C, Panzenboeck U. Gestational diabetes mellitus modulates cholesterol homeostasis in human fetoplacental endothelium. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:968-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | ALTamimi JZ, AlFaris NA, Al-Farga AM, Alshammari GM, BinMowyna MN, Yahya MA. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p66Shc axis and activation of FOXO-3a. J Nutr Biochem. 2021;87:108515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Singh S, Sharma S. Dynamin-related protein-1 as potential therapeutic target in various diseases. Inflammopharmacology. 2017;25:383-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tang WX, Wu WH, Qiu HY, Bo H, Huang SM. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol. 2013;26:1073-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Dai W, Jiang L. Dysregulated Mitochondrial Dynamics and Metabolism in Obesity, Diabetes, and Cancer. Front Endocrinol (Lausanne). 2019;10:570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Harish G, Mahadevan A, Pruthi N, Sreenivasamurthy SK, Puttamallesh VN, Keshava Prasad TS, Shankar SK, Srinivas Bharath MM. Characterization of traumatic brain injury in human brains reveals distinct cellular and molecular changes in contusion and pericontusion. J Neurochem. 2015;134:156-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Maiese K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015:875961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 17. | Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653-2658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 784] [Cited by in F6Publishing: 877] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 19. | Sarina, Li DF, Feng ZQ, Du J, Zhao WH, Huang N, Jia JC, Wu ZY, Alamusi, Wang YY, Ji XL, Yu L. Mechanism of Placenta Damage in Gestational Diabetes Mellitus by Investigating TXNIP of Patient Samples and Gene Functional Research in Cell Line. Diabetes Ther. 2019;10:2265-2288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Fisher JJ, McKeating DR, Cuffe JS, Bianco-Miotto T, Holland OJ, Perkins AV. Proteomic Analysis of Placental Mitochondria Following Trophoblast Differentiation. Front Physiol. 2019;10:1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 22. | Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107:579-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 23. | Chaube R, Werstuck GH. Mitochondrial ROS versus ER ROS: Which Comes First in Myocardial Calcium Dysregulation? Front Cardiovasc Med. 2016;3:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Cawyer CR, Horvat D, Leonard D, Allen SR, Jones RO, Zawieja DC, Kuehl TJ, Uddin MN. Hyperglycemia impairs cytotrophoblast function via stress signaling. Am J Obstet Gynecol. 2014;211:541.e1-541.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Le ZY, Dong S, Zhang R, Cai XP, Gao A, Xiao R, Yu HL. Placental mitochondrial biogenesis and function was slightly changed by gestational hypercholesterolemia in full-term pregnant women. J Dev Orig Health Dis. 2018;9:395-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Diaz-Morales N, Rovira-Llopis S, Bañuls C, Escribano-Lopez I, de Marañon AM, Lopez-Domenech S, Orden S, Roldan-Torres I, Alvarez A, Veses S, Jover A, Rocha M, Hernandez-Mijares A, Victor VM. Are Mitochondrial Fusion and Fission Impaired in Leukocytes of Type 2 Diabetic Patients? Antioxid Redox Signal. 2016;25:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Görlach A, Dimova EY, Petry A, Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, Palmeira CM, Kietzmann T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015;6:372-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Fisher JJ, Bartho LA, Perkins AV, Holland OJ. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin Exp Pharmacol Physiol. 2020;47:176-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Abbade J, Klemetti MM, Farrell A, Ermini L, Gillmore T, Sallais J, Tagliaferro A, Post M, Caniggia I. Increased placental mitochondrial fusion in gestational diabetes mellitus: an adaptive mechanism to optimize feto-placental metabolic homeostasis? BMJ Open Diabetes Res Care. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 873] [Cited by in F6Publishing: 876] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 31. | Menini S, Iacobini C, Ricci C, Oddi G, Pesce C, Pugliese F, Block K, Abboud HE, Giorgio M, Migliaccio E, Pelicci PG, Pugliese G. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia. 2007;50:1997-2007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Pagnin E, Fadini G, de Toni R, Tiengo A, Calò L, Avogaro A. Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab. 2005;90:1130-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, Liu JJ, Lu YB, Zhang ZQ, Yang RF, Zhang R, Cai H, Liu DP, Liang CC. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 34. | Ranieri SC, Fusco S, Panieri E, Labate V, Mele M, Tesori V, Ferrara AM, Maulucci G, De Spirito M, Martorana GE, Galeotti T, Pani G. Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2010;107:13420-13425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Pesaresi MG, Amori I, Giorgi C, Ferri A, Fiorenzo P, Gabanella F, Salvatore AM, Giorgio M, Pelicci PG, Pinton P, Carrì MT, Cozzolino M. Mitochondrial redox signalling by p66Shc mediates ALS-like disease through Rac1 inactivation. Hum Mol Genet. 2011;20:4196-4208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells. 2015;33:542-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Ulivieri C. Cell death: insights into the ultrastructure of mitochondria. Tissue Cell. 2010;42:339-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Galvan DL, Badal SS, Long J, Chang BH, Schumacker PT, Overbeek PA, Danesh FR. Real-time in vivo mitochondrial redox assessment confirms enhanced mitochondrial reactive oxygen species in diabetic nephropathy. Kidney Int. 2017;92:1282-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |