Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.286
Peer-review started: September 8, 2022
First decision: November 12, 2022
Revised: November 23, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: February 15, 2023
Processing time: 159 Days and 10.9 Hours
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a newly discovered oncogene. It is an active cell proliferation regulatory factor that inhibits tumor apoptosis in gastric cancer (GC) cells. CIP2A is functionally related to chemoresistance in various types of tumors according to recent studies. The underlying mechanism, however, is unknown. Further, the primary treatment regimen for GC is oxaliplatin-based chemotherapy. Nonetheless, it often fails due to chem
The goal of this study was to examine CIP2A expression and its association with oxaliplatin resistance in human GC cells.
Immunohistochemistry was used to examine CIP2A expression in GC tissues and adjacent normal tissues. CIP2A expression in GC cell lines was reduced using small interfering RNA. After confirming the silencing efficiency, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium and flow cytometry assays were used to evaluate cell proliferation and apoptosis caused by oxaliplatin treatment. Further, the key genes and protein changes were verified using real-time quantitative reverse transcription PCR and Western blotting, respectively, before and after intervention. For bioinformatics analysis, we used the R software and Bioconductor project. For statistical analysis, we used GraphPad Prism 6.0 and the Statistical Package for the Social Sciences software version 20.0 (IBM, Armonk, United States).
A high level of CIP2A expression was associated with tumor size, T stage, lymph node metastasis, Tumor Node Metastasis stage, and a poor prognosis. Further, CIP2A expression was higher in GC cells than in normal human gastric epithelial cells. Using small interfering RNA against CIP2A, we discovered that CIP2A knockdown inhibited cell proliferation and significantly increased GC cell sensitivity to oxaliplatin. Moreover, CIP2A knockdown enhanced oxaliplatin-induced apoptosis in GC cells. Hence, high CIP2A levels in GC may be a factor in chemoresistance to oxaliplatin. In human GC cells, CIP2A regulated protein kinase B phosphorylation, and chemical inhibition of the protein kinase B signaling pathway was significantly associated with increased sensitivity to oxaliplatin. Therefore, the protein kinase B signaling pathway was correlated with CIP2A-enhanced chemoresistance of human GC cells to oxaliplatin.
CIP2A expression could be a novel therapeutic strategy for chemoresistance in GC.
Core Tip: Gastric cancer (GC) is primarily treated with oxaliplatin-based chemotherapy. Patients who receive chemotherapy often develop resistance. Cancerous inhibitor of protein phosphatase 2A (CIP2A) is a novel oncogene. Recent studies suggested that CIP2A is linked to chemoresistance in various cancers. The purpose of this study was to look into the relationship between CIP2A expression and oxaliplatin resistance in GC. The findings revealed that GC tissues have higher CIP2A expression than matched adjacent normal gastric tissues, and CIP2A expression plays an important role in the chemoresistance of GC, suggesting a new treatment strategy for GC.
- Citation: Zhao YX, Ma LB, Yang Z, Wang F, Wang HY, Dang JY. Cancerous inhibitor of protein phosphatase 2A enhances chemoresistance of gastric cancer cells to oxaliplatin. World J Gastrointest Oncol 2023; 15(2): 286-302
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/286.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.286
Gastric cancer (GC) is the most common solid tumor originating from the digestive system and is one of the most severe and fatal malignancies worldwide[1]. GC has a high mortality rate of 75.0% and accounts for 8.8% of all cancer-related deaths[2]. Advanced-stage GC is associated with rapid metastatic growth, relapse, and a poor prognosis with a 5-year survival rate of 30%-50%. Clinically, GC is treated via surgical resection and chemotherapy, which is a viable option[3]. The primary treatment for GC is neoadjuvant or adjuvant therapy. Chemoresistance is a major challenge with few benefits. Further, the aggressiveness of GC is attributed in part to intrinsic and extrinsic chemoresistance[4]. As a result, identifying the molecular mechanism of chemoresistance in GC is critical.
S-1 (tegafur, gimeracil, and oteracil potassium capsules) or capecitabine in combination with oxaliplatin is currently used as adjuvant therapy for GC in various East Asian institutions[5,6]. Oxaliplatin is a third-generation platinum analog commonly used to treat GC, resulting in a large amo
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is an oncogene that inhibits c-Myc degradation. CIP2A has been shown to facilitate the proliferation of various cancer cells[18]. Moreover, CIP2A is overexpressed in a variety of cancers, including breast, head and neck, prostate, lung, and digestive system cancers[18-20]. In human GC, cancer tissues had higher CIP2A levels than noncancerous tissues. As a result, CIP2A may play an oncogenic role in human GC progression and is correlated with a poor prognosis[21-23]. Recent studies have linked increased CIP2A expression to doxorubicin resistance in human breast and colon cancer cells[24]. Moreover, CIP2A overexpression is associated with cisplatin chemoresistance in human non–small cell lung carcinoma, and Akt phosphorylation may play a role in this process[25]. The effect of CIP2A on oxaliplatin resistance in human GC is unknown.
The current study sought to investigate CIP2A expression in tumor tissue and its relationship to clinicopathological features and prognosis in GC patients. We investigated the expression of CIP2A and its relationship with oxaliplatin resistance in human GC cells as well as the possible mechanisms involved.
Between January 2012 and December 2015, 108 paired primary gastric carcinoma tissue and adjacent normal tissue (> 5 cm from the tumor margin and noncancerous tissues determined by the pathologist) specimens were collected from patients undergoing D2 radical resection at the Department of Gastroenterological and Oncological Surgery of the First Hospital of Lanzhou University. Prior to surgery, none of the patients received chemotherapy, radiotherapy, targeted therapy, or immunotherapy. Patients with GC ranged in age from 26-years-old to 78-years-old (mean: 57.3 ± 6.8 years). Table 1 shows the clinicopathological characteristics of patients. The Tumor Node Metastasis stage of the tumor was determined using the 8th edition of the American Joint Committee on Cancer staging manual[26]. All patients were followed up for at least 5 years after surgery. Further, following surgery, all patients received six cycles of S-1 (tegafur, gimeracil, and oteracil potassium capsules) combined with oxaliplatin. To detect the mRNA and protein expression of CIP2A, 18 frozen GC tissue and paired normal tissue specimens were selected. All pathological results were evaluated independently by two specialized pathologists who were blinded. The ethics review board of the First Hospital of Lanzhou University approved this study, and each participant provided written informed consent. All experiments were performed in accordance with the principles of the Declaration of Helsinki.
| Prognostic variables | Number | Expression of CIP2A | χ2 | P value | |
| Low | High | ||||
| Sex | 1.766 | 0.184 | |||
| Male | 71 | 27 | 44 | ||
| Female | 37 | 19 | 18 | ||
| Age, yr | 0.175 | 0.676 | |||
| < 60 | 68 | 30 | 38 | ||
| ≥ 60 | 40 | 16 | 24 | ||
| Tumor location | 0.097 | 0.756 | |||
| Proximal + middle | 37 | 15 | 22 | ||
| Distal | 71 | 31 | 40 | ||
| Histological grade | 0.001 | 0.973 | |||
| G1 + G2 | 26 | 11 | 15 | ||
| G3 | 82 | 35 | 47 | ||
| Tumor size in cm | 5.975 | 0.015a | |||
| < 5 | 84 | 41 | 43 | ||
| ≥ 5 | 24 | 5 | 19 | ||
| T stage | 5.472 | 0.019a | |||
| T1–T2 | 36 | 21 | 15 | ||
| T3–T4 | 72 | 25 | 47 | ||
| N stage | 12.428 | 0.000a | |||
| N0 | 47 | 29 | 18 | ||
| N1–N3 | 61 | 17 | 44 | ||
| TNM stage | 5.168 | 0.023a | |||
| I + II | 69 | 35 | 34 | ||
| III + IV | 39 | 11 | 28 | ||
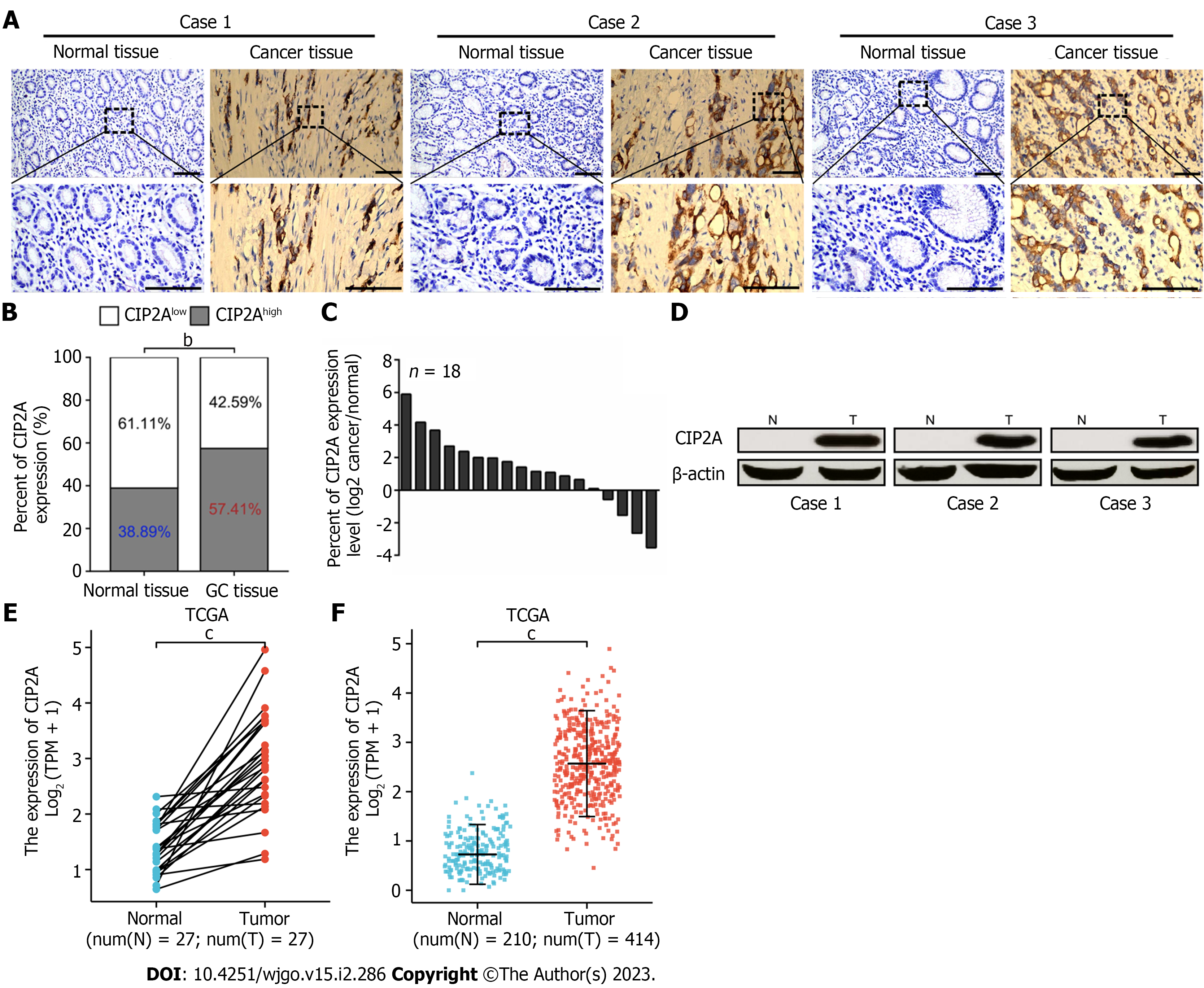
The expression of CIP2A in 108 pairs of GC tissue and matched adjacent normal tissue samples was evaluated via immunohistochemistry (IHC) using the SP method. Formaldehyde-fixed and paraffin-embedded 4-μm-thick samples were deparaffinized with xylene and rehydrated with graded ethanol. Antigen retrieval was performed by boiling in a pressure cooker. The endogenous peroxidase activity was blocked with H2O2. Primary antibodies (CIP2A antibody 1:500, Santa Cruz Biotechnology, United States) were added to the sections and incubated for 1 h at 37 °C in the dark. The secondary antibodies were then added at 37 °C for 30 min. Next, DAB (3,3′-Diaminobenzidine) chromogenic reagent was added to develop, and hematoxylin was added for staining. In the negative control group, phosphate-buffered solution (PBS) was used instead of the primary antibody. The IHC score of each slide was calculated by multiplying the intensity of staining by the average percentage of positive cells[27]. The staining intensity scores were classified as follows: colorless (no staining), 0; light yellow (weak staining), 1; yellow-brown (moderate staining), 2; and brown (strong staining), 3. The average percentages of positive cells were as follows: 1 = 1%-25%, 2 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%. Based on the Statistical Package for Social Sciences software version 20.0, the optimal cutoff IHC score was set as 6. In the final analysis, samples with a score of ≥ 6 were classified as CIP2Ahigh expression, whereas those with a score of < 6 were classified as CIP2Alow expression.
The Cancer Genome Atlas (TCGA) and The National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) were used to obtain data on CIP2A mRNA expression. Moreover, R software and the Bioconductor project were used to analyze and process data on CIP2A mRNA expression. Data on the mRNA expression of CIP2A were processed with log2 and standardized with R software. For the survival analysis of CIP2A, the online tool Kaplan–Meier plotter (http://www.kmplot.com/gastric) was used to assess the prognostic value of CIP2A in GC patients. The Kaplan–Meier plotter database contains 875 patients with clinicopathologic information about GC from the National Center for Biotechnology Information Gene Expression Omnibus 208853 dataset. Moreover, the survival data were analyzed online. The Kaplan–Meier survival curves were drawn using GraphPad (GraphPad Prism 6.0, La Jolla, CA, United States).
Human GC cell lines MKN-45 and AGS, as well as normal human gastric epithelial cells (GES-1) (Chinese Academy of Sciences, China), were cultured in RPMI-1640 (Hyclone Laboratories Inc., United States) supplemented with 1% penicillin and streptomycin (North China Pharmaceutical Company, Inc., China) and 10% fetal bovine serum (Hyclone Laboratories Inc., United States). Oxaliplatin was purchased from the Hengrui Medicine Co., Ltd. (Jiangsu, China). Further, Invitrogen Inc. (Carlsbad, CA, United States) provided the unique CIP2A small interfering RNA (siRNA) and negative control. The CIP2A siRNA sequence is 5’-GACAACUGUCAAGUGUACCACUCUU-3’[28]. To deliver the siRNA into the MKN-45 and AGS cells, LipofectamineTM 2000 (Invitrogen Inc., Carlsbad, CA, United States) was used based on the manufacturer’s instructions. In addition, MK-2206 was acquired from Cell Signaling Inc. (InvivoGen, San Diego, CA, United States).
Cell proliferation was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium (MTT) assay. Cultured cells were separated in 96-well plates for 12 h (5000 cells/well). Moreover, 20 μL/well of the MTT reagent was added to each sample after treatment. The sample was then incubated at 37 °C for 4 h before being washed with PBS. Following that, 200 μL of dimethyl sulfoxide was added. The 490-nm optical density was evaluated. The rate of cell proliferation was calculated as the score of surviving cells. Cell viability was measured as a percentage of survival (control group: 100%).
Total RNA was extracted in an RNase-free environment using TRIzol reagent (Invitrogen, United States), and cDNA was obtained using PrimeScript™ RT Master Mix (Takara Biotechnology Co., China) according to the manufacturer’s instructions. Real-time quantitative reverse transcription (RT-q) PCR was performed using the 7500 Fast PCR System (Applied Biosystems, CA, United States) with SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., China). The reactions were carried out using the 20-μL reaction system per the manufacturer’s instructions. Moreover, glyceraldehyde-3-phosphate dehydrogenase was used as the housekeeping gene. The primer sequences were as follows: CIP2A (accession no. NM020890) sense 5’-GGCACTTGGAGGTAATTTCT-3’, anti-sense 5’-CTGGTTTCAATGTCTACTG
PBS and radio immunoprecipitation assay lysis buffer (Beyotime Biotechnology, China) was used to treat the cells, which were supplemented with 1 mmol/L phenylmethanesulfonyl fluoride. They were centrifuged for 15 min at 12000 × g at 4 °C. The supernatant was then collected, and the protein concentration was determined using the BCA protein assay (Beyotime Biotechnology, China). Using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, an equal amount of sample (50 μg) was isolated and moved into the polyvinylidene fluoride membrane. The samples were then blocked with 5% nonfat milk and incubated with the following primary antibodies: CIP2A (2G10-3B5, Santa Cruz Biotechnology, United States), phospho-Akt (Ser473, Santa Cruz Biotechnology, United States), Akt (Cell Signaling Technology, Inc., United States), and β-actin (Zhongshan Golden Bridge Biotech, China). β-actin was used as an internal control. After that, samples were incubated with secondary antibodies (Zhongshan Golden Bridge Biotech, China) (1:5000). The SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., United States) was used to obtain the results. Moreover, data were analyzed using Quantity One (Bio-Rad Inc.).
After 48 h of siRNA treatment, the GC cells were treated with a specific dose of oxaliplatin (2 μg/mL) for 24 h. They were then collected, washed with PBS, and suspended with propidium iodide annexin V-fluorescein 5-isothiocyanate (BD Pharmingen, United States) in the binding buffer of annexin V. Fluorescence was detected using a flow cytometer (BD Biosciences, San Jose, CA, United States) after 20 min in the dark at room temperature. The cells were then counted using Flow Cytometry Cell Quest, and data were analyzed using Magnetic Cell Sorting Quant.
All examinations were performed in triplicate, and the results were presented as mean ± standard deviation. To compare absorbance values and percentages of apoptotic and viable cells, the two-tailed Student’s t test was used. The Pearson’s χ2 test was used to examine the relationship between CIP2A and clinicopathological features. Moreover, survival analysis was conducted using the Kaplan–Meier method, and the difference in survival curves was examined using the log-rank test. We also ran univariate and multivariate Cox proportional hazards regression analyses. All statistical analyses were performed using GraphPad Prism 6.0 and the Statistical Package for the Social Sciences software version 20.0 (IBM, Armonk, United States). SigmaPlot 10.0 (Systat Software Inc., United States) was utilized to display the results. Further, P values of < 0.05 (aP < 0.05, bP < 0.01, cP < 0.001) were considered significant.
To investigate the clinical value of CIP2A in GC, we assessed the expression of CIP2A in 108 pairs of GC tissue and matched normal tissue samples using IHC and hematoxylin and eosin staining (Figure 1A). CIP2A was found in the nucleus and, more specifically, the cytoplasm of GC cells (Figure 1A). CIP2A expression was found to be significantly higher in tumor tissues. Meanwhile, CIP2A expression was absent or significantly reduced in adjacent normal gastric tissues (Figure 1A). CIP2A expression in GC tissues was significantly higher than that in adjacent normal gastric tissues (Figure 1B). RT-qPCR and Western blot analysis were performed to detect the expression of CIP2A in 18 pairs of fresh GC tissue and adjacent normal gastric tissue samples. Results showed that the expression of CIP2A in GC tissues was significantly higher than that in adjacent normal gastric tissues (Figure 1C and D). This finding supported the IHC analysis results of paraffin-embedded tissues (Figure 1A). The mRNA expression of CIP2A was then assessed using The Cancer Genome Atlas data from paired (n = 27, P < 0.05, Figure 1E) and unpaired databases (n = 210, normal samples; n = 414, tumor samples, P < 0.05, Figure 1F). CIP2A expression was significantly higher in GC tissues than in adjacent normal gastric tissues (Figure 1).
We further investigated the relationship between CIP2A expression and clinicopathological features in 108 patients with GC. As shown in Table 1, high CIP2A expression was associated with tumor size (P = 0.015), T stage (P = 0.019), N stage (P = 0.000), and Tumor Node Metastasis stage (P = 0.023) but not with sex (P = 0.184), age (P = 0.676), tumor location (P = 0.756), and histological grade (P = 0.973).
According to these findings, CIP2A expression was significantly higher in GC tissues. CIP2A could thus be an oncogene that promotes tumor development in GC.
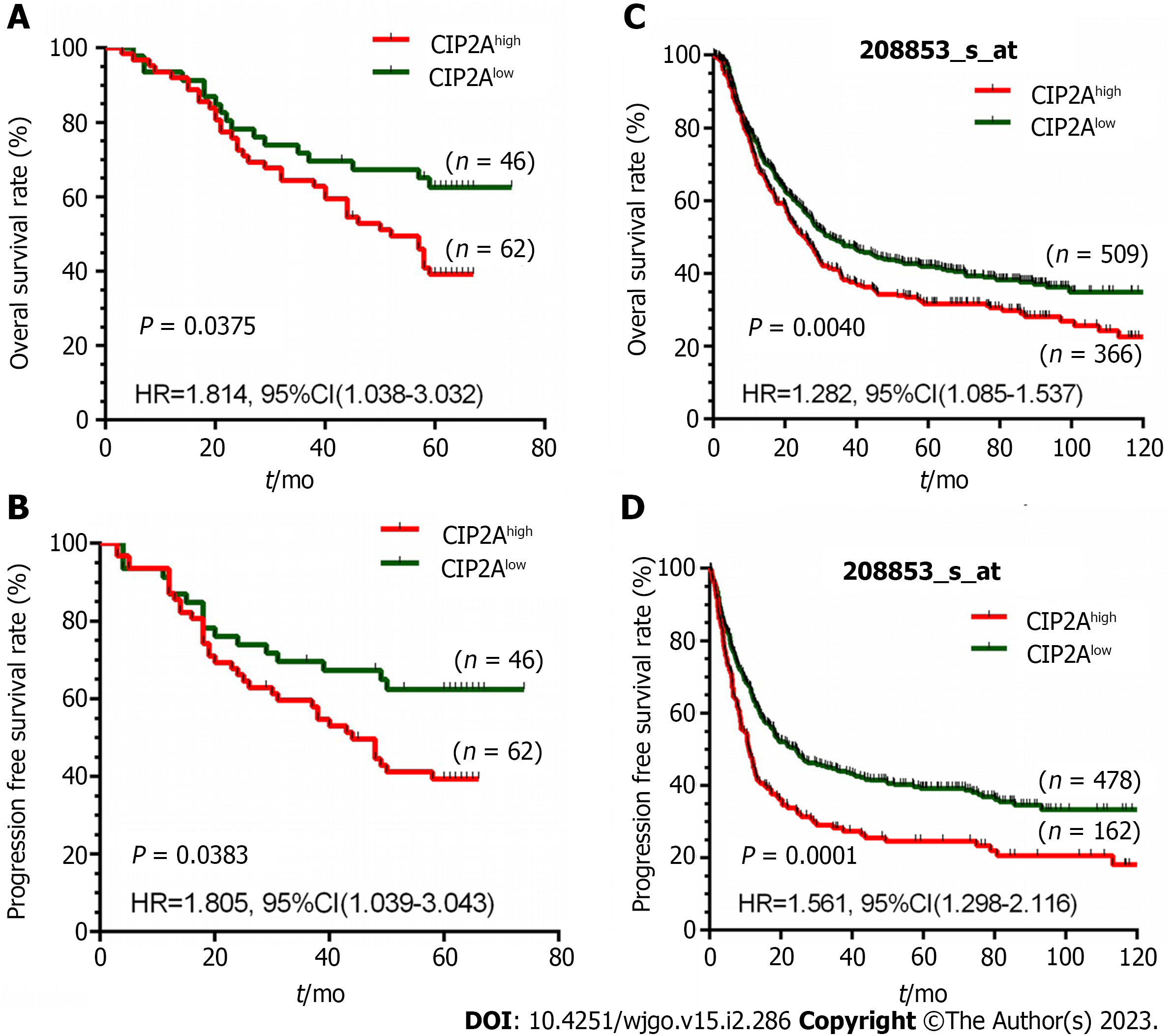
We investigated the prognostic value of CIP2A expression in GC patients. In 108 GC patients followed up for a median of 58 mo (range: 3–74 mo), 61 (56.48%) died and 47 (43.52%) survived. According to the Kaplan–Meier survival analysis, patients with high CIP2A expression had a shorter overall survival (hazard ratio: = 1.814, 95% confidence interval = 1.038–3.032, P = 0.0375, Figure 2A) and progression-free survival (hazard ratio = 1.805, 95% confidence interval = 1.039–3.043, P = 0.0383, Figure 2B) than those with low CIP2A expression. Based on the National Center for Biotechnology Information Gene Expression Omnibus 208853 dataset, a similar trend was discovered, confirming the prognostic significance of CIP2A expression in patients with GC (Figure 2C and D).
The Cox proportional risk regression model was used to evaluate CIP2A expression and the prognostic factors in GC patients. Univariate analysis showed that tumor size, T stage, N stage, Tumor Node Metastasis stage, and CIP2A expression (Tables 2 and 3) were significantly correlated with prognosis in patients with GC. According to the multivariate analysis, a significant increase in CIP2A expression was associated with lower overall survival and progression-free survival rates in patients with GC. Further, the expression of CIP2A was an independent prognostic factor in patients with GC (P = 0.046 and 0.042, Tables 2 and 3). As a result, CIP2A could be an important oncoprotein in the deve
| Prognostic variables | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95% confidence interval | P value | Hazard ratio | 95% confidence interval | P value | |
| Sex | 0.99 | 0.584–1.679 | 0.97 | - | - | - |
| Age | 1.372 | 0.820–2.296 | 0.229 | - | - | - |
| Tumor location | 0.925 | 0.545–1.569 | 0.772 | - | - | - |
| Histological grade | 0.736 | 0.420–1.288 | 0.283 | - | - | - |
| Tumor size | 2.417 | 1.399–4.176 | 0.004a | 0.87 | 0.504–1.504 | 0.619 |
| T stage | 3.184 | 1.687–6.007 | 0.000a | 2.651 | 1.295–5.423 | 0.008a |
| N stage | 2.034 | 1.196–3.457 | 0.009a | 1.414 | 0.709–2.818 | 0.325 |
| TNM stage | 1.946 | 1.168–3.243 | 0.011a | 0.912 | 0.469–1.774 | 0.786 |
| CIP2A expression | 2.319 | 1.288–3.927 | 0.003a | 1. 802 | 1.012–3.210 | 0.046a |
| Prognostic variables | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95% confidence interval | P value | Hazard ratio | 95% confidence interval | P value | |
| Sex | 0.973 | 0.574–1.650 | 0.919 | - | - | - |
| Age | 1.363 | 0.814–2.281 | 0.239 | - | - | - |
| Tumor location | 0.913 | 0.538–1.548 | 0.735 | - | - | - |
| Histological grade | 0.73 | 0.417–1.278 | 0.27 | - | - | - |
| Tumor size | 2.43 | 1.407–4.197 | 0.004a | 0.869 | 0.504–1.499 | 0.614 |
| T stage | 3.15 | 1.669–5.943 | 0.000a | 2.644 | 1.294–5.420 | 0.009a |
| N stage | 2.044 | 1.202–3.476 | 0.008a | 1.451 | 0.728–2.891 | 0.29 |
| TNM stage | 1.917 | 1.150–3.194 | 0.013a | 0.88 | 0.452–1.713 | 0.88 |
| CIP2A expression | 2.309 | 1.282–3.911 | 0.003a | 1.821 | 1.021–3.247 | 0.042a |
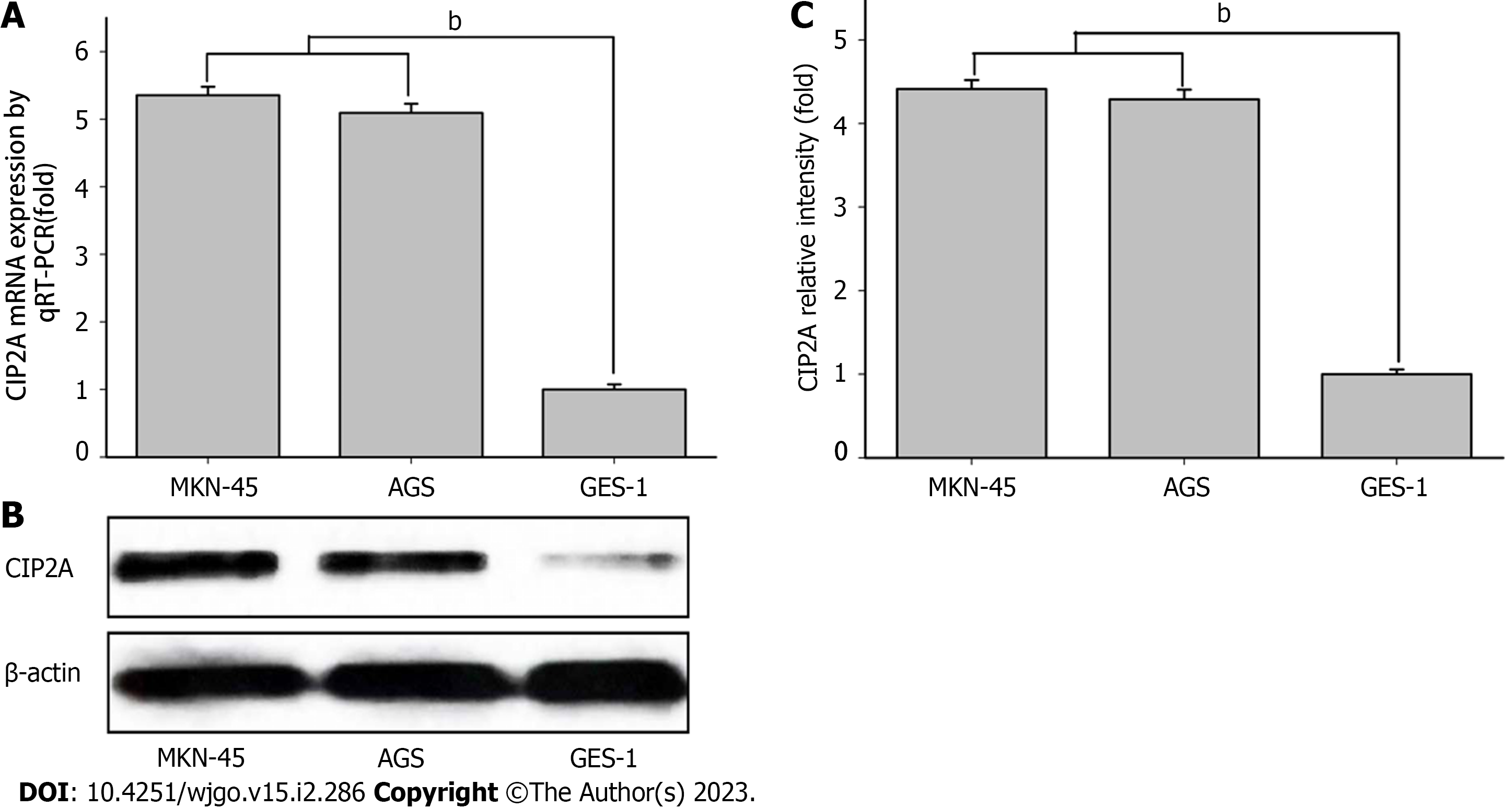
CIP2A has been shown to promote the proliferation of the human GC cell lines AGS and MKN-45. However, its molecular mechanism remains unknown. To determine the expression of CIP2A in AGS, MKN-45, and GES-1, RT-qPCR and immunoblotting were performed. The mRNA expression of CIP2A in human GC cell lines AGS and MKN-45 was higher than in the human GC cell line GES-1 (Figure 3A). According to the protein expression analysis, CIP2A expression was higher in the human GC cell lines AGS and MKN-45 (Figure 3B and C). Due to their high expression of CIP2A, AGS and MKN-45 were selected.
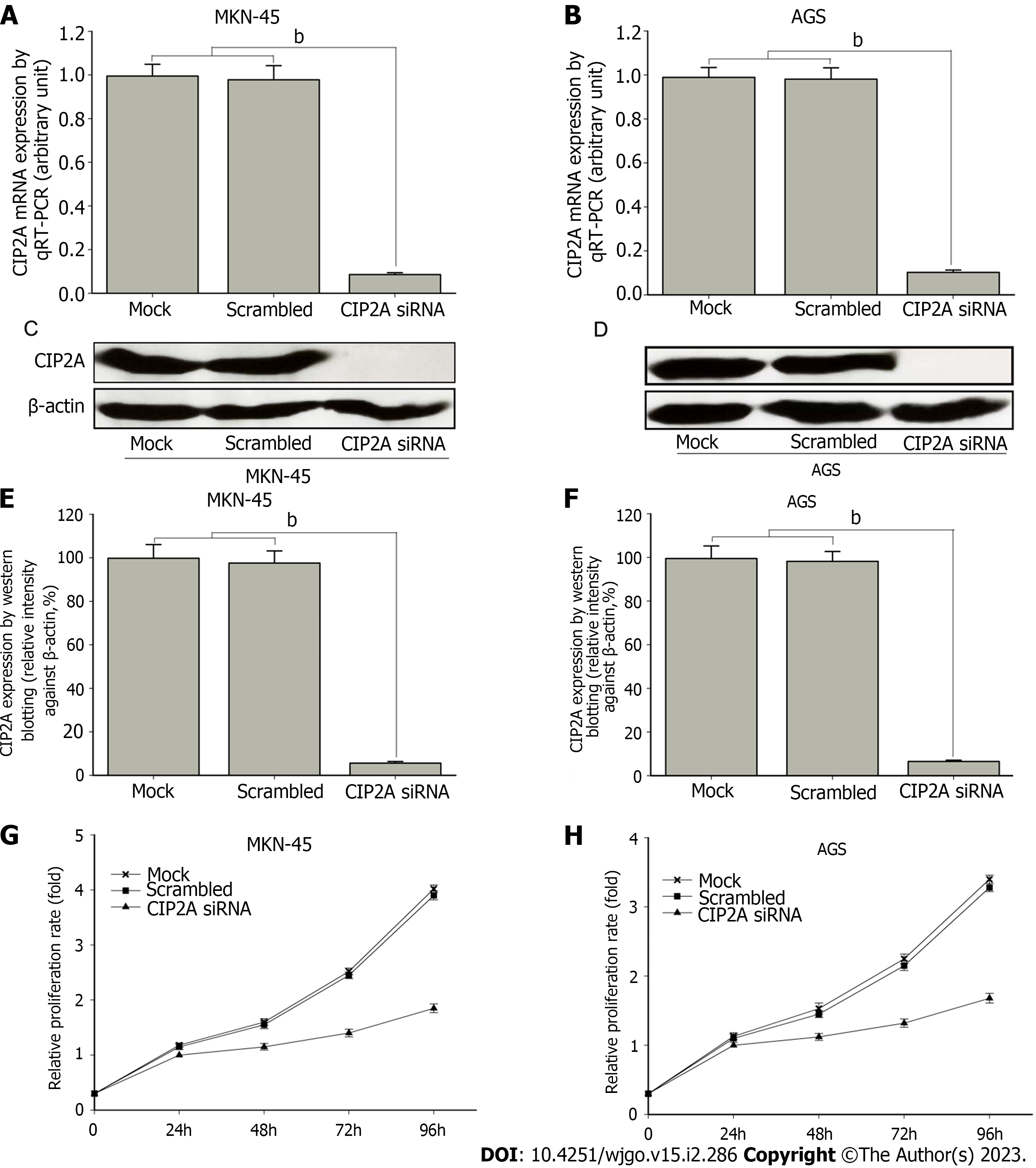
To assess the role of CIP2A in the growth of human GC cell lines, siRNA targeting CIP2A was transfected into AGS and MKN-45. RT-qPCR revealed that the mRNA expression of CIP2A knockdown in MKN-45 cells was 90% lower than that of scrambled siRNA and mock cells (P < 0.01). Meanwhile, the mRNA expression of CIP2A knockdown in AGS cells was 88% lower than that of scrambled siRNA and mock cells (P < 0.01). There was no statistically significant difference between the last two samples (Figure 4A and B). These findings were confirmed by immunoblotting (Figure 4C-F). Based on the MTT assay, the downregulation of CIP2A expression significantly reduced the proliferation of AGS and MKN-45 cells. When compared to scrambled siRNA and mock cells, CIP2A siRNA significantly reduced the rate of AGS and MKN-45 cell proliferation (P < 0.05) (Figure 4G and H).
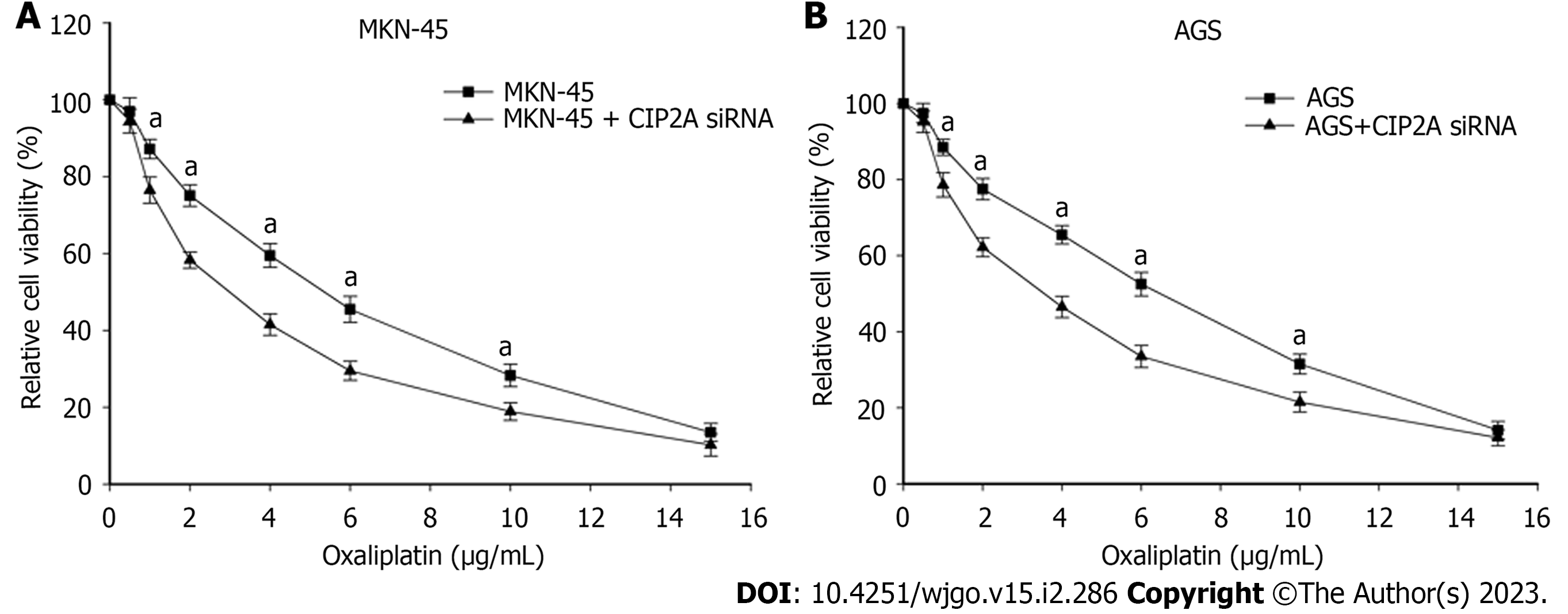
CIP2A may be overexpressed in human GC cells. Thus, there could be a correlation between CIP2A expression and oxaliplatin sensitivity. To test this theory, CIP2A knockdown cells were treated with oxaliplatin at various concentrations. CIP2A knockdown increased susceptibility to oxaliplatin significantly (Figure 5A and B). The half maximal inhibitory concentrations of oxaliplatin in CIP2A-downregulated MKN-45 and AGS cells were 2.9 μg/mL and 3.6 μg/mL, respectively. In the control sample, the concentrations were 5.3 μg/mL and 6.2 μg/mL, respectively (P < 0.05). The susceptibility of MKN-45 and AGS cells to oxaliplatin were strengthened by 45% and 42%, respectively. Thus, cells with downregulated CIP2A expression were more sensitive to oxaliplatin treatment.
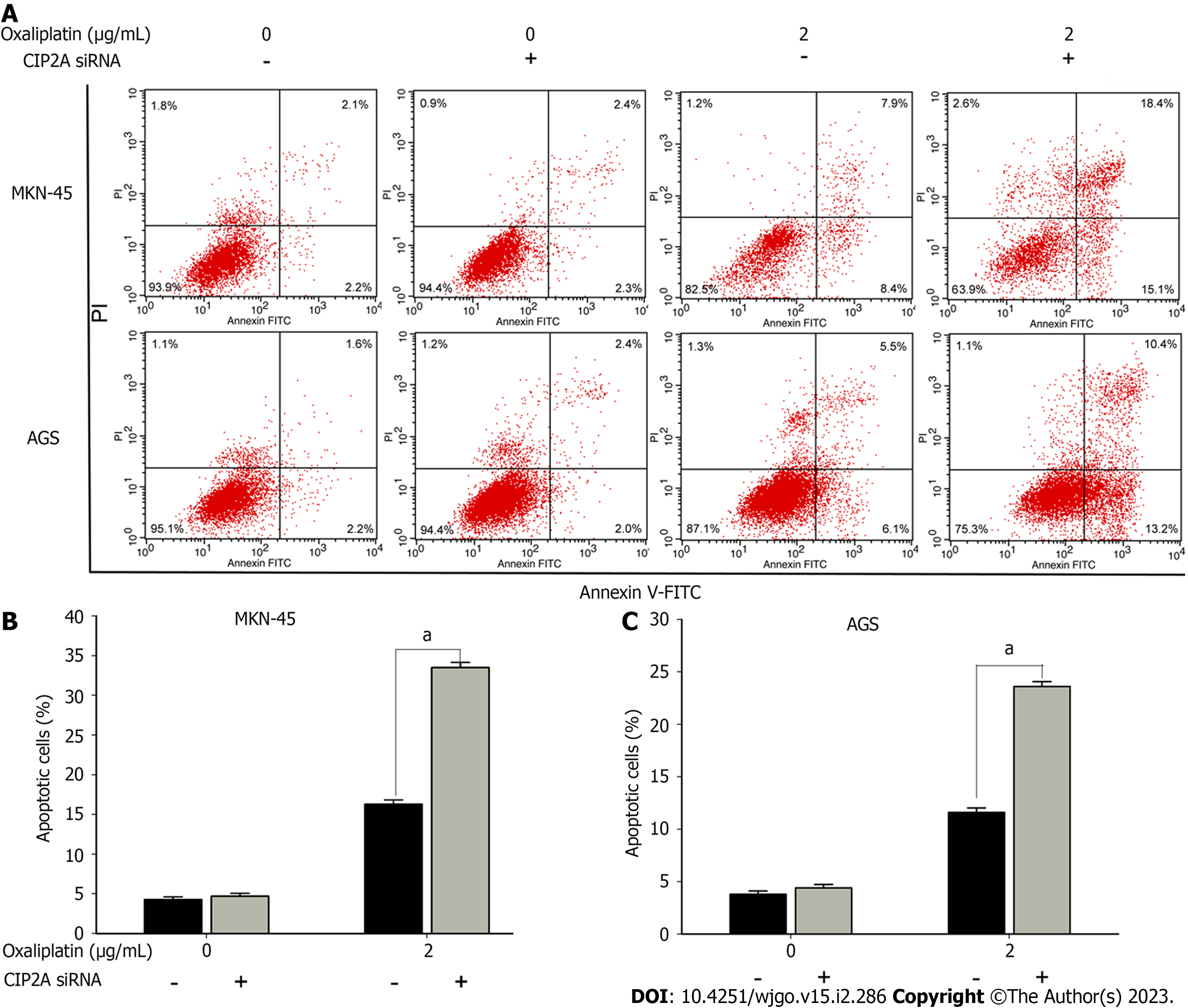
Flow cytometric analysis was used to determine whether CIP2A knockdown promoted cell apoptosis. Following oxaliplatin treatment, propidium iodide and annexin V staining were performed. Interestingly, CIP2A knockdown significantly enhanced apoptosis caused by oxaliplatin (2 μg/mL). The apoptosis rates of CIP2A-downregulated MKN-45 and AGS cells added to oxaliplatin exhibited 33.5% and 23.6%, respectively. Control rates in oxaliplatin-treated cells were 16.3% and 11.6%, respectively (P < 0.05). In addition, siRNA transfection of CIP2A did not increase the apoptosis rate (Figure 6A-C). As a result, CIP2A knockdown caused cell apoptosis. Hence, high CIP2A expression could be a factor of oxaliplatin resistance in GC cells.
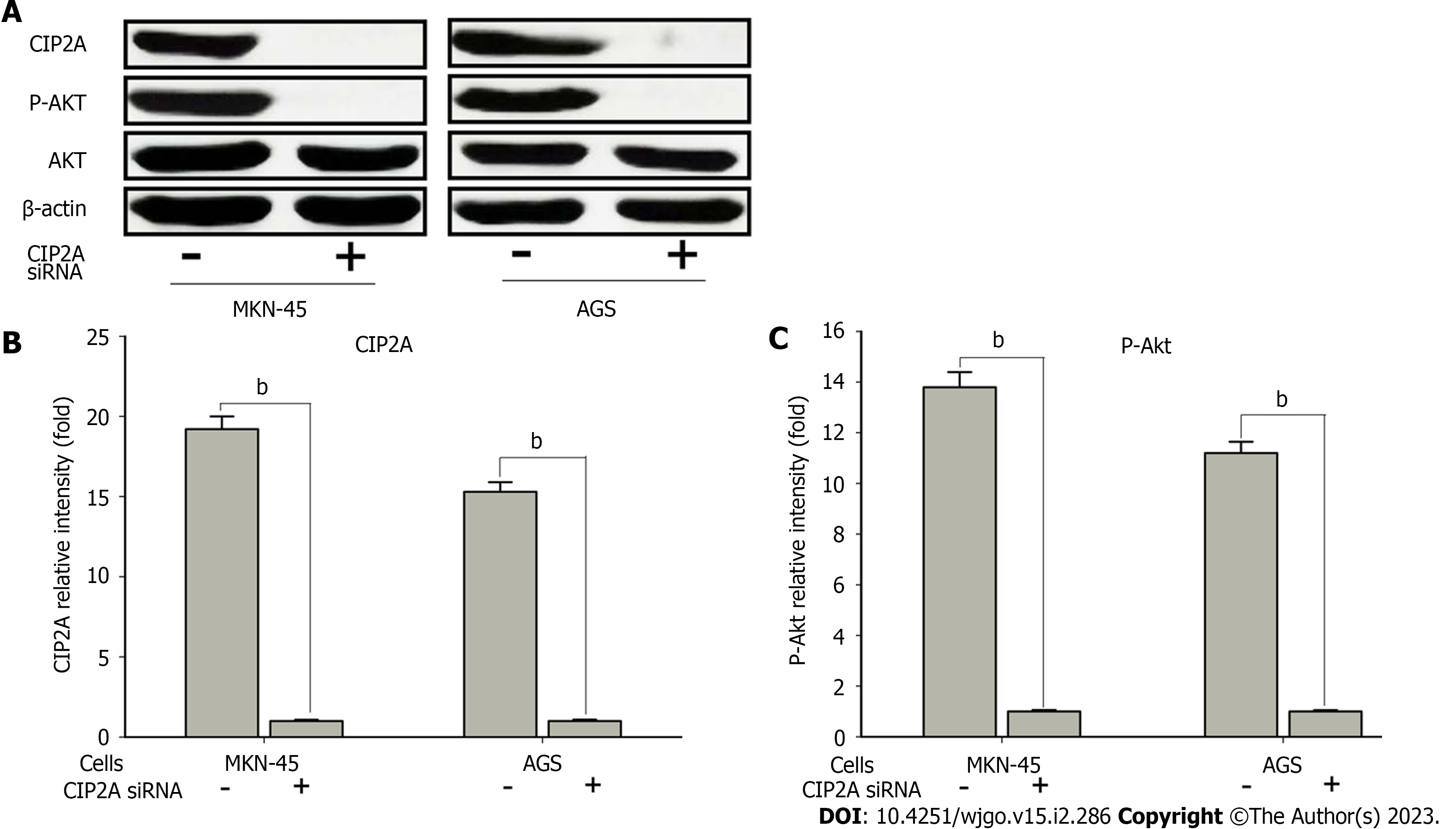
To validate the potential mechanisms by which CIP2A promoted the chemoresistance of human GC cells, the protein expression and phosphorylation level of Akt signaling in CIP2A knockdown AGS and MKN-45 cells were evaluated. According to the findings, CIP2A knockdown significantly reduced Akt phosphorylation levels (Ser473). However, it had no effect on the level of Akt protein expression (Figure 7A-C). Based on these findings, CIP2A influenced Akt activity in human GC cells, and CIP2A knockdown decreased cell proliferation and increased sensitivity to oxaliplatin in human GC cells by decreasing the Akt activity.
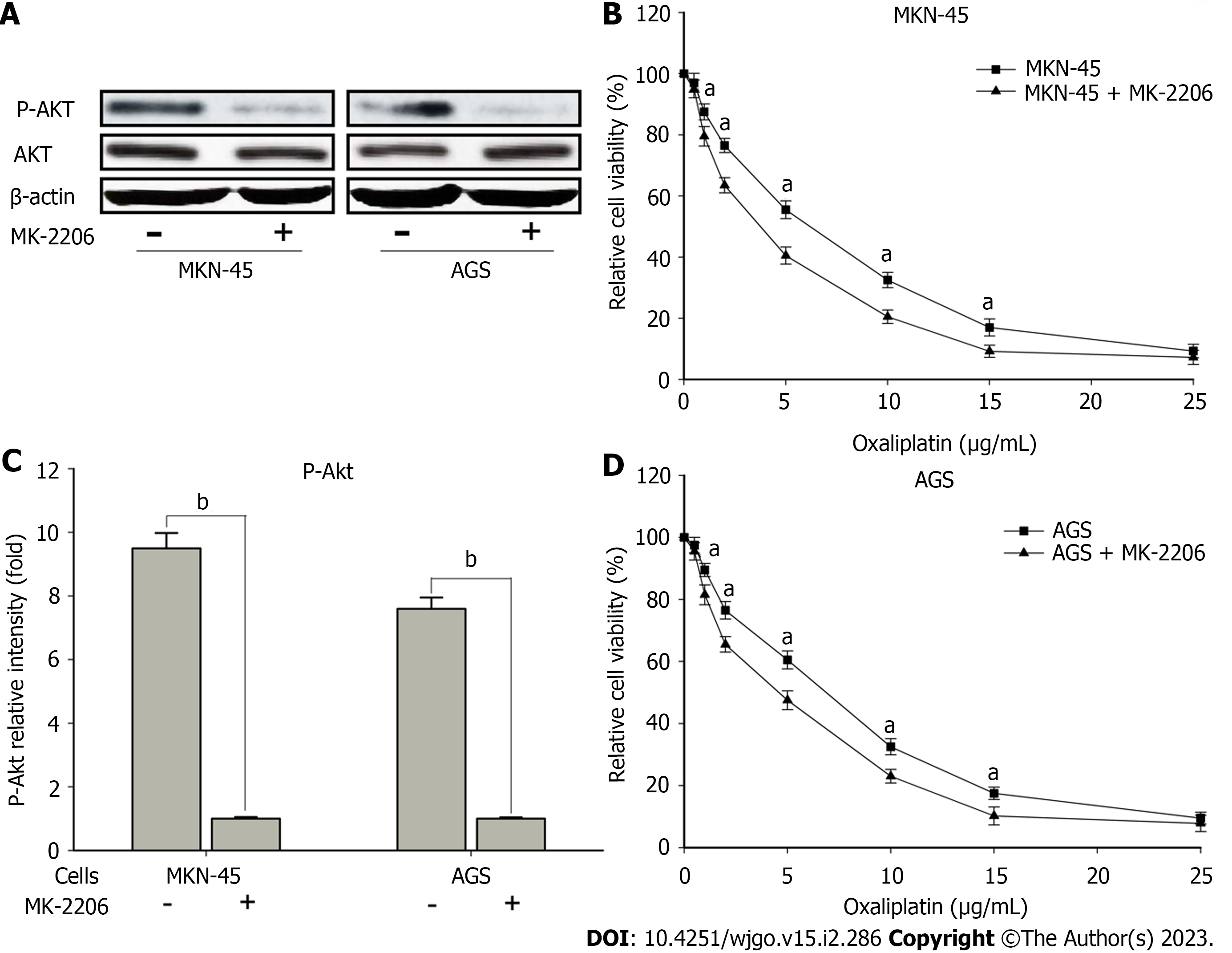
Moreover, to validate the effects of Akt signaling in CIP2A with respect to sensitivity to oxaliplatin, MK-2206, an allosteric Akt inhibitor, was used to pretreat the high expression CIP2A human GC cell line. The expression of the indicated proteins and sensitivity to oxaliplatin were assessed using immunoblotting and the MTT assay, respectively. Pretreatment with MK-2206 reduced p-Akt levels (Figure 8A and B) while increasing sensitivity to oxaliplatin (Figure 8C and D) in MKN-45 and AGS cells. Thus, Akt signaling may play a role in sensitizing CIP2A overexpression in human GC cells exposed to oxaliplatin.
When first diagnosed, most patients have middle- and late-stage GC. The only curative treatment option for gastric tumors is surgical resection. However, patients frequently relapse after resection. Therefore, after 1B resection, combination therapy has become the standard treatment for advanced-stage disease[29]. As the first-line chemotherapy regimen, a platinum–fluoropyrimidine-based treatment is often used[30]. Oxaliplatin, as the third-generation platinum derivative, has been used successfully to treat GC[31,32]. According to the CLASSIC trial, XELOX (capecitabine and oxaliplatin) is superior to observation alone after D2 radical gastrectomy. Hence, chemotherapy is effective[33]. Although patients initially respond well to oxaliplatin, they eventually develop resistance[34,35]. Tumor cells can gain resistance to the cytotoxic effects of oxaliplatin, similar to other anticancer drugs[36]. However, its specific molecular mechanism remains unknown, and it must be investigated further.
CIP2A promotes cell proliferation and tumorigenesis in numerous types of tumors by maintaining c-Myc[18,22,37]. In addition, CIP2A overexpression is correlated with a poor prognosis[21,37]. Interes
Using The Cancer Genome Atlas data analysis, we discovered that the expression of CIP2A in GC tissues was significantly higher than that in adjacent normal gastric tissues. We also confirmed consistent results in GC tissue specimens. Survival analysis revealed that CIP2A expression was significantly correlated with overall survival and progression-free survival in patients with GC. Moreover, CIP2A expression in GC cells was significantly higher compared to GES-1 cells. CIP2A overexpression has previously been observed in several GC cells[38], and this finding is similar to the current study. As a result, MKN-45 and AGS were studied further because they have high CIP2A expression[18,39-41].
We performed siRNA knockdown of CIP2A expression to investigate its biological function in GC cells. Results showed that CIP2A silencing reduced the growth rate of MKN-45 and AGS cells, indicating that CIP2A plays an important role in GC cell proliferation. A similar finding has been reported in our previous studies[42]. To investigate the association between CIP2A expression and drug sensitivity, we knocked down CIP2A in GC cells and tested their sensitivity to oxaliplatin treatment. Previous studies have shown that the knockdown of CIP2A significantly increased the susceptibility of GC cells to oxaliplatin. Based on some reports, CIP2A can promote the proliferation of colon cancer cells, and CIP2A knockdown significantly increased sensitivity to oxaliplatin in colon cancer cells[43,44]. As a result, GC cells with CIP2A expression downregulation were more sensitive to oxaliplatin treatment.
Oxaliplatin has the ability to cause apoptosis in GC cells[45,46]. Nevertheless, the underlying mechanism is unknown. Previous studies have shown that CIP2A plays an important role in lung cancer cell apoptosis when treated with cisplatin[47]. The biological impact of CIP2A is a common phenomenon in tumor cells. According to a recent study, the downregulation of CIP2A expression in MKN-45 and AGS cells increased apoptosis and oxaliplatin sensitivity, which could be a cause of oxaliplatin resistance in GC. Therefore, CIP2A knockdown made GC cells more susceptible to oxaliplatin-induced apoptosis, enhancing the cytotoxic effect of oxaliplatin.
According to a previous study, inhibiting the Akt pathway sensitizes GC cells to apoptosis caused by cisplatin[48]. Therefore, we discovered that signaling was correlated with the biological behaviors of CIP2A. Since the phosphorylation of Akt is a critical step in phosphoinositide 3-kinase/Akt signaling activation[49], we confirmed its association with CIP2A expression. CIP2A knockdown caused the phosphorylation of Akt in both MKN-45 and AGS cells, according to the findings. Moreover, the chemical inhibition of the Akt signaling pathway increased oxaliplatin sensitivity in human GC cells. Several studies have found that the downregulation of CIP2A expression can improve the efficacy of chemotherapeutic drugs and inhibit Akt signaling in colorectal and lung cancers[26,38]. As a result, the association between CIP2A and the Akt signaling pathway might be involved in the biological functions of GC cells. This interaction could be correlated with oxaliplatin resistance in GC.
A high expression of CIP2A can promote chemoresistance to oxaliplatin in GC, and Akt signaling may play a role in this mechanism. The inhibition of CIP2A significantly improved sensitivity to oxaliplatin in human GC cells. As a result, suppressing CIP2A expression may be an indirect strategy for more effectively treating patients with GC. Nevertheless, further clinical trials on the role of this signaling pathway should be conducted.
Cancerous inhibitor of protein phosphatase 2A (CIP2A) plays a key role in various types of tumors, which may be related to the resistance of gastric cancer (GC) cells to oxaliplatin.
The mechanism of drug resistance in gastric cancer needs to be further studied, and CIP2A expression in GC cells and the mechanism of oxaliplatin resistance may be a breakthrough.
To explore the expression of the CIP2A in human GC cells and its correlation with oxaliplatin resistance.
Immunohistochemistry was used to examine CIP2A expression in GC tissues and adjacent normal tissues. CIP2A gene expression in GC cell lines was reduced using small interfering RNA. After confirming the silencing efficiency, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium and flow cytometry assays were used to evaluate cell proliferation and apoptosis, respectively, caused by oxaliplatin treatment. Further, the key genes and protein changes were verified using real-time quantitative reverse transcription PCR and Western blotting, respectively, before and after intervention. For bioinformatics analysis, we used the R software and Bioconductor project. For statistical analysis, we used GraphPad Prism 6.0 and the Statistical Package for the Social Sciences software version 20.0.
High CIP2A expression was associated with tumor size, T stage, lymph node metastasis, Tumor Node Metastasis stage, and poor prognosis. CIP2A knockdown inhibited cell proliferation and significantly increased the susceptibility of GC cells to oxaliplatin. CIP2A regulated the phosphorylation of protein kinase B, and chemical inhibition of the protein kinase B signaling pathway was significantly associated with increased sensitivity to oxaliplatin.
CIP2A expression was closely related to chemotherapy resistance of GC cells. The protein kinase B signaling pathway was correlated with CIP2A-enhanced chemoresistance of human GC cells to oxaliplatin.
Regulation of CIP2A expression may be one of the key points in the treatment of GC chemotherapy resistance.
The authors would like to express their gratitude to Doctor Lei Jiang (The Sixth Department of General Surgery, The First Hospital of Lanzhou University, Gansu, China) for the critical revision of this manuscript. The authors also greatly appreciate the experimental assistance provided by Doctor Yu-Ping Wang (The Department of Gastroenterology, The First Hospital of Lanzhou University, Gansu, China).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aurello P, Italy; Hori T, Japan; Rakić M, Croatia S-Editor: Liu GL L-Editor: Filipodia P-Editor: Cai YX
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11942] [Article Influence: 2985.5] [Reference Citation Analysis (4)] |
| 2. | Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Carlson RW, Anderson BO, Burstein HJ, Carter WB, Edge SB, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Ljung BM, Kiel K, Marks LB, McCormick B, Nabell LM, Pierce LJ, Reed EC, Silver SM, Smith ML, Somlo G, Theriault RL, Ward JH, Winer EP, Wolff AC. Invasive breast cancer. J Natl Compr Canc Netw. 2007;5:246-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, Grivel JC. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 380] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 5. | Xiao C, Qian J, Zheng Y, Song F, Wang Q, Jiang H, Mao C, Xu N. A phase II study of biweekly oxaliplatin plus S-1 combination chemotherapy as a first-line treatment for patients with metastatic or advanced gastric cancer in China. Medicine (Baltimore). 2019;98:e15696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Peng J, Tan C, Zeng X, Liu S. Cost-effectiveness analysis of capecitabine monotherapy vs capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. PloS One. 2018;13:e0199553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel vs fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1648] [Article Influence: 274.7] [Reference Citation Analysis (0)] |
| 8. | Jiang Y, Liu W, Li T, Hu Y, Chen S, Xi S, Wen Y, Huang L, Zhao L, Xiao C, Huang X, Han Z, Liu H, Qi X, Yang Y, Yu J, Cai S, Li G. Prognostic and Predictive Value of p21-activated Kinase 6 Associated Support Vector Machine Classifier in Gastric Cancer Treated by 5-fluorouracil/Oxaliplatin Chemotherapy. EbioMedicine. 2017;22:78-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Yudushkin I. Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Wang S, Liu F, Zhu J, Chen P, Liu H, Liu Q, Han J. DNA Repair Genes ERCC1 and BRCA1 Expression in Non-Small Cell Lung Cancer Chemotherapy Drug Resistance. Med Sci Monit. 2016;22:1999-2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Lu ZN, Shi ZY, Dang YF, Cheng YN, Guan YH, Hao ZJ, Tian B, He HW, Guo XL. Pantoprazole pretreatment elevates sensitivity to vincristine in drug-resistant oral epidermoid carcinoma in vitro and in vivo. Biomed Pharmacother. 2019;120:109478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Qiu N, He YF, Zhang SM, Zhan YT, Han GD, Jiang M, He WX, Zhou J, Liang HL, Ao X, Xia HM, Li J, Yang YY, He ZM, Zou ZZ, Li HS. Cullin7 enhances resistance to trastuzumab therapy in Her2 positive breast cancer via degrading IRS-1 and downregulating IGFBP-3 to activate the PI3K/AKT pathway. Cancer Lett. 2019;464:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Hudis C, Swanton C, Janjigian YY, Lee R, Sutherland S, Lehman R, Chandarlapaty S, Hamilton N, Gajria D, Knowles J, Shah J, Shannon K, Tetteh E, Sullivan DM, Moreno C, Yan L, Han HS. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li J, Zhang Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2017;36:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 15. | Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R, Tang XJ. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol. 2012;18:979-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Luo HY, Wei W, Shi YX, Chen XQ, Li YH, Wang F, Qiu MZ, Li FH, Yan SL, Zeng MS, Huang P, Xu RH. Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep. 2010;23:1735-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Guo Q, Jing FJ, Qu HJ, Xu W, Han B, Xing XM, Ji HY, Jing FB. Ubenimex Reverses MDR in Gastric Cancer Cells by Activating Caspase-3-Mediated Apoptosis and Suppressing the Expression of Membrane Transport Proteins. Biomed Res Int. 2019;2019:4390839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grènman R, Kast J, Kallunki T, Sears R, Kähäri VM, Westermarck J. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 506] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 19. | Khanna A, Pimanda JE. Clinical significance of cancerous inhibitor of protein phosphatase 2A in human cancers. Int J Cancer. 2016;138:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | De P, Carlson J, Leyland-Jones B, Dey N. Oncogenic nexus of cancerous inhibitor of protein phosphatase 2A (CIP2A): an oncoprotein with many hands. Oncotarget. 2014;5:4581-4602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia J, Xu D. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722-3728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C, Westermarck J, Ristimäki A. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Li Y, Wang M, Zhu X, Cao X, Wu Y, Fang F. Prognostic Significance of CIP2A in Esophagogastric Junction Adenocarcinoma: A Study of 65 Patients and a Meta-Analysis. Dis Markers. 2019;2019:2312439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Choi YA, Park JS, Park MY, Oh KS, Lee MS, Lim JS, Kim KI, Kim KY, Kwon J, Yoon DY, Moon EY, Yang Y. Increase in CIP2A expression is associated with doxorubicin resistance. FEBS Lett. 2011;585:755-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Wei L, Qu W, Sun J, Wang X, Lv L, Xie L, Song X. Knockdown of cancerous inhibitor of protein phosphatase 2A may sensitize NSCLC cells to cisplatin. Cancer Gene Ther. 2014;21:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | He X, Wu W, Lin Z, Ding Y, Si J, Sun LM. Validation of the American Joint Committee on Cancer (AJCC) 8th edition stage system for gastric cancer patients: a population-based analysis. Gastric Cancer. 2018;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Reiner A, Spona J, Reiner G, Schemper M, Kolb R, Kwasny W, Függer R, Jakesz R, Holzner JH. Estrogen receptor analysis on biopsies and fine-needle aspirates from human breast carcinoma. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Am J Pathol. 1986;125:443-449. [PubMed] [DOI] [Full Text] |
| 28. | Liu L, Wu N, Wang Y, Zhang X, Xia B, Tang J, Cai J, Zhao Z, Liao Q, Wang J. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J Exp Clin Cancer Res. 2019;38:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Seo S, Ryu MH, Ryoo BY, Park Y, Park YS, Na YS, Lee CW, Lee JK, Kang YK. Clinical significance of MET gene amplification in metastatic or locally advanced gastric cancer treated with first-line fluoropyrimidine and platinum combination chemotherapy. Chin J Cancer Res. 2019;31:620-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 582] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 33. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 776] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 34. | Peinert S, Grothe W, Stein A, Müller LP, Ruessel J, Voigt W, Schmoll HJ, Arnold D. Safety and efficacy of weekly 5-fluorouracil/folinic acid/oxaliplatin/irinotecan in the first-line treatment of gastrointestinal cancer. Ther Adv Med Oncol. 2010;2:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Bang YJ, Kang YK, Ng M, Chung HC, Wainberg ZA, Gendreau S, Chan WY, Xu N, Maslyar D, Meng R, Chau I, Ajani JA. A phase II, randomised study of mFOLFOX6 with or without the Akt inhibitor ipatasertib in patients with locally advanced or metastatic gastric or gastroesophageal junction cancer. Eur J Cancer. 2019;108:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Lin L, Li X, Pan C, Lin W, Shao R, Liu Y, Zhang J, Luo Y, Qian K, Shi M, Bin J, Liao Y, Liao W. ATXN2L upregulated by epidermal growth factor promotes gastric cancer cell invasiveness and oxaliplatin resistance. Cell Death Dis. 2019;10:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Qu W, Li W, Wei L, Xing L, Wang X, Yu J. CIP2A is overexpressed in esophageal squamous cell carcinoma. Med Oncol. 2012;29:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Zhang Y, Huang P, Liu X, Xiang Y, Zhang T, Wu Y, Xu J, Sun Z, Zhen W, Zhang L, Si Y, Liu Y. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci. 2018;137:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Zhao D, Liu Z, Ding J, Li W, Sun Y, Yu H, Zhou Y, Zeng J, Chen C, Jia J. Helicobacter pylori CagA upregulation of CIP2A is dependent on the Src and MEK/ERK pathways. J Med Microbiol. 2010;59:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Zhao Y, Li Y, Han J, Liu T, Guan Q, Zhao P, Guo L, Liu K, He D. Helicobacter pylori enhances CIP2A expression and cell proliferation via JNK2/ATF2 signaling in human gastric cancer cells. Int J Mol Med. 2014;33:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Li X, Yuan J, Cao Q, Xie A, Chen J. MicroRNA3835p inhibits the proliferation and promotes the apoptosis of gastric cancer cells by targeting cancerous inhibitor of PP2A. Int J Mol Med. 2020;46:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Mu R, Gao YP, Dong J, Zhu L, Ma Y, Li YH, Zhang HQ, Han D, Zhang Y, McInnes IB, Zhang J, Shen B, Yang G, Li ZG. A Cytomegalovirus Peptide-Specific Antibody Alters Natural Killer Cell Homeostasis and Is Shared in Several Autoimmune Diseases. Cell Host Microbe. 2016;19:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Liu CY, Hsu CC, Huang TT, Lee CH, Chen JL, Yang SH, Jiang JK, Chen WS, Lee KD, Teng HW. ER stress-related ATF6 upregulates CIP2A and contributes to poor prognosis of colon cancer. Mol Oncol. 2018;12:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Teng HW, Yang SH, Lin JK, Chen WS, Lin TC, Jiang JK, Yen CC, Li AF, Chen PC, Lan YT, Lin CC, Hsu YN, Wang HW, Chen KF. CIP2A is a predictor of poor prognosis in colon cancer. J Gastrointest Surg. 2012;16:1037-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Li Q, Ren L, Zhang Y, Gu Z, Tan Q, Zhang T, Qin M, Chen S. P38 Signal Transduction Pathway Has More Cofactors on Apoptosis of SGC-7901 Gastric Cancer Cells Induced by Combination of Rutin and Oxaliplatin. Biomed Res Int. 2019;2019:6407210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Zhang P, Shi L, Zhang T, Hong L, He W, Cao P, Shen X, Zheng P, Xia Y, Zou P. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell Oncol (Dordr). 2019;42:847-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Feng FF, Cheng P, Sun C, Wang H, Wang W. Inhibitory effects of polyphyllins I and VII on human cisplatin-resistant NSCLC via p53 upregulation and CIP2A/AKT/mTOR signaling axis inhibition. Chin J Nat Med. 2019;17:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Zhou L, Wu Y, Guo Y, Li Y, Li N, Yang Y, Qin X. Calycosin Enhances Some Chemotherapeutic Drugs Inhibition of Akt Signaling Pathway in Gastric Cells. Cancer Invest. 2017;35:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Wang C, Lin C, Tao Q, Zhao S, Liu H, Li L. Evaluation of calcium-binding protein A11 promotes the carcinogenesis of hypopharygeal squamous cell carcinoma via the PI3K/AKT signaling pathway. Am J Transl Res. 2019;11:3472-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |