Published online Feb 15, 2020. doi: 10.4239/wjd.v11.i2.42
Peer-review started: July 1, 2019
First decision: August 2, 2019
Revised: November 27, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: February 15, 2020
Insulin resistance (IR) is the main complication found in 35%-80% of women with polycystic ovary syndrome (PCOS). However, there is no definite consensus regarding which marker to use for its assessment in PCOS women. Research has shown that hyperinsulinemia is correlated with increased bone mass. Given that most women with PCOS are insulin resistant, which is independent from body fat and characterized by hyperinsulinemia, it could be hypothesized that there would be an increased bone mass in the patient as a result. Subsequently, increased bone mass could be measured using the wrist circumference method.
To assess the wrist circumference as an easy-to-detect marker of IR in Congolese women with PCOS.
Seventy-two Congolese women with PCOS and seventy-one controls from the same ethnic group, were enrolled in the study (mean age 24.33 ± 5.36 years). Fasting biochemical parameters, and the Homeostasis Model Assessment of insulin resistance (HOMA-IR) and body composition were evaluated. The non-dominant wrist circumference was measured manually, as was the waist circumference (WC), hip circumference, height and weight. Calculated measures included evaluation of body mass index (BMI), Waist-to-Height (WHtR) and Waist-to-hip ratio (WHR). In addition, body composition was assessed by Bioelectrical Impedance Analysis using a body fat analyzer.
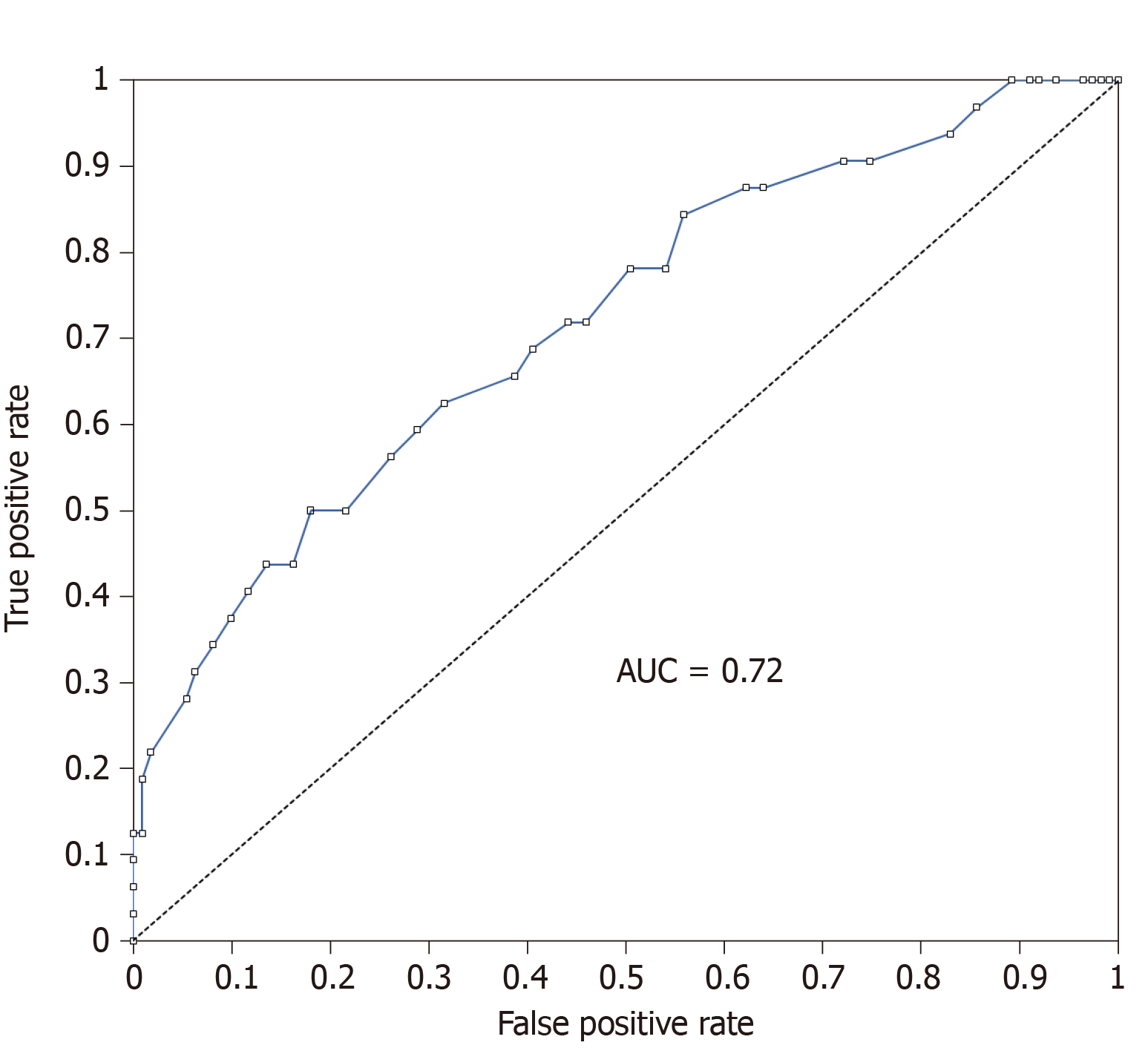
The non-dominant wrist circumference was more closely correlated with HOMA-IR (r = 0.346; P = 0.003) and was the best anthropometrical marker correlated with IR (P = 0.011 ) compared with other anthropometrical markers in women with PCOS: Dominant Wrist Circumference (r = 0.315; P = 0.007), Waist Circumference (WC) (r = 0.259; P = 0.028), BMI (r = 0.285; P = 0.016), WHR (r = 0.216; P = 0,068) and WHtR (r = 0.263; P = 0.027). The diagnostic accuracy of the non-dominant wrist circumference for the presence or absence of IR using Receiver-operating characteristic (ROC) curve analysis showed that the area under the ROC curve was 0.72. A cutoff value for the non-dominant wrist circumference of 16.3 cm was found to be the best predictor of IR in Congolese women with PCOS.
Non-dominant wrist circumference is, to date, the best anthropometrical marker of IR in Sub-Saharan African women with PCOS. It could be suggested as an easy-to-detect marker for assessing IR.
Core tip: In a previous study, we found that insulin resistance (IR), which is independent of the body fat and central distribution in women with polycystic ovary syndrome (PCOS), is commonly found among the African population. Among various markers of IR, the role of anthropometric indicators is obvious in developing countries. Therefore, a more appropriate method that should be easy to perform is sought. In the present study, we found for the first time that non-dominant wrist circumference is not only a marker of IR, but the best anthropometric marker known to date for the assessment of IR in women with PCOS.
- Citation: Amisi CA, Ciccozzi M, Pozzilli P. Wrist circumference: A new marker for insulin resistance in African women with polycystic ovary syndrome. World J Diabetes 2020; 11(2): 42-51
- URL: https://www.wjgnet.com/1948-9358/full/v11/i2/42.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i2.42
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting women of reproductive age[1]. Insulin resistance (IR), the major complication for women with PCOS, is strongly associated with an increased risk of cardiovascular diseases and diabetes and affects 35%-80% of women[2-4]. Early detection of IR could help prevent these complications. However, there is no consensus regarding the most accurate method to predict IR in women with PCOS.
In recent decades, several structural body components have been evaluated in relation to IR[5-9]. Waist circumference (WC), body mass index (BMI), waist-to-hip ratio (WHR), waist-to-height ratio (WHtR) are examples. However, because they are all based on body fat assessment, they cannot accurately predict IR in women with PCOS. Indeed, IR in PCOS in particular is independent of the BMI and body fat distribution[2,3]. Consequently, a more appropriate method that should be easy to perform is sought.
Among various markers of IR, the role of anthropometric indicators is obvious in developing countries[9]. Because of poverty and a lack of suitable laboratories, biological evaluation of IR based on the measurement of insulin or other biological markers is difficult. Therefore, finding a marker that is not based on the measurement of fat, which is anthropometric and easy to perform, has become a challenge. Meeting this challenge has been the basis of our research.
Indeed, in a previous study, we found that IR in women with PCOS, although appearing to be a Western pathology when we look at the currently available literature, is commonly found among the African population[3]. Moreover, one of two Congolese women with PCOS is insulin-resistant[3]. However, many live with this burden and its consequences, and it is not well-treated. Recently, publications have reported on the bone system as a new endocrine organ[10,11]. Indeed, bone has been found to be involved in glucose metabolism via osteocalcin (OC) effects on insulin[11-16]. Osteocalcin, the hormone secreted by osteoblasts, exerts an endocrine regulation of sugar homeostasis by improving insulin sensitivity. It has been shown that in the presence of IR, its compensatory hyperinsulinaemia is associated with increased bone mass, which could be detected by a simple measurement of the wrist circumference (WrC)[17]. Wide WrC has been associated with IR[17-19]. Subsequently, the WrC has been proposed as a new easy-to-detect marker of IR in young obese people[17,20-21].
Given that most women with PCOS are insulin-resistant, independent of fat and characterized by hyperinsulinaemia[2,3,22], we hypothesized that in the presence of IR in Congolese women with PCOS, hyperinsulinaemia might induce increased bone mass that could be detected by the measurement of WrC. Wide WrC should be associated with IR in Congolese women with PCOS. Thus, WrC could be proposed as a new easy-to-detect marker for IR assessment in women with PCOS. The aim of this study was to assess the wrist circumference in Congolese women with PCOS in relation to IR using the Homeostasis Model Assessment as a biological reference marker.
This prospective case-control study was performed from October 2015 to December 2016 in Kinshasa, the capital city of the Democratic Republic of Congo, and involved 72 women with PCOS and 71 controls. Because the prevalence of PCOS in the Democratic Republic of Congo is unknown, we have taken into account the global prevalence, which varies between 5%-10%, to determine our sample size. Because PCOS is largely unknown in the Democratic Republic of Congo, numerous real cases of PCOS are not found among outpatient clinics. Due to popular beliefs, many PCOS patients are often discouraged from seeing a doctor for numerous reasons: (1) The doctor visit is expensive; (2) Hirsutism is seen as a beautiful trait. In fact, women never consult for hirsutism; (3) Spaniomenorrhoea and/or amenorrhoea are sometimes seen as a normal phenomenon in a woman’s life, especially before marriage; and (4) Lack of efficacy of previous treatment received. For these reasons, our PCOS patients were recruited not only from outpatient clinics but also from universities and the community by means of personal interviews and advertising.
The patients were women with PCOS, all African and of reproductive age and from a Congolese ethnic group, without hormonal treatment for the 2 months preceding the study. The study protocol was approved by the ethical committee of the Università Campus Bio-Medico di Roma. Written informed consent was obtained from each subject before entry into the study. PCOS was defined according to the Rotterdam 2003 consensus[1], by the presence of at least two of the following three features: (1) Clinical and/or biochemical signs of hyperandrogenism; (2) Oligomenorrhoea and/or anovulation; and (3) Polycystic ovaries. Clinical hyperandrogenism was defined by a Ferriman-Gallwey score > 8. Age-matched control women came from the same ethnic group. They were of reproductive age, non-hirsute, without a personal or family history of hirsutism and/or endocrine disorders, and not on medical treatment.
Women were excluded if they: (1) Refused to participate in the study; (2) Were pregnant or became pregnant during the study period; (3) Were in the peripubertal period; (4) Were in menopause; or (5) Were using any contraception method, hormonal treatment or insulin sensitizers.
All women underwent a physical examination including an evaluation of the blood pressure, weight, height, wrist circumference, abdominal and hip circumference. All anthropometric measurements were taken by the same examiner to minimize error.
Blood pressure was measured after a 10-minute rest in the sitting position, with the feet on the floor and the arm supported at heart level. Normal values were considered to be < 130/85 mm Hg[1]. The non-dominant wrist circumference was measured with the subjects in a seated position using a tape measure positioned over Lister’s tubercle of the distal radius and over the distal ulna[23].
The WC was measured at the end of a normal expiration, at a level parallel to the floor, at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest, in the mid axillary line, using stretch‐resistant tape, with the women relaxed and standing with the feet close together, arms at the side and body weight evenly distributed[24]. The hip circumference was measured at a level parallel to the floor, at the largest circumference of the buttocks[24]. Calculated measures included the BMI, WHtR and WHR.
In addition, body composition was assessed by Bioelectrical Impedance Analysis using a body fat analyser (OMRON BF 511®). This device measures the impedance of each body segment to 50 kHz. It uses electrical impedance with height, weight, age and gender information to generate body composition data (body fat percentage, visceral fat level and skeletal muscle percentage). The assessment was performed in the morning after a fast of ≥ 3 h, with the patient wearing a light dress and stripped of all metal objects. The women were instructed not to practice vigorous exercise before the test. The BMI was classified according to the WHO criteria as[25]: (1) Normal: 18.5-24.9 kg/m2; (2) Overweight: 25.0-29.9 kg/m2; (3) Obese: (a) Class I: 30.0-35.0 kg/m2; (b) Class II: 35.1-39.9 kg/m2; (c) Class III: > 40.0 kg/m2. The WHR was classified as normal if < 0.85 and substantially increased if ≥ 0.85[24]. The WC was classified as normal if < 80 cm and substantially increased if ≥ 80 cm[24].
The biological measurements included the fasting glucose and insulin. A fasting blood sample was collected from the PCOS and control subjects in the morning and was centrifuged immediately. The serum was stored at -20 °C until analysis. The blood glucose concentration was determined on the day of blood collection by the glucose oxidase method using a glucometer (Freestyle). Insulin was measured using the ELISA method. IR was assessed by Homeostasis Model Assessment of insulin resistance (HOMA-IR) using the following formula:
HOMA-IR = [(glucose in mmol/L) × insulin in µU/mL]/22.5[26,27]
Where glucose is in mmol and has been transformed from mg/dL by the following formula:
Glucose in mmol/L = Glucose (in mg/dL)/18
The normal value of HOMA-IR was ≤ 2.74 mol × µU/L², as previously reported[3].
All women with HOMA-IR > 2.74 mol × µU/L², were insulin-resistant[3].
The statistical analysis was performed using SPSS statistical software (version 16.0). Qualitative data were expressed as the frequency (n = number) and proportion (%). Continuous data are expressed as the mean ± standard deviation. The Kolmogorov-Smirnov was used for the normality analysis of the parameters. Student’s t-test and Chi-square test were used for the comparisons between groups and subgroups of continuous and categorical variables, respectively. Non-parametric tests were used for variables not normally distributed. Logistic regression was performed to analyze the association between the study variables. The odds ratio (OR) was presented with their 95% confidence interval (CI). Statistical significance was expressed as aP < 0.05, bP < 0.001.
Table 1 and Table 2 show the characteristics of our study population. The 72 Women with PCOS and 71 controls participated at all stages of the study. The mean age was similar in both groups and subgroups. There was a significant difference for the Ferriman-Gallwey (F-G) score between women with PCOS and controls as well as for insulinaemia and HOMA-IR (P <0.001). Before performing logistic regression, we studied the correlation between anthropometrical parameters and HOMA-IR. We found that non-dominant wrist circumference was more closely correlated with HOMA-IR (r = 0.346; P = 0.003) than dominant wrist circumference (r = 0.315; P = 0.007), WC (r = 0.259; P = 0.028), BMI (r = 0.285; P = 0.016), WHR (r = 0.216; P = 0.068) or WHtR (r = 0.263; P = 0.027).
| Parameters | PCOS (n = 72) | Controls (n = 71) | P value |
| Age (yr) | 24.4 ± 5.2 | 24.25 ± 5.5 | 0.869 |
| Menarche age (yr) | 12.97 ± 1.9 | 12.86 ± 1.8 | 0.723 |
| Ferriman-Gallwey Score | 8.62 ± 6.2 | 2.32 ± 2.3 | < 0.001 |
| Systolic blood pressure (mm Hg) | 109.24 ± 17.26 | 100.86 ±15.27 | 0.003 |
| Diastolic blood pressure (mm Hg) | 71.84 ± 14.11 | 64.96 ± 12.53 | 0.003 |
| BMI (kg/m²) | 25.23 ± 5.55 | 23.07 ± 4.52 | 0.013 |
| WC (cm) | 84.28 ± 13.94 | 78.63 ± 11.60 | 0.009 |
| WHR | 0.81 ± 0.06 | 0.80 ± 0.05 | 0.101 |
| WHtR | 0.51 ± 0.08 | 0.48 ± 0.07 | 0.018 |
| Dominant Wrist Circumference (cm) | 15.98 ± 1.15 | 15.53 ± 0.87 | 0.009 |
| Non-Dominant Wrist Circumference (cm) | 15.80 ± 1.18 | 15.33 ± 0.87 | 0.009 |
| Weight (kg) | 66.9 ± 16.3 | 60.8 ± 11.8 | 0.013 |
| Body fat (%) | 35.05 ± 9.6 | 32.37 ± 8 | 0.076 |
| Muscle (%) | 26.6 ± 4.5 | 27.9 ± 3 | 0.042 |
| Visceral fat | 4.67 ± 2 | 3.97 ± 1.7 | 0.031 |
| Glucose (mg/dL) | 86.51 ± 10.3 | 84.3 ± 8.04 | 0.155 |
| Insulin (µU/L) | 14.91 ± 15.4 | 6.34 ± 3.8 | < 0.001 |
| HOMA-IR (mol × µU/L²) | 3.40 ± 4.03 | 1.33 ± 0.83 | < 0.001 |
| Parameters | PCOS IR+ (n = 28) | PCOS IR- (n = 44) | P value |
| Age (yr) | 25.07 ± 5.67 | 23.98 ± 4.89 | 0.388 |
| Menarche age (yr) | 12.71 ± 2.03 | 13.14 ± 1.85 | 0.367 |
| Ferriman-Gallwey Score | 8.04 ± 6.91 | 9.00 ± 5.87 | 0.528 |
| Systolic blood pressure (mm Hg) | 114.08 ± 22.20 | 106.59 ± 13.43 | 0.087 |
| Diastolic blood pressure (mm Hg) | 74.62 ± 17.42 | 70.32 ± 11.89 | 0.232 |
| BMI (kg/m²) | 27.39 ± 6.27 | 23.90 ± 4.66 | 0.016 |
| WC (cm) | 90.09 ± 15.76 | 80.58 ± 11.36 | 0.008 |
| WHR | 0.84 ± 0.07 | 0.80 ± 0.06 | 0.014 |
| WHtR | 0.54± 0.09 | 0.49 ± 0.07 | 0.014 |
| Dominant wrist circumference (cm) | 16.49 ± 1.27 | 15.66 ± 0.95 | 0.005 |
| Non-dominant wrist circumference (cm) | 16.33 ± 1.20 | 15.45 ± 1.03 | 0.002 |
| Weight (kg) | 72.88 ± 18.56 | 63.26 ± 13.86 | 0.025 |
| Body fat (%) | 38.50 ± 9.44 | 32.93 ± 9.22 | 0.017 |
| Muscle (%) | 26.30 ± 3.42 | 26.90 ± 5.09 | 0.591 |
| Visceral fat | 5.48 ± 2.15 | 4.16 ± 1.75 | 0.010 |
| Glucose (mg/dL) | 93.04 ± 10.34 | 82.36 ± 7.99 | < 0.001 |
| Insulin (µU/L) | 26.22 ± 19.99 | 7.71 ± 2.99 | < 0.001 |
| HOMA-IR (mol × µU/L²) | 6.26 ± 5.32 | 1.57 ± 0.63 | < 0.001 |
In contrast, we found a strong correlation between the dominant WrC, non-dominant WrC, WC, BMI, weight, WHtR and WHR. Because of the severe collinearity and high correlation, we could not use these parameters in the same regression model. Logistic regression analysis was then performed. First, we performed logistic regression using binary HOMA-IR (IR+: HOMA-IR > 2.74; IR-: HOMA-IR ≤ 2.74) as the dependent variable. The non-dominant WrC was significantly associated with IR (P =0.011) (Table 3).
| B | SE | Wald | df | P value | Exp (B) | |
| Age | 0.031 | 0.055 | 0.311 | 1 | 0.577 | 1.031 |
| Height | -0.052 | 0.045 | 1.323 | 1 | 0.250 | 0.950 |
| Diastolic blood pressure (mm Hg) | -0.004 | 0.021 | 0.028 | 1 | 0.866 | 0.996 |
| Non-dominant wrist circumference | 0.737 | 0.291 | 6.424 | 1 | 0.011 | 2.089 |
| Constant | -4.384 | 6.785 | 0.417 | 1 | 0.518 | 0.012 |
Next, we compared our results using other anthropometrical markers. We replaced the non-dominant WrC with the WC in the model. We observed that the significance disappeared (P = 0.065) (Table 4). We observed the same phenomenon when we replaced the non-dominant wrist circumference with BMI (P = 0.070) (Table 5) and then WHR (P = 0.239) (Table 6) and WHtR (P = 0.068) (Table 7). It appeared that non-dominant wrist circumference was the best anthropometric marker correlated with IR.
| B | SE | Wald | df | P value | Exp (B) | |
| Diastolic blood pressure (mm Hg) | -0.008 | 0.023 | 0.118 | 1 | 0.731 | 0.992 |
| Height | -0.020 | 0.042 | 0.222 | 1 | 0.637 | 0.980 |
| Age | 0.020 | 0.055 | 0.133 | 1 | 0.715 | 1.020 |
| WC | 0.048 | 0.026 | 3.408 | 1 | 0.065 | 1.049 |
| Constant | -1.383 | 6.739 | 0.042 | 1 | 0.837 | 0.251 |
| B | SE | Wald | df | P value | Exp (B) | |
| Diastolic blood pressure (mm Hg) | -0.004 | 0.022 | 0.034 | 1 | 0.854 | 0.996 |
| Height | -0.010 | 0.041 | 0.060 | 1 | 0.807 | 0.990 |
| Age | 0.036 | 0.054 | 0.451 | 1 | 0.502 | 1.037 |
| BMI | 0.104 | 0.057 | 3.286 | 1 | 0.070 | 1.109 |
| Constant | -2.215 | 6.653 | 0.111 | 1 | 0.739 | 0.109 |
| B | SE | Wald | df | P value | Exp (B) | |
| Diastolic blood pressure (mm Hg) | 0.003 | 0.022 | 0.025 | 1 | 0.873 | 1.003 |
| Height | -0.002 | 0.041 | 0.003 | 1 | 0.956 | 0.998 |
| Age | 0.019 | 0.057 | 0.110 | 1 | 0.740 | 1.019 |
| WHR | 6.009 | 5.107 | 1.384 | 1 | 0.239 | 407.060 |
| Constant | -5.891 | 7.309 | 0.650 | 1 | 0.420 | 0.003 |
| B | S.E. | Wald | df | P value | Exp (B) | |
| Diastolic blood pressure (mm Hg) | -0.008 | 0.023 | 0.121 | 1 | 0.728 | 0.992 |
| Height | 0.004 | 0.041 | 0.012 | 1 | 0.914 | 1.004 |
| Age | 0.020 | 0.055 | 0.134 | 1 | 0.714 | 1.021 |
| WHtR | 7.878 | 4.312 | 3.338 | 1 | 0.068 | 2.638E3 |
| Constant | -5.335 | 6.920 | 0.594 | 1 | 0.441 | 0.005 |
We then assessed the diagnostic accuracy of non-dominant wrist circumference for the presence or absence of IR using Receiver-operating characteristic (ROC) curve analysis (Figure 1). As shown, the area under the ROC curve was 0.72. This result indicates that non-dominant wrist circumference has a 72% chance of predicting the presence of IR in women with PCOS. In our search of a cutoff value for non-dominant wrist circumference, we found 16.3 cm to be the best predictor of IR in Congolese women with PCOS.
To our knowledge, this is the first study that provides evidence that non-dominant wrist circumference can be used as a marker for IR in women with PCOS. Current recommendations for the management of PCOS suggest that given the association with IR, all women with PCOS should be evaluated for the risk of metabolic syndrome and its components, including type 2 diabetes, hypertension, hyperlipidaemia, and the possible risk of clinical events, including acute myocardial infarction and stroke[28]. However, a non-consensus method is provided for the assessment of IR. In contrast, the problem in Sub-Saharan African countries is the diagnosis of IR. The biological evaluation of IR is not only extremely expensive and complicated but also available only in a few laboratories in capital cities. Anthropometrical parameters are, therefore, most recommended[10].
We aimed to assess the non-dominant wrist circumference in relation to IR in Congolese women with PCOS using the Homeostasis Model Assessment for IR as the reference biological index. This choice regarding HOMA-IR was determined by the precedent study performed in Congolese women with PCOS[3] but also because HOMA-IR is an extensively validated marker[4,29-32]. Making a comparison between women with PCOS and the controls, we found a significant difference regarding dominant and non-dominant wrist circumference. This observation corroborates that of Esmaeilzadeh et al[33], who found that adolescent girls with PCOS have a higher mean wrist circumference compared to those without PCOS.
We found a positive and significant correlation between non-dominant wrist circumference and IR among women with PCOS. Moreover, non-dominant wrist circumference was the strongest marker associated with HOMA-IR, whereas the WC, BMI, WHR and WHtR were poorly associated with this parameter. This observation was predictable because IR in women with PCOS is independent of body fat[2,3]. Our observation makes non-dominant wrist circumference the best anthropometric marker of IR known to date. The novelty of the WrC as a marker of IR is that it is based on an assessment of IR on bone, not fat[17,20].
This observation also highlights the impact of IR on bone in women with PCOS, opening a new research perspective on this complication in such women. Indeed, to date, many publications that have studied the impact of PCOS on bone have mainly focused on the study of bone mineral density[34-38]. Our study focuses on the impact of IR and PCOS status on the hormonal interaction between bone and insulin, suggesting a close relationship among them. Therefore, it is necessary to understand the pathophysiological mechanism of this association to develop appropriate preventive strategies.
Recently, wrist circumference has attracted much attention[17-20,33]. Many authors have found a strong association between the wrist circumference and cardio-vascular risk. Capizzi et al[17], studying overweight and obese adolescents, was the first to report that wrist circumference is strongly correlated with fasting insulin levels and insulin-resistance. Mohebi et al[39] evaluated the effect of wrist circumference on the risk of incident hypertension and cardiovascular disease (CVD) in an adult population and found that in non-centrally obese women, an increase in wrist circumference was independently associated with both hypertension and cardiovascular disease. Amini et al[20], in a study conducted among 1709 participants, found that the association of wrist circumference with cardiometabolic risk factors was significantly positive with waist circumference (P = 0.001), BMI (P = 0.001), and LDL-C (P = 0.01) but significantly inverse with HDL-C (P = 0.001). He suggested that measurement of the wrist circumference can serve as an easy-to-detect clinical marker to identify individuals at risk of cardiometabolic disorders and can be used in large epidemiological studies.
In the present study, we found, for the first time, that non-dominant wrist circumference is not only a marker of IR, but the best anthropometric marker known to date, for the assessment of IR in women with PCOS. However, we recognise some limitations of our study, among them, the limited size of our study population. We recommend large-scale studies to validate our observations.
Polycystic ovary syndrome (PCOS) is insulin-resistant and strongly associated with an increased risk of cardiovascular diseases and diabetes in women. Early detection of insulin resistance (IR) could prevent these complications. There is no consensus regarding methods to predict IR in women with PCOS. Some structural body components have been evaluated in relation to IR in PCOS, and IR seems to be independent. In this article we tried for a new easy detectable marker for IR in women affected by PCOS.
We tried to develop a new easy marker for IR in women with PCOS to improve the diagnosis of IR in Sub-Saharan African women.
Our aim was to assess the wrist circumference in women affected by PCOS and living in Kinshasa, the capital city of the Democratic Republic of Congo, in relation to IR using the Homeostasis Model Assessment as a biological reference marker.
This study was a prospective case-control study performed from October 2015 to December 2016 in Kinshasa. Seventy-two women with PCOS and 71 controls were enrolled. Parametric and non-parametric statistical test have been used where appropriated. The statistical analysis was performed using SPSS statistical software (version 16.0).
In this study we have found a significant difference for the Ferriman-Gallwey (F-G) score between the women with PCOS and controls as well as for insulinaemia and HOMA-IR (P <0.001). A strong correlation between the dominant WrC, non-dominant WrC, WC, BMI, Weight, WHtR and WHR have been found. The Receiver-Operating Characteristic (ROC) curve analysis, showed that the non-dominant wrist circumference has a 72% chance of predicting the presence of IR in women with PCOS.
In the present study for the first time, we showed that the non-dominant wrist circumference is both, a marker of IR, and the best anthropometric marker known, to date for the assessment of IR in women with PCOS.
This article could open new perspectives between IR and bone homeostasis in women with PCOS.
The authors would like to acknowledge Mrs. Luciana Valente for her involvement in performing the insulinaemia analysis. We also express appreciation to all the women who participated in this study for their enthusiastic support, especially to Mazze Tshimanga.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barzilay J S-Editor: Wang J L-Editor: A E-Editor: Qi LL
| 1. | Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3717] [Cited by in F6Publishing: 3761] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 2. | Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 975] [Cited by in F6Publishing: 964] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 3. | Amisi C, Mputu L, Mboloko E, Bieleli E, Pozzili P. [Biological insulin resistance in Congolese woman with polycystic ovary syndrome (PCOS)]. Gynecol Obstet Fertil. 2013;41:707-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the Homeostasis Model Assessment. Fertil Steril. 2005;83:1454-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64:16-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 422] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 6. | Vazquez G, Duval S, Jacobs DR, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 611] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 7. | Chouraki V, Wagner A, Ferrières J, Kee F, Bingham A, Haas B, Ruidavets JB, Evans A, Ducimetière P, Amouyel P, Dallongeville J. Smoking habits, waist circumference and coronary artery disease risk relationship: the PRIME study. Eur J Cardiovasc Prev Rehabil. 2008;15:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Mansour AA, Al-Jazairi MI. Cut-off values for anthropometric variables that confer increased risk of type 2 diabetes mellitus and hypertension in Iraq. Arch Med Res. 2007;38:253-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: Analysis of data from the British National Diet And Nutrition Survey of adults aged 19-64 years. Obes Facts. 2009;2:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Schwetz V, Pieber T, Obermayer-Pietsch B. The endocrine role of the skeleton: background and clinical evidence. Eur J Endocrinol. 2012;166:959-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Veldhuis-Vlug AG, Fliers E, Bisschop PH. Bone as a regulator of glucose metabolism. Neth J Med. 2013;71:396-400. [PubMed] [Cited in This Article: ] |
| 13. | Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Confavreux CB. Bone: from a reservoir of minerals to a regulator of energy metabolism. Kidney Int. 2011;79121:S14-S19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008;396:66-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. J Clin Endocrinol Metab. 2007;92:4161-4164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Capizzi M, Leto G, Petrone A, Zampetti S, Papa RE, Osimani M, Spoletini M, Lenzi A, Osborn J, Mastantuono M, Vania A, Buzzetti R. Wrist circumference is a clinical marker of insulin resistance in overweight and obese children and adolescents. Circulation. 2011;123:1757-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Derakhshan A, Tohidi M, Hajebrahimi MA, Saadat N, Azizi F, Hadaegh F. Sex-specific incidence rates and risk factors of insulin resistance and β-cell dysfunction: a decade follow-up in a Middle Eastern population. Diabet Med. 2017;34:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhäger JC, Lamberts SW, Grobbee DE. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone. 1996;18:545-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Amini A, Soltanian N, Iraj B, Askari G, Ebneyamin S, Ghias M, Hajian H, Zahed A, Amini M. Association of wrist circumference with cardio metabolic risk factors. J Pak Med Assoc. 2012;62:S34-S36. [PubMed] [Cited in This Article: ] |
| 21. | Jahangiri Noudeh Y, Hadaegh F, Vatankhah N, Momenan AA, Saadat N, Khalili D, Azizi F. Wrist circumference as a novel predictor of diabetes and prediabetes: results of cross-sectional and 8.8-year follow-up studies. J Clin Endocrinol Metab. 2013;98:777-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS). Hum Reprod. 2012;27:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 23. | Nyland J, Fried A, Maitra R, Johnson DL, Caborn DN. Wrist circumference is related to patellar tendon thickness in healthy men and women. Clin Imaging. 2006;30:335-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 2010;64:2-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | WHO/FAO Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:i-viii, 1-149, backcover. [PubMed] [Cited in This Article: ] |
| 26. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22373] [Cited by in F6Publishing: 23300] [Article Influence: 597.4] [Reference Citation Analysis (0)] |
| 27. | Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3281] [Cited by in F6Publishing: 3439] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 28. | Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society. American Association Of Clinical Endocrinologists, American College Of Endocrinology, And Androgen Excess And Pcos Society Disease State Clinical Review: Guide To The Best Practices In The Evaluation And Treatment Of Polycystic Ovary Syndrome - Part 2. Endocr Pract. 2015;21:1415-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 29. | Sawathiparnich P, Weerakulwattana L, Santiprabhob J, Likitmaskul S. Obese adolescent girls with polycystic ovary syndrome (PCOS) have more severe insulin resistance measured by HOMA-IR score than obese girls without PCOS. J Med Assoc Thai. 2005;88 Suppl 8:S33-S37. [PubMed] [Cited in This Article: ] |
| 30. | Cebeci F, Onsun N, Mert M. Insulin resistance in women with hirsutism. Arch Med Sci. 2012;8:342-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Lankarani M, Valizadeh N, Heshmat R, Peimani M, Sohrabvand F. Evaluation of insulin resistance and metabolic syndrome in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:504-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wei HJ, Young R, Kuo IL, Liaw CM, Chiang HS, Yeh CY. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: a cross-sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864-1868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Esmaeilzadeh S, Delavar MA, Amiri M, Khafri S, Pasha NG. Polycystic ovary syndrome in Iranian adolescents. Int J Adolesc Med Health. 2014;26:559-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Katulski K, Slawek S, Czyzyk A, Podfigurna-Stopa A, Paczkowska K, Ignaszak N, Podkowa N, Meczekalski B. Bone mineral density in women with polycystic ovary syndrome. J Endocrinol Invest. 2014;37:1219-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | McBreairty LE, Zello GA, Gordon JJ, Serrao SB, Pierson RA, Chizen DR, Chilibeck PD. Women With Polycystic Ovary Syndrome Have Comparable Hip Bone Geometry to Age-Matched Control Women. J Clin Densitom. 2018;21:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Attlee A, Nusralla A, Eqbal R, Said H, Hashim M, Obaid RS. Polycystic ovary syndrome in university students: occurrence and associated factors. Int J Fertil Steril. 2014;8:261-266. [PubMed] [Cited in This Article: ] |
| 37. | To WW, Wong MW. A comparison of bone mineral density in normal weight and obese adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2012;25:248-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Mario FM, do Amarante F, Toscani MK, Spritzer PM. Lean muscle mass in classic or ovulatory PCOS: association with central obesity and insulin resistance. Exp Clin Endocrinol Diabetes. 2012;120:511-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Mohebi R, Mohebi A, Sheikholeslami F, Azizi F, Hadaegh F. Wrist circumference as a novel predictor of hypertension and cardiovascular disease: results of a decade follow up in a West Asian cohort. J Am Soc Hypertens. 2014;8:800-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |