Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.625
Peer-review started: March 5, 2021
First decision: March 29, 2021
Revised: April 3, 2021
Accepted: May 25, 2021
Article in press: May 25, 2021
Published online: June 15, 2021
Hepatopancreatoduodenectomy (HPD) is the simultaneous combination of hepatic resection, pancreaticoduodenectomy, and resection of the entire extrahepatic biliary system. HPD is not a universally accepted due to high mortality and morbidity rates, as well as to controversial survival benefits.
To evaluate the current role of HPD for curative treatment of gallbladder cancer (GC) or extrahepatic cholangiocarcinoma (ECC) invading both the hepatic hilum and the intrapancreatic common bile duct.
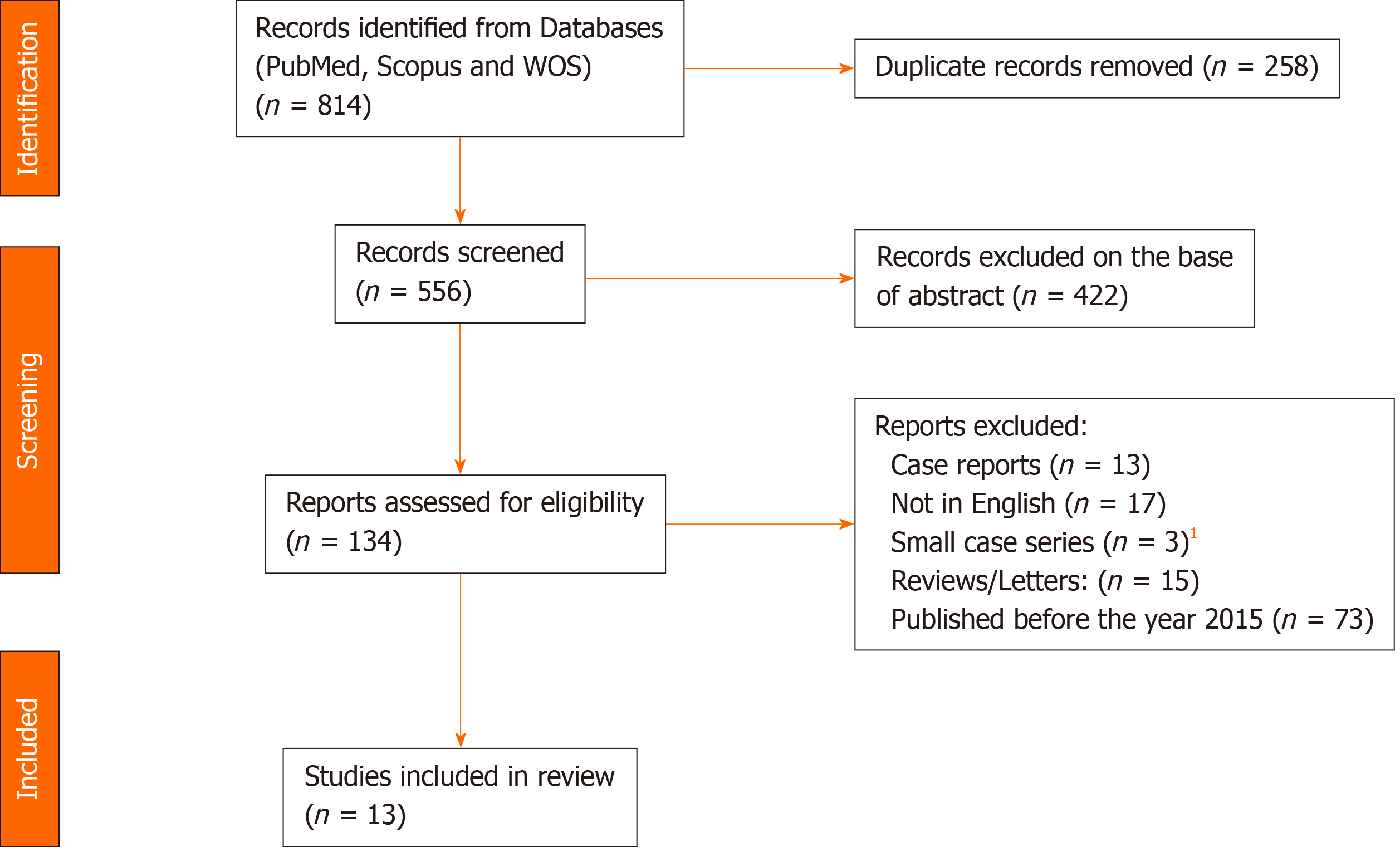
A systematic literature search using the PubMed, Web of Science, and Scopus databases was performed to identify studies reporting on HPD, using the following keywords: ‘Hepatopancreaticoduodenectomy’, ‘hepatopancreatoduodenectomy’, ‘hepatopancreatectomy’, ‘pancreaticoduodenectomy’, ‘hepatec
This updated systematic review, focusing on 13 papers published between 2015 and 2020, found that rates of morbidity for HPD have remained high, ranging between 37.0% and 97.4%, while liver failure and pancreatic fistula are the most serious complications. However, perioperative mortality for HPD has decreased compared to initial experiences, and varies between 0% and 26%, although in selected center it is well below 10%. Long term survival outcomes can be achieved in selected patients with R0 resection, although 5–year survival is better for ECC than GC.
The present review supports the role of HPD in patients with GC and ECC with horizontal spread involving the hepatic hilum and the intrapancreatic bile duct, provided that it is performed in centers with high experience in hepatobiliary-pancreatic surgery. Extensive use of preoperative portal vein embolization, and preoperative biliary drainage in patients with obstructive jaundice, represent strategies for decreasing the occurrence and severity of postoperative complications. It is advisable to develop internationally-accepted protocols for patient selection, preoperative assessment, operative technique, and perioperative care, in order to better define which patients would benefit from HPD.
Core Tip: Hepatopancreatoduodenectomy (HPD) is a complex operation that may achieve curative treatment for selected patients with locally advanced gallbladder cancer and extrahepatic cholangiocarcinoma. However, it represents a surgical procedure with high morbidity and mortality rates, that should be performed in centers with high experience in hepatobiliary-pancreatic surgery. Internationally-accepted protocols on selection criteria, preoperative assessment, operative technique, and perioperative care, are needed in order to better define which patients would benefit from HPD.
- Citation: Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, Porcu A. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: A systematic review. World J Gastrointest Oncol 2021; 13(6): 625-637
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/625.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.625
Gallbladder cancer (GC) and extrahepatic cholangiocarcinoma (ECC) are tumours with dismal prognosis. Resection provides the only chance of cure, although this kind of surgery is technically challenging due to the complexity of biliary and vascular anatomy of the hepatobiliary-pancreatic region, and the necessity to perform extended hepatic resection[1-4]. In general, biliary cancers have various modes of local extension, including a ‘horizontal spread’ involving the entire extrahepatic biliary tree. Hepatopancreatoduodenectomy (HPD) is the simultaneous combination of hepatic resection, pancreaticoduodenectomy (PD), and resection of the entire extrahepatic biliary system that has been used for curative treatment of selected patients with GC and ECC invading both the hepatic hilum and the intrapancreatic common bile duct, historically considered as unresectable tumours[1]. The combination in the same operation of hepatic resection and PD, both of which belong to the category of major surgical oncology procedures, has known a limited spread due to high mortality and morbidity rates registered in the initial experiences, as well as to controversial survival benefits. A systematic review of safety and efficacy of HPD for biliary cancer published in 2015 from Zhou et al[5] just found 18 studies including 397 patients.
To date, HPD is not universally recognized as a surgical option in patients with locally advanced GC and ECC. However, although it remains a debated surgical operation currently performed in few centers with high expertise in hepatobiliary-pancreatic surgery, perioperative mortality has gradually decreased and encouraging survival outcomes have been observed in recent years.
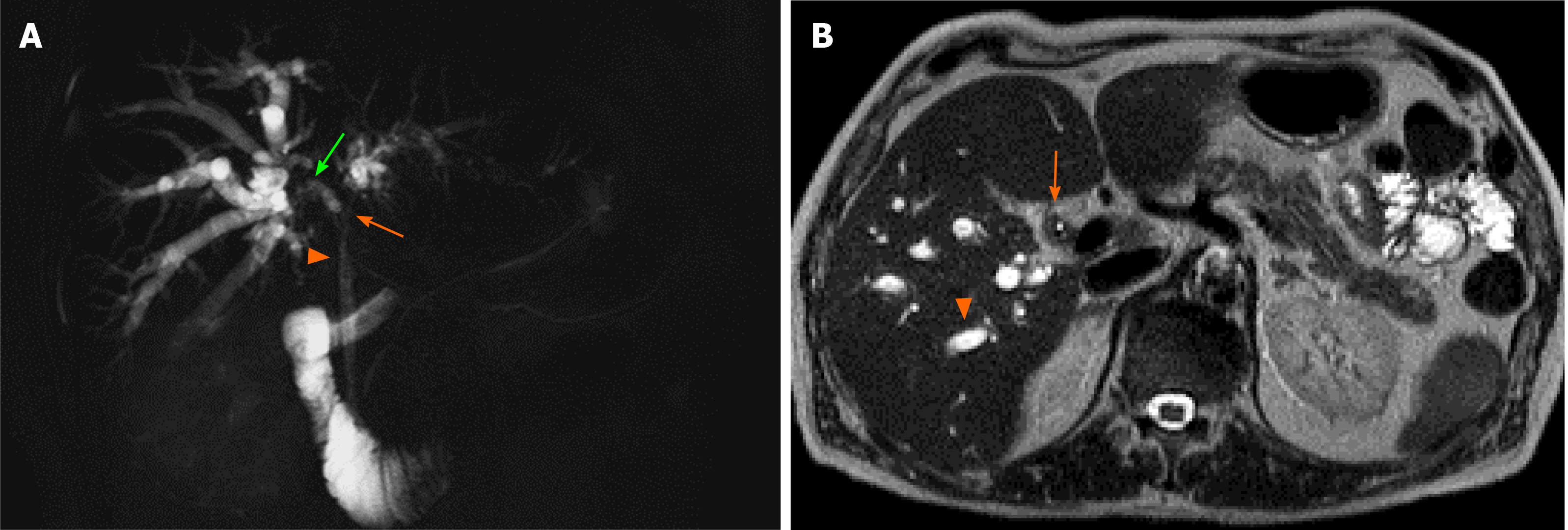
Drawing on a recent case of HPD carried out at our institution (illustrated in Figure 1), the present paper aims to make a review of new insights on the use of this surgical intervention, focusing on current indications, mortality, morbidity, and survival outcomes of patients who received HPD for GC or ECC.
HPD was described for the first time in 1974 from Kasumi et al[6], for treatment of a patient with GC involving the duodenum. The patient overcame the operation but died for cancer recurrence 5 months later. Subsequently, Takasaki et al[7], described 5 cases of extended right lobectomy combined with PD for gallbladder carcinoma. During the 80es and the 90es, HPD was performed in some institutions in Japan mostly for advanced GC and ECC, with reported high mortality and morbidity rates, and poor survival outcomes. In general, it should be recognized that Japanese surgeons have contributed significantly to the evolution of extended surgery for hepatobiliary-pancreatic malignancies[1,2]. Since the results of those procedures had been published essentially in Japanese journals, HPD had for years limited diffusion in the rest of the world[2]. It was not until the start of the 2000's that limited patient series on the use of that procedure were published also from American, European, and Asian institutions other than Japanese ones[8-11]. Looking at review articles, two main papers reported on the results of HPD published until the year 2015[1,5]. The appearance in the literature of new cohort studies in the last six years partially prompted the present review (Table 1).
| Ref. | Country | No. of patients submitted to HPD | Time frame | Inclusion criteria | Main conclusions |
| Tran et al[23], 2015 | United States | 1072 | 2005-2013 | ECC, GC, pancreatic cancer, benign pancreatic disease NET, secondary liver cancer | A synchronous hemihepatectomy (or trisectionectomy) with PD remains a high morbid combination and should be reserved for patients who have undergone extremely cautious selection. |
| Fukami et al[15], 2016 | Japan | 38 | 1994-2014 | ECC, GC | Major HPD with resection of the hepatic artery can be a preferable option for ECC with acceptable perioperative morbidity and mortality, as well as long-term survival. This procedure for GC should not be performed. |
| Fernandes et al[8], 2016 | Brazil | 35 | 2004-2014 | ECC, GC, NET, secondary liver cancer/liver direct infiltration | Major liver resection with PD is associated to very high mortality. Efforts to ensure a remnant liver over 40%-50% of the total liver volume is the key to obtain patient survival. |
| Aoki et al[21], 2016 | Japan | 52 | 1994-2014 | ECC, GC | HPD can be safely performed using the presently reported surgical strategies with acceptable short and long-term outcomes. |
| Dai et al[13], 2017 | China | 12 | 1998-2014 | ECC, GC, HCC, liver sarcoma | Morbidity and mortality after HPD were significant. With R0 resection, the 5-year OS and DFS rates were 27.8% and 29.6%, respectively. |
| Lee et al[41], 2018 | Korea | 22 | 2004-2013 | ECC, GC | HPD for GC and ECC can be performed with acceptable mortality and morbidity rates. GC patients who underwent HPD showed comparable survival rates compared with ECC patients. |
| Welch et al[9], 2019 | United States | 23 | 2014-2016 | ECC, GC, pancreatic cancer, NET, liver cancer, other malignancy, benign disease | The morbidity and mortality after HPD are significantly higher than after major hepatectomy or PD alone. Centralization of HPD to a very few centers may be a strategy to improve outcomes. |
| Mizuno et al[37], 2019 | Japan | 38 | 1996-2016 | GC | HPD for GC is associated with poor OS, high morbidity and mortality rates compared to hepatic resection. Although HPD may eradicate locally spreading GC, the procedure is questioned from an oncological view. |
| D’Souza et al[10], 2019 | Sweden3 | 66 | 2003-2018 | ECC, GC | HPD, although associated with substantial perioperative mortality, can offer a survival benefit in patient subgroups with ECC and GC. To achieve negative resection margins is paramount for an improved survival. |
| Toyoda et al[43], 2019 | Japan | 100 | 2001-2017 | ECC | Presurgical cholangiographic classification, diffuse or localized type, is a tumor-related factor closely associated with survival; therefore, it may be a useful feature for patient selection prior to HPD for ECC. |
| Liu et al[11], 2020 | China | 16 | 2007-2017 | ECC | The radical resection of ECC combined with the partial resection of the pancreatic head in some selected patients can actually replace HPD as a surgical treatment for ECC with distal bile duct involvement. |
| Shimizu et al[28], 2020 | Japan | 37 | 1990-2019 | ECC | HPD is a valid treatment option for extensive cholangiocarcinoma, offering long-term survival benefit at the cost of relatively high but acceptable morbidity and mortality. HPD is advocated in selected patients provided that it is considered possible to achieve R0 resection. |
| Oba et al[42], 2020 | Japan | 36 | 1998-2018 | ECC | Invasive tumor thickness could be measured using simple methods and may be used to stratify postoperative prognosis in patients with ECC. |
A systematic literature search using the PubMed, Web of Science, and Scopus databases was performed in January 2021 to identify studies in English reporting on HPD during the time-frame 2015-2020, with the aim of focusing on the most recent insights in the use of this complex procedure. The following keywords were used and combined for the search: ‘Hepatopancreaticoduodenectomy’, ‘hepatopancreatoduodenectomy’, ‘hepatopancreatectomy’, ‘pancreaticoduodenectomy’, ‘hepatecto
In contrast to classic meta-analyses, the outcomes were defined as the percentages of outcomes of interest without comparison (morbidity and mortality) in cohorts of patients receiving HPD for GC or ECC. Overall proportions can be estimated from the weighted mean of percentages measured in each study. The weight in this case is derived from the number of subjects included in the studies (resumed in Table 1) out of the total number of subjects in all studies, which is inverse of the variance in the classic meta-analyses.
At the time of this review, a total of 13 studies were found in which HPD was used for treatment of either GC or ECC. HPD represents the only curative treatment for GC and ECC (the latter also known as ‘Klatskin tumour’, or ‘hilar cholangiocarcinoma’ or ‘peri-hilar cholangiocarcinoma’), having extensive horizontal tumor spread with infiltration of the hepatic hilum and the intrapancreatic bile duct, due to the tissue invasion via the lymphatics and perineural spaces[3,4]. While CG and ECC represent the main indication for HPD, in a minority of cases this surgical approach has been used also in patients having benign disease, liver cancer, neuroendocrine tumours (especially pancreatic neuroendocrine tumor metastatic to the liver) and other malignancies[8,9,12-14]. However, for the purposes of this study, survival outcomes of HPD only for the treatment of GC and ECC were considered.
HPD undoubtedly represents the most complex operation in the hepatobiliary-pancreatic region, and to date still remains a controversial procedure[5,9]. In the majority of cases, HPD includes a major hepatic resection (at least three Coinaud’s segments), being right hepatectomy with simultaneous PD the most common combination in HPD[1,8,15]. Usually, also the segment I is included in the liver resection during HPD in order to increase the rate of R0 resection, especially in cases of ECC of Bismuth-Corlette type III–IV extending to the pancreato-duodenum, since the caudate lobe is involved by tumour[3,10]. Segmental hepatic resection or metastasectomy associated to PD (like in cases of PD for neuroendocrine tumours with limited hepatic metastases), or PD associated to hepatic resection without extirpation of the hilar bile duct (like in cases of GC with retropancreatic lymph node involvement) should not be considered as pure HPD. In fact, genuine HPD consists in removal of the entire extrahepatic biliary system with the adjacent liver and the pancreatoduodenum [1]. Also a two-stage procedure in which the pancreatic and liver resections were performed at two different occasions not separated more than 2 months in time, can be barely defined as pure HPD[10].
Variations in surgical steps of HPD have been described. Nonetheless, meticulous preparation of the hepatic inflow vessels represents the first step, in order to achieve preservation of the future liver remnant after hepatic resection. Usually, pylorus-preserving (or subtotal stomach-preserving) PD precedes the hepatic resection, and the tumor is removed en bloc by HPD[1,8]. A frozen section histologic examination at the proximal bile duct margin and distal ductal stump is performed like in standard PD. Clearance of the lymph nodes of the hepatoduodenal ligament and pancreaticoduodenal region is necessary in all cases. Reconstruction of the digestive tract is carried out with a Roux-en-Y jejunal limb.
Other authors prefer a ‘liver first’ approach for HPD, in which liver transection precedes PD, because this method may facilitate a curability assessment of the liver side, especially when doubts exist about the proximal extension of the tumour, allowing for an extended hepatectomy to be planned[12,16,17].
To note, reconstruction of the portal vein or hepatic artery or both is required in 20%–30% of cases during HPD[18]. Vascular resection/reconstruction during PD or hepatic resection is a complex procedure performed in centers with expertise in hepatobiliary-pancreatic surgery. In particular, venous resection has increased the number of resectable patients with pancreatic cancer[19]. Infiltration of the portal-mesenteric axis is no longer a contraindication for PD, and portal resection/ reconstruction can be effectively carried out with direct suture, or using autologous or synthetic graft. The results of a recent meta-analysis demonstrated that PD plus venous resection has inferior survival outcomes and higher 30-d mortality when compared with standard PD, nonetheless that operation can obtain better survival outcomes when compared to nonoperative treatments in patients with portal-mesenteric invasion from pancreatic head adenocarcinoma[19]. For extension, venous resection has been used when necessary also during HPD[10,14]. On the other hand, the role arterial resection in surgical treatment of pancreatic and bile duct cancer remains controversial, although the prognostic value of hepatectomy with simultaneous resections of the portal vein and hepatic artery in patients with advanced ECC has been reported by some authors[2]. In this regard, Fukami et al[15] and Ota et al[20] performed HPD with hepatic artery resection/reconstruction (the so-called hepato-ligamento-pancreatoduodenectomy) in patients with ECC having macroscopic hepatoduodenal ligament invasion. Fukama e al did not observe any significant difference in 2-year survival between the patients with (12) and without (26) hepatic artery resection (P = 0.465). The same authors advised against the use of that procedure for GC[15].
Ideally, such a complex operation like HPD should be carefully planned preoperatively, taking into account the risk/benefit balance. In the European experience described by D’souza et al[10], in 46% of the patients, the decision to perform HPD was taken intraoperatively, while in the series from Aoki et al[21] the operative procedure was switched to an HPD in 25% of cases. Not surprisingly, intraoperative switch to HPD has been associated to a decreased recurrence-free survival.
HBP is a skill-demanding procedure with high morbidity and mortality rates. In the review of Zhou et al[5], the perioperative mortality associated to HPD was 10.3%. However, recent studies published between 2015 and 2020 showed significant differences among Eastern and Western countries, also reflecting the existing differences in mortality rates (12% vs 3%) after resection of ECC without HPD[10] (Table 2).
| Ref. | Total n of patients | Morbidity (%) | Perioperative mortality (%) |
| Tran et al[23], 2015 | 107 | 87.53 | 18.24 |
| Fukami et al[15], 2016 | 38 | 44.71 | 13.5 |
| Fernandes et al[8], 2016 | 35 | 97.4 | 34.2 |
| Aoki et al[21], 2016 | 52 | 37.01 | 0 |
| Dai et al[13], 2017 | 12 | 83.3 | 25.0 |
| Lee et al[41], 2018 | 22 | 68.2 | 4.5 |
| Welch et al[9], 2019 | 23 | 87.0 | 26.0 |
| Mizuno et al[37], 2019 | 38 | 87.01 | 18.0 |
| D’Souza et al[10], 2019 | 66 | 50.01 | 15.0 |
| Toyoda et al[43], 2019 | 100 | 81.01 | 0%2 |
| Liu et al[11], 2020 | 16 | 62.5 | 12.5 |
| Shimizu et al[28], 2020 | 37 | 51.41 | 5.4 |
In a recent study investigating the safety-related outcomes of hepatobiliary-pancreatic surgeries performed in Japan after establishment of the ‘Japanese Society of Hepato-Biliary-Pancreatic Surgery board certification system for expert surgeons’, a mortality rate of 7.6% for HPD was registered[22]. Higher mortality rates for HPD were observed in United States (18.2%), Brasil (34.2%), and Europe (15%)[8,10,13,23]. However, it should be recognized that rates of mortality in selected centers from Japan were well below 5%. In fact, recent reports documented a mortality of 2.4%[24] or even no mortality in patients who underwent HPD for GC or ECC[14,21].
The morbidity rates associated to HPD were historically around 80%[5]. The largest single center report of 85 HPD cases for cholangiocarcinoma at the University of Nagoya published in 2012 found a high morbidity (76% of patients with Clavien-Dindo 3 or higher complications), in spite of considerable low operative mortality (2.4%)[24]. Similar results were reported from Utsumi et al[14] in a study on 17 patients, where morbidity rate was 88.3% and mortality rate 0%. D’souza et al[10] found postoperative complications Clavien-Dindo 3–4 in 50% of patients, with a higher rate in patients with ECC (63%) than in those with GC (35%). Welch et al[9], in their study promoted from the American College of Surgeons-National Surgical Quality Improvement Program, reported an overall morbidity and mortality for HPD of 87% and 26%, respectively. To note, morbidity and mortality rates were significantly higher when compared to both major hepatectomy (51% and 7.6%) and PD (52% and 1.4%), respectively (Table 2).
Hepatic failure, pancreatic fistula, biliary fistula and sepsis are the most common and serious postoperative complications of HPD, and also are important predictors of mortality[5,8,9]. The conspicuous blood losses associated to HPD undoubtedly play an important role in the occurrence of perioperative complications[2].
Interestingly, hepatic failure is the most common cause of perioperative death[5,9], although different definitions of that condition were encountered in the studies. Most HPDs include a major hepatectomy with removal of a large amount of hepatic mass, which exposes to the risk of leaving an insufficient liver remnant. An effective strategy for improving the safety and feasibility of major hepatectomy has become the preoperative portal vein embolization, that induces atrophy of the segments to be resected and compensatory contralateral hypertrophy of the remnant liver[17,23]. Ebata et al[24], among 85 patients receiving HPD, performed preoperative portal vein embolization in 78.8% of cases. In the experience of Fukami et al[15], criteria for preoperative portal vein embolization before HPD were right hepatectomy with a future remnant liver volume less than 40%. In spite of preoperative portal vein embolization, in some cases a desirable future liver remnant cannot be achieved, and volume increases and rapid tumour progression can occur while waiting for surgery. In those cases, HPD including liver parenchymal sparing surgery such as mesohepatectomy or central liver resection, may be used instead of typical major hepatectomy[25,26]. It should be taken into account that postoperative performance of the remnant liver is not only a matter of volume, in fact it is related also to the underlying liver function that need to be assessed with clinical examination, biochemistry, and other liver function tests[3]. The technique of Associating Liver Partition and Portal vein for Staged hepatectomy (ALLPS)[27], that has been used to rapidly enhance the volume of the liver remnant, is associated to considerable mortality and morbidity rates, and has no place in patients candidates to HPD[2,25].
According to Shimuzu et al[28], the indications for HPD in patients 70 years or older should be carefully considered, because they may require greater liver remnant volume in order to avoid the occurrence of postoperative liver failure.
In the pathogenesis of hepatic failure after HPD, also preoperative hyperbilirubinemia plays an important role[14]. The effects of the biliary stasis on the liver remnant include impaired function of hepatocyte mitochondria, impaired activity of microsomial mixed function oxidase, and in general increased predisposition to endotoxemia[5,29]. The role of preoperative biliary drainage of jaundiced patients scheduled for PD remains questioned[11]. However, authors suggested that biliary drainage may be appropriate before HPD, especially when major hepatectomy is planned[10,11,30,31].
Another primary concern in patients undergoing HPD is the occurrence of pancreatic fistula[32-34]. Postoperative pancreatic fistula is associated with other serious complications (especiallly intraabdominal hemorrhage and formation of abscesses) and mortality after PD[10,17,34]. Hepatic hilar clamping during liver resection, that usually follows PD, may induce venous congestion in the remnant pancreas that might facilitate pancreatic fistula formation[15]. To prevent a pancreatic fistula after HPD different methods have been used such external drainage of pancreatic juice by inserting a tube into the main pancreatic duct[35], that can also be followed by second-stage pancreatojejunostomy[36], and wrapping an omental flap around the dissected gastroduodenal artery[17]. Fukami et al[15] routinely employed an external pancreatojejunostomy stent in their series including 38 HPDs. Other possible complications, which can originate from the combination of hepatic resection and PD were delayed gastric emptying, hemorrhage, multi-organ failure, liver abscess, suppurative cholangitis, peritonitis, metabolic acidosis, portal vein thrombosis, sepsis, and hepaticojejunostomy leakage[5,8-10,20]. Some authors have proposed technical variants like ‘pancreatic sparing resection’ during HPD with the aim to reduce mortality and morbidity linked to HPD[11,20], but no conclusions can be drawn at this stage due to the paucity of reports.
High body mass index is a known independent risk factor for morbidity after HPD[22]. Since body mass index of Japanese people is lower than Western people, this finding might partially explain the better outcomes observed in Japanese series.
A careful patient selection and a multidisciplinary approach are essential issues to limit the occurrence and severity of complications of HPD[37]. An accurate assessment of nutritional status can be useful to stratify the perioperative risk of complications in order to optimize preoperative conditions as much as possible[8].
In summary, from the recent literature one can argue that HPD including simultaneous major hepatic resection and PD remains an intervention with a high risk of complications, although low perioperative mortality rates can be reached in institutions with high expertise. Centralization in centers of excellence of patients who can benefit from HPD may be a strategy to improve outcomes[9,38].
While patients with GC and ECC have in general a poor prognosis, long survival outcomes can be achieved in selected patients with R0 resection, since it has been demonstrated that negative margin is the most prognostic factor influencing long term survival after resection[11]. HPD carried out with curative intent with free margins has been reported to obtain acceptable survival outcomes, although important differences exist between GC and ECC, having the former a worst prognosis. For that reason, some authors have underscored that HPD can be considered an acceptable option for ECC, but have questioned its utility in patients with GC[1,2]. In fact, some authors underscored that no patients who received HPD for advanced GC survived after 5 years in their experience[39,40]. On the contrary, Mizuno et al among 38 patients with GC submitted to HPD reported a 5-year survival of 11%[37] . To note, two study reported comparable survival between patients who underwent HPD for GD or ECC [21,41].
In general, advancement in multimodality treatment of biliary cancer has led to improvement in survival after HPD in both GC and ECC in the last ten years. Zhou et al[5] in a review including studies published until 2014, reported that the 5-year overall survival in patients who underwent HPD with R0 resection ranged between 18% and 68.8% (median 51.3%), while it was 0% in those with R1 or R2 resection. The median 5-year survival rate of patients receiving HPD was 33% and 10.4% for patients with ECC and GC, respectively. In another review from Ebata et al[1], including the studies published between 2000 and 2013, the 5-year survival rates were 12%-64% for ECC and 0%-25% for GC[1]. It is important to look with attention at more recent cohort studies on HPD, in that better survival outcomes were observed. In a multicenter study from Europe published in 2019, 3-year overall survival after HPD was cholangiocarcinoma 80% for ECC and 30% for GC (P = 0.018). The authors argued that more advanced T-stage for the GC might partially explain the worse survival[10]. Fukami et al[15] observed a 2-year overall survival of 71% and 39%, with a median survival time 42.3 and 13,5 months (P = 0.465) between patients with GC and ECC who underwent HPD plus hepatic artery resection and HPD without hepatic artery resection, respectively. The survival of the patients with CG was significantly worse than patients with ECC (P = 0.001). One of the most important reports on the use of HPD for advanced ECC was that form the Shinshu University (Japan) on 37 consecutive patients. The 1-, 3-, and 5-year overall survival rates were 83%, 48%, and 37%, respectively. Interestingly, in patients with R0 resection, 5-year overall survival was comparable between patients who had undergone major HPD and major hepatectomy alone (41% vs 40%)[28].
The survival outcomes of papers published in the time frame 2015-2020 were resumed in Table 3.
In summary, recent reports have noted good survival results, provided that R0 resection was achieved, although survival for GC remains worse than that for ECC.
Prognostic factors in patients with biliary cancer undergoing HPD remain to be clarified, and may somehow differ from those receiving major hepatectomy[42]. In a recent study including 100 patients, pathologic vascular invasion, pancreatic invasion, nodal metastasis, and margin status were not prognostic factors from the standpoint of long-term survival. Instead, presurgical cholangiographic classification, differentiating between “diffuse” or “localized” type, seems to be a tumor-related factor closely associated with survival probability. According to Toyoda et al[43], that cholangiographic classification may be effective to stratify patients candidates to HPD according to long-term survival probability.
Surgical resection with free margins remains the only possibility of cure able to achieve significant survival outcomes in patients with biliary cancer. In fact, systemic therapy and/or local treatments alternative to surgery demonstrated limited efficacy. The present review supports the role of HPD in patients with GC and ECC with horizontal spread involving the hepatic hilum and the intrapancreatic bile duct, although several aspects need to be clarified. HPD has had a limited diffusion, mainly due to the limited number of patients operated on with high mortality rates, and also because of questionable survival benefit. However, recent reports have showed improved operative results in centers with expertise in hepatobiliary-pancreatic surgery, due to advances in surgical techniques and perioperative patient care. Mortality rates in patients operated on in centers of excellence for this procedure were less than 10%, although morbidity rates remained high[11,21]. Indubitably, the team’s expertise in advanced hepatobiliary-pancreatic surgery, and specifically in HPD procedure, plays a pivotal role in obtaining satisfactory results in terms of perioperative outcomes. As for oncological outcomes, recent reports have showed acceptable 5-year survival of 25% and 18%-40%, for GC and ECC, respectively. It is our view that the survival outcomes of patients receiving HPD should not be compared with those patients who had standard hepatic resection, but rather with those who receive nonoperative or palliative treatments. In this regard, authors observed a significantly better prognosis of patients receiving HPD for GC than those of the unresectable group[44].
The improved results in terms of perioperative morbidity and mortality, as well as the encouraging survival outcomes, have led to attach importance to HPD as a curative treatment in selected patients with biliary cancer, although it is not currently considered a standard procedure worldwide. Meticulous patients' selection is fundamental in order to obtain a R0 resection, that should represent the oncological objective of the procedure. From a risk/benefit perspective, we believe that R1 or R2 resection should not be an option in such a complex procedure as HPD. Prevention of hepatic failure with precise preoperative evaluation of the remnant liver function plays a key role in the success of HPD. According to centers’ practice, methods such as 99mTc labeled galactosyl human serum albumin liver scintigraphy, computed tomography volumetry, or indocyanine green kinetics, can be used to quantitatively assess hepatic function. Probably, a remnant liver over 40%-50% of the total liver volume should be maintained to ensure patient survival[8].Extensive use of preoperative portal vein embolization, and preoperative biliary drainage in patients with obstructive jaundice, represent strategies for decreasing the occurrence and severity of postoperative complications[25,26].
We recognize that the present review has some limitations, in that it includes only articles published in English in the time-lapse 2015-2020. Moreover, in the included studies, the indications for the HPD procedure were heterogeneous. However, this work has some points of strength since it addresses the insights from the most recent experiences in the use of HPD, thus it may be useful as an update review for best practices in the clinical setting.
It is plausible that the growing experience in HPD in selected centers will give impetus to further research on the use of that approach in the near future. To note, the exact role of HPD in patients with locally extended biliary cancer still remains to be defined and the combination of HPC with a multimodality approach with adjuvant/ neoadjuvant treatments needs to be explored[31,45]. The indications for HPD slightly differ between Western and Eastern countries, and need to be standardized. Differences also exist in preoperative work up and operative technique among the institutions. Furthermore, survival outcomes for both GC and ECC in the different studies are difficult to compare due to heterogeneous methodologies and patients’ inclusion criteria; also the results of the present review suggest that the role HPD may differ in the treatment of those two conditions. It is advisable to develop internationally-accepted protocols on selection criteria, preoperative assessment, operative technique, perioperative care, information sharing and data collection in order to better define which patients would benefit from HPD.
In conclusion, the present study suggests that HPD does have a definite role in the treatment of patients with GC and ECC with horizontal spread, although some aspects of the procedure remain to be elucidated. Surgeons’ experience and careful patients’ selection have a pivotal role in achieving R0 resection and acceptable oncological outcomes.
Hepatopancreatoduodenectomy (HPD) is a challenging procedure that can be used for treatment of gallbladder cancer or extrahepatic cholangiocarcinoma invading the hepatic hilum and the intrapancreatic common bile duct. Due to high mortality and morbidity rates, as well as to controversial survival benefits, HPD is not a universally accepted procedure.
The aim of this review was to consolidate the evidence currently available on HPD for the treatment of gallbladder cancer and extrahepatic cholangiocarcinoma in a systematic fashion.
The main outcomes of interest were morbidity rates, mortality rates and survival outcomes after HPD for treatment of gallbladder cancer or extrahepatic cholangiocarcinoma.
A systematic literature search was performed in PubMed, Web of Science, and Scopus databases to identify studies reporting on HPD during the time-frame 2015-2020.
Thirteen studies were included in this systematic review. Mortality rates varied among studies from Eastern and Western countries. In selected centers from Japan with high expertise in the hepatobiliary surgery, mortality rates were below 10%. Morbidity rates, albeit variable, were reported in more than 50% of patients. Five-year survival after HPD was higher in patients with extrahepatic cholangiocarcinoma than gallbladder carcinoma, and can be considered acceptable in cases were a R0 resection was obtained.
The present review supports the role of hepatopancreaticoduodenectomy in selected patients with gallbladder cancer and extrahepatic cholangiocarcinoma, provided that a R0 resection is achieved. Preoperative portal vein embolization and preoperative biliary drainage in jaundiced patients represent strategies for decreasing the occurrence and severity of postoperative complications.
The present review may be useful as a reference for best practices in the clinical setting, since it addresses the insights from the most recent experiences in the use of heptopancreaticoduodenectomy. Internationally-accepted protocols on selection criteria, preoperative assessment, operative technique, and perioperative care, are warranted to identify patients who would benefit from HPD.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khachfe H S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Nagino M. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci. 2014;21:550-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Nagino M. Fifty-year history of biliary surgery. Ann Gastroenterol Surg. 2019;3:598-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Capobianco I, Rolinger J, Nadalin S. Resection for Klatskin tumors: technical complexities and results. Transl Gastroenterol Hepatol. 2018;3:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Feo CF, Ginesu GC, Barmina M, Pinna A, Scanu AM, Cossu ML, Fancellu A, Porcu A. Curative Resection for Hilar Cholangiocarcinoma: Single-Center Experience with Long-Term Follow-Up. Am Surg. 2018;84:9-10. [PubMed] [Cited in This Article: ] |
| 5. | Zhou Y, Zhang Z, Wu L, Li B. A systematic review of safety and efficacy of hepatopancreatoduodenectomy for biliary and gallbladder cancers. HPB (Oxford). 2016;18:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Kasumi F, Takagi K, Konishi T, Sakamoto G. Treatment of gallbladder cancer. Jpn J Gastroenterol Surg. 1976;9:170-177. [Cited in This Article: ] |
| 7. | Takasaki K, Kobayashi S, Muto S, Akimoto K, Toda S, Asado Y. (1980) Our experience (5 cases) of extended right lobectomy combined with pancreatoduodenectomy for carcinoma of the gallbladder. Tan to Sui (J Bil Pancr). 1980;1:923-932. [Cited in This Article: ] |
| 8. | Fernandes Ede S, Mello FT, Ribeiro-Filho J, Monte-Filho AP, Fernandes MM, Coelho RJ, Matos MC, Souza AA, Torres OJ. THE LARGEST WESTERN EXPERIENCE WITH HEPATOPANCREATODUODENECTOMY: LESSONS LEARNED WITH 35 CASES. Arq Bras Cir Dig. 2016;29:17-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Welch JC, Gleeson EM, Karachristos A, Pitt HA. Hepatopancreatoduodenectomy in North America: are the outcomes acceptable? HPB (Oxford). 2020;22:360-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | D'Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D'Hondt M, Dasari B, Ferrero A, Franken LC, Fusai G, Guglielmi A, Hagendoorn J, Hidalgo Salinas C, Hoogwater FJH, Jorba R, Karanjia N, Knoefel WT, Kron P, Lahiri R, Langella S, Le Roy B, Lehwald-Tywuschik N, Lesurtel M, Li J, Lodge JPA, Martinou E, Molenaar IQ, Nikov A, Poves I, Rassam F, Russolillo N, Soubrane O, Stättner S, van Dam RM, van Gulik TM, Serrablo A, Gallagher TM, Sturesson C; E-AHPBA scientific and research committee. Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford). 2020;22:1339-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Liu F, Hu HJ, Ma WJ, Wang JK, Ran CD, Regmi P, Li FY. Is radical resection of hilar cholangiocarcinoma plus partial resection of pancreatic head justified for advanced hilar cholangiocarcinoma? ANZ J Surg. 2020;90:1666-1670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Iacono C, De Bellis M, Guglielmi A. ASO Author Reflections: Hepatopancreatoduodenectomy: Why, When, and How? Ann Surg Oncol. 2020;27:3358-3359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Dai WC, Chok KS, Cheung TT, Chan AC, Chan SC, Lo CM. Hepatopancreatoduodenectomy for advanced hepatobiliary malignancies: a single-center experience. Hepatobiliary Pancreat Dis Int. 2017;16:382-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Utsumi M, Sadamori H, Shinoura S, Umeda Y, Yoshida R, Nobuoka D, Takagi K, Fujiwara T, Yagi T. Risk factors of morbidity and predictors of long-term survival after hepatopancreatoduodenectomy for biliary cancer. Hepatogastroenterology. 2014;61:2167-2172. [PubMed] [Cited in This Article: ] |
| 15. | Fukami Y, Kaneoka Y, Maeda A, Takayama Y, Onoe S. Major hepatopancreatoduodenectomy with simultaneous resection of the hepatic artery for advanced biliary cancer. Langenbecks Arch Surg. 2016;401:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Ishii T, Seo S, Ito T, Ogiso S, Fukumitsu K, Masui T, Taura K. Liver Transection-First Approach in Hepatopancreatoduodenectomy for Hilar Cholangiocarcinoma: A Safe and Secure Technique for the Early Assessment of Curable Resection and Vascular Reconstruction. Ann Surg Oncol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Chiba N, Abe Y, Yokozuka K, Hikita K, Kobayashi T, Sano T, Tomita K, Tsutsui R, Kawachi S. Surgical Technique of Pancreatic Parenchyma Transection-Delayed Approach (PPTDA) in Hepatopancreatoduodenectomy for Hilar Cholangiocarcinoma. J Gastrointest Surg. 2019;23:613-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hemming AW. Hepatopancreatoduodenectomy in North America: acceptable outcomes? HPB (Oxford). 2020;22:358-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Fancellu A, Petrucciani N, Porcu A, Deiana G, Sanna V, Ninniri C, Perra T, Celoria V, Nigri G. The Impact on Survival and Morbidity of Portal-Mesenteric Resection During Pancreaticoduodenectomy for Pancreatic Head Adenocarcinoma: A Systematic Review and Meta-Analysis of Comparative Studies. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Ota T, Araida T, Yamamoto M, Takasaki K. Operative outcome and problems of right hepatic lobectomy with pancreatoduodenectomy for advanced carcinoma of the biliary tract. J Hepatobiliary Pancreat Surg. 2007;14:155-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Aoki T, Sakamoto Y, Kohno Y, Akamatsu N, Kaneko J, Sugawara Y, Hasegawa K, Makuuchi M, Kokudo N. Hepatopancreaticoduodenectomy for Biliary Cancer: Strategies for Near-zero Operative Mortality and Acceptable Long-term Outcome. Ann Surg. 2018;267:332-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Otsubo T, Kobayashi S, Sano K, Misawa T, Ota T, Katagiri S, Yanaga K, Yamaue H, Kokudo N, Unno M, Fujimoto J, Miura F, Miyazaki M, Yamamoto M. Safety-related outcomes of the Japanese Society of Hepato-Biliary-Pancreatic Surgery board certification system for expert surgeons. J Hepatobiliary Pancreat Sci. 2017;24:252-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Tran TB, Dua MM, Spain DA, Visser BC, Norton JA, Poultsides GA. Hepato-pancreatectomy: how morbid? HPB (Oxford). 2015;17:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 24. | Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, Nagino M. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 2012;256:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Nagaraj K, Goto Y, Kojima S, Sakai H, Hisaka T, Akagi Y, Okuda K. Central hepatopancreatoduodenectomy-oncological effectiveness and parenchymal sparing option for diffusely spreading bile duct cancer: report of two cases. BMC Surg. 2021;21:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Mizuno T, Kanemoto H, Sugiura T, Okamura Y, Uesaka K. Central hepatectomy with pancreatoduodenectomy for diffusely spread bile duct cancer. J Hepatobiliary Pancreat Sci. 2015;22:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 930] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 28. | Shimizu A, Motoyama H, Kubota K, Notake T, Fukushima K, Ikehara T, Hayashi H, Yasukawa K, Kobayashi A, Soejima Y. Safety and Oncological Benefit of Hepatopancreatoduodenectomy for Advanced Extrahepatic Cholangiocarcinoma with Horizontal Tumor Spread: Shinshu University Experience. Ann Surg Oncol. 2021;28:2012-2025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Maguchi H, Takahashi K, Katanuma A, Osanai M, Nakahara K, Matuzaki S, Urata T, Iwano H. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:441-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Torres OJM, Alikhanov R, Li J, Serrablo A, Chan AC, de Souza M Fernandes E. Extended liver surgery for gallbladder cancer revisited: Is there a role for hepatopancreatoduodenectomy? Int J Surg. 2020;82S:82-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Mizuno T, Ebata T, Nagino M. Advanced hilar cholangiocarcinoma: An aggressive surgical approach for the treatment of advanced hilar cholangiocarcinoma: Perioperative management, extended procedures, and multidisciplinary approaches. Surg Oncol. 2020;33:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Gouillat C, Gigot JF. Pancreatic surgical complications--the case for prophylaxis. Gut. 2001;49 Suppl 4:iv32-iv39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Fancellu A, Ginesu GC, Feo CF, Cossu ML, Puledda M, Pinna A, Porcu A. Pancreatic head excavation for tissue diagnosis may reduce unnecessary pancreaticoduodenectomies in the setting of chronic pancreatitis. Hepatobiliary Pancreat Dis Int. 2017;16:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, Ramacciato G, Melis M. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Zhou Y, Yang C, Wang S, Chen J, Li B. Does external pancreatic duct stent decrease pancreatic fistula rate after pancreatic resection? Pancreatology. 2011;11:362-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Miwa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, Kusama K, Ogawa S, Soeda J, Miyagawa S. Is major hepatectomy with pancreatoduodenectomy justified for advanced biliary malignancy? J Hepatobiliary Pancreat Surg. 2007;14:136-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, Watanabe N, Ando M, Nagino M. Major hepatectomy with or without pancreatoduodenectomy for advanced gallbladder cancer. Br J Surg. 2019;106:626-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Endo I, Hirahara N, Miyata H, Yamamoto H, Matsuyama R, Kumamoto T, Homma Y, Mori M, Seto Y, Wakabayashi G, Kitagawa Y, Miura F, Kokudo N, Kosuge T, Nagino M, Horiguchi A, Hirano S, Yamaue H, Yamamoto M, Miyazaki M. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: An analysis of patients registered in the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci. 2021;28:305-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Sakamoto Y, Nara S, Kishi Y, Esaki M, Shimada K, Kokudo N, Kosuge T. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 2013;153:794-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Kaneoka Y, Yamaguchi A, Isogai M. Hepatopancreatoduodenectomy: its suitability for bile duct cancer versus gallbladder cancer. J Hepatobiliary Pancreat Surg. 2007;14:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Lee EC, Han SS, Lee SD, Park SJ. Is Hepatopancreatoduodenectomy an Acceptable Operation for Biliary Cancer? Am Surg. 2018;84:703-711. [PubMed] [Cited in This Article: ] |
| 42. | Oba M, Nakanishi Y, Amano T, Okamura K, Tsuchikawa T, Nakamura T, Noji T, Asano T, Tanaka K, Hirano S. Stratification of Postoperative Prognosis by Invasive Tumor Thickness in Perihilar Cholangiocarcinoma. Ann Surg Oncol. 2021;28:2001-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Toyoda Y, Ebata T, Mizuno T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, Watanabe N, Nagino M. Cholangiographic Tumor Classification for Simple Patient Selection Prior to Hepatopancreatoduodenectomy for Cholangiocarcinoma. Ann Surg Oncol. 2019;26:2971-2979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Uemura S, Miyata T, Kato Y, Uesaka K. Is combined pancreatoduodenectomy for advanced gallbladder cancer justified? Surgery. 2016;159:810-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Nagino M, Ebata T, Yokoyama Y, Igami T, Mizuno T, Yamaguchi J, Onoe S, Watanabe N. Hepatopancreatoduodenectomy with simultaneous resection of the portal vein and hepatic artery for locally advanced cholangiocarcinoma: Short- and long-term outcomes of superextended surgery. J Hepatobiliary Pancreat Sci. 2021;28:376-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |