Published online Apr 15, 2016. doi: 10.4251/wjgo.v8.i4.341
Peer-review started: September 16, 2015
First decision: October 21, 2015
Revised: November 23, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: April 15, 2016
Helicobacter pylori (H. pylori) is one of the most widespread infections in humans worldwide that chronically infects up to 50% of the world’s population. The infection is involved in the pathogenesis of chronic active gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma and gastric cancer, therefore, it has been classified as class I definite carcinogen by the World Health Organization. Despite the established etiological role of H. pylori, its actual distribution and association with related diseases is controversial and there is a large intercountry variation especially among Asian countries. H. pylori infection is more frequent in developing countries like India, Pakistan, and Bangladesh as compared to developed Asian countries like Japan, China and South Korea. However, the frequency of gastric cancer is comparatively lower in India, Pakistan, and Bangladesh with that of Japan, China and South Korea. Such phenomenon of clinical diversity, defined as enigma, is judged by genetic variability of the infecting H. pylori strains, differences in the host genetic background in various ethnic groups, and environmental factors such as dietary habits. Most of the studies have so far focused on the bacterial factor while environmental issues, including dietary components, were not given due attention as one of the factors related with H. pylori associated gastric carcinogenesis. The dietary factor has been suggested to play an important role in H. pylori related carcinogenesis, and in this respect several studies have corroborated the intake of various dietary components as modulatory factors for gastric cancer risk. In this review, such studies, from in vitro experiments to clinical trials, are being focused in detail with respect to enigma associated with H. pylori. It may be conceivably concluded from the available evidence that dietary factor can be a game changer in the scenario of Asian enigma, particularly in high risk population infected with virulent H. pylori strains, however further affirmation studies are desperately needed to achieve conclusive outcomes.
Core tip: Despite the established etiological role of Helicobacter pylori (H. pylori), its actual distribution and association with related diseases is controversial, especially among Asian countries, a phenomenon termed as Asian enigma. This is judged by genetic variability of the infecting H. pylori strains, diversity in the host genetic background, and environmental factors such as diet. Amongst these, the dietary factor was not given much attention. In this review, dietary components are focused in detail with respect to H. pylori-associated enigma with a specific emphasis and comparison of dietary ingredients between Asian countries in order to critically evaluate its role in Asian enigma.
- Citation: Zaidi SF. Helicobacter pylori associated Asian enigma: Does diet deserve distinction? World J Gastrointest Oncol 2016; 8(4): 341-350
- URL: https://www.wjgnet.com/1948-5204/full/v8/i4/341.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i4.341
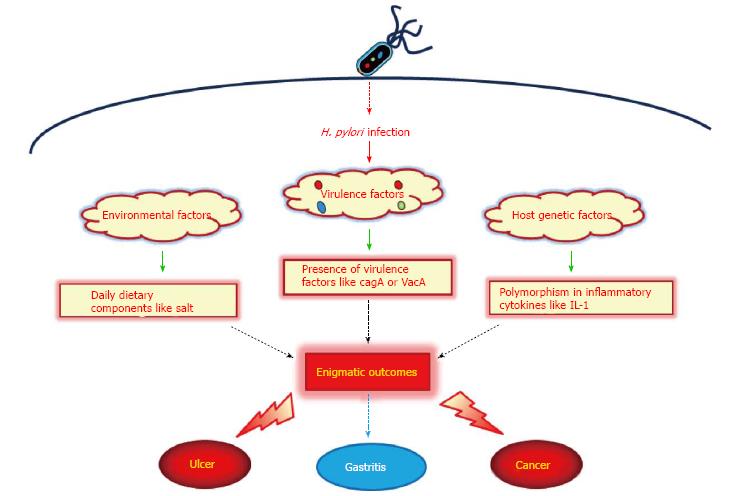
Helicobacter pylori (H. pylori) have been found in the stomachs of humans in all parts of the world. It is one of the most common bacterial infections worldwide, infecting more than half of the world’s population[1]. H. pylori infection in the stomach induces mucosal inflammatory response and oxidative stress that leads to diverse clinical outcomes in humans such as gastritis, peptic ulcer and gastric cancer[2]. There is a strong correlation between prevalence of infection and socioeconomic status[3]. In some developing countries 70%-90% of the population is infected with H. pylori, whereas in high-income countries the prevalence is 25%-50%. Most infections are acquired in childhood[4,5]. However, the incidence of H. pylori infection is declining, and today only 10% of children in high income countries are infected[6,7]. Regardless of the established etiological role of H. pylori, its actual distribution and association with related diseases is controversial especially in Asian countries. H. pylori infection is more frequent in developing Asian countries like Thailand, Malaysia, India, Pakistan, and Bangladesh, whereas, the occurrence of gastric cancer is comparatively lower than in developed Asian countries like Japan, China, and South Korea[8-10]. Such phenomenon of clinical diversity, defined as Enigma, is judged by genetic variability of the infecting H. pylori strains with respect to virulence factors, difference in the host genetic makeup, and environmental factors such as dietary habits (Figure 1)[11].
Epidemiological studies indicate that Asian countries have a high prevalence of H. pylori infection as compared to Western countries, with a correspondingly high incidence of severe gastroduodenal (GD) diseases, especially gastric neoplasia[8]. However, the frequency of H. pylori infection differs markedly between and within populations of different Asian countries. In developing countries like India, Pakistan, Bangladesh and Thailand, infection with H. pylori is more frequent among the general population[9]. In contrast, in more industrialized and developed regions of Asia like Japan, China, and South Korea, frequency of H. pylori infection has been reported to be somewhat lower[8]. Singapore is also in the same category and the prevalence of H. pylori infection is quite low compared to developing countries[10].
Although the overall incidence of gastric cancer is declining, it is still the world’s second most widespread malignancy, having been overtaken by lung cancer[12]. There is a marked variation internationally in gastric cancer incidence with highest rates reported from Japan[8,13]. It is noteworthy that despite Japan being a developed country with a lower prevalence of H. pylori infection, it has the highest frequency of gastric cancer. The annual incidence of gastric cancer in Japan is around 100 times higher than those in India. Similarly, the frequency of gastric cancer is quite high in China despite a lower frequency of H. pylori infection[8]. Contrary, people living in underdeveloped countries of Asia with high frequency of H. pylori infection have a lower incidence of gastric cancer[8,14]. Some studies from India revealed no correlation between H. pylori infection and gastric cancer, while studies from China and Japan are consistent with previous findings, affirming association between H. pylori infection and gastric cancer[15-17]. It has also been observed that frequency of gastric cancer varies within regions of a particular country; for example, in Japan and India[16,18]. These corroborations support a potential role of other factors in the diverse and contradictory presentation of gastric diseases in different regions and populations.
The above mentioned evidences point out the fact that only H. pylori infection is solely not enough to cause life threatening conditions like gastric cancer, highlighting the importance of factors behind enigma, like genetic variability of the infecting strain and infected individual, along with life style habits.
As much has been focused on the genetic differences of host and agent, therefore, in this review the basics of these factors will be covered, while more emphasis will be on environmental factors including life style and dietary components, to critically evaluate their plausible role in H. pylori associated enigma.
H. pylori is a micro-aerophil gram-negative bacterium with several flagella required for bacterial motility[19]. Numerous biochemical factors are produced by H. pylori that are important to the organism’s survival, virulence and initiation of pathophysiological effects in the infected host. Multiple pathways are modulated after H. pylori infection either through direct interaction or via virulent factors injected in the host cell which leads to the pathological outcome. Some of the important virulence factors are mentioned below that are responsible for bacterial colonization and pathogenesis, which are postulated to play some role in enigmatic diversity of clinical outcomes.
It is postulated that all strains of H. pylori are not pathogenic and the genetic diversity of H. pylori virulence factors has been linked with clinical outcome. The most pathogenic and well-established cluster of virulence in H. pylori is the cytotoxin associated pathogenicity island (cag PAI); this being a 27-gene locus that is present in a majority of the clinical strains found in Europe, United States and Japan. H. pylori strains with the cag PAI have been shown to be more virulent, with an increased risk of development of GD disorders like duodenal ulcers and gastric adenocarcinoma, than strains lacking the gene complex[20]. The cytotoxin associated gene A (cagA) is located in the cag PAI, and encodes for the cagA protein. Inside the host cell the cagA interferes with cell signaling pathways and induces cytoskeletal rearrangements[21,22]. It has been suggested that cag PAI positive strains are involved in the activation of transcription factor nuclear factor-kappa B (NF-κB), resulting in production of inflammatory or carcinogenic molecules such as interleukin-8 (IL-8) or activation-induced cytidine deaminase (AID)[21-23]. The in vitro observation of large vacuoles in the cytoplasm of cells incubated with H. pylori lead to the discovery of the Vacuolating cytotoxin A (VacA)[24]. VacA induces apoptosis in epithelial cells, but it is still not clarified why vacuolation is required for this type of apoptosis. The VacA protein inserts itself into the epithelial cell membrane and forms a channel through which bicarbonate and organic anions can be released[25]. Several studies have documented the genetic polymorphism in VacA gene and have concluded that the presence of both cagA and VacA genotype s1/m1 is more associated with severe disease outcomes[26-28]. The gene babA2 encodes for the protein BabA, which is an outer-membrane protein. H. pylori strains that possess the bab2 gene are associated with an increased incidence of gastric adenocarcinoma. BabA-expressing strains adhere more tightly to epithelial cells, which might promote pathogenesis[29]. Another virulence factor, iceA, has been linked with peptic ulceration and increased mucosal IL-8 secretion[30,31]. Although the above mentioned studies provide evidence for the linkage of genetic variation in H. pylori with the disease outcomes, it is still not always conclusive due to controversial results. Genotyping analysis of H. pylori from an Indian population showed high prevalence of pathogenic strains in both adults and children with GD diseases as well as in control subjects[9,32]. However, the incidence of gastric cancer is quite low in India as discussed above. Hence other factors behind enigma play simultaneous role in producing the final outcome of H. pylori infection.
As discussed above, the basis for the diverse clinical outcomes of enigmatic scenario particularly in Asian countries cannot be entirely explained on the strain genetic diversity, as most patients are infected by more virulent strains than in Western countries[8]. Another reason behind enigma is the host genetic makeup or ethnicity. Infection with any microorganism results in immune response leading to expression of inflammatory cytokines. Polymorphism in the genes of these cytokines may affect the dynamics of response to any infection, including that of H. pylori. Few studies have reported such polymorphism in the gene cluster of IL-1, a proinflammatory cytokine with a potent acid inhibitory effect, and its association with gastric cancer risk[33,34]. Polymorphisms in other candidate cytokine genes, such as tumor necrosis factor-alpha and IL-10, may enhance or suppress inflammation of the gastrointestinal (GI) mucosa resulting in different disease outcomes[35,36]. Besides inflammatory cytokines, gastric atrophy associated with reduced acid secretion is also documented to be linked with the ethnic background. For example, the Japanese population has much lower acid secretion as compared to the western population[37]. As reported, gastric atrophy occurs more readily in subjects with lower acid secretion than in those with high acid secretion[38]. It was further confirmed by Kuipers et al[39] that the use of proton pump inhibitors, drugs that reduce gastric acid secretion, in the patients of reflux esophagitis aggravated the severity of H. pylori-associated gastritis. This may be one of the reasons that the Japanese have high incidence of gastric cancer, as gastric atrophy is a precancerous lesion. In short, the above findings support the role of genetic makeup or ethnicity in H. pylori-associated enigmatic disease outcome but still further studies are required from other Asian countries like Malaysia, India or Pakistan to conclude the precise role of polymorphisms in specific genes or combinations of genes for disturbances in acid secretory output and gastric cancer risks in Asian countries.
Stomach and intestines are among the parts in our body that are exposed at maximum to our daily diet and of course environmental factors like personal hygiene can equally affect them. Hence, it is postulated that diet may play a critical role in GD disorders like peptic ulcer or gastric carcinoma. When talking about enigma associated with H. pylori under the light of recent scientific and molecular studies, dietary components can modulate the pathogenic processes by simple anti-oxidant to complex anti-carcinogenic activities. The paradoxical clinical outcomes after H. pylori infection might be crucially regulated by dietary habits of the population, especially when talking about the Asian population under Asian enigma. It has been known since ancient times that healthy diet prevents digestive problems and avoid chronic malaise, but it was not long ago when the dietary factor was brought into focus with respect to H. pylori associated pathogenesis. It was first reported by Holcombe[40] back in 1992 in his leading article on African enigma that environmental factors including dietary habits can influence the outcome of H. pylori infection. Several studies from various countries thereafter followed Holcombe’s hypothesis and documented the plausible role of dietary components in disease outcome.
So as to discuss about Asian enigma, two sets of populations can be broadly classified as mentioned above; one with low incidence of gastric cancer and high prevalence of H. pylori such as the South Asian population including India, Pakistan, and Bangladesh and the second with high incidence of gastric cancer and low prevalence of H. pylori such as the East Asian population including Japan, China and Korea. Among the latter, Japanese researchers initiated the focus on the dietary factor in their population to uncover the plausible role of diet in Asian enigma not only by comparing it with western dietary habit but also within different regions of Japan[8]. Both aggravating (negative) and alleviating (positive) effects of diet have been documented with respect to H. pylori associated pathogenesis but majority of the studies from Japan linked dietary components as a negative regulator. This may be due to the fact that the Japanese population is well recognized for high prevalence and mortality due to gastric cancer and the diet therein might be a negative regulator. However, it is interesting to note that different regions of Japan have a variable incidence of gastric cancer. For example, northern regions in Japan such as Akita prefecture have a higher prevalence of gastric cancer than the Southern region of Okinawa prefecture[41]. While looking at the dietary pattern in Akita, it was found that salt has been highly consumed in this region almost double to that of Okinawa[42]. This may further provide the convincing evidence that in a population of similar host genetic makeup and H. pylori strains, it may be due to different dietary habits, like high salt intake in that region that can lead to enigmatic outcomes.
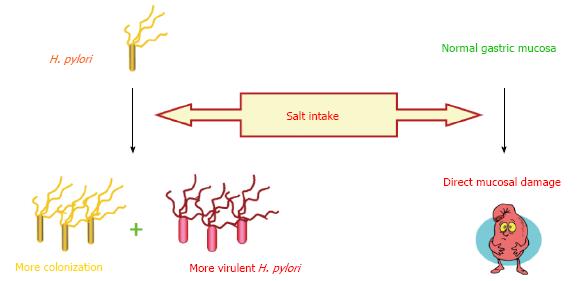
High salt consumption is one of the most extensively investigated dietary component which is well known to increase the risk of gastric cancer[43,44]. Furthermore, interesting data was published in a prospective study of a Japanese population which demonstrated the aggravating effect of high-salt diet in H. pylori infected subjects with an increase in gastric cancer risk, when compared with infected subjects with lower consumption of salt[45]. This may be one of the leading clues for the potential role of diet in H. pylori associated enigma. In vivo studies have also shown that high salt not only directly damages the gastric mucosa but also increases the colonization of H. pylori in stomach resulting in loss of parietal cells, atrophy, and intestinal metaplasia[46-48]. A recent study conducted in Mongolian gerbils provided the evidence that both cagA+ H. pylori strains and a high salt diet are synergistically significant risk factors for gastric adenocarcinoma compared with mutant cagA strain and a regular diet[49]. An interesting finding was revealed by Loh et al[49] that changes in salt concentration altered the gene expression in H. pylori especially cagA which was later observed in H. pylori infected gerbils as well[50]. These findings provide evidence that salt can aggravate gastric mucosal damage in multiple ways, shown in Figure 2, which might form the basis of different disease outcomes in a genetically similar population like in Japan. It may be intriguing to evaluate the effect of high salt intake in different populations infected with H. pylori in a single study along with the genetic status of H. pylori. This may help in identifying the role of host genetic makeup in the presence of high salt intake and H. pylori infection in the process of gastric carcinogenesis.
Other than salt, high consumption of pickled, preserved, and smoked food in the Japanese population has also been postulated as a risk factor for gastric cancer[43]. Pickled vegetables are also very much common in the East Asian region such as Japan, China, and Korea and are almost a part of daily cuisine. Nitrosamines are dietary carcinogens found in pickled and smoked food and their intake increased the risk of gastric cancer[51,52]. It has been documented that nitrosamine can augment H. pylori associated gastric carcinogenesis not only in gerbils but in rhesus monkeys as well[53,54]. A recent study in H. pylori-infected Chinese population documented the similar finding; that high intake of sodium, heme iron, and red meat increase the risk of gastric cancer while abundant consumption of fresh fruits decrease the risk[55]. Another important source of carcinogens in the East Asian population is fermented products such as soybean pastes and kimchi, a traditional Korean dish made of fermented cabbage[51,56]. Nan et al[57] documented the increase in gastric cancer risk in Korean subjects consuming kimchi and soybean pastes. This may again be due to the presence of nitrosated compounds in high quantity in such fermented products which have been reported to augment H. pylori associated carcinogenesis[51,58]. In short, it can be retrieved from the above mentioned studies that the continuous intake of these salty, pickled, and fermented foods in the East Asian population might be a crucial factor in H. pylori-associated enigma of gastric cancer.
When it comes to the South Asian region, including India, Pakistan, and Bangladesh, dietary habits are quite different from those in East Asian population. It is due to this difference in dietary pattern that many researchers have postulated dietary habits as one of the important factors behind H. pylori-associated disease outcome and Asian enigma[8,9,59]. One of the reasons behind this speculation is the low incidence of gastric cancer despite high prevalence of virulent H. pylori strains in South Asia[60]. Although host genetic factors may also interplay in pathogenesis, diet can be quite crucial when comparing East with South Asian countries. As discussed above, some ingredients in the East Asian population diet are negative (aggravating) regulator in H. pylori associated pathogenesis, while in the case of the South Asian region, one can hypothesize that diet might be functioning as a positive regulator in alleviating H. pylori-linked diseases. This seems to be true to some extent under the light of available evidence. Consumption of salty, pickled, and fermented food is comparatively low in the South Asian region than in East Asian countries. Furthermore, the use of spices in daily cuisine and frequent over the counter use of herbal or traditional medicines can be a hallmark in different disease outcomes[61]. It is interesting to note that the daily cuisine and dietary patterns in South Asian countries like India, Pakistan, and Bangladesh are quite alike, which to some extent, is a true copy of the similarity in dietary habits between East Asian nations such as Japan, China, and South Korea.
Several studies from India have documented the plausible role of diet on the incidence of gastric cancer in different regions[8,9]. Although the overall prevalence of gastric cancer in India is quite low when compared with Japan, it is worth to note that the frequency of gastric cancer differs in different regions of India, most probably due to differences in dietary habits of these regions; a pattern similar to that in Japan, as discussed above. Southern and eastern parts of India have high frequency of gastric cancer compared to the northern region. This is attributed to be due to frequent consumption of non-vegetarian food, like fish with excess spices and high salt. Contrarily, northern regions have a wheat-based vegetarian diet[9]. A similar pattern is seen in the Kashmir region of India where esophageal and gastric cancer frequency is higher than other districts[62]. This is again supposed to be due to high intake of dietary amines and nitrate in the Kashmir district[63]. Another study by Mathew et al[64] from south India documented the increase risk of gastric cancer with a high intake of rice, spicy, and high-temperature food. One of the limitations in these studies is the lack of consideration of H. pylori co-infection in the study subjects. However, one study by Phukan et al[65] from South Indian region of Mizoram demonstrated the association of dietary components (fermented and smoked meat) with H. pylori infection with an increased risk of gastric cancer. These facts further ascertain the role of diet in the diverse prevalence of gastric cancer in the Indian population, thus highlighting its importance in enigma.
Several studies have shown protective effects of spices and medicinal plants against numerous diseases including H. pylori and GI disorders[66-70]. It is worth mentioning here that in South Asian countries like India, Pakistan, Nepal, and Bangladesh, spices are also used as medicinal herbs as they possess medicinal values in the traditional system of medicine of these countries. In addition, many of these dietary spices are prescribed for the treatment of GI diseases[67,71]. O’Mahony et al[70] first reported the bactericidal and anti-adhesive activities of culinary herbs from Malaysia against H. pylori. Later, we reported in a series of studies the potential effect of commonly used spices and medicinal plants from Pakistan on H. pylori and its associated pathogenesis[71-76]. All of the medicinal plants/spices used in these studies are also prescribed for the treatment of GI disorders. The results of these studies are very convincing, which reveals not only bactericidal (anti-H. pylori) activities of these plants but also anti-inflammatory effects against H. pylori-initiated pathological events. Spices like turmeric, nutmeg, mace, cardamom, black caraway, cumin, etc. which are part of daily cuisine in South Asian countries exhibited promising anti-H. pylori activities[71]. We further documented that the plants, not having anti-H. pylori activity, can inhibit H. pylori-induced IL-8 secretion or reactive oxygen species (ROS) generation in gastric epithelial cells. Both IL-8 and ROS are reported to play an important role in H. pylori-linked pathological sequel[74]. Among the spices that inhibit IL-8 secretion, cinnamon showed most strong activity, and cinnamaldehyde was found to be the major reason for this effect[76]. The major limitation with these studies was the use of only in vitro assays, which points out the dire need of in vivo or clinical trials with these herbs.
Some of the dietary components and spices have not only been examined against H. pylori in in vitro/in vivo assays but also in clinical trials. The most promising effects were demonstrated by the spice named turmeric or curcuma and its active ingredient curcumin. In in vitro assays, both turmeric and curcumin showed significant anti-H. pylori activity[71,77]. Curcumin has also been documented to eradicate H. pylori in C57BL/6 infected mice and also reduced the level of gastric damage[78]. Anti-inflammatory activity of curcumin was also documented by Foryst-Ludwig et al[79] by decreasing H. pylori-induced NF-κB activation and the subsequent release of IL-8. We also demonstrated that curcumin not only blocked NF-κB activation but also suppressed the anomalous expression of AID, an enzyme highly linked with H. pylori-induced gastric carcinogenesis[23]. Sintara et al[80] reported the role of curcumin in Sprague-Dawley rats by reducing the gastric inflammation by inhibiting NF-κB. Later, a clinical study demonstrated that curcumin based triple therapy significantly improved dyspeptic symptoms and reduced serologic signs of gastric inflammation even after 2 mo of the therapy[81]. Hence, it can be postulated that the use of spices like turmeric/cinnamon can, not only suppress H. pylori colonization, but also halt the inflammatory cascade initiated by H. pylori, ultimately preventing carcinogenesis.
Another commonly consumed cuisine ingredient in South Asian countries is garlic. Garlic or allium has revealed promising activities against H. pylori-induced gastritis model of Mongolian gerbils by decreasing the degree of gastritis[82]. However, it has been described in the same study that H. pylori was not eradicated by the garlic extract treatment. A clinical trial was also conducted using garlic in H. pylori infected subjects revealing no effect on eradication of the bacteria[83]. This suggests that garlic might only be helpful in attenuating H. pylori-induced pathological pathways while not killing the bacteria itself in in vivo and clinical settings. Overall these studies signify the potential role of dietary ingredients including spices in South Asian countries in modulating H. pylori associated pathological processes. However, it cannot be overlooked that the excessive and continued use of a spicy diet may act as an aggravating factor in H. pylori-infected individuals, a question still needed to be affirmed in the region of South Asia. Last but not the least, it is important to note that high amount of spices are always accompanied with high amount of salt to give proper taste. So it might be the salt as the main offender in these spicy diets rather than spices itself. It will be interesting to see the effect of a high intake of spices with or without excessive salt either by in vivo experiments or clinical trials in H. pylori-infected subjects.
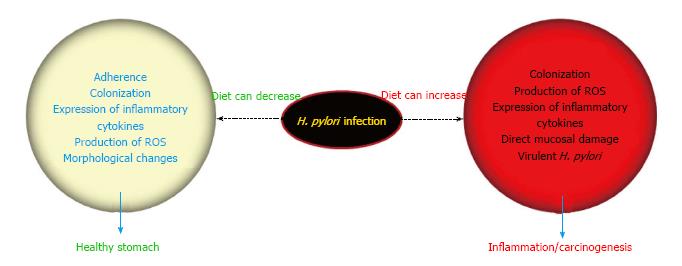
H. pylori associated pathological outcome is a result of chronic inflammation from years to decades, and as H. pylori alone is not enough to cause associated diseases, several factors can play an important role in the final outcome. Each of the factors discussed above behind enigma is plausibly exerting some influence in the variable incidence of gastric cancer in Asian countries. Along with genetic status of both H. pylori and host, diet can also modulate the clinical outcome by aggravating or alleviating H. pylori-linked pathogenic processes (Figure 3). In this review, plausible mechanisms by which diet can manipulate the enigmatic consequences of H. pylori infection are discussed. As evident from the high prevalence of H. pylori infection among South Asian countries in the studies mentioned earlier, these dietary ingredients can barely eradicate H. pylori in the doses that are consumed on a daily basis. However, it is quite evident from the discussion that the dietary factor can modulate H. pylori-linked pathogenesis either by decreasing the colonization of H. pylori or via hampering the production of inflammatory/carcinogenic mediators released after H. pylori infection. This can end up in perplexing clinical outcomes leading to enigma. Besides the negative or aggravating role of salt among daily dietary components, it may be too earlier to firmly pose any other ingredient as a positive or negative regulator in the scenario of Asian enigma. There is no doubt concerning the role of H. pylori as a significant and intriguing factor in causing chronic gastritis that may later predispose the infected host to develop gastric cancer, but the nature of our daily diet can undeniably define the future course of disease outcome.
Further large scale comparative studies are required on a national and international level, not only to identify perpetrators in our dietary habits but also to develop effective ways for preventing this cancer in high risk populations, as chemoprevention seems to be the most practical way of controlling this deadly disease. It is needless to mention that while designing clinical trials or in vivo models, the triad of causing factors, i.e., virulence of H. pylori, host genetic make up, and dietary habits should be considered, otherwise exhaustive efforts will again end up with no conclusive results.
P- Reviewer: Garcia-Compean D, Lakatos PL, Osawa S S- Editor: Wang JL L- Editor: A E- Editor: Lu YJ
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3302] [Cited by in F6Publishing: 3116] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 2. | Muhammad JS, Sugiyama T, Zaidi SF. Gastric pathophysiological ins and outs of helicobacter pylori: a review. J Pak Med Assoc. 2013;63:1528-1533. [PubMed] [Cited in This Article: ] |
| 3. | Mégraud F. Epidemiology of Helicobacter pylori infection. Gastroenterol Clin North Am. 1993;22:73-88. [PubMed] [Cited in This Article: ] |
| 4. | Rowland M, Daly L, Vaughan M, Higgins A, Bourke B, Drumm B. Age-specific incidence of Helicobacter pylori. Gastroenterology. 2006;130:65-72; quiz 211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Rowland M, Kumar D, Daly L, O’Connor P, Vaughan D, Drumm B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology. 1999;117:336-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495-1501. [PubMed] [Cited in This Article: ] |
| 7. | Fiedorek SC, Malaty HM, Evans DL, Pumphrey CL, Casteel HB, Evans DJ, Graham DY. Factors influencing the epidemiology of Helicobacter pylori infection in children. Pediatrics. 1991;88:578-582. [PubMed] [Cited in This Article: ] |
| 8. | Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 10. | Fock KM. Helicobacter pylori infection--current status in Singapore. Ann Acad Med Singapore. 1997;26:637-641. [PubMed] [Cited in This Article: ] |
| 11. | Sugiyama T, Asaka M. Helicobacter pylori infection and gastric cancer. Med Electron Microsc. 2004;37:149-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2651] [Cited by in F6Publishing: 2570] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 13. | Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ. 1992;45-173. [PubMed] [Cited in This Article: ] |
| 14. | Misra V, Pandey R, Misra SP, Dwivedi M. Helicobacter pylori and gastric cancer: Indian enigma. World J Gastroenterol. 2014;20:1503-1509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Ghoshal UC, Tiwari S, Dhingra S, Pandey R, Ghoshal U, Tripathi S, Singh H, Gupta VK, Nagpal AK, Naik S. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Asaka M, Kato M, Kudo M, Katagiri M, Nishikawa K, Yoshida J, Takeda H, Miki K. Relationship between Helicobacter pylori infection, atrophic gastritis and gastric carcinoma in a Japanese population. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S7-10. [PubMed] [Cited in This Article: ] |
| 17. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County, Fujian Province, China. World J Gastroenterol. 2000;6:374-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 49] [Cited by in F6Publishing: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mohandas KM, Nagral A. Epidemiology of digestive tract cancers in India. II. Stomach, and gastrointestinal lymphomas. Indian J Gastroenterol. 1998;17:24-27. [PubMed] [Cited in This Article: ] |
| 19. | Goodwin CS, Armstrong JA. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur J Clin Microbiol Infect Dis. 1990;9:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] [Cited in This Article: ] |
| 21. | Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, Naumann M, Meyer TF. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 345] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 22. | Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 263] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Zaidi SF, Yamamoto T, Refaat A, Ahmed K, Sakurai H, Saiki I, Kondo T, Usmanghani K, Kadowaki M, Sugiyama T. Modulation of activation-induced cytidine deaminase by curcumin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2009;14:588-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 223] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Boquet P, Ricci V, Galmiche A, Gauthier NC. Gastric cell apoptosis and H. pylori: has the main function of VacA finally been identified? Trends Microbiol. 2003;11:410-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Miehlke S, Kirsch C, Agha-Amiri K, Günther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 27. | Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710-1714. [PubMed] [Cited in This Article: ] |
| 29. | Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778-12783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 482] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Peek RM, Thompson SA, Donahue JP, Tham KT, Atherton JC, Blaser MJ, Miller GG. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998;110:531-544. [PubMed] [Cited in This Article: ] |
| 31. | van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 398] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Singh M, Prasad KN, Yachha SK, Krishnani N. Genotypes of Helicobacter pylori in children with upper abdominal pain. J Gastroenterol Hepatol. 2003;18:1018-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1690] [Cited by in F6Publishing: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 34. | Camargo MC, Mera R, Correa P, Peek RM, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 35. | Jang WH, Yang YI, Yea SS, Lee YJ, Chun JH, Kim HI, Kim MS, Paik KH. The -238 tumor necrosis factor-alpha promoter polymorphism is associated with decreased susceptibility to cancers. Cancer Lett. 2001;166:41-46. [PubMed] [Cited in This Article: ] |
| 36. | Sturlan S, Oberhuber G, Beinhauer BG, Tichy B, Kappel S, Wang J, Rogy MA. Interleukin-10-deficient mice and inflammatory bowel disease associated cancer development. Carcinogenesis. 2001;22:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Kinoshita Y, Kawanami C, Kishi K, Nakata H, Seino Y, Chiba T. Helicobacter pylori independent chronological change in gastric acid secretion in the Japanese. Gut. 1997;41:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Kuipers EJ, Lee A, Klinkenberg-Knol EC, Meuwissen SG. Review article: the development of atrophic gastritis--Helicobacter pylori and the effects of acid suppressive therapy. Aliment Pharmacol Ther. 1995;9:331-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 473] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 40. | Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 241] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Tsubono Y, Takahashi T, Iwase Y, Iitoi Y, Akabane M, Tsugane S. Nutrient consumption and gastric cancer mortality in five regions of Japan. Nutr Cancer. 1997;27:310-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Watanabe S. Nihonjin no gann. Tokyo: Kanehara Syuppan 1995; 60-64. [Cited in This Article: ] |
| 43. | Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 298] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 44. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823-4828. [PubMed] [Cited in This Article: ] |
| 47. | Toyoda T, Tsukamoto T, Hirano N, Mizoshita T, Kato S, Takasu S, Ban H, Tatematsu M. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008;23:593-599. [PubMed] [Cited in This Article: ] |
| 48. | Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709-4715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258-2267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Seel DJ, Kawabata T, Nakamura M, Ishibashi T, Hamano M, Mashimo M, Shin SH, Sakamoto K, Jhee EC, Watanabe S. N-nitroso compounds in two nitrosated food products in southwest Korea. Food Chem Toxicol. 1994;32:1117-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del Giudice G, Palli D, Saieva C. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497-1501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Tsukamoto T, Mizoshita T, Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. 2007;35:636-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Liu H, Merrell DS, Semino-Mora C, Goldman M, Rahman A, Mog S, Dubois A. Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in nonhuman primates. Gastroenterology. 2009;137:1367-1379.e1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Epplein M, Zheng W, Li H, Peek RM, Correa P, Gao J, Michel A, Pawlita M, Cai Q, Xiang YB. Diet, Helicobacter pylori strain-specific infection, and gastric cancer risk among Chinese men. Nutr Cancer. 2014;66:550-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Kim JH, Shin HS. Effects of main raw material and jeot-kal (fermented fish sauce) on formation of N-nitrosamines during kimchi fermentation. J Food Hyg Safety. 1997;12:333-339. [Cited in This Article: ] |
| 57. | Nan HM, Park JW, Song YJ, Yun HY, Park JS, Hyun T, Youn SJ, Kim YD, Kang JW, Kim H. Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol. 2005;11:3175-3181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 72] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Cover TL, Peek RM. Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes. 2013;4:482-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Muhammad JS, Zaidi SF, Sugiyama T. Epidemiological ins and outs of helicobacter pylori: a review. J Pak Med Assoc. 2012;62:955-959. [PubMed] [Cited in This Article: ] |
| 61. | Zaidi SF, Muhammad JS, Usmanghani K, Sugiyama T. Review: Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pak J Pharm Sci. 2015;28:1171-1176. [PubMed] [Cited in This Article: ] |
| 62. | Khuroo MS, Zargar SA, Mahajan R, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut. 1992;33:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 122] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Siddiqi M, Kumar R, Fazili Z, Spiegelhalder B, Preussmann R. Increased exposure to dietary amines and nitrate in a population at high risk of oesophageal and gastric cancer in Kashmir (India). Carcinogenesis. 1992;13:1331-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev. 2000;9:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Phukan RK, Narain K, Zomawia E, Hazarika NC, Mahanta J. Dietary habits and stomach cancer in Mizoram, India. J Gastroenterol. 2006;41:418-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Gilani AH, Rahman AU. Trends in ethnopharmocology. J Ethnopharmacol. 2005;100:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 67. | Zaidi SF, Sugiyama T. Antibacterial and anti-inflammatory effects of medicinal plants or spices against Helicobacter pylori: A review. Helicobacter Res. 2013;17:430-436. [Cited in This Article: ] |
| 68. | Usmanghani K, Saeed A, Alam MT. Indusynic Medicine. Pakistan: Research Institute of Indusyunic Medicine 1997; 285-287. [Cited in This Article: ] |
| 69. | Thompson Coon J, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2002;16:1689-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | O’Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, Basset C. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol. 2005;11:7499-7507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 106] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 71. | Zaidi SF, Yamada K, Kadowaki M, Usmanghani K, Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J Ethnopharmacol. 2009;121:286-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 72. | Zaidi SF, Yoshida I, Butt F, Yusuf MA, Usmanghani K, Kadowaki M, Sugiyama T. Potent bactericidal constituents from Mallotus philippinensis against clarithromycin and metronidazole resistant strains of Japanese and Pakistani Helicobacter pylori. Biol Pharm Bull. 2009;32:631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Zaidi SF, Ahmed K, Yamamoto T, Kondo T, Usmanghani K, Kadowaki M, Sugiyama T. Effect of resveratrol on Helicobacter pylori-induced interleukin-8 secretion, reactive oxygen species generation and morphological changes in human gastric epithelial cells. Biol Pharm Bull. 2009;32:1931-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Zaidi SF, Muhammad JS, Shahryar S, Usmanghani K, Gilani AH, Jafri W, Sugiyama T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J Ethnopharmacol. 2012;141:403-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Yakoob J, Abbas Z, Khan R, Usman MW, Zaidi SF, Sugiyama T, Hamid S, Awan S, Shamim K, Jafri W. Anti-Helicobacter pylori activity and Inhibition of Helicobacter pylori-induced release of IL-8 in AGS cells by plant extracts. J Med Plant Res. 2013;7:970-979. [Cited in This Article: ] |
| 76. | Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, Sugiyama T. Anti-inflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull. 2015;38:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179-4181. [PubMed] [Cited in This Article: ] |
| 78. | De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 79. | Foryst-Ludwig A, Neumann M, Schneider-Brachert W, Naumann M. Curcumin blocks NF-kappaB and the motogenic response in Helicobacter pylori-infected epithelial cells. Biochem Biophys Res Commun. 2004;316:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol. 2010;16:4039-4046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 81. | Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, Comparato G, Fanigliulo L, Morana E. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Iimuro M, Shibata H, Kawamori T, Matsumoto T, Arakawa T, Sugimura T, Wakabayashi K. Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett. 2002;187:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Graham DY, Anderson SY, Lang T. Garlic or jalapeño peppers for treatment of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1200-1202. [PubMed] [Cited in This Article: ] |