Published online May 15, 2022. doi: 10.4251/wjgo.v14.i5.1057
Peer-review started: December 24, 2021
First decision: March 13, 2022
Revised: March 26, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 15, 2022
Pancreatic accessory spleen (PAS) is an uncommon congenital abnormality of the spleen. Spleen hamartoma (SH) is also rare. Moreover, hamartoma in the PAS has not been reported thus far. We report the first case here.
A 26-year-old male presented with a one-month history of left upper quadrant abdominal pain, and computerized tomography (CT) examination suggested a mass in the pancreas tail. The patient then attended our hospital for diagnosis and treatment. Ultrasonography, CT, and magnetic resonance imaging revealed a solid mass with cystic degeneration growing from the tail of the pancreas. The tumor marker carbohydrate antigen 19-9 (CA19-9) increased to 96.7 U/mL (normal range 0-37 U/mL). An epidermoid cyst in a PAS was considered preoperatively. However, a malignant tumor cannot be ruled out. We performed laparoscopic surgery, and two pancreatic masses were found growing from the pancreatic tail. The two masses were so closely connected that preoperative imaging examinations suggested only one mass. We carefully isolated the masses from the splenic artery and vein. A laparoscopic spleen-preserving distal pancreatectomy was successfully performed. On pathological examination, the masses were well-defined, homogeneous red-tan, 4 × 3, and 4.5 × 1.5 in size, respectively. One of them was cystically degenerated. On microscopical examination, the mass contained unorganized small slit-like vascular channels enclosing red blood cells and lined with plump endothelial cells. No area of cytologic atypia was identified. Focal lymphoid aggregates were found in the intravascular areas. White pulp or fibrosis was not observed. The final diagnosis was pancreatic accessory SH with cystic degeneration. After the operation, CA19-9 was reduced to normal. The patient recovered well, and the 34-mo follow-up period was uneventful.
Here, we report the first case of pancreatic accessory SH. A laparoscopic spleen-preserving distal pancreatectomy was successfully performed. The patient recovered well and had a good prognosis.
Core Tip: Pancreatic accessory spleen (PAS) is an uncommon congenital abnormality of the spleen. Spleen hamartoma (SH) is also rale. Moreover, hamartoma in the PAS has not been reported thus far. Here, we report the first case of pancreatic accessory SH. The tumor marker carbohydrate antigen 19-9 was abnormal. A precise diagnosis was challenging to obtain preoperatively, and a malignant tumor could not be ruled out. We successfully performed laparoscopic spleen-preserving distal pancreatectomy. The patient recovered well and had a good prognosis.
- Citation: Xu SY, Zhou B, Wei SM, Zhao YN, Yan S. Successful treatment of pancreatic accessory splenic hamartoma by laparoscopic spleen-preserving distal pancreatectomy: A case report. World J Gastrointest Oncol 2022; 14(5): 1057-1064
- URL: https://www.wjgnet.com/1948-5204/full/v14/i5/1057.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i5.1057
An accessory spleen is a congenital defect caused by fusion failure of the splenic anlage during embryology. Approximately 10%-15% of the general population experiences this event. The splenic hilum is the most common location of an accessory spleen. Tissue is not commonly found in the pancreas (only 16% of cases)[1]. A pancreatic accessory spleen (PAS) is not always recognized preoperatively. Radiologically, the pancreatic accessory spleen appears to be a well-defined, solitary, and hypervascular lesion. The differential diagnosis includes well-differentiated adenocarcinoma, mucinous cystic neoplasm, neuroendocrine neoplasm, solid pseudopapillary tumor, or metastatic tumor to the pancreas[2]. PAS is a benign lesion and rarely causes any symptoms. It is rarely diagnosed preoperatively, and most cases are identified only after surgical resection.
Splenic hamartoma (SH), which was first described in 1861 by Rokitansky, is a rare benign lesion of the spleen[3]. SH is a very rare benign vascular lesion, with fewer than 200 cases reported in the English literature thus far[4]. SH occurs equally in men and women, and adults are the most affected population[5]. SH consists of disorganized sinusoid-like channels, such as red pulp tissue, the lining cells of which can be highlighted by CD8 immunopositivity. In contrast, no white pulp elements are observed in the lesion[6]. SH is generally a single lesion, and multiple lesions are rare. Patients are usually asymptomatic and diagnosed incidentally, while patients with large hamartomas can have symptoms such as nonspecific abdominal pain, thrombocytopenia, splenomegaly, fever, and night sweats[7]. To date, hamartoma in the PAS has not been reported. Here, we report the first case of pancreatic accessory SH. After laparoscopic spleen-preserving distal pancreatectomy, the patient recovered well and had a good prognosis.
A 26-year-old male attended a local hospital because of left upper quadrant abdominal pain.
Laboratory examination revealed carbohydrate antigen 19-9 (CA19-9) 41.7 U/mL (normal range 0-37 U/mL), and computerized tomography (CT) examination suggested a mass in the pancreatic tail. The patient was then referred to our hospital for diagnosis and treatment.
There was no other significant medical history. There was no relevant history, including past interventions and outcomes.
The patient did not have a history of smoking or drinking alcohol. There was no relevant family history.
The patient’s vital signs were stable. The abdomen was soft and nondistended without evidence of a palpable mass.
Levels of the tumor markers CA19-9 were abnormal, 41.7 U/mL, 96.7 U/mL in the local hospital and our hospital, respectively. Tumor markers alpha-fetoprotein, CA125, and carcinoembryonic antigen were in the normal range. Other blood tests, fecal examinations, and coagulation function were normal.
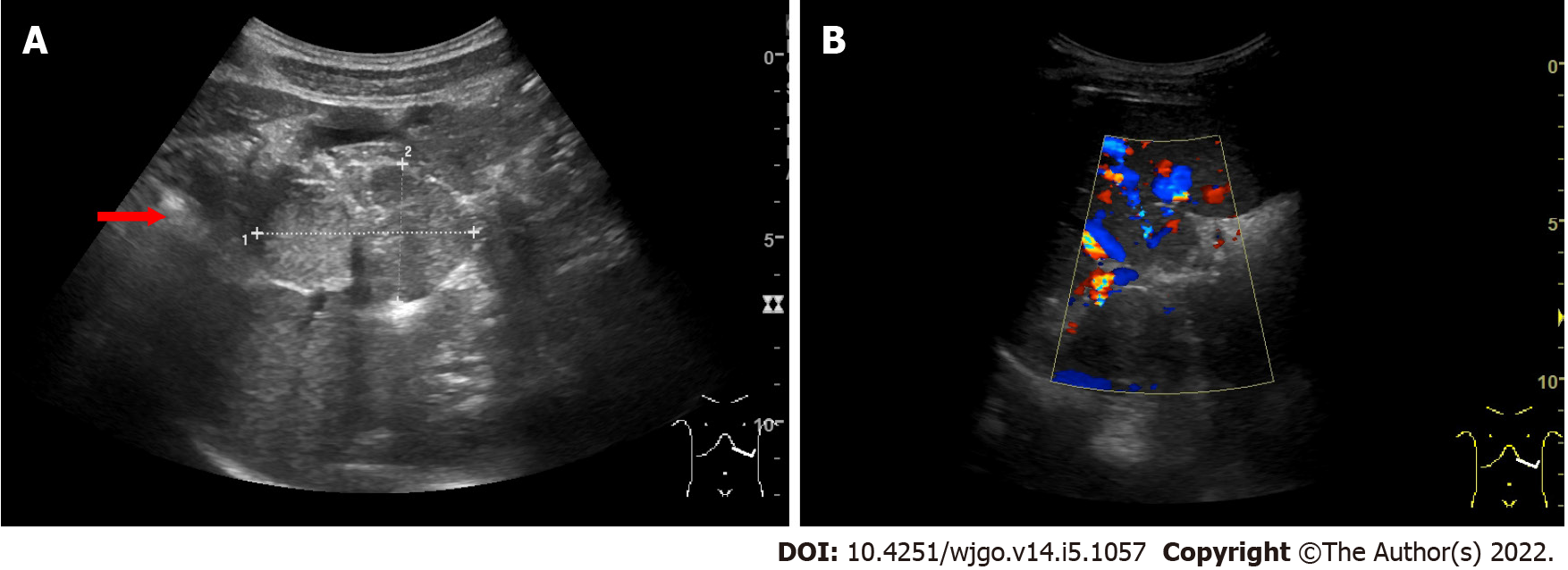
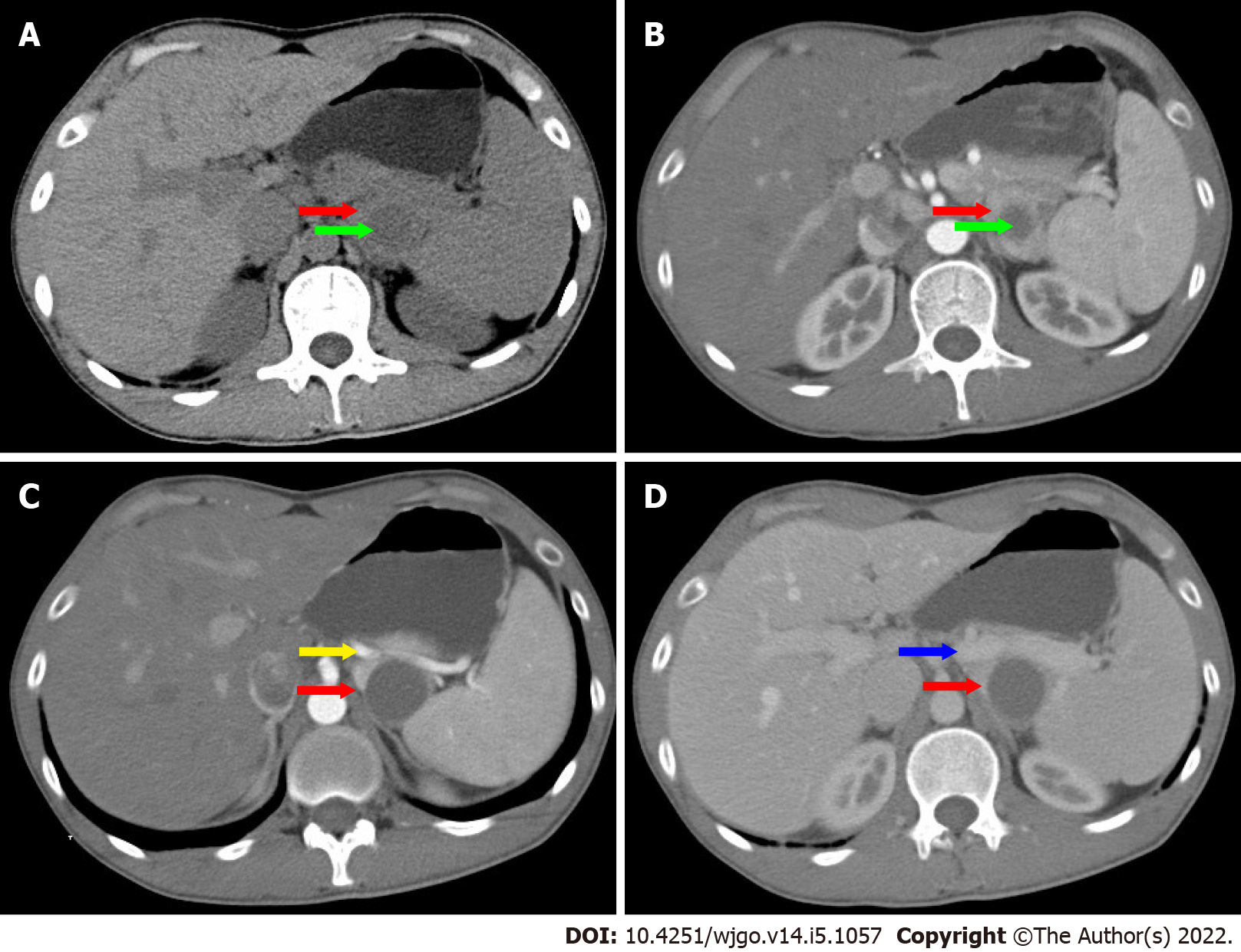
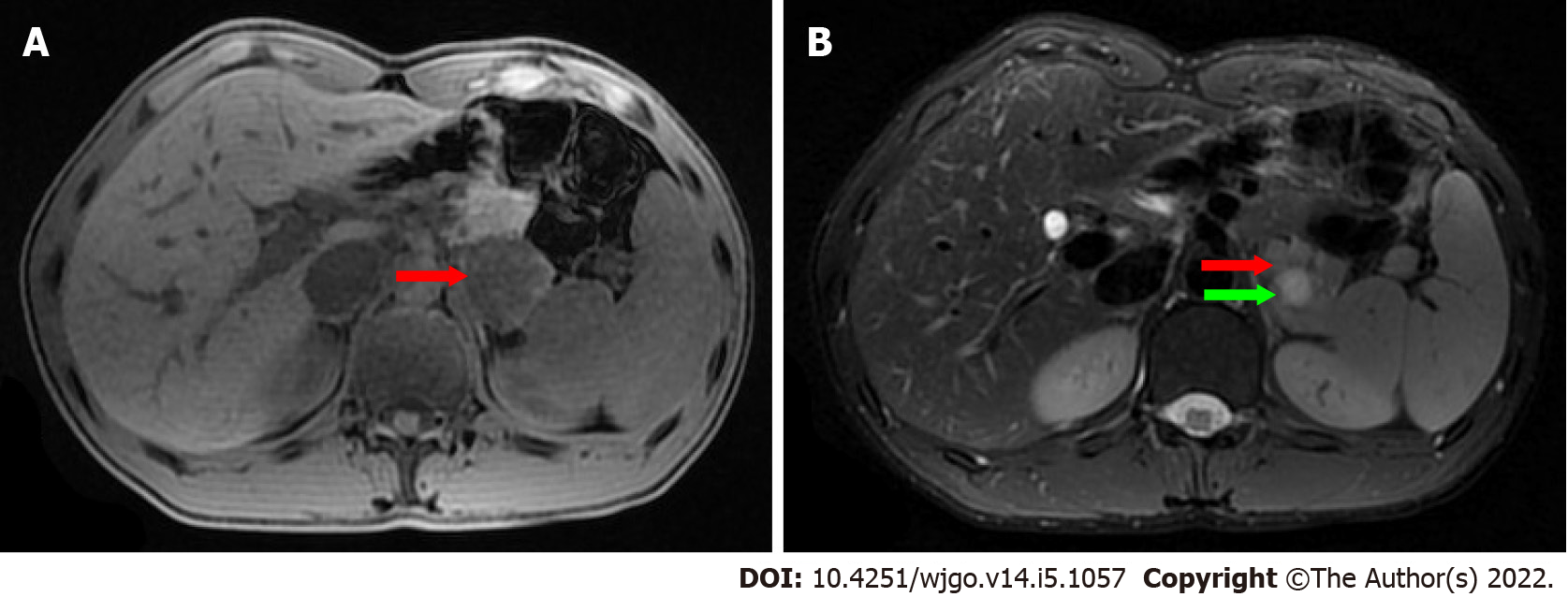
On ultrasonography (US), a well-defined, inhomogeneous echoic mass with a size of 5.9 cm × 3.8 cm was seen growing from the tail of the pancreas with both hyperechoic solid and hypoechoic cystic parts, dominated by a solid part (Figure 1A). Color Doppler flow imaging showed dotted blood flow signals in the mass (Figure 1B). On CT imaging, a well-defined, 6.8 cm × 3.7 cm mass with cystic change was found growing from the tail of the pancreas. The mass was isointense relative to the normal splenic parenchyma on plain scanning (Figure 2A). After enhancement, the enhancement of the parenchyma was similar to that of the spleen (Figure 2B), and the mass was close to both the splenic artery (Figure 2C) and splenic vein (Figure 2D). The mass was isointense on T1-weighted magnetic resonance imaging (MRI) (Figure 3A). On T2-weighted MRI, heterogeneous hyperintensity was found, and the signal in the middle of the tumor was higher (Figure 3B). According to these results, an epidermoid cyst in a PAS was considered preoperatively. However, a malignant tumor cannot be ruled out.
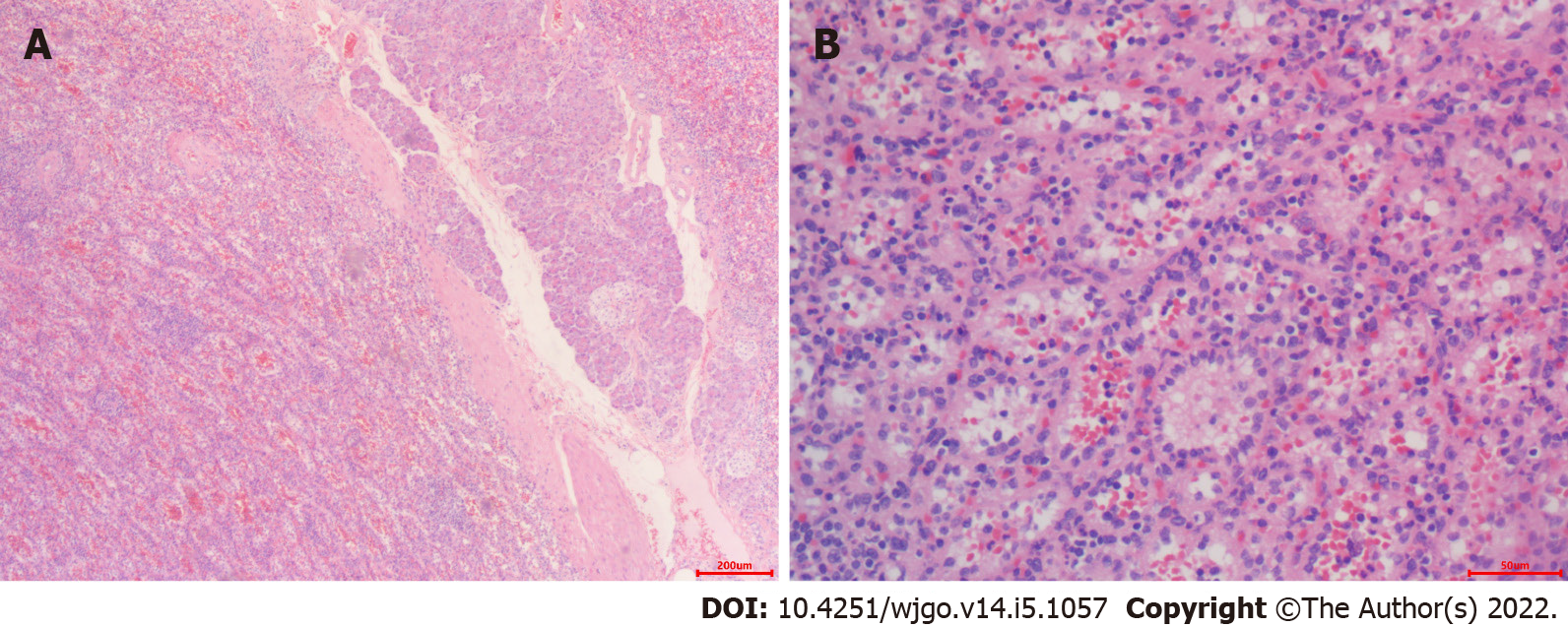
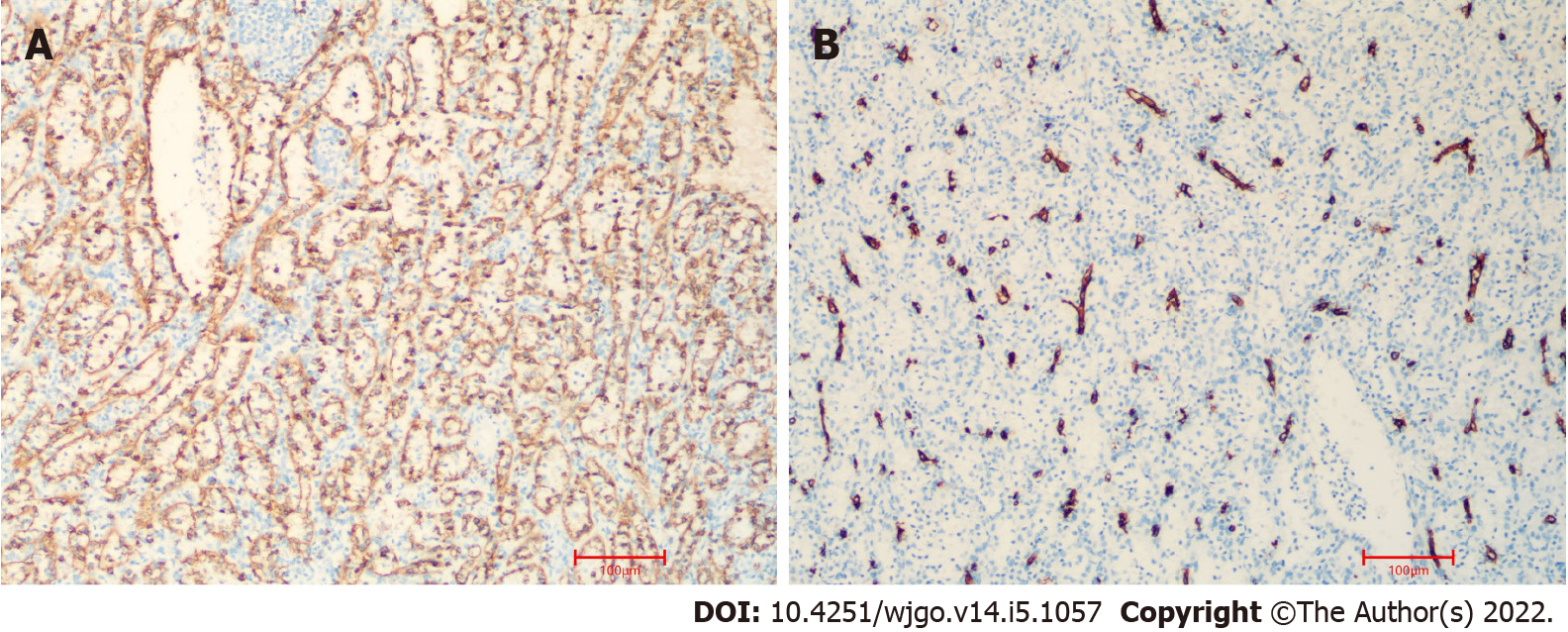
On pathological examination of surgical specimen, two well-defined, homogeneous red-tan masses were found to be 4 × 3 and 4.5 × 1.5 in size. The former was cystically degenerated. Hemorrhage or necrosis was not found. On microscopical examination, the mass grew from the pancreas (Figure 4A). It contained unorganized small slit-like vascular channels enclosing red blood cells and lined with plump endothelial cells. No area of cytologic atypia was identified. Focal lymphoid aggregates were found in the intravascular areas (Figure 4B). White pulp or fibrosis was not observed. On immunohistochemical staining, CD8 was positive in the lining cells and scattered lymphocytes (Figure 5A); CD34 was positive in vascular lining cells (Figure 5B).
The final diagnosis was pancreatic accessory SH with cystic degeneration.
After sufficient preparation and consent from the patient and his family members, the surgery was performed. The patient was placed in a 45° head high position. Following anesthesia and sterilization of the surgery field, sterile drapes were whisked onto the patient's body. Subsequently, a 10 mm long incision was made in the superior border of the umbilicus. A CO2 pneumoperitoneum was set up with 15 mmHg intra-abdominal pressure using a Veress needle, and a 10 mm trocar puncture was made to insert a laparoscopic lens. Under direct vision, 10 mm and 5 mm incisions were made in the left and right abdomen, respectively, and corresponding trocars were implanted in each incision. Then, we inserted surgical instruments to operate the surgery. We exposed the pancreas with an ultrasonic scalpel. Two red pancreatic masses were found, close to each other and growing from the pancreatic tail. The upper and lower margins of the middle pancreas were isolated. We dissociated the superior mesenteric artery and portal vein from the lower margin of the pancreas and opened the posterior pancreatic passage along the portal vein sulcus. The body and tail of the pancreas were isolated toward the splenic hilum. We dissected the distal pancreas, including the masses, with a cutting closure device. The masses were close to both the splenic artery and vein. We carefully isolated the masses from the blood vessels. Finally, the distal pancreas and tumor were removed entirely, and the spleen was preserved by laparoscopic surgery.
The patient recovered well with no pancreatic leakage postoperatively, and CA19-9 was reduced to normal. The patient left our hospital three days later. During the 34-mo follow-up period, the patient remained well without any complications.
PAS is an uncommon congenital abnormality of the spleen with an incidence of approximately 2%[8]. It is a benign lesion, and patients are usually asymptomatic. It is difficult to accurately diagnose preoperatively, and they are usually misdiagnosed as hypervascular pancreatic neoplasms. SH is a rare benign “tumor”, and fewer than 200 cases have been reported thus far. Moreover, hamartoma in PAS has not been reported. In the present study, we report the first case. SH can occur in any age group, usually asymptomatically with no sex predilection[9]. They are usually found incidentally during physical examination or at autopsy. The tumors vary in size, ranging from a few millimeters to a maximum of 20 cm, with a median size of 5 cm. Women often encounter larger masses, suggesting a hormonal influence[4].
Obtaining an accurate diagnosis of SH through the use of US, CT, and MRI is challenging[10]. On US, the lesion is usually solid and hyperechoic, relative to normal splenic tissue. Color Doppler flow imaging shows dotted blood flow signals in the lesion. It appears on CT as an isodense or hypoattenuating solid lesion compared to the normal splenic tissue. On MRI imaging, SH can show different findings depending on whether it is fibrous or not. Most SHs appear on MRI as isointense lesions on T1-weighted image and heterogeneously hyperintense on T2-weighted image.
Although the use of multiple radiologic imaging techniques makes it possible to detect SH, definitive diagnosis still depends on pathological examination. SHs usually are well demarcated from normal spleen and present as dark red, solitary or multiple masses. Histologically, SH can be classified into white and red pulp subtypes, consisting of lymphoid tissue and a complex of sinuses, respectively[4]. The majority of SHs are mixtures of the different characteristics. Immunohistochemically, the lining cells of sinusoid-like channels are CD8-positive[11]. The cells are also positive for CD31, CD34, factor VIII–related antigen, and vimentin[12-14].
The pathogenesis of SH is still controversial. One hypothesis is that the tumor is a congenital malformation of the red pulp, excessive and disorganized growth of abnormally formed red pulp, a neoplasm, or a reactive lesion to prior trauma[15,16]. SH has also been associated with other hamartomatous masses, such as tuberous sclerosis[17]. In some cases, SHs are combined with hematological disorders. But, the clear relationship between them has not been studied[18].
The radiological differential diagnosis of SH includes lymphoma, inflammatory myofibroblastic and metastatic tumors[19]. Pathologically, SH should be differentially diagnosed from other splenic vascular tumors, such as hemangioma, lymphangioma and so on[6]. Most patient of SH are asymptomatic. When a malignant tumor can not be eliminated, splenectomy may be a good choice for patient with an accidentally found splenic lesion[20]. In the present case, one lesion was found in the tail of the pancreas, and a pancreatic accessory spleen with epidermoid cystic change was considered preoperatively by multiple imaging examinations.
Abnormally elevated CA19-9 often occurs in patients with malignancies or inflammation of the pancreatic, biliary and gynecological systems[21]. SH is a rare benign “tumor”, and fewer than 200 cases have been reported thus far. To date, only one study has reported the elevation of serum CA19-9 in a case of spleen hamartoma[22]. However, normalization was not reported after resection. In our case, the patient was symptomatic with left upper quadrant abdominal pain. In addition, laboratory examination revealed that CA19-9 was abnormally elevated (96.7 U/mL). A malignant tumor cannot be ruled out. Therefore, we successfully performed laparoscopic spleen-preserving distal pancreatectomy for the patient. The final diagnosis was pancreatic accessory splenic hamartoma with cystic degeneration. After resection, the serum CA19-9 level was reduced to normal, and CA19-9 immunostaining of the tissue was negative. As the case is extremely rare, the mechanism behind the occasional elevation of serum CA19-9 in the case of spleen hamartoma has not been studied thus far. In our case, the elevation of serum CA19-9 might be caused by the inflammation of the cystic degeneration of pancreatic accessory SH.
A PAS is an uncommon congenital abnormality. SH is also rale. Moreover, hamartoma in the PAS has not been reported thus far. Here, we report the first case. The tumor marker CA19-9 was abnormal, and a malignant tumor could not be ruled out preoperatively. We successfully performed laparoscopic spleen-preserving distal pancreatectomy, and the patient recovered well and had a good prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dias E, Portugal; Sugiyama Y, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Wadham BM, Adams PB, Johnson MA. Incidence and location of accessory spleens. N Engl J Med. 1981;304:1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol. 2010;83:668-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Eker T, Kocaay AF, Sevim Y, Çakmak A. Splenic hamartoma is a rare cause of abdominal pain: Case report and literature review. Turk J Surg. 2017;33:294-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Cheng N, Chen J, Pan Y, Jiang Y, Zhou J, Shao C. Splenic hamartoma with bizarre stromal cells: a case report and literature review. Diagn Pathol. 2018;13:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Falk S, Stutte HJ. Hamartomas of the spleen: a study of 20 biopsy cases. Histopathology. 1989;14:603-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lee H, Maeda K. Hamartoma of the spleen. Arch Pathol Lab Med. 2009;133:147-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Gonzalez Urquijo M, Rodarte-Shade M, Rangel-Rangel R, Castillo-Meraz JA, Rodriguez-Tejeda JR, Gil-Galindo G. A giant splenic hamartoma associated with hematologic disorders: A case report. Ann Med Surg (Lond). 2018;36:199-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Zhu HX, Lou WH, Kuang TT, Wang DS. Post-splenectomy intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas. Int J Surg Case Rep. 2014;5:1151-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Lam KY, Yip KH, Peh WC. Splenic vascular lesions: unusual features and a review of the literature. Aust N Z J Surg. 1999;69:422-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Sim J, Ahn HI, Han H, Jun YJ, Rehman A, Jang SM, Jang K, Paik SS. Splenic hamartoma: A case report and review of the literature. World J Clin Cases. 2013;1:217-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 11. | Zukerberg LR, Kaynor BL, Silverman ML, Harris NL. Splenic hamartoma and capillary hemangioma are distinct entities: immunohistochemical analysis of CD8 expression by endothelial cells. Hum Pathol. 1991;22:1258-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Yigit N, Covey S, Tam W. Massive splenic hamartoma with bizarre stromal cells. Int J Hematol. 2015;101:315-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Ramdall RB, Alasio TM, Cai G, Yang GC. Primary vascular neoplasms unique to the spleen: littoral cell angioma and splenic hamartoma diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2007;35:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Conlon S, Royston D, Murphy P. Splenic hamartoma. Cytopathology. 2007;18:200-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Silverman ML, LiVolsi VA. Splenic hamartoma. Am J Clin Pathol. 1978;70:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Levy AD, Abbott RM, Abbondanzo SL. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology. 2004;230:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Darden JW, Teeslink R, Parrish A. Hamartoma of the spleen: a manfestation of tuberous sclerosis. Am Surg. 1975;41:564-566. [PubMed] [Cited in This Article: ] |
| 18. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Wang JH, Ma XL, Ren FY, Zuo CJ, Tian JM, Wang ZF, Zheng JM. Multi-modality imaging findings of splenic hamartoma: a report of nine cases and review of the literature. Abdom Imaging. 2013;38:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Havlik RJ, Touloukian RJ, Markowitz RI, Buckley P. Partial splenectomy for symptomatic splenic hamartoma. J Pediatr Surg. 1990;25:1273-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, Talia KL, Park KJ, McCluggage WG. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am J Surg Pathol. 2016;40:636-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Fujii T, Obara T, Shudo R, Tanno S, Maguchi H, Saitoh Y, Ura H, Kohgo Y. Splenic hamartoma associated with thrombocytopenia. J Gastroenterol. 1997;32:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |