Published online Feb 15, 2017. doi: 10.4251/wjgo.v9.i2.70
Peer-review started: August 10, 2016
First decision: September 12, 2016
Revised: October 16, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: February 15, 2017
To investigate the outcomes of liver and pancreatic resections for renal cell carcinoma (RCC) metastatic disease.
This is a retrospective, single centre review of liver and/or pancreatic resections for RCC metastases between January 2003 and December 2015. Descriptive statistical analysis and survival analysis using the Kaplan-Meier estimation were performed.
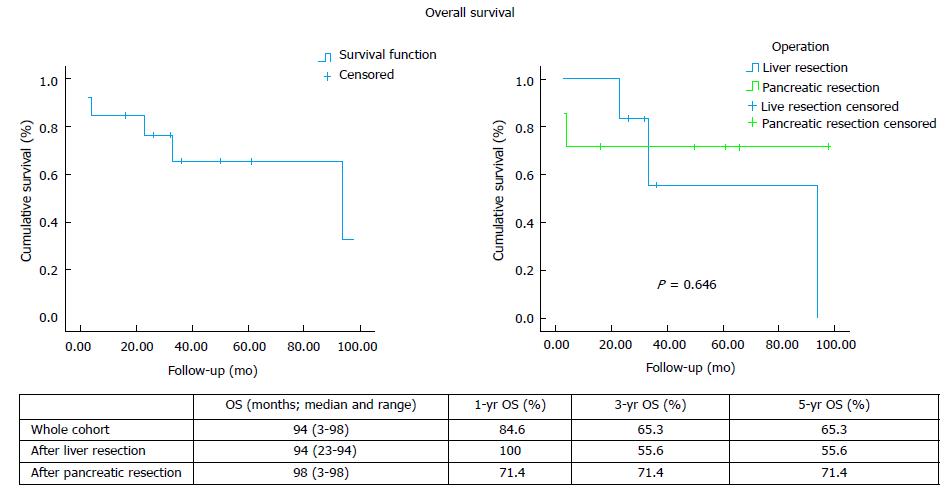
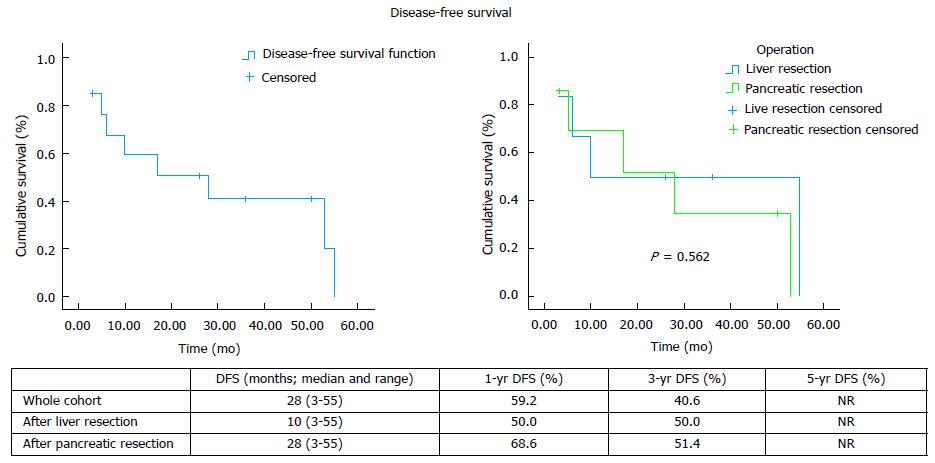
Thirteen patients had 7 pancreatic and 7 liver resections, with median follow-up 33 mo (range: 3-98). Postoperative complications were recorded in 5 cases, with no postoperative mortality. Three patients after hepatic and 5 after pancreatic resection developed recurrent disease. Median overall survival was 94 mo (range: 23-94) after liver and 98 mo (range: 3-98) after pancreatic resection. Disease-free survival was 10 mo (range 3-55) after liver and 28 mo (range 3-53) after pancreatic resection.
Our study shows that despite the high incidence of recurrence, long term survival can be achieved with resection of hepatic and pancreatic RCC metastases in selected cases and should be considered as a management option in patients with oligometastatic disease.
Core tip: The evidence on the role of surgery in management of renal cell carcinoma (RCC) metastatic disease to the liver and pancreas remains limited due to the rare nature of the disease. We have treated 13 patients in our institution, achieving median overall survival of 94 mo (range: 23-94) after liver and 98 mo (range: 3-98) after pancreatic resection. Disease-free survival was 10 mo (range: 3-55) after liver and 28 mo (range: 3-53) after pancreatic resection. Long term survival can be achieved with resection of hepatic and pancreatic RCC metastases in selected cases and should be considered in patients with oligometastatic disease.
- Citation: Chatzizacharias NA, Rosich-Medina A, Dajani K, Harper S, Huguet E, Liau SS, Praseedom RK, Jah A. Surgical management of hepato-pancreatic metastasis from renal cell carcinoma. World J Gastrointest Oncol 2017; 9(2): 70-77
- URL: https://www.wjgnet.com/1948-5204/full/v9/i2/70.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i2.70
Renal cell carcinoma (RCC) is one of the commonest causes of cancer related mortality, with 5-year survival ranging between 10%-90% depending on the stage of the disease[1]. Metastatic disease is present at the time of diagnosis in almost a third of patients, while 20%-50% of patients with localised disease will develop metastases after nephrectomy[1,2]. The most common site of metastatic disease is lung, followed by lymph nodes, bones and liver[3-6]. Hepatic metastasis has been reported in 41% of patients on autopsy[7], while pancreatic metastases are rarer, representing less than 5% of RCC metastases[1,8,9].
Current management strategies of metastatic RCC include surgical resection, chemotherapy [including newer agents such as tyrosine kinase inhibitors (TKI)], radiotherapy and hormonal therapy. However, the results are still poor with a median 5-year survival 5%-20%[3,4,10-14]. The role of surgery is still not clear as published data are limited. Nonetheless, a survival benefit has been reported in selected cases rendering surgical resection a management option in cases of oligometastatic disease.
We present our centre’s experience in the surgical management of hepato-pancreatic RCC metastases.
This is a retrospective review of all the patients who had liver and/or pancreatic resection for RCC metastasis in our institution, a tertiary regional referral centre for the surgical management of hepatic and pancreatic malignancies, between January 2003 and December 2015. Data from the pre- and post-operative period were collected from the hospital’s electronic records and histopathological database. Follow-up data were obtained based on the most recent oncology clinic or electronic medical entry. It is our institution’s policy for all patients to provided informed consent for research at the time of the operation.
All patients with suspected RCC metastatic disease to the liver or pancreas were managed by the hepatopancreatobiliary multidisciplinary team. Preoperative staging was based on imaging with contrast enhanced computer tomography (ceCT) of the chest, abdomen and pelvis. During the later part of the study (after 2011), positron emission tomography CT was used in the staging of all patients and magnetic resonance imaging of the liver in all cases with suspected liver metastasis. Tissue diagnosis was obtained in all cases of suspected pancreatic metastasis by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). The diagnosis of liver metastatic disease was predominantly based on three dimensional imaging, with half of the cases (n = 3) also undergoing CT- or ultrasound-guided guided core biopsy.
All patients received standard peri-operative care and data regarding the post-operative complications were recorded. Pancreatic fistula was defined and graded using the recommendation of the International Study Group on pancreatic fistula, as drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than 3 times the serum amylase activity[15].
No patient received any neoadjuvant therapy. The post-operative follow-up consisted of standard imaging surveillance with ceCT of the chest, abdomen and pelvis. No patient received any adjuvant treatment. Patients diagnosed with disease recurrence received appropriate individualised care (radiation, chemotherapy or palliative care). Survival analysis was performed using the Kaplan-Meier estimation (software package SPSS for Windows version 13.0; SPSS Inc., Chicago, IL, United States).
Thirteen patients were treated by our team for metastatic RCC to the liver or pancreas over the 13-year period. The majority of the primary tumours (n = 12) were of the clear cell histological type, while 1 was adenocarcinoma. Median follow-up was 33 mo (range: 3-98). Cohort descriptive characteristics, as well as the individual case characteristics can be found on Tables 1 and 2 respectively. Fourteen resections for metastatic disease were performed, 7 pancreatic and 7 liver resections. One patient had a laparoscopic distal pancreatectomy and splenectomy followed by a laparoscopic non-anatomical liver resection. In 2 cases the patients were found to have low volume pulmonary metastases during pre-operative staging. In both cases the multidisciplinary team decision was to proceed with the major abdominal operation first and consider subsequent management of the pulmonary metastases after the patients were fully recovered. All resections were histologically R0 with the exception of one total pancreatectomy which had a clear histological margin of less than 1 mm (R1). In only one pancreatectomy case nodal metastasis was confirmed histologically. The overall median length of stay was 15 d (range: 4-28); 18 d (range: 8-28) for pancreatic and 9 d (range: 4-27), for liver resections.
| Patient demographics and primary disease (RCC) | |
| Age | 63 yr (range: 44-80) |
| Gender: male:female | 7:6 |
| Nephrectomy: right:left | 6:7 |
| Hepatic and pancreatic metastatic disease | |
| Total cases | 14 |
| Synchronous | 3 (2 liver, 1 pancreas) |
| Metachronous | 11 |
| Time between nephrectomy and diagnosis of metachronous disease | 57 mo (range: 4-292) |
| Liver | 29 mo (range: 4-129) |
| Pancreas | 80 mo (range: 10-292) |
| Resection of metastases | |
| Total cases | 14 |
| Hepatic resections | 7 |
| Right hepatectomy | 1 (with IVC resection) |
| Non-anatomical hepatectomy | 6 (1 with en block diaphragmatic resection) |
| Pancreatic resections: | 7 |
| PD | 3 |
| TP + S | 2 |
| DP + S | 2 |
| Post-operative complications | 5 |
| Hepatic resections | 1 |
| Chylous ascites | 1 (managed with parenteral nutrition) |
| Pancreatic resections | 4 |
| Pancreatic fistula | 2 (grade B) |
| Fluid collection | 2 (managed with radiological-guided drainage and antibiotics) |
| Bleeding | 2 (operative management) |
| Patient | Age, gender | Site of metastasis | Time nephrectomy to metastasis (mo) | Other metastasis pre-operatively | Operation | Post-operative complications | Follow-up (mo) | Status at last follow-up | Site of recurrence | Time from resection of metastasis to recurrence (mo) |
| 1 | 50, female | Pancreas | 73 | Lungs (resected) | PD | Bleeding, pancreatic fistula | 50 | Alive, disease-free | - | - |
| 2 | 61, female | Pancreas | 110 | - | PD | Collection | 66 | Alive, recurrence | Right iliac bone (radiation) | 28 |
| 3 | 64, male | Pancreas | 93 | - | TP + S | - | 98 | Alive, recurrence | Pancreatic bed recurrence and abdominal LN (Pazopanib) | 53 |
| 4 | 66, male | Pancreas | 292 | - | TP | - | 4 | Death from disease | Brain (radiation) | 3 |
| 5 | 63, male | Pancreas | 0 | - | Laparoscopic DP + S | - | 61 | Alive, disease-free | Liver | 17 |
| Liver | 28 | Laparoscopic non-anatomical | - | - | ||||||
| 6 | 63, female | Pancreas | 10 | - | DP + S | Pancreatic fistula | 16 | Alive, recurrence | Right kidney and lung (Pazopanib) | 5 |
| 7 | 80, female | Pancreas | 26 | - | PD | Bleeding, collection | 3 | Death, no evidence of disease | - | - |
| 8 | 65, male | Liver | 29 | - | Non-anatomical | - | 94 | Death, no evidence of disease | Lungs (resected) | 55 |
| 9 | 44, female | Liver | 0 | - | Non-anatomical | Chylous scites | 23 | Death, recurrence | Liver (Pazopanib) | 6 |
| 10 | 70, male | Liver | 4 | Right kidney | Non-anatomical + right partial nephrectomy | - | 33 | Death, recurrence | Lung and spine (radiation and Sunitinib) | 3 |
| 11 | 61, female | Liver | 0 | Right adrenal, lungs (resected) | Non-anatomical | - | 36 | Alive, disease-free | - | - |
| 12 | 59, male | Liver | 40 | Adrenal (in specimen of RCC resection) Lungs | Non-anatomical + diaphragm | - | 32 | Alive, recurrence | Liver (Pazopanib) | 10 |
| 13 | 65, male | Liver | 129 | Lungs | Right hepatectomy + IVC resection | - | 26 | Alive, with disease, no recurrence | - | - |
Eight patients developed recurrent disease after hepatic (n = 3) and pancreatic (n = 5) resection, with only 1 case of local recurrence (Table 2). The median overall survival and the 1-, 3- and 5-year rates for the whole cohort, as well as separately after liver and pancreatic resection, are shown on Figure 1. The median disease-free survival and the 1-, 3- and 5-year rates for the whole cohort, as well as separately after liver and pancreatic resection, are shown on Figure 2.
The published evidence on the management of RCC metastatic disease is scarce. Long-term survival with targeted treatment, including surgical resection, has been reported. Differences have also been noted in the literature with regards to the management approach and outcomes of RCC metastases to the liver and the pancreas, while results from the use of the novel chemotherapy agents TKIs in liver metastatic disease are non-existent. Data on the role of systemic therapy in combination with operative management in a neoadjuvant and/or adjuvant setting are scarce and limited to case series. Following liver resection, overall survival has been reported between 16 and 142 mo after liver resection and disease-free survival between 7 and 30 mo (Table 3). Our experience demonstrates comparable results, with 94 mo (range: 23-94) median overall survival and 10 mo (range: 3-55) median disease free survival after hepatectomy.
| Ref. | Patient No. and site | Synchronous (n and %) | Time nephrectomy to metastasis (mo, median and range | Pre-op treatment (n and %) | Post-op treatment (n and %) | Mortality (%) | Morbidity (%) | OS (mo; median and range) | 1-yr OS (%) | 3-yr OS (%) | 5-yr OS (%) | DFS (mo; median and range) | 1-yr DFS (%) | 3-yr DFS (%) | 5-yr DFS (%) |
| Ruys et al[4] | 33, liver | 10 (30) | 50 (7-360) | 12 (36) | - | 0 | 54.5 | 33 (4-224) | 78 | 47 | 43 | 10 (1-54) | 49 | 18 | 11 |
| Hatzaras et al[5] | 43, liver | 9 (21) | 17 (2-189) | 5 (12) | 25 (58) | 2.3 | 23.3 | NR | 94.2 | 62.1 | - | 15.5 (3-76) | 79.5 | 27.3 | - |
| Staehler et al[10] | 68, liver | 19 (28) | - | - | 54 (80) | 0 | 20.1 | 142 (115-169) | - | - | 62.2 | - | - | - | - |
| Aloia et al[22] | 19, liver | 5 (26) | 53 (9-137) | - | - | 5.3 | 32 | 36 (-) | - | 52 | 26 | 13 (-) | - | 25 | 25 |
| Langan et al[23] | 10, liver | 5 (50) | - | 4 (40) | 4 (40) | 0 | 30 | 24 (3-254) | 79 | 45 | 34 | 7.2 (-) | - | - | - |
| Alves et al[24] | 14, liver | - | - | - | - | - | - | 26 (-) | 69 | 26 | - | - | - | - | - |
| Karavias et al[25] | 6, liver | 5 (83) | - | 0 | 2 (33) | 0 | - | NR | NR | NR | NR | NR | 66.7 | 66.7 | 66.7 |
| Kawata et al[26] | 4, liver | 2 (50) | 4 (2-6) | 2 (50) | 4 (100) | 0 | - | 30 (12-40) | 75 | 37.5 | NR | 30 (12-30) | 50 | 50 | NR |
| Thelen et al[27] | 31, liver | 6 (19) | - | - | - | 3.2 | 12.9 | 48 (-) | 82.2 | 54.3 | 38.9 | 27 (-) | 76.2 | 38.1 | 26.1 |
| Yezhelyev et al[28] | 13, liver | 13 (100) | 0 | - | - | - | - | 16 (1-34) | - | - | - | - | - | - | - |
| Santoni et al[6] | 44, pancreas | 1 (2) | - | 6 (14) | - | - | - | 103 (-) | - | - | - | 27 (3-167) | - | - | - |
| Bassi et al[8] | 17, pancreas | - | 126 (--276) | - | - | 0 | 47 | 22 (9-35) | - | - | - | 16 (3-30) | - | - | - |
| Schwartz et al[9] | 62, pancreas | 2 (3) | 120 (0-300) | 3 (5) | - | 6.4 | - | - | - | 72 | 63 | 26 (5-166) | - | 54 | 35 |
| Konstadinidis et al[29] | 20, pancreas | 1 (5) | 104 (0-264) | - | - | - | - | 104 (15-144) | - | - | 61 | - | - | - | - |
| Eidt et al[30] | 7, pancreas | 0 | 169 (108-240) | - | 12 (100) | 0 | - | 65 (5-86) | 85.7 | 85.7 | 85.7 | 65 (5-86) | 85.7 | 85.7 | 85.7 |
| Ghavamian et al[31] | 11, pancreas | 0 | 108 (18-295) | - | - | - | - | 120 (5-120) | 90.9 | 90.9 | 80.8 | NR | 90.9 | 90.9 | 80.8 |
| Reddy et al[32] | 21, pancreas | 3 (14) | 112 (-) | - | - | 0 | - | 58 (4-220) | - | - | - | - | - | - | - |
| Tanis et al[17] | 10, pancreas | 0 | 107 (5-228) | - | 1 (10) | - | - | 69 (6-69) | 88.9 | 66.7 | 66.7 | 35 (6-69) | 100 | 58 | 38.9 |
| Zerbi et al[20] | 23, pancreas | 0 | - | - | - | 0 | 47.8 | NR | 100 | 88 | 88 | 44 (33-52) | - | - | - |
| Kassabian et al[21] | 5, pancreas | 0 | 12 (4-15) | - | - | - | - | - | - | - | 67 | - | - | - | - |
| Untch et al[33] | 27, pancreas | - | - | - | - | - | - | 96 (-) | - | - | - | - | - | - | - |
With regards to pancreatic metastases, initial data from published case series (Table 3) and literature reviews[1,16,17] suggested that surgical management offers superior results with median overall survival between 22-120 mo. Survival rates have been reported at 67%-91% at 3 years and 61%-88% at 5 years. On the contrary, non-surgical management resulted in significantly worse 3-year and 5-year survival of 21% and 0%-47% respectively[16-18]. However, more recent data suggest that the use of TKI-based chemotherapy conferred similar overall survival to surgery[6]. Even though the median survival achieved with resection was about 1 year longer compared to TKI-based chemotherapy (86 mo vs 103 mo), it did not reach statistical significance (P = 0.201). Since no complete disease response was observed with chemotherapy, the study concluded that surgery may be a better option in the subgroup of patients with good prognostic criteria and no other concomitant metastases as it can radically treat the disease. In our series, after pancreatic resection the median overall survival was 93 mo (range: 3-98) and 3- and 5-year rates 71.4%, which is comparable to the published data. Likewise, we recorded median disease-free survival at 28 mo (range: 3-53), which is comparable to the published reports of median disease-free survival between 16 and 65 mo after pancreatic resection for RCC metastases (Table 3).
Liver and pancreatic resections carry a significant risk for perioperative morbidity and a small risk for mortality. Morbidity after resection of RCC liver metastases has been reported between 13% and 55% and after pancreatic resection as high as 47% (Table 3). Mortality has been reported between 0% and 5.3% after liver and between 0% and 6.4% after pancreatic resections for RCC metastases. Therefore, a careful patient selection for surgical management should be employed. Patients with disseminated or very aggressively progressing disease are not considered good surgical candidates for liver or pancreas metastasectomies, as this may substantially impact their quality of life without adding any survival benefit. Similarly, patients with significant medical history and/or poor performance status, that would not tolerate a major resection, should be considered for non-surgical management. In our cohort, all patients had Karnofsky performance score 80-100 and were deemed fit for major surgery after clinical and laboratory assessment by the multidisciplinary team. Postoperative complications were recorded in 5 cases (one liver and 4 pancreatic resections), with no postoperative mortality.
In this study, the median time interval between nephrectomy for the primary tumour and the diagnosis of metastatic disease was 57 mo (range: 4-292) (Table 1). Specifically for liver metastasis, with the exception of the 2 cases of synchronous metastatic disease, this period was of 29 mo (range: 4-129), which is comparable to reports in the literature of a median 4-53 mo (Table 3). For pancreatic metastasis this period was 80 mo (range: 10-292), somewhat shorter than most published case series (Table 3) and reviews[1,16,17], reporting a period between 104-169 mo after nephrectomy. A possible explanation could be the implementation of more intensive follow-up protocols for these patients in the recent years after the resection of the primary tumour. Furthermore, due to the rare nature of RCC metastasis to the pancreas all cases were confirmed with EUS-FNA. The need for tissue diagnosis was further supported by the fact that in almost all of our patients (6 out of 7) the pancreatic metastasis was the first and only site of metastatic disease. The use of immunohistochemistry (e.g., cytokeratin, vimentin, CD10, synaptophysin, CK7, CK20 and CA19) in addition to the standard histopathological stains can be useful in facilitating the diagnosis and is strongly advisable[19,20].
The evidence on the management of RCC metastatic disease to the liver and pancreas remains inconclusive. The rare nature of the disease precludes randomised studies and therefore current evidence is drawn from published case series. Regardless of the small size of our cohort, our data show that despite the high incidence of recurrence, long term survival can be achieved with resection of hepatic and pancreatic RCC metastases in selected cases and should be considered as a management option in patients with oligometastatic disease.
Renal cell carcinoma (RCC) is one of the commonest causes of cancer related mortality. Metastatic disease is present at the time of diagnosis in almost a third of patients, while 20%-50% of patients with localised disease will develop metastases after nephrectomy. The role of surgery in the management of metastatic RCC is still not clear as published data are limited.
This is a retrospective single centre experience in the surgical management of patients with metastatic RCC to the liver and pancreas.
Surgical management of liver and pancreas RCC metastases can be performed with low morbidity and mortality. In our series, postoperative complications were recorded in 5 cases (out of a total of 14), with no postoperative mortality. Median overall survival was 94 mo (range: 23-94) after liver and 98 mo (range: 3-98) after pancreatic resection. Disease-free survival was 10 mo (range 3-55) after liver and 28 mo (range 3-53) after pancreatic resection.
The authors’ study shows that despite the high incidence of recurrence, long term survival can be achieved with resection of hepatic and pancreatic RCC metastases in selected cases and should be considered as a management option in patients with oligometastatic disease.
The authors have well described these cases and the review of the literature about this topic has been conducted sufficiently in depth.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chetty R, Luchini C, Sperti C S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Ballarin R, Spaggiari M, Cautero N, De Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, De Marco G, D’Amico G. Pancreatic metastases from renal cell carcinoma: the state of the art. World J Gastroenterol. 2011;17:4747-4756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 114] [Cited by in F6Publishing: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 549] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 3. | Pfannschmidt J, Hoffmann H, Muley T, Krysa S, Trainer C, Dienemann H. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg. 2002;74:1653-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Ruys AT, Tanis PJ, Nagtegaal ID, van Duijvendijk P, Verhoef C, Porte RJ, van Gulik TM. Surgical treatment of renal cell cancer liver metastases: a population-based study. Ann Surg Oncol. 2011;18:1932-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Hatzaras I, Gleisner AL, Pulitano C, Sandroussi C, Hirose K, Hyder O, Wolfgang CL, Aldrighetti L, Crawford M, Choti MA. A multi-institution analysis of outcomes of liver-directed surgery for metastatic renal cell cancer. HPB (Oxford). 2012;14:532-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Santoni M, Conti A, Partelli S, Porta C, Sternberg CN, Procopio G, Bracarda S, Basso U, De Giorgi U, Derosa L. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann Surg Oncol. 2015;22:2094-2100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer. 1981;48:1487-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Bassi C, Butturini G, Falconi M, Sargenti M, Mantovani W, Pederzoli P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. 2003;90:555-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Schwarz L, Sauvanet A, Regenet N, Mabrut JY, Gigot JF, Housseau E, Millat B, Ouaissi M, Gayet B, Fuks D. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann Surg Oncol. 2014;21:4007-4013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Staehler MD, Kruse J, Haseke N, Stadler T, Roosen A, Karl A, Stief CG, Jauch KW, Bruns CJ. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol. 2010;28:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH. Kidney cancer, version 3.2015. J Natl Compr Canc Netw. 2015;13:151-159. [PubMed] [Cited in This Article: ] |
| 12. | Aben KK, Heskamp S, Janssen-Heijnen ML, Koldewijn EL, van Herpen CM, Kiemeney LA, Oosterwijk E, van Spronsen DJ. Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands. Eur J Cancer. 2011;47:2023-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Culp SH, Tannir NM, Abel EJ, Margulis V, Tamboli P, Matin SF, Wood CG. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 2010;116:3378-3388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261-2266. [PubMed] [Cited in This Article: ] |
| 15. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3282] [Cited by in F6Publishing: 3393] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 16. | Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Tanis PJ, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96:579-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008;15:1161-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Gajendra S, Sachdev R, Mohapatra I, Goel R, Goel S. Metastatic Renal Cell Carcinoma: An Unusual Cause of Bleeding Pancreatic Mass. J Clin Diagn Res. 2015;9:ED15-ED17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Mikami S, Oya M, Mizuno R, Kosaka T, Ishida M, Kuroda N, Nagashima Y, Katsube K, Okada Y. Recent advances in renal cell carcinoma from a pathological point of view. Pathol Int. 2016;66:481-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Kassabian A, Stein J, Jabbour N, Parsa K, Skinner D, Parekh D, Cosenza C, Selby R. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Aloia TA, Adam R, Azoulay D, Bismuth H, Castaing D. Outcome following hepatic resection of metastatic renal tumors: the Paul Brousse Hospital experience. HPB (Oxford). 2006;8:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Langan RC, Ripley RT, Davis JL, Prieto PA, Datrice N, Steinberg SM, Bratslavsky G, Rudloff U, Kammula US, Stojadinovic A. Liver directed therapy for renal cell carcinoma. J Cancer. 2012;3:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Alves A, Adam R, Majno P, Delvart V, Azoulay D, Castaing D, Bismuth H. Hepatic resection for metastatic renal tumors: is it worthwhile? Ann Surg Oncol. 2003;10:705-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Karavias DD, Tepetes K, Karatzas T, Felekouras E, Androulakis J. Liver resection for metastatic non-colorectal non-neuroendocrine hepatic neoplasms. Eur J Surg Oncol. 2002;28:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Kawata N, Hirakata H, Yuge H, Kodama M, Sugimoto S, Yagasaki H, Mochida J, Fujimura K, Takimoto Y. Cytoreductive surgery with liver-involved renal cell carcinoma. Int J Urol. 2000;7:382-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Thelen A, Jonas S, Benckert C, Lopez-Hänninen E, Rudolph B, Neumann U, Neuhaus P. Liver resection for metastases from renal cell carcinoma. World J Surg. 2007;31:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Yezhelyev M, Master V, Egnatashvili V, Kooby DA. Combined nephrectomy and major hepatectomy: indications, outcomes, and recommendations. J Am Coll Surg. 2009;208:410-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C, Warshaw AL, Ferrone CR. Metastatic tumors in the pancreas in the modern era. J Am Coll Surg. 2010;211:749-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Eidt S, Jergas M, Schmidt R, Siedek M. Metastasis to the pancreas--an indication for pancreatic resection? Langenbecks Arch Surg. 2007;392:539-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Ghavamian R, Klein KA, Stephens DH, Welch TJ, LeRoy AJ, Richardson RL, Burch PA, Zincke H. Renal cell carcinoma metastatic to the pancreas: clinical and radiological features. Mayo Clin Proc. 2000;75:581-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N, Wolfgang CL. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol. 2008;15:3199-3206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Untch BR, Allen PJ. Pancreatic metastasectomy: the Memorial Sloan-Kettering experience and a review of the literature. J Surg Oncol. 2014;109:28-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |