Published online Nov 15, 2014. doi: 10.4251/wjgo.v6.i11.420
Revised: October 3, 2014
Accepted: October 23, 2014
Published online: November 15, 2014
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) of the liver is a safe procedure in the diagnosis and staging of hepatobiliary malignancies with a minimal major complication rate. EUS-FNA is useful for liver lesions poorly accessible to other imaging modalities of the liver. EUS-guided FNA of biliary neoplasia and malignant biliary stricture is superior to the conventional endoscopic brushing and biopsy.
Core tip: The present article reviews the usefulness of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in patients with focal liver and biliary tract lesions. We conducted MEDLINE search using the terms “endoscopic ultrasound-guided fine needle aspiration”, “focal liver lesions” and “biliary tract lesions”, “EUS and biliary stricture”, EUS and focal liver mass”, “EUS and cholangiocarcinoma” and “EUS and gallbladder” to retrieve articles published between 1999 to 2014.
- Citation: Hammoud GM, Almashhrawi A, Ibdah JA. Usefulness of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of hepatic, gallbladder and biliary tract Lesions. World J Gastrointest Oncol 2014; 6(11): 420-429
- URL: https://www.wjgnet.com/1948-5204/full/v6/i11/420.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i11.420
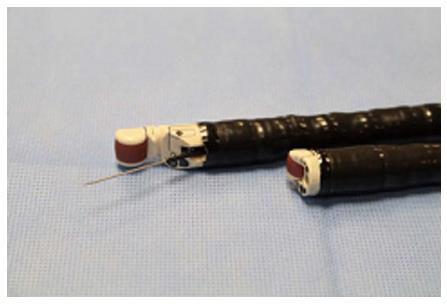
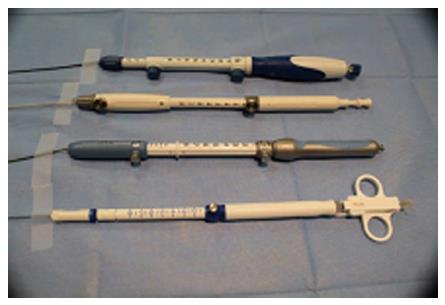
Endoscopic ultrasonography (EUS) has become an indispensable diagnostic and therapeutic procedure in the field of gastroenterology coupling endoscopy with high frequency echo sonography. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is performed using the curved linear array echoendoscope (Figure 1) using various needles (Figure 2). The recently introduced forward viewing linear echoendoscope is gaining momentum in endoscopic ultrasound-guided interventions (Figure 1). EUS-FNA is minimally invasive that is utilized for procurement of tissue from unresectable tumors. EUS-guided fine needle aspiration is used increasingly for the diagnosis of mediastinal, pancreatic and gastric tumors, however, not much is known about EUS-FNA in hepatic lesions. EUS imaging of the liver is currently limited to the left lobe, the proximal right lobe, the hilum and part of the intrahepatic biliary tract. EUS-FNA may be considered as an alternative to liver percutaneous biopsy in patients at high risk of bleeding or with small lesions of the liver uncharacterized by cross-sectional abdominal imaging. EUS-guided biliary drainage (EUS-BD) was developed using a curved linear array echoendoscope for cases with failed endoscopic biliary drainage. Table 1 summarizes the use of endoscopic ultrasound-guided fine needle aspiration in the diagnosis and management of hepatic, gallbladder and biliary tract lesions.
| Diagnosis of focal malignant and benign liver lesions |
| Diagnosis of malignant biliary stricture and neoplasia |
| Preoperative staging of hepatocellular carcinoma and lymph node metastasis |
| Ablation of focal malignant and benign liver lesions |
| Liver biopsy |
| Fluid acquisition and biopsy of peritoneal and omental deposits |
| Drainage of intrahepatic and extrahepatic biliary tree |
| Drainage of hepatic abscesses |
Focal liver lesions include simple liver cyst, focal nodular hyperplasia, hepatic adenoma, hepatic hemangioma, regenerative nodular hyperplasia, biliary cystadenoma, intrahepatic cholangiocarcinoma, hepatocellular carcinoma and metastatic liver lesions. The majority of these lesions can be diagnosed with certainty by cross-sectional abdominal imaging and by percutaneous liver biopsy. However, small lesions less than 1-cm in diameter may not be well characterized by abdominal ultrasound (US), computed tomography (CT) and/or magnetic resonance imaging (MRI). In general, the lowest ultrasound frequency available should be used to maximize penetration. EUS-guided liver biopsy using a 19-gauge FNA needle (non-Trucut) and EUS-guided Trucut needle appear to be feasible, safe and provide excellent diagnostic yield and specimen adequacy[1-3]. In a retrospective study by DeWitt et al[4], EUS-FNA of liver lesions that range from 3-40 mm in size was performed in 77 patients[4]. Of these lesions, 58% were diagnostic for malignancy, 33% were benign, and 9% were nondiagnostic. In a study by tenBerge et al[5], EUS-FNA was used to sample liver lesions in 167 patients. The indications were pancreatic mass in 37%, liver metastasis of unknown origin in 20%, esophageal, gastric and liver masses. EUS-FNA of the liver revealed malignancy in patients when abdominal ultrasonography-guided FNA and CT-guided FNA have failed. Crowe et al[6] compared 34 percutaneous computerized tomographic-guided fine needle aspiration liver biopsies and 16 EUS-FNA liver biopsies showed comparable results. These studies and others suggest that EUS-FNA is feasible and comparable to US/CT-guided biopsy in the diagnosis of patients with focal liver lesions.
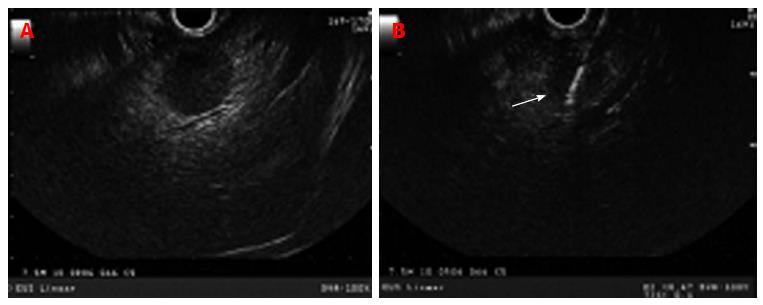
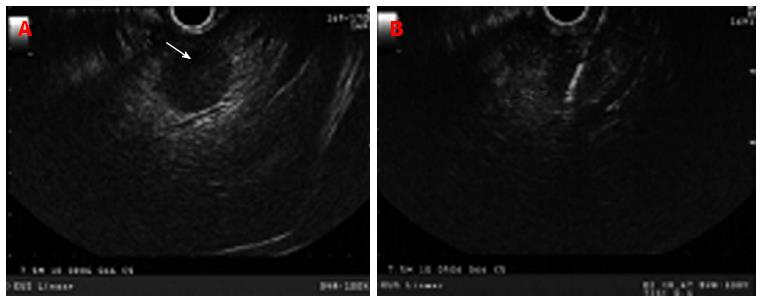
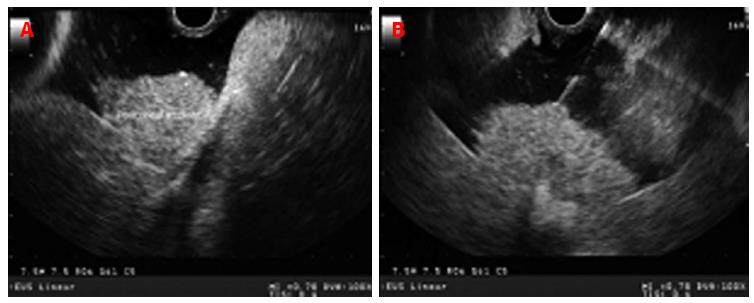
EUS can provide high resolution imaging of the left hepatic lobe to detect unsuspected metastatic disease during staging and may deter from unnecessary surgery[7,8]. EUS-FNA of liver lesions can provide useful information for future management. Hepatic metastasis is generally echo-poor without a distinct border such as the one seen in pancreatic and colon metastasis (Figure 3) or echo-rich such as seen in metastatic neuroendocrine tumors and renal cell carcinoma (Figure 4). EUS-FNA can detect tumors less than 3 mm in size[7]. Solid liver lesions accessible by EUS may be safely sampled by EUS-FNA. The use of stylet during FNA does not appear to confer any advantage with regards to the adequacy of specimen or diagnostic yield of malignancy[9]. In a prospective study of 132 subjects with newly diagnosed tumors, the diagnostic accuracy of EUS/EUS-FNA and CT scan in detecting hepatic metastasis was 98% and 92%, respectively (P = 0.0578)[10]. In a large single-center experience, the sensitivity of EUS-FNA for the diagnosis of liver cancer ranged from 82% to 94%[4]. In a prospective study of 41 patients, 33 of whom had clinical findings suggestive of liver malignancies, EUS-FNA provided biopsy specimens in 40/41 patients[11]. Combining histological and cytological features had a sensitivity of 94%, specificity of 100%, negative predictive value of 78%, and positive predictive value of 100%[11]. These data suggest that EUS-FNA is a sensitive diagnostic procedure in patients with focal malignant liver lesions especially to those confined to left hepatic lobe.
EUS-FNA may be useful in the diagnosis of focal liver lesions, early hepatocellular carcinoma, and evaluation of perihepatic adenopathy[12-15]. Hepatocellular carcinoma (HCC) may appear on EUS images either as hypoechoic or hyperechoic[16]. Burrel et al[17] showed that lesions smaller than 1cm in diameter are missed in a significant percentage (70%) of the patients by modalities such as CT imaging[14,18] and magnetic resonance imaging[18]. EUS and EUS-FNA are particularly valuable for the preoperative staging of hepatocellular and metastatic liver carcinoma. In a study by Awad et al[18], EUS identified liver lesions 0.3-14 cm in size in all 14 study patients with hepatocellular cancer and metastatic lesions who underwent both dynamic CT scans and EUS[18]. Moreover, in 28% of the patients, EUS identified new lesions less than 0.5 cm in size. In a prospective single-center study evaluating 17 patients who underwent cross-sectional imaging and EUS, 9 had liver tumors[16]. EUS-FNA established a tissue diagnosis in 8 of the 9 cases. The diagnostic accuracy of transabdominal ultrasonography, abdominal CT, MRI, and EUS/EUS-FNA were 38%, 69%, 92%, and 94%, respectively[16]. Another retrospective study evaluated the sensitivity and complications of EUS-FNA of liver nodules in 14 patients, performed by single endoscopist[19]. Twenty-one percent of the cases were hepatocellular carcinoma. The sensitivity of diagnosis of malignant liver lesions utilizing cytology was 78.5%. However, combining clinical course and pathology increased the sensitivity to 100%. These data suggest that EUS has an excellent diagnostic accuracy in patients with HCC.
Moreover, EUS-guided fine needle aspiration of portal vein thrombus to detect malignancy has been described in literature[20,21]. More recently, a newly developed promising technique utilizing real time-sonoelastography (RTE) by EUS might improve the characterization and differentiation between benign and malignant focal liver lesions[22].
The use of EUS-FNA in screening for HCC is limited by the semi-invasive nature of the procedure as well as its inability to evaluate all liver segments at this time[13]. Nevertheless, EUS can provide an additional option for treatment in patients with hepatocellular carcinoma who are difficult to treat utilizing percutaneous ablative therapy such as endoscopic ultrasound-guided ethanol injection[23,24] and EUS-guided Nd:YAG laser ablation of a caudate lobe hepatocellular carcinoma[25].
Large hepatic cysts are amenable to percutaneous drainage or surgical resection. EUS-guided ethanol injection has been shown to be effective in treating patients with large hepatic cysts especially in the left hepatic lobe. In a retrospective study evaluating 17 patients with 19 hepatic cysts (median cyst volume before therapy was 368.9 mL)[26], ten cysts were drained by the percutaneous approach and 8 cysts underwent EUS-guided aspiration and lavage treatment. During 15-mo follow-up, the cysts showed nearly 100% reduction in the EUS-guided group compared to 97% reduction in the percutaneous group. Furthermore, EUS-FNA has also shown excellent success rates in selected patients with hepatic abscesses. In a recent review of the literature by Singhal et al[27], seven studies have reported 100% technical and clinical success rates of EUS-guided drainage of hepatic abscesses in patients refractory or not amenable to percutaneous drainage.
EUS-guided paracentesis is valuable in the cytologic diagnosis and staging of malignant ascites[28,29]. EUS frequently identifies ascites missed by other imaging modalities and may identify malignancy[30]. It is particularly useful when CT imaging does not identify abnormalities[31]. EUS-FNA can be performed safely for therapeutic paracentesis[32]. In a retrospective single center study that evaluated 101 patients who underwent EUS-guided paracentesis, the specificity, sensitivity, positive and negative predictive values, and diagnostic accuracy were 100%, 80%, 100%, 95% and 96%, respectively[29]. Furthermore, EUS-FNA can be used effectively and safely to obtain tissue from the peritoneum for diagnosis of tuberculous peritonitis[33]. EUS-FNA allows the sampling of peritoneal metastatic lesions, which appear on EUS images as hyperechoic compared to surrounding anechoic ascitic fluid (Figure 5). In a small study involving 12 patients with undiagnosed ascites, peritoneal deposits noted in 10 (83.3%) patients[34]. The cytological results were positive for malignancy in 6 of those patients, while the remaining four patients had inflammatory cells.
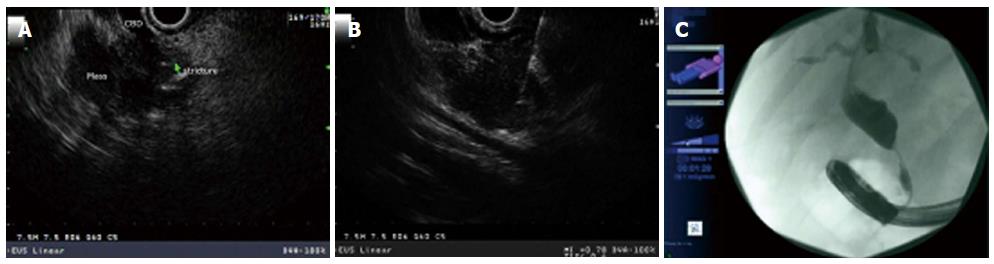
Preoperative tissue diagnosis is required for hilar neoplasia [cholangiocarcinoma (CCA)] to avoid risk of unnecessary extensive surgery. Endoscopic transpapillary brush cytology and forceps biopsy are used for the pathological diagnosis of malignant biliary strictures. Endoscopic retrograde cholangiography (ERC) is currently the main diagnostic procedure performed to obtain sampling of the biliary tree. However, the sensitivity and specificity of obtaining a sample in biliary neoplasia is variable. EUS is capable of visualizing the hilum at the duodenal bulb by tracing the common bile duct (CBD) towards the liver hilum. In a meta-analysis of 36 studies by Garrow et al[35] EUS has a sensitivity of 78% and specificity of 84% in detecting malignant biliary strictures. Nayar et al[36] reported on 32 patients who underwent 36 procedures for hilar lesions. The overall sensitivity, accuracy, specificity, positive predictive value and negative predictive value of EUS-FNA were 52%, 68%, 100%, 100% and 54%, respectively. Fritscher-Ravens et al[37] prospectively evaluated 44 patients with hilar strictures diagnosed by CT and/or Endoscopic retrograde cholangiopancreaticography (ERCP) that were suspicious for hilar cholangiocarcinoma but had inconclusive tissue diagnosis. The sensitivity, accuracy, and specificity of EUS-FNA in this study were 89%, 91%, and 100%, respectively. Moreover, EUS and EUS-FNA changed preplanned surgical approach in about half of these patients[37]. The above studies suggest that hilar neoplasia can be sampled by EUS-FNA although the accuracy and sensitivity were not robust. Moreover, EUS-FNA may be considered in evaluating regional lymph nodes to evaluate for metastasis in patients with unresectable hilar cholangiocarcinoma[38,39]. EUS-FNA in patients with cholangiocarcinoma did not appear to adversely affect the overall survival[40].
The sensitivity of EUS-FNA is much higher in distal malignant biliary strictures than proximal strictures. Malignant distal biliary strictures are most commonly secondary to pancreatic malignancy and/or distal bile duct cholangiocarcinoma (Figure 6). In a recent prospective comparative one-year study of 51 patients who underwent EUS and ERCP in the same session for evaluation of malignant biliary obstruction[41], EUS-FNA was superior to ERCP in tissue sampling for evaluating suspected malignant biliary obstruction, especially for pancreatic masses with an overall accuracy and sensitivity of 94% and 94% for EUS-FNA, and 53% and 50% for ERCP sampling, respectively. In an observation study of prospectively collected data of 228 patients with biliary strictures who underwent EUS[42]. Cholangiocarcinoma was detected in eighty-one, Fifty-one of the patients (63%) had distal and 30 (37%) had proximal CCA. The overall sensitivity of EUS-FNA for the diagnosis of CCA was 73% and was significantly higher in distal compared to proximal CCA (81% vs 59%, respectively; P = 0.04). Furthermore, a retrospective analysis of 342 patients who underwent EUS-FNA after presenting with biliary stricture and obstructive jaundice[43] showed an overall 92.4% accuracy of EUS-FNA for diagnosing malignancy with 91.5% sensitivity and 80.9% negative predictive value. These studies and others demonstrate the higher sensitivity of EUS-FNA in distal biliary stricture. Moreover, EUS-FNA appears equivalent to ERCP sampling for biliary tumors and indeterminate strictures[41] and may provide a diagnosis of malignancy when ERCP sampling is indeterminate[44]. Moreover, EUS-FNA can have a role in diagnosing other lesions that may mimic cholangiocarcinoma and present either as a mass or with obstructive jaundice. Such lesions as epithelial vs nonepithelial tumors, neuroendocrine tumors, lymphoma, and metastasis from other primaries[45,46].
ERCP is currently the standard of care for biliary drainage, however the failed cannulation rates ranges 3% to 5% in experienced hands. EUS-guided biliary drainage includes EUS-guided choledochoduodenostomy[47], hepaticogastrostomy[48], and EUS-guided transpapillary rendezvous biliary drainage[49]. The procedure technique has been described as follows[50]: the linear-array EUS scope is placed against the cardia or lesser curve of the stomach in a patient with dilated left intrahepatic biliary tree for hepaticogastrostomy or against the bulb of the duodenum for choledochoduodenostomy. The dilated bile duct or left intrahepatic duct which appears as hyperechoic structure running alongside the portal venous system without Doppler flow signals is then identified and punctured using a 19-guage or 22-guage needle. The stylet is then removed followed by contrast injection to visualize the biliary tree under fluoroscopy. A 0.035’’ or 0.021’’ guidewire is subsequently passed via the FNA needle into the bile duct or dilated intrahepatic duct. The needle knife is then used to make an incision of the gastric or duodenal wall under EUS guidance for preparation of dilation of the transmural tract. Dilation can be performed using 4.5F to 5F ERCP cannula, 4-mm or 6-mm dilating biliary balloon. A plastic biliary stent or self-expandable fully covered metal stent can then be placed[51,52]. In a large multicenter, nonrandomized retrospective study of 240 patients who underwent EUS-guided bile duct access and drainage[53], success was achieved in 87% of the cases. Similarly, in extrahepatic and intrahepatic approaches, the success rate was 84.3% vs 90.4%; respectively.
EUS-FNA has gained momentum in sampling gallbladder masses for diagnostic and staging purposes with accuracy reaching 100% in early stages. Sadamoto et al[54] reported EUS accuracy of 100% for in situ tumors (Tis), 76% for T1, 85% for T2, and 93% for T3 and T4 lesions. In one series, EUS-FNA provided accurate diagnosis of six patients with obstructive jaundice (five with gallbladder adenocarcinomas) where CT scans mostly failed to detect the causing lesions[55]. Jacobson et al[56] described similar findings in four out of five patients diagnosed with adenocarcinoma of the gallbladder. Meara et al[57] reported sensitivity of 80% and specificity of 100% in diagnosing gallbladder wall lesions.
EUS and transabdominal US are usually viewed as good tools to evaluate gallbladder polyps with superior sensitivities for EUS 97% vs transabdominal US 71% in one study[58]. Diagnostic distinction between malignant and non-malignant polyps for the purpose of staging and determining next steps management, remains mostly dependent on the ultrasonographic features of the polyps rather than tissue sampling[54]. No reports of the use of EUS-FNA in approaching gallbladder polyps were found. Endoscopic ultrasound-guided transmural gallbladder drainage with placement of self-expandable stent has been reported and is technically successful for the management of acute cholecystitis in high risk patients[59-61]. Table 2 summarizes the sensitivity, specificity and diagnostic accuracy in the reported studies.
| Study, year, number | Sensitivity (%) | Specificity (%) | Diagnosticaccuracy (%) |
| Focal malignant liver lesions | |||
| DeWitt et al[4], 2003, n = 77 | 82-94 | - | - |
| Hollerbach et al[11], 2003, n = 44 | 94 | 100 | - |
| Singh et al[16], 2007, n = 17 | 89 | 100 | 94 |
| CT (71) | 67 | 69 | |
| MR (86) | 100 | 92 | |
| Prachayakul et al[19], 2012, n = 14 | 78.5 | - | - |
| Malignant biliary tract and gallbladder lesions | |||
| Garrow et al[35], 2007, 36 studies, n = 3532 | 78 | 84 | 90 |
| Nayar et al[36], 2011, n = 32 | 52 | 100 | 68 |
| Fritscher-Ravens et al[37], 2004, n = 44 | 89 | 100 | 91 |
| Weilert et al[41], 2014, n = 51 | 94 | 100 | 94 |
| ERCP brushing (50) | 100 | 53 | |
| Mohamadnejad et al[42], 2011, n = 228 | 73 | - | - |
| ERCP brushing (27) | - | ||
| Tummala et al[43], 2013, n = 342 | 91.5 | - | 92.4 |
| Meara et al[57], 2006, n = 53 | 80 | 100 | - |
| ERCP brushing (13) | 75 | - | |
EUS-FNA can provide an excellent diagnostic accuracy in distinguishing between benign and malignant ampullary tumors in comparison to surface biopsy with duodenoscopy, and/or intra-ampullary biopsy, and/or brush cytology with ERCP, and/or intra-ampullary biopsy after endoscopic sphincterotomy (100% vs 70%)[62]. Furthermore, the diagnostic accuracy of EUS-FNA for ampullary tumors supersedes that without EUS-FNA. In a retrospective study by Roberts et al[63], rates of diagnostic accuracy in high-grade dysplasia, low-grade dysplasia, and adenocarcinoma were 20%, 72%, and 96%, respectively, in the non-EUS group, and 50%, 93%, and 100%, respectively, in the EUS group.
The diagnostic accuracy of EUS-FNA is dependent on how the sample is processed after acquisition. The presence of a rapid on-site cytology evaluation (ROSE) by a cytopathologist in the vicinity where the sample is obtained has been shown to improve the diagnostic yield of the procedure[64]. ROSE may allow a less number of needle passes and ensure adequacy of the sample obtained by onsite staining prior to completion of procedure. In general, the diagnostic yield of EUS-FNA with ROSE in most studies exceeds 90%. Meara et al[57] reported on 53 cases undergone EUS-FNA from 46 bile duct and seven gallbladder lesions where ROSE was available. All cases initially diagnosed as suspicious/malignant were confirmed on the final cytological interpretation. The specificity for EUS-FNA was 100% with sensitivity rates of 80% and 87% from clinically suspected malignancies of gallbladder and biliary tract, respectively. A retrospective study by Jhala et al[65] provided on-site diagnosis of malignancy on 485 EUS-FNA of the pancreas (n = 305), lymph nodes (n = 91), biliary tree (n = 47), liver (n = 15), gastrointestinal tract (n = 19), and adrenal gland (n = 8). A significantly higher degree of concordance was noted for unequivocal diagnosis of malignancy vs no malignancy (98.9% vs 67.2%) between on-site and final cytologic diagnosis. These studies have demonstrated ROSE by cytopathologist and interpretation significantly improves the diagnostic yield of EUS-FNA.
In a retrospective questionnaire sent to 130 EUS-FNA centers across the world[5]. 167 cases of EUS-FNA of the liver were reported by 21 centers. A complication was reported in 6 (4%) of the 167 cases including the following: death in 1 patient, bleeding (1), fever (2), and pain (2)[5]. EUS-guided liver biopsy appears to be safe and associated with no significant complications[2-4,66]. Several studies have reported no adverse events related to EUS-FNA of bile duct strictures, gallbladder and masses[41,42,56,57,67]. However, EUS-FNA of malignant biliary lesions was reported to have a risk of bleeding, infection, or pancreatitis in less than 2% of the cases[68]. Hemobilia was reported in 1.3% of patients who underwent EUS-FNA of malignant biliary stricture[42]. Bacteremia after EUS-FNA is rare. However, prophylactic antibiotics should be given prior and after EUS-FNA of biliary tract in patients with biliary obstruction. EUS-guided diagnostic abdominal paracentesis was not associated with any complication in one study[28]. Bile peritonitis has been reported after inadvertent biliary puncture during EUS-FNA[69]. Complications of EUS-guided biliary drainage included pneumoperitoneum 5%, bleeding 11%, bile leak/peritonitis 10%, and cholangitis 5%[53]. Needle track tumor seeding has been reported and is a risk after EUS-FNA of malignant biliary neoplasia[70,71]. EUS-FNA of malignant biliary stricture is considered a contraindication in patients eligible for liver transplantation. Cholecystitis and bile peritonitis have been reported after EUS-FNA of gallbladder lesions[72]. Bleeding after EUS-FNA of solid tumor is rare and appears as an expanding extraluminal echopoor region adjacent to the sampled lesion[73].
The head of pancreas and CBD are not visualized after Roux-en-Y surgery and Billroth II surgery if the afferent limb is not intubated. Presence of vascular structures or collaterals in needle path may limit EUS-FNA of focal lesions. Because the right liver lobe is farther away from the probe, it is generally not seen except in small parts. The presence of pneumobilia, fatty infiltration, calcifications and extensive fibrosis may interfere with ultrasound beam and images. Endosonographer’s experience, time consumed to image the liver and patient’s body habitus are of critical importance to clearly identify and diagnose focal liver lesions. The miss rate for resectable pancreaticobiliary malignancy by EUS-FNA is rather small. Moreover, EUS and EUS-FNA may not be widely available and require an expertise with dedicated echosonographer in the field. With improving resolution and widespread use of EUS with dedicated formal training, small liver metastasis and other focal liver lesions are being increasingly detected. EUS does not use intravenous contrast to evaluate the nature of focal liver lesions and thus correlation with other cross-sectional imaging such as CT and/or MR is needed. However, the technology has dramatically improved. The use of color and power Doppler imaging, three-dimensional imaging, electronic scanning, tissue harmonic imaging, elastography, and recently contrast-enhanced images have improved the diagnostic capability. The depth of tumor infiltration and differentiation between infiltrating or exophytic lesions can now be assessed with greater accuracy[74-76].
Endoscopic ultrasound-guided fine needle aspiration of the liver, gallbladder and biliary tract is feasible and provides an excellent diagnostic accuracy. The presence of ROSE has increased the diagnostic yield. EUS-FNA is capable to differentiate between focal benign or malignant liver lesions. The widespread of EUS and increase formal training have enhanced the diagnostic and therapeutic armamentarium of EUS in hepatobiliary disorders. EUS-FNA should be considered as an adjunct to other cross-sectional imaging in the differentiation between benign and focal hepatobiliary disorders. EUS-guided interventions such as fine-needle injections, tumor ablative therapies and biliary drainage have increased the application of EUS and is considered as an adjunct to other modalities.
P- Reviewer: Arcidiacono PG, Chen JQ, Scherubl H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Stavropoulos SN, Im GY, Jlayer Z, Harris MD, Pitea TC, Turi GK, Malet PF, Friedel DM, Grendell JH. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Gleeson FC, Clayton AC, Zhang L, Clain JE, Gores GJ, Rajan E, Smyrk TC, Topazian MD, Wang KK, Wiersema MJ. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Dewitt J, McGreevy K, Cummings O, Sherman S, Leblanc JK, McHenry L, Al-Haddad M, Chalasani N. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest Endosc. 2009;69:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | DeWitt J, LeBlanc J, McHenry L, Ciaccia D, Imperiale T, Chappo J, Cramer H, McGreevy K, Chriswell M, Sherman S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [PubMed] [Cited in This Article: ] |
| 6. | Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Prasad P, Schmulewitz N, Patel A, Varadarajulu S, Wildi SM, Roberts S, Tutuian R, King P, Hawes RH, Hoffman BJ. Detection of occult liver metastases during EUS for staging of malignancies. Gastrointest Endosc. 2004;59:49-53. [PubMed] [Cited in This Article: ] |
| 8. | McGrath K, Brody D, Luketich J, Khalid A. Detection of unsuspected left hepatic lobe metastases during EUS staging of cancer of the esophagus and cardia. Am J Gastroenterol. 2006;101:1742-1746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Wani S, Gupta N, Gaddam S, Singh V, Ulusarac O, Romanas M, Bansal A, Sharma P, Olyaee MS, Rastogi A. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409-2414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Singh P, Mukhopadhyay P, Bhatt B, Patel T, Kiss A, Gupta R, Bhat S, Erickson RA. Endoscopic ultrasound versus CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Bissonnette J, Paquin S, Sahai A, Pomier-Layrargues G. Usefulness of endoscopic ultrasonography in hepatology. Can J Gastroenterol. 2011;25:621-625. [PubMed] [Cited in This Article: ] |
| 13. | Thuluvath PJ. EUS-guided FNA could be another important tool for the early diagnosis of hepatocellular carcinoma. Gastrointest Endosc. 2007;66:274-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Hollerbach S, Reiser M, Topalidis T, König M, Schmiegel W. Diagnosis of hepatocellular carcinoma (HCC) in a high-risk patient by using transgastric EUS-guided fine-needle biopsy (EUS-FNA). Z Gastroenterol. 2003;41:995-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kimura H, Matsubayashi H, Fukutomi A, Asakura K, Sasaki K, Yamaguchi Y, Ono H. Lymph node metastasis diagnosed by EUS-FNA in four cases with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:237-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, Ulf Westblom T. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc. 2007;66:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Awad SS, Fagan S, Abudayyeh S, Karim N, Berger DH, Ayub K. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601-604; discussion 601-604. [PubMed] [Cited in This Article: ] |
| 19. | Prachayakul V, Aswakul P, Kachintorn U. EUS guided fine needle aspiration cytology of liver nodules suspicious for malignancy: yields, complications and impact on management. J Med Assoc Thai. 2012;95 Suppl 2:S56-S60. [PubMed] [Cited in This Article: ] |
| 20. | Storch I, Gomez C, Contreras F, Schiff E, Ribeiro A. Hepatocellular carcinoma (HCC) with portal vein invasion, masquerading as pancreatic mass, diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Dig Dis Sci. 2007;52:789-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Lai R, Stephens V, Bardales R. Diagnosis and staging of hepatocellular carcinoma by EUS-FNA of a portal vein thrombus. Gastrointest Endosc. 2004;59:574-577. [PubMed] [Cited in This Article: ] |
| 22. | Sandulescu L, Padureanu V, Dumitrescu C, Braia N, Streba CT, Gheonea DI, Cazacu S, Ciurea T, Rogoveanu I, Saftoiu A. A pilot study of real time elastography in the differentiation of focal liver lesions. Curr Health Sci J. 2012;38:32-35. [PubMed] [Cited in This Article: ] |
| 23. | Nakaji S, Hirata N, Iwaki K, Shiratori T, Kobayashi M, Inase M. Endoscopic ultrasound (EUS)-guided ethanol injection for hepatocellular carcinoma difficult to treat with percutaneous local treatment. Endoscopy. 2012;44 Suppl 2 UCTN:E380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | DiMaio CJ, Krishnan S, Roayaie S. EUS-guided ethanol ablation for management of metastatic hepatocellular carcinoma. J Interv Gastroenterol. 2014;4:13-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Di Matteo F, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd: YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest Endosc. 2011;73:632-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Lee S, Seo DW, Paik WH, Park DH, Lee SS, Lee SK, Kim MH. Ethanol lavage of huge hepatic cysts by using EUS guidance and a percutaneous approach. Gastrointest Endosc. 2014;May 30; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Singhal S, Changela K, Lane D, Anand S, Duddempudi S. Endoscopic ultrasound-guided hepatic and perihepatic abscess drainage: an evolving technique. Therap Adv Gastroenterol. 2014;7:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Shibukawa G, Sato A, Sato M, Ikeda T, Watanabe K. An automated spring-loaded needle for endoscopic ultrasound-guided abdominal paracentesis in cancer patients. World J Gastrointest Endosc. 2014;6:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wardeh R, Lee JG, Gu M. Endoscopic ultrasound-guided paracentesis of ascitic fluid: a morphologic study with ultrasonographic correlation. Cancer Cytopathol. 2011;119:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Chu KM. EUS could detect ascites missed by CT scan. Gut. 2006;55:1524; author reply 1524. [PubMed] [Cited in This Article: ] |
| 32. | Varadarajulu S, Drelichman ER. EUS-guided therapeutic paracentesis. Gastrointest Endosc. 2008;67:758-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Kocaman O, Danalioğlu A, İnce AT, Tozlu M, Şentürk H. Diagnosis of tuberculous peritonitis using endoscopic ultrasound-guided fine-needle aspiration biopsy of the peritoneum. Turk J Gastroenterol. 2013;24:65-69. [PubMed] [Cited in This Article: ] |
| 34. | Rana SS, Bhasin DK, Srinivasan R, Singh K. Endoscopic ultrasound-guided fine needle aspiration of peritoneal nodules in patients with ascites of unknown cause. Endoscopy. 2011;43:1010-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Nayar MK, Manas DM, Wadehra V, Oppong KE. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862-1865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 37. | Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51. [PubMed] [Cited in This Article: ] |
| 38. | Gleeson FC, Rajan E, Levy MJ, Clain JE, Topazian MD, Harewood GC, Papachristou GI, Takahashi N, Rosen CB, Gores GJ. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Pollack MJ, Gholam PM, Chak A. EUS-FNA in unresectable cholangiocarcinoma: a novel indication. Gastrointest Endosc. 2008;67:444-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | El Chafic AH, Dewitt J, Leblanc JK, El Hajj II, Cote G, House MG, Sherman S, McHenry L, Pitt HA, Johnson C. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013;45:883-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:244-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Fletcher ND, Wise PE, Sharp KW. Common bile duct papillary adenoma causing obstructive jaundice: case report and review of the literature. Am Surg. 2004;70:448-452. [PubMed] [Cited in This Article: ] |
| 46. | Penn I. Primary malignancies of the hepato-biliary-pancreatic system in organ allograft recipients. J Hepatobiliary Pancreat Surg. 1998;5:157-164. [PubMed] [Cited in This Article: ] |
| 47. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 48. | Giovannini M, Dotti M, Bories E, Moutardier V, Pesenti C, Danisi C, Delpero JR. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003;35:1076-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 50. | Yamao K, Hara K, Mizuno N, Sawaki A, Hijioka S, Niwa Y, Tajika M, Kawai H, Kondo S, Shimizu Y. EUS-Guided Biliary Drainage. Gut Liver. 2010;4 Suppl 1:S67-S75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos). Gastrointest Endosc. 2010;72:1279-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 53. | Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 54. | Sadamoto Y, Kubo H, Harada N, Tanaka M, Eguchi T, Nawata H. Preoperative diagnosis and staging of gallbladder carcinoma by EUS. Gastrointest Endosc. 2003;58:536-541. [PubMed] [Cited in This Article: ] |
| 55. | Varadarajulu S, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration in the evaluation of gallbladder masses. Endoscopy. 2005;37:751-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Jacobson BC, Pitman MB, Brugge WR. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest Endosc. 2003;57:251-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Meara RS, Jhala D, Eloubeidi MA, Eltoum I, Chhieng DC, Crowe DR, Varadarajulu S, Jhala N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: analysis of 53 cases. Cytopathology. 2006;17:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Sugiyama M, Xie XY, Atomi Y, Saito M. Differential diagnosis of small polypoid lesions of the gallbladder: the value of endoscopic ultrasonography. Ann Surg. 1999;229:498-504. [PubMed] [Cited in This Article: ] |
| 59. | Choi JH, Lee SS, Choi JH, Park do H, Seo DW, Lee SK, Kim MH. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 60. | Widmer J, Singhal S, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided endoluminal drainage of the gallbladder. Dig Endosc. 2014;26:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014;May 13; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 62. | Ogura T, Hara K, Hijioka S, Mizuno N, Imaoka H, Niwa Y, Tajika M, Kondo S, Tanaka T, Shimizu Y. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for tumors of the ampulla of vater? -an initial study. Endosc Ultrasound. 2012;1:84-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Roberts KJ, McCulloch N, Sutcliffe R, Isaac J, Muiesan P, Bramhall S, Mirza D, Marudanayagam R, Mahon BS. Endoscopic ultrasound assessment of lesions of the ampulla of Vater is of particular value in low-grade dysplasia. HPB (Oxford). 2013;15:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697-705. [PubMed] [Cited in This Article: ] |
| 65. | Jhala NC, Eltoum IA, Eloubeidi MA, Meara R, Chhieng DC, Crowe DR, Jhala D. Providing on-site diagnosis of malignancy on endoscopic-ultrasound-guided fine-needle aspirates: should it be done? Ann Diagn Pathol. 2007;11:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Mathew A. EUS-guided routine liver biopsy in selected patients. Am J Gastroenterol. 2007;102:2354-2355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 68. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. [PubMed] [Cited in This Article: ] |
| 69. | Di Matteo F, Shimpi L, Gabbrielli A, Martino M, Caricato M, Esposito A, De Cicco ML, Coppola R, Costamagna G. Same-day endoscopic retrograde cholangiopancreatography after transduodenal endoscopic ultrasound-guided needle aspiration: do we need to be cautious? Endoscopy. 2006;38:1149-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos). Gastrointest Endosc. 2012;76:1024-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Kim HJ, Lee SK, Jang JW, Kim TG, Ryu CH, Park do H, Lee SS, Seo DW, Kim MH. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology. 2012;59:1691-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc. 2001;53:221-225. [PubMed] [Cited in This Article: ] |
| 74. | Hirooka Y, Itoh A, Kawashima H, Ohno E, Itoh Y, Nakamura Y, Hiramatsu T, Sugimoto H, Sumi H, Hayashi D. Contrast-enhanced endoscopic ultrasonography in digestive diseases. J Gastroenterol. 2012;47:1063-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Park CH, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Kim H, Hwang HK, Lee WJ, Song SY. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Hirooka Y, Naitoh Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hashimoto S, Hayakawa T. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest Endosc. 1998;48:406-410. [PubMed] [Cited in This Article: ] |