Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.509
Peer-review started: February 21, 2021
First decision: April 19, 2021
Revised: May 2, 2021
Accepted: May 15, 2021
Article in press: May 15, 2021
Published online: June 15, 2021
This review describes woodchucks chronically infected with the woodchuck hepatitis virus (WHV) as an animal model for hepatocarcinogenesis and treatment of primary liver cancer or hepatocellular carcinoma (HCC) induced by the hepatitis B virus (HBV). Since laboratory animal models susceptible to HBV infection are limited, woodchucks experimentally infected with WHV, a hepatitis virus closely related to HBV, are increasingly used to enhance our understanding of virus-host interactions, immune response, and liver disease progression. A correlation of severe liver pathogenesis with high-level viral replication and deficient antiviral immunity has been established, which are present during chronic infection after WHV inoculation of neonatal woodchucks for modeling vertical HBV transmission in humans. HCC in chronic carrier woodchucks develops 17 to 36 mo after neonatal WHV infection and involves liver tumors that are comparable in size, morphology, and molecular gene signature to those of HBV-infected patients. Accordingly, woodchucks with WHV-induced liver tumors have been used for the improvement of imaging and ablation techniques of human HCC. In addition, drug efficacy studies in woodchucks with chronic WHV infection have revealed that prolonged treatment with nucleos(t)ide analogs, alone or in combination with other compounds, minimizes the risk of liver disease progression to HCC. More recently, woodchucks have been utilized in the delineation of mechanisms involved in innate and adaptive immune responses against WHV during acute, self-limited and chronic infections. Therapeutic interventions based on modulating the deficient host antiviral immunity have been explored in woodchucks for inducing functional cure in HBV-infected patients and for reducing or even delaying associated liver disease sequelae, including the onset of HCC. Therefore, woodchucks with chronic WHV infection constitute a well-characterized, fully immunocompetent animal model for HBV-induced liver cancer and for preclinical evaluation of the safety and efficacy of new modalities, which are based on chemo, gene, and immune therapy, for the prevention and treatment of HCC in patients for which current treatment options are dismal.
Core Tip: Hepatitis B virus-induced liver tumors are hard to treat with currently available interventions and the prognosis of hepatocellular carcinoma (HCC) in patients remains still poor. Immunocompetent woodchucks are a useful animal model for human HCC, because multiple tumors at different stages develop spontaneously and secondary to viral infection. This similarity to human hepatocarcinogenesis and the animal’s vascular architecture allowing catheterization with human-sized products have increased the preclinical use of this model to improve existing imaging (ultrasound, magnetic resonance imaging, and positron-emission tomography) and ablation techniques (embolization and radiotherapy) and to evaluate interventions (chemo, gene, and immune therapy) intended to treat human HCC.
- Citation: Suresh M, Menne S. Application of the woodchuck animal model for the treatment of hepatitis B virus-induced liver cancer. World J Gastrointest Oncol 2021; 13(6): 509-535
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/509.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.509
Infection of adult humans with the hepatitis B virus (HBV) usually leads to self-limited liver disease (i.e., acute hepatitis B) and viral resolution, as the virus is controlled via a strong antiviral immune response[1,2]. Progression to chronic HBV infection is observed infrequently and occurs only in 5% of infected, healthy adults[3]. However, HBV infection acquired at birth by mother-to-child transfer or during early childhood in unvaccinated infants persists in 95% of individuals[3]. Persistent HBV infection then leads to chronic liver disease (i.e., chronic hepatitis B) that is associated with a diminished or impaired immune response unable to control the virus[1,2]. The immunodeficiencies developed overtime during the persistence of HBV infection are further responsible for the progression of liver disease to liver cirrhosis and hepatocellular carcinoma (HCC) later in life[1,4]. Estimates indicate that approximately 257 million people worldwide are chronic carriers of HBV[5]. Without antiviral treatment and/or liver transplantation, these individuals will die, because end-stage HCC has a low five-year survival rate of about 10%[6]. The therapeutic interventions available for the treatment of chronic HBV infection and associated liver disease sequelae are suboptimal, as they rarely induce viral clearance or significantly lower the risk of HCC development and either require lifelong administration or are associated sometimes with severe adverse effects[4,7-10]. HCC has a high mortality rate because it is frequently asymptomatic and medical attention is often sought when removal by surgery (i.e., hepatectomy) is limited or impossible[11,12]. The poor prognosis of HCC at an advanced stage is mainly due to its unresponsiveness to chemotherapy [11,13-16]. Although tyrosine kinase inhibitors such as sorafenib have demonstrated survival benefits among patients with advanced liver cancer, the prognosis of patients with HCC remains dismal, with tumor recurrence rates of 50% after three years[17]. Thus, chronic HBV infection is a major source of human HCC, which is the fifth most common cancer in the world and the third leading cause of cancer deaths[11,18-20]. Compared to uninfected individuals, the lifetime risk of developing HCC is significantly increased by 15- to 20-fold in patients positive for the HBV surface antigen (HBsAg), and can reach 100-fold in individuals with high levels of HBV replication and serum positivity for HBV e antigen (HBeAg)[20]. The HCC lifetime risk remains increased even after spontaneous clearance of the infection[21]. Therefore, the large reservoir of chronic HBV carriers could benefit immensely from the development of more effective and safer antiviral and anticancer therapies that cure the infection, eliminate the risk of liver disease progression, and/or eradicate established HCC.
Woodchuck hepatitis virus (WHV) infects naturally the Eastern woodchuck
Early progress in the development of the woodchuck as an animal model for HBV infection involved basic studies on virological response and liver tumor development that are associated with experimental WHV infection of neonatal and adult woodchucks. Thereafter, neonatal WHV inoculation progressing to chronic viral infection during adulthood has been initially applied for the evaluation of conventional vaccines and nucleos(t)ide analogs for safety and efficacy against HBV[30,34-37]. More recently, the neonatal inoculation model of chronic WHV infection has been increasingly used for the development of immunomodulators, including those stimulating pathogen recognition receptors (PRRs) or blocking immune checkpoint markers[34,38,39]. While some of these studies provided evidence for the prevention and treatment of liver disease progression[37,38], evaluation of interventions directly targeting liver tumors in woodchucks for the treatment of HCC is limited. Since immunopathogenesis and liver disease progression to HCC induced by WHV parallels HBV infection in humans more so than in any other animal model currently available for HBV research[30,34,38-40], woodchucks with established liver tumors have been further applied in the improvement of imaging and ablation techniques and in the evaluation of new therapeutic approaches for the treatment of human HCC. The purpose of this review is to highlight the woodchuck as an animal model for hepatitis virus-induced carcinogenesis and treatment of HCC in patients with chronic HBV infection.
Inoculation of adult woodchucks with WHV almost always results in the acute, self-limited (i.e., resolved) outcome of infection[33,41-44]. Although virtually 100% of hepatocytes in the liver become infected with WHV[45], antiviral control is achieved by strong innate and adaptive immune responses. In the liver, innate immune response is activated within hours after experimental infection and partially inhibits WHV replication[46], although the infection expands further and reaches a peak thereafter. After a lack phase of immune response induction probably due to the “stealth-like behavior” of hepatitis viruses[38,47], a second, more marked, suppression of WHV replication is observed that is mediated by a non-cytolytic mechanism of viral clearance involving type I and II interferons (IFNs)[48]. IFN-α and IFN-β are most likely produced by activated PRRs after sensing of viral DNA and RNA in the liver, while IFN-γ is mainly secreted by natural killer (NK) cells. These antiviral cytokines inhibit the transcription of viral pre-genomic RNA from the episomal, covalently-closed circular (ccc) DNA genome in the nucleus of infected hepatocytes, block its packaging into nucleocapsids, prevent viral replication through upregulation of a ribonuclease, and/or impede synthesis of viral relaxed-circular (rc) DNA within these core particles during maturation, as shown for HBV in cell culture[49-53]and animal models[54-56]. However, these antiviral cytokines do not affect the levels of WHV e and surface antigens (WHeAg and WHsAg) in the periphery of woodchucks[48,57]. This is followed by a cytolytic mechanism of viral clearance leading to a nearly complete loss of both serum viremia and antigenemia, as well as of intrahepatic WHV cccDNA[48,57]. This mechanism involves killing of infected hepatocytes via mainly cytolytic T lymphocytes (CTLs), apoptosis, and regeneration of hepatocytes, resulting in transient, moderate to marked hepatic inflammation and liver injury[48,58-60]. In addition, virus-neutralizing, protective antibodies against WHsAg, as well as antibodies against WHV core antigen (WHcAg) and WHeAg, are elicited by B-cells[48,61]. The concerted actions of the immune system then lead to an almost complete shutdown of viral replication in the liver and clearance of the virus from the periphery, although residual amounts of replication-competent WHV and viral cccDNA often remain detectable in serum and in liver, spleen, and blood cells after resolution[45,61-64]. Truncated and thus replication-incompetent WHV DNA is found integrated into the chromosomal DNA of hepatocytes[65-67]. Such viral DNA is typically rearranged and targets different sites within the cellular DNA, suggesting that these integration events may contribute to hepatocarcinogenesis. The presence of unintegrated and integrated virus appears to correlate with an overall lifetime risk of HCC development in 5%-20% of woodchucks after resolution of acute WHV infection[64,68].
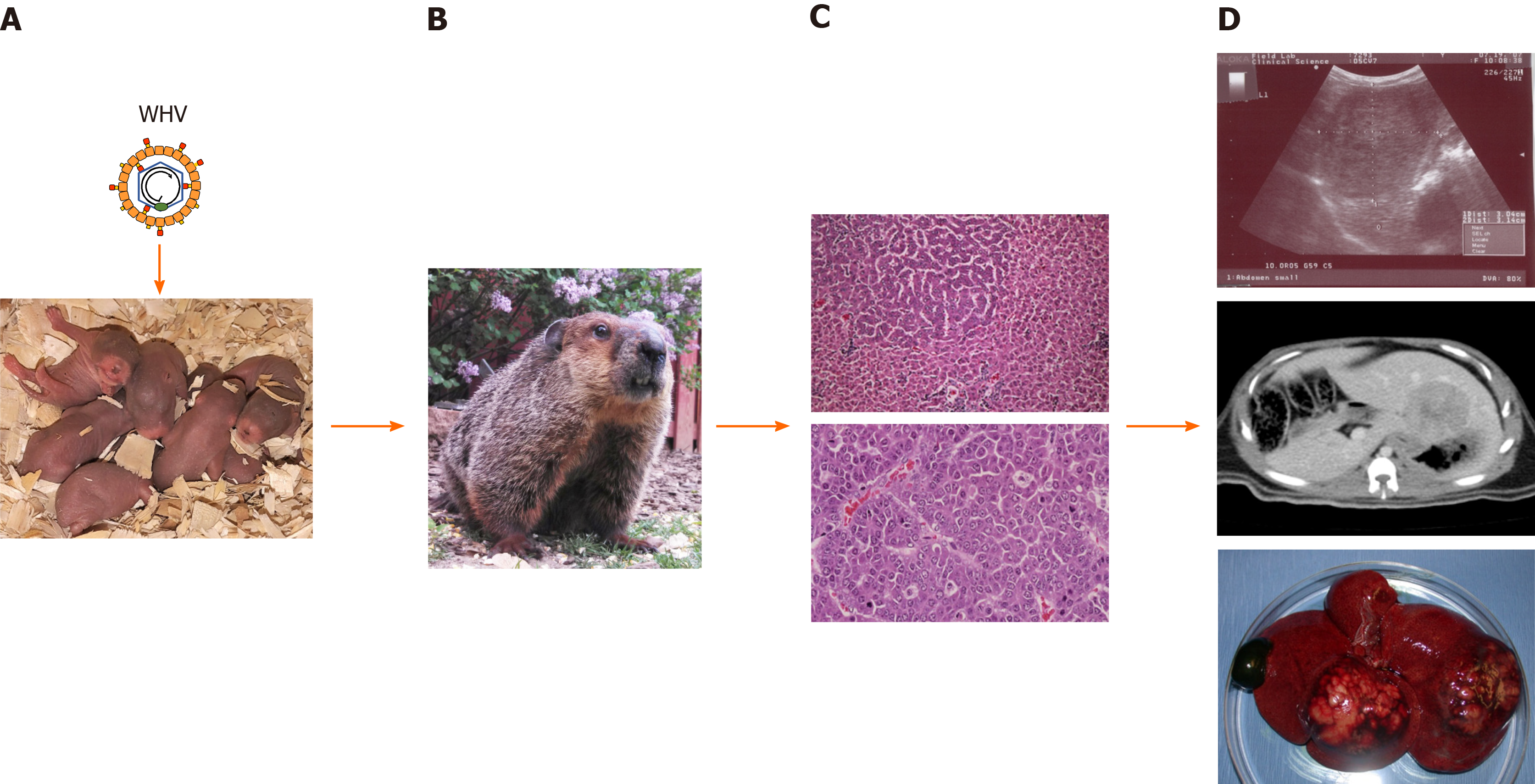
This is in contrast to the inoculation of neonatal woodchucks with WHV (Figure 1), which leads to the chronic outcome of infection in approximately 60%-75% of animals later in life, and thus models the effect of age on the outcome of HBV infection in humans[31,33,41]. Persistent WHV infection in these animals involves an ongoing viral replication in liver, minimal to moderate hepatic inflammation and liver injury, and high levels of viral DNA and antigens in the periphery. Compared to the virion levels in patients with chronic HBV infection that are in the range of 109-1010 particles per mL[28], WHV virions often reach 10- to 100-fold greater concentrations in woodchucks with established chronic infection (i.e., 1010-1011 particles/mL), while subviral particles containing WHsAg are produced in large excess. Like in human HBV infection[69], a WHV core-related antigen (WHcrAg), including the classical WHcAg and WHeAg, and additionally, the WHV precore-related antigen (WPreC), is produced during infection in woodchucks, with elevated levels present in chronic WHV carriers[57]. The high loads of circulating WHeAg and WHsAg produced during chronic WHV infection in woodchucks are thought to be responsible for the immunological tolerance to the virus at the T- and B-cell level[30,34,39,40], and are further associated with the liver disease progression to chronic hepatitis B and liver cancer[31,70,71]. HCC develops in all animals over a median period of 2 to 2½ years after neonatal inoculation, and the median life expectancy is approximately 6 mo that is similar to the situation in patients with HCC[37,68,72]. More specifically, HCC develops in 50% of woodchucks after 29 mo of chronic WHV infection, in 95% of animals after 3 years, and in 100% of animals by 5 years[73,74]. Thus, chronicity as an outcome of neonatal WHV infection appears to result from a suboptimal or unsuccessful immune response relatively early during the acute phase of infection[30,75,76]. During the later stage of chronic WHV infection, and comparable to chronic HBV infection in patients [1,2,77,78], a limited type I but a moderate type II IFN response is present in liver[76,79]. Persistent WHV infection is further characterized by the inhibition of antigen presentation to immune cells[80], increases in hepatocyte cytotoxicity via perforin-granzyme B and Fas ligand-Fas death pathways[81,82], induction of molecules linked to T-cell exhaustion (i.e., immune checkpoint markers)[79,83], and elevated levels of suppressor of cytokine signaling (SOC3)[79]. Since neutrophils accumulate in woodchuck liver[79], these cells may be responsible for the intrahepatic recruitment of mononuclear inflammatory cells via neutrophil-derived metalloproteinases, as observed in a transgenic mouse model of acute hepatitis B and in patients with chronic hepatitis B[84,85]. Liver disease then appears to progress to HCC due to the reduced immune-mediated clearance of WHV-infected hepatocytes by both non-cytolytic and cytolytic mechanisms[30,76], continuing chronic microinflammation [43,86-88], and viral integration events[67,72,89-91]. However, as described in more detail below, these deficiencies in humoral and cellular immune responses present in chronic WHV carrier woodchucks can be altered by different means leading to a functional cure (defined as a loss of viral DNA and surface antigen in serum, with or without seroconversion to virus-neutralizing antibodies[10]) that delays or even prevents HCC onset.
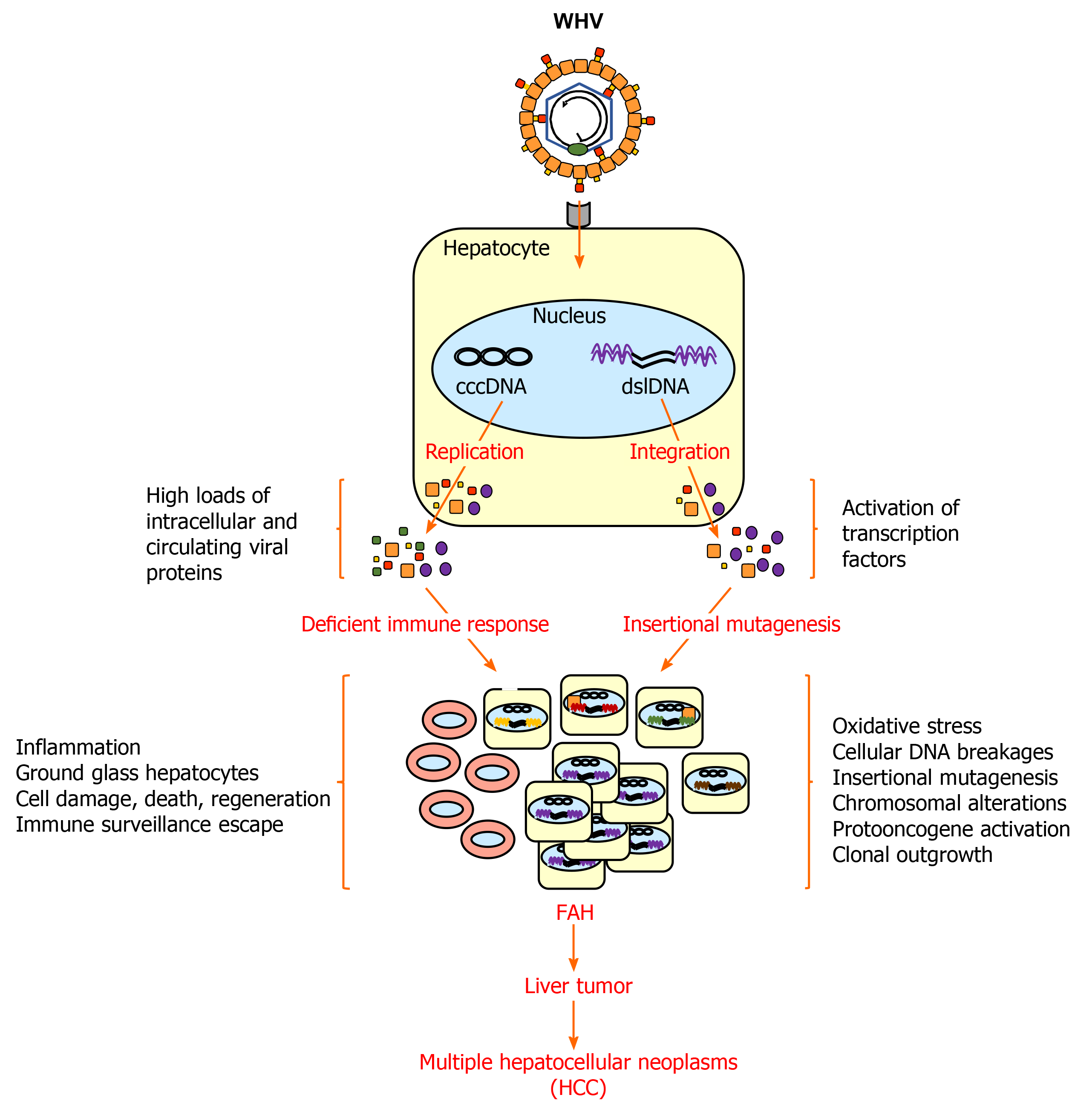
Virus-induced hepatocarcinogenesis in chronic WHV carrier woodchucks is a multistage process (Figure 2). Chronic hepatitis B in these animals is characterized by the mild infiltration of mononuclear cells into portal tracts, sometimes extending beyond the limiting plate[31]. Liver cells with cytoplasmic inclusions are present, which correspond to the ground glass hepatocytes found in the liver of patients with chronic HBV infection and that contain HBV surface antigen (HBsAg)[92]. In HBV transgenic mice, these HBsAg-containing ground glass hepatocytes cluster and form nodules and are seen as preneoplastic lesions[93]. In addition, scattered parenchymal hepatocellular necrosis with neutrophils and macrophages, as well as bile duct proliferation, are usually observed in woodchucks, and in some cases early fibrosis was noted but hepatic cirrhosis and ascites is essentially absent[32,86,87,94,95]. Clinical manifestation of cirrhosis, however, is also absent in a minority of human HCCs due to chronic HBV infection and approximately 20% of HCCs involve non-cirrhotic livers[96,97]. Neoplasia in woodchuck liver then progresses from foci of altered hepatocytes (FAHs) to neoplastic nodules and HCCs[88,98-100]. These altered hepatocytes often harbor viral DNA integrations[65], as also noted in HBV-infected patients[101,102]. They further have a selective regeneration or survival advantage[65] and may be able to escape immune surveillance due to limited intracellular WHV replication and/or presentation of viral epitopes to immune cells. FAHs are detected as early as 6 mo after neonatal WHV inoculation, while small liver tumors occur as early as 3 mo thereafter[68]. Metastasis of HCC outside of the liver is essentially absent in woodchucks[32,87,94,103], except for rare cases of pulmonary metastasis in a few animals[86,94]. The hepatic neoplasms present in woodchucks are typically well-differentiated, trabecular HCCs, although various histologic types are found in different animals or in different tumors in the same animal[32,94,104]. A comparison of intratumoral transcriptional profiles in woodchucks and HBV-infected patients established that WHV-induced HCC shares molecular characteristics with two subtypes of human HCC[79]. One HCC signature present in woodchucks correlated well with the human HCC subclass of poor prognosis (‘‘poor survival subclass’’) that is characterized by low-level cluster of differentiation (CD) 8+ T-cell and NK-cell infiltration[105]. The second HCC signature in woodchucks was associated with the S2 subclass, a well-defined human HCC subtype[106], which is characterized by the activation of the MYC protooncogene, expression of alpha-fetoprotein (AFP) and epithelial cell adhesion molecule (EpCAM), and a relative suppression of IFN-responsive genes. The observation that HCC develops in all chronic WHV carrier woodchucks provides direct experimental evidence for the oncogenicity of WHV, and by analogy of HBV, as well as other hepatitis viruses naturally infecting several ground squirrel species[24,68]. However, infection with California ground squirrel hepatitis virus (GSHV) leads to less frequent liver cancer development and the HCC onset is much later seen than in chronic WHV infection[107]. This lower oncogenic potential of GSHV was further demonstrated in a comparative study of woodchucks infected as neonates with both WHV and GSHV, as GSHV-induced HCC developed at an later age than WHV-induced HCC in the same host[71]. Since immune cell infiltration into the liver is present during chronic WHV infection, as described above, this continuing chronic inflammatory response likely plays a role in the development of WHV-induced HCC, in addition to viral integration events and proteins, as also observed for HBV-induced HCC[108-111].
Important features of hepatitis virus-induced hepatocarcinogenesis have been investigated in woodchucks and are described here in more detail. Since replication-incompetent WHV DNA is integrated into the chromosomal DNA of woodchuck liver tumor cells, which is comparable to the HBV DNA integration in human HCCs[112-115], a direct molecular role of hepatitis viruses in hepatocarcinogenesis is conceivable. The main substrate for integration is viral double-stranded linear (dsl) DNA, which is sometimes produced by the viral polymerase instead of rcDNA within the nucleocapsid[114]. Integration occurs after recycling of nucleocapsids to the nucleus for replenishment of the cccDNA pool[28]. Initial integration in hepatocytes, at least in vitro, is mediated by virus-induced oxidative stress resulting in oxidation-dependent cellular DNA breakages[116]. The integrated viral DNA cannot serve as the source of the progeny virus, but the produced RNA transcripts for the surface and X antigens can become abundantly or even predominantly present, when compared to the viral RNAs transcribed from the cccDNA genome[117]. Thus, integration-derived RNA transcripts may serve as a considerable source for viral antigens with similar function as replication-derived viral proteins and may influence the course of chronic infection and liver disease progression by interfering with the antiviral immunity[117,118].
WHV DNA integrates into the woodchuck genome at multiple sites within hours after experimental infection[67]. Although it does not appear that there is a preferential integration site for hepatitis viruses[101,113], WHV integrates often into or near the MYC family of protooncogenes in most woodchuck HCCs[68]. Integration close to the N-MYC2 gene or in the b3n and win downstream loci then leads to activated N-MYC genes and overexpression of their transcripts in malignant hepatocytes[89,119-122]. In coordination with N-MYC, the insulin-like growth factor-2 (IGF2) is also overexpressed in woodchuck FAHs and HCCs[123,124]. IGF2 blocks apoptosis of malignant liver cells, and thus may allow hepatocytes which otherwise might die to survive, to form FAHs, and to progress to liver tumors[123]. WHV DNA integration further causes N-MYC2 rearrangements, especially in large but less differentiated liver tumors, suggesting that these genetic alterations provide initially a proliferative stimulus or growth advantage for transformed hepatocytes[104]. However, compared with woodchucks that naturally acquired WHV infection, animals experimentally infected with WHV as neonates have more WHV DNA integrations near the N-MYC2 Locus[121]. Although the exact role of N-MYC2 rearrangements and transcripts remains to be elucidated, it was shown that transgenic mice carrying the N-MYC2 gene under the control of WHV regulatory sequences develop liver cancer, including hepatocellular adenomas and HCCs[125].
Like in human HCCs[126], woodchuck liver tumors express small non-protein-coding RNAs or microRNAs at elevated levels, such as miR-17-92 polycistron and miR-21[127]. Knockdown of these microRNAs in human- and woodchuck-derived hepatoma cell lines resulted in a 55% reduction of cell proliferation and anchorage-independent growth, as well as in a suppression of cellular antiapoptotic function. This suggests that onco-microRNAs are involved in the maintenance of malignant hepatocyte transformation during hepatitis virus-induced hepatocarcinogenesis.
Among all viral proteins, the X antigen, a multifunctional transactivator of viral and cellular genes and essential for the establishment of WHV infection in woodchucks[128], has been implicated as a cofactor in the malignant transformation of hepatocytes[129]. HBV replication and liver cell transformation by the HBV X antigen (HBxAg) are associated with the induction of the mitotic polo-like kinase 1 (PLK1) and a parallel downregulation of chromatin remodeling components, including polycomb repressive complex 2 subunit (SUZ12) and zinc finger MYM-type protein 2 (ZMYM2 or ZNF198)[130]. This inverse relationship of PLK1 and SUZ12 was also identified in woodchuck liver tumors[131]. SUZ12 targets many hepatic cancer stem cell markers and proliferation genes. Since expression of these genes is reduced in normal hepatocytes, they are also named “SUZ12 repressed” genes. During WHV-induced hepatocarcinogenesis, the SUZ12 repressed genes encoding BMP, activin membrane-bound inhibitor homolog (BAMBI), and EpCAM, as well as the proliferation gene PLK1, are selectively upregulated in woodchuck tumor cells. Furthermore, metastatic tumor antigen 1 (MTA1), a component of the nucleosome remodeling histone deacetylase complex involved in regulating transcription and chromatin remodeling, is associated with tumor invasiveness and poor prognosis in HBV-induced HCC[132]. Comparable to human HCC, the presence of MTA1 is increased in woodchuck liver tumors, its expression is regulated by the WHV X antigen (WHxAg), and the protein is essential for nuclear factor-kappa B (NF-κB) signaling and tumor progression induced by WHV[133].
Altered expression of vascular endothelial growth factor (VEGF) in the liver is used as a prognostic marker for human HCC[134] and therapeutic interventions targeting this protein or its receptors (VEGFR1/R2) can improve the clinical outcome of HCC in patients[135]. In woodchucks, WHV-induced hepatocarcinogenesis is associated with elevated VEGFR2 expression and increased ligation of VEGF to VEGFR2[136]. This VEGF-driven angiogenesis is accompanied by changes in the liver vasculature, extracellular matrix, and basement membrane, as the number of vessels positive for laminin and platelet endothelial cell adhesion molecule (PECAM1) increased while the number of collagen IV-positive blood vessels declined. This suggests that woodchucks with liver tumors can be utilized in the preclinical evaluation of VEGF-directed therapies for human HCC.
Matrix metalloproteinases (MMPs) play a central role in tumor invasion and metastasis during HBV-induced hepatocarcinogenesis[137]. For obtaining insight in the mechanisms involved in extracellular matrix remodeling in human HCCs, the expression of MMPs was investigated in woodchuck liver tumors[138]. High levels of several MMP transcripts were detected, and especially the transcript and protein levels of MMP-9 correlated with liver disease progression and tumor differentiation in woodchucks, while the protein’s gelatinase activity increased during hepatocarcinogenesis. These results are comparable to findings in human liver tumors where the MMP-9 protein level was used for characterizing a more invasive and metastatic type of HCC with poor prognosis[139,140]. Since the gelatinase activities of woodchuck MMP-2 and MMP-9 could be inhibited by a commercially available drug, the use of MMP inhibitors for treatment of human HCC may be a possible treatment option and could be evaluated in woodchucks.
Hepatitis delta virus (HDV), a natural subviral agent of HBV, is known to contribute to HBV-induced hepatocarcinogenesis and to increase the overall risk of HCC in patients during concomitant infection[141-143]. Since HDV only needs the HBsAg for virion envelopment[143], persistence of HDV infection may be independent of HBV replication if integration-derived viral surface antigen can be used, as demonstrated in cell culture[118]. The contribution of HDV to HCC induction and development remains to be elucidated; however, one possible mechanism was revealed in woodchucks[144]. Intravenous inoculation of woodchucks with liver tumors using WHsAg-enveloped HDV demonstrated that malignant hepatocytes are susceptible to HDV infection. Thus, it appears likely that HDV may influence the fate of HCCs by actively replicating in tumor cells and changing the expression of host genes.
Overall, these studies demonstrated that WHV-induced hepatocarcinogenesis in woodchucks has strong similarity to HBV-induced liver carcinogenesis in humans. The features of HCC that are associated with chronic hepatitis virus infection make the woodchuck animal model unique. It further distinguishes woodchucks from other animal models, in which HCC is induced by either a chemical carcinogen, a transgene, or by transplantation of established tumor cell lines into immune-deficient or immune-compatible hosts. Additional advantages of the woodchuck model are the outbred nature of the animals and the heterogeneity of liver tumors that resemble the situation of HBV-infected patients with HCC. These studies further indicated an important role of viral DNA integration, activation of protooncogenes, microRNAs, and the viral X antigen in the malignant transformation of hepatocytes.
As described above, liver tumors develop in woodchucks with chronic WHV infection and HCC is fatal in 100% of cases. Tumor progression is usually monitored by serial ultrasonography (US)[86,145,146] and to a lesser degree by repeated magnetic resonance imaging (MRI)[147,148]. Changes in liver enzymes are also used for determining the degree of liver injury due to tumor development[104]. Especially, gamma-glutamyl transferase was validated as an oncogenic biomarker in woodchucks, as increases in this liver enzyme correlate with the onset of HCC[149]. In addition, elevated levels of AFP were linked with WHV-induced hepatocarcinogenesis in woodchucks[150].
The woodchuck model of HCC has been utilized in the development of new imaging agents for enhancing the detection of hepatic neoplasms by different imaging techniques (Table 1). In the beginning, several contrast agents were evaluated for both gray scale and color Doppler US, including those that use microbubble technology, alone and in combination with hypobaric activation, a vascular imaging agent, or an agent taken up by the reticuloendothelial (RE) system. These agents facilitated tumor localization in the liver and improved measurements of tumor growth and regression in untreated versus treated woodchucks by increasing the sensitivity of US. Furthermore, iron oxide as a contrast agent for the detection of HCC by MRI was tested in woodchucks, either following parenteral administration for uptake by the RE system or intravenous injection as an arabinogalactan conjugate for targeting the asialoglycoprotein receptor that is highly upregulated on normal hepatocytes but not on liver tumor cells. Hepatic imaging with 99mTc-sulfur colloid also detected HCCs after uptake by the RE system and concentration in woodchuck liver. More recently, woodchucks were applied in the improvement of positron-emission tomography (PET) techniques for the early detection of human HCC by comparing radiotracers for uptake into liver tumors and surrounding hepatic tissues. HCC localization and response to radiotherapy was also assessed with MRI by applying contrast agents typically used in patients for visualizing lesions with abnormal vascularity. HCC detection and response to anticancer treatment was further tested by computed tomography (CT) with contrast agents for human use. MRI and/or CT techniques were also applied for generating a virtual three-dimensional (3D) model of the woodchuck hepatic vascular tree[151], as well as for producing virtual and printable 3D models of the woodchuck liver containing tumors that allowed accurate co-localization of imaging with histopathology[152].
| Imaging technique | Contrast agent | Brand name | Ref. |
| Ultrasonography | Air-filled albumin microspheres | [195] | |
| Cyanacrylate polymer microparticles | SHU563A | [196] | |
| Dodecafluoropentane emulsion | EchoGen | [197,198] | |
| Galactose microparticles/palmitic acid | Levovist | [199-201] | |
| Perflexane-filled lipid microspheres | Imagent | [202] | |
| Perfluoropropane-filled albumin microspheres | Optison/FS069 | [95,200,201,203,204] | |
| Scintigraphy | 99mTc-sulfur colloid | [205] | |
| Positron-emission tomography | [1-11C]acetate | [206-208] | |
| [1-14C]acetate | [207] | ||
| [N-methyl-11C]choline | [206,209,210] | ||
| [18F]clofarabine | [211] | ||
| [18F]fluoro-ethylcholine | [210] | ||
| Anti-1-amino-3-[18F]fluoro-cyclobutyl-1-carboxylic acid | [212] | ||
| 2-deoxy-2-[18F]fluoro-D-glucose | [208,209,213] | ||
| 6-deoxy-6[18F]fluoro-D-glucose | [206,209,210] | ||
| L-[S-methyl-11C]methionine | [212] | ||
| 3-deoxy-3-[18F]fluoro-thymidine | [214] | ||
| Magnetic resonance imaging | Gadolinium | Gadavist or Omniscan | [157,179,185] |
| Gadopentetate dimeglumine | Magnevist | [158,189] | |
| Iron oxide | [215,216] | ||
| Computed tomography | Biodegradable radiopaque fiducial markers based on polymers and iodine | Ioversol, Isovue-370, Optiray300, or Optiray350 | [152,153,159,186,206] |
| Diatrizoic acid | Angiografin | [190] | |
| Meglumine iotroxate | Biliscopin | [151] | |
| Iohexol | Omnipaque or Omnipaque350 | [157,188] |
The woodchuck model was further applied in the evaluation of new techniques developed for gaining less-invasive access to liver tumors for the treatment of HCC in patients. For improving percutaneous liver biopsy techniques, needle-based diffuse optical spectroscopy (DOS) was tested in woodchucks[153]. This established that tissue blood and lipid content and oxygenation level declined, while tissue density increased, when the needle crossed the margin from healthy hepatic parenchyma to liver tumors, indicating that these measurements could be used in real-time as a primary discriminator of normal liver and HCC.
For the treatment of human HCC, chemoembolization and radioembolization via intra-arterial therapies (IAT), alone and in combination with immunotherapy, hold great promise. For the testing of IAT, rather diverse animal species, including mice, rats, rabbits, and pigs, are commonly used as preclinical models of HCC[154-156]. Translation of IAT from these animal models into patients, however, is limited due to the dissimilarity in liver disease development and the size of the vascular system that make arterial access either impossible or challenging, and often requires a surgical cut down for the use of human-size products[155]. This situation is different in woodchucks, because the size of the animals greatly facilitates IAT and other experimental approaches of intratumoral injection. Woodchucks also possess a hepatic arterial anatomy that can be accessed via the femoral artery and allows catheterization with clinically used microcatheters[151]. Accordingly, three studies explored IAT in woodchucks with or without liver tumors[157-159]. In these studies, arterial access via the femoral artery with human standard catheters allowed delivery of contrast agents for the localization of HCCs by CT or MRI. Catheterization further permitted delivery of embolic particles routinely used in patients into liver tumors by angiography. Lobar embolization with 355–500 µm polyvinyl alcohol (PVA) particles (Boston Scientific) was successful in woodchucks without liver tumors[158]. In addition, liver tumor embolization for the targeted delivery of 100-300 µm PVA microspheres (LC- Bead; BTG, London, United Kingdom) produced a heterogeneous distribution of embolic particles in the hepatic neoplasms[157]. Moreover, chemoembolization with drug-eluting embolic 70-150 µm radiopaque PVA microspheres (LC Bead LUMI; BGT) loaded with doxorubicin resulted in a targeted drug delivery into liver tumors[159]. Doxorubicin is an anticancer drug that stops the growth of tumor cells by blocking topoisomerase II and that generates reactive oxygen species for the induction of apoptosis[160].
There is also interest in assessing the biomedical utility of nanomaterials in immunocompetent animal models for the treatment of human HCC. In particular, tumor-associated macrophages within the environment of solid tumors are a preferred target of nanoparticle-based applications, as the balance of inflammatory (tumoricidal) and immunoregulatory (tumor promoting) macrophages controls tumor development, progression, and metastasis[161]. One study evaluated the distribution and clearance of 60 nanometer gold particles into woodchuck liver and tumors after a single intravenous injection at a dose of 14 mg/kg[162]. Although these nanoparticles accumulated to some degree in the spleen after systemic administration, they were mainly found in the lysosome of immunoregulatory macrophages within the liver, as well as in liver resident macrophages. Nanoparticles were further detected in liver tumors and their accumulation in immunoregulatory macrophages was significantly greater in the periphery than in the tumor core. The study concluded that nanoparti
Overall, these studies established that woodchucks with liver tumors are a useful preclinical animal model for the evaluation of transarterial embolotherapies for the treatment of human HCC. They further demonstrated the feasibility of nanoparticle-based delivery of chemotherapeutics or immunomodulators into tumors and assessment of anticancer effects by CT, MRI, or PET imaging.
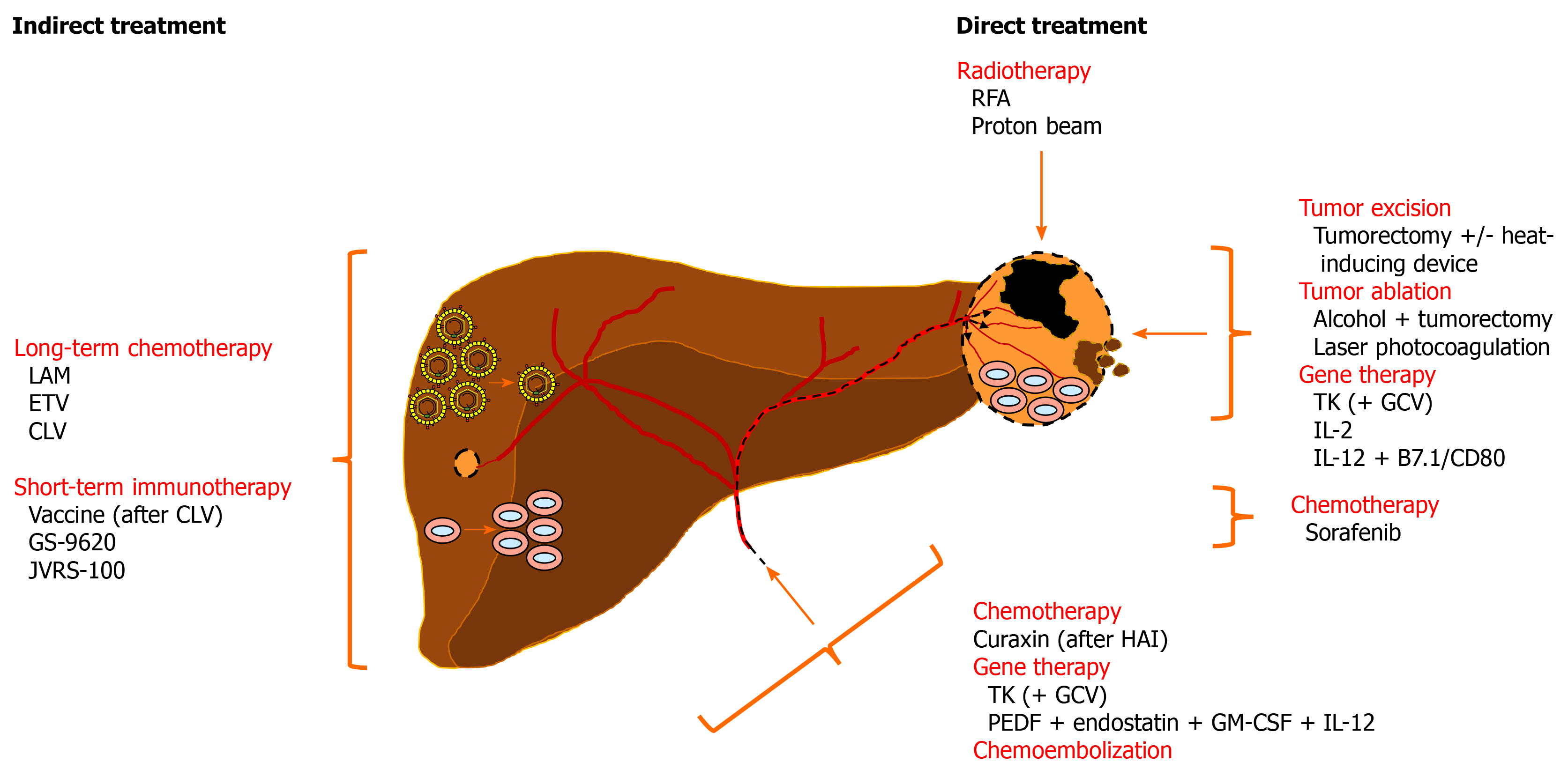
Woodchucks have been utilized in the evaluation of anticancer effects mediated indirectly by treatment with antiviral drugs or immunomodulators and directly by radiotherapy, tumor excision and ablation, gene therapy, or anticancer drugs (Figure 3).
Woodchucks with chronic WHV infection were applied in the preclinical evaluation of antiviral drugs being developed for the treatment of HBV-infected patients [30,35,37,68,163]. Among these drugs, nucleos(t)ide analogs that suppress viral replication in the liver, and thus reduce viremia levels in the periphery, were assessed in woodchucks mainly as a single agent but also in combination (Table 2). Many of these nucleos(t)ide analogs are now approved by national regulatory agencies for administration to patients. While most woodchuck studies were focused on testing nucleos(t)ide analogs for safety and antiviral efficacy during short-term treatment, some studies were extended for the additional evaluation of effects against liver disease progression.
| Antiviral drug | Abbreviation | Brand name | Ref. |
| Adefovir dipivoxil | ADV | Hepsera | [217-219] |
| Clevudine1 | CLV or L-FMAU | Levovir and Revovir | [172,220-222] |
| Emtricitabine | FTC | Coviracil | [219,221,223,224] |
| Entecavir1 | ETV | Baraclude | [171,225-228] |
| Lamivudine1 | LAM or 3TC | Epivir | [164,165,194,219,229] |
| Telbivudine | LdT | Tyzeka | [230-232] |
| Tenofovir alafenamide | TAF | Vemlidy | [233] |
| Tenofovir disoproxil fumarate | TDF | Viread | [219] |
| Valtorcitabine | LdC | [230-232] |
Lifelong, oral treatment of woodchucks with lamivudine, starting at an age of 8 mo and by applying two separate drug doses (i.e., 5 mg/kg/d for approximately 10 mo and then 15 mg/kg/d for up to 50 mo in surviving animals), produced a 4-5 Log10 reduction in viremia and the antiviral effect was sustained for 1⅔ years while treatment continued[164]. Woodchucks experienced a significant delay in the onset of HCC and death due to severe liver cancer. In particular, lamivudine treatment delayed the development of liver tumors by 24 mo (until an animal age of 32 mo) and extended HCC-free survival by 12 mo (until an animal age of 44 mo). However, when oral lamivudine treatment was initiated in older woodchucks at an age of 13-19 mo and with relatively high doses (i.e., 40 mg/kg/d for 3 mo and/or 200 mg/kg/d for up to 15 mo), the shorter treatment duration and the less pronounced antiviral effect (~2.5 Log10 decline in viremia) failed to delay hepatocarcinogenesis[165]. Almost all woodchucks developed liver tumors while receiving lamivudine and needed to be euthanized between 12 and 19 mo of treatment due to end-stage HCC (at an animal age of 26-38 mo). Complicating in both studies was the selection of lamivudine-resistant WHV mutants during treatment. These mutations occurred frequently in the B domain of the WHV polymerase gene[166,167] and were identical to those reported in lamivudine-treated patients, in addition to mutations in the C domain of the HBV polymerase gene[168-170].
Long-term, oral treatment of woodchucks with entecavir for 14 or 36 mo, starting at an animal age of 8 mo and then continuing with a lower dosing frequency from 10 mo of age onward (i.e., 0.5 mg/kg/d for two months and then 0.5 mg/kg/wk for 12 or 34 mo), resulted in a 5-8 Log10 reduction in serum WHV DNA in 60% or 80% of animals, respectively[171]. The levels of serum WHsAg and intrahepatic WHV cccDNA declined alongside and in parallel with the marked reductions in viremia. An emergence of entecavir-resistant mutants was not observed during the study. Since woodchucks with a sustained antiviral effect stayed negative for signs of liver tumors for up to 2⅓ years after drug withdrawal, entecavir treatment prevented the development of liver cancer in a majority of animals (i.e., up to 80% HCC-free survival).
Delayed HCC onset and prolonged survival was also achieved during long-term, oral treatment of woodchucks with clevudine for 32 wk at a dose of 10 mg/kg/d, starting at an animal age of 1-2 years[37,172]. Thereafter, half of the placebo- or clevudine-treated woodchucks received intramuscularly four doses of a conventional, alum-adsorbed WHsAg vaccine that was administered monthly after drug withdrawal. Combination treatment with clevudine and vaccine reduced viremia by up to 9 Log10, with undetectable serum WHV DNA in many animals. The antiviral effect was sustained for more than 1 year after treatment cessation in 75% of woodchucks and prevented HCC onset in 38% of animals. However, once HCC was established, the growth rates (i.e., volume doubling times) of liver tumors were similar to those of control animals. Importantly, initiation of clevudine treatment at an animal age of 1 year, and independent of vaccination, produced a more pronounced anticancer effect than a treatment begin at an animal age of 2 years. The development of liver tumors in the younger cohort of woodchucks was further delayed and HCC-free survival increased after 3 (50% vs 0%) and 4 years (25% vs 0%). Moreover, vaccination of these animals without initial clevudine treatment improved the B- and T-cell responses to WHsAg, the protein on which the vaccine was based, but had no effect on viral replication or liver enzyme levels. In combination with clevudine, however, vaccination enhanced these B- and T-cell responses based on the higher titers of virus-neutralizing antibodies and the greater proliferation capability to stimulation with WHsAg. In addition, combination treatment broadened the antiviral immunity to include T-cell responses to other viral antigens, such as WHcAg, WHeAg, and WHxAg, while liver enzyme levels normalized.
Woodchucks were further applied in the preclinical evaluation of immunomodulating compounds being developed for the treatment of HBV-infected patients. The immunomodulators tested so far in woodchucks suppressed WHV replication in the liver and reduced viremia and antigenemia in the periphery at varying degrees. In some instances, the antiviral effect was sustained after the end of treatment, and seroconversion to antibodies against WHsAg and/or WHeAg was achieved in a subset of animals, indicating that a functional cure was induced. Immunomodulators were administered as single agents but more often in combination with nucleos(t)ide analogs and/or inhibitors of viral gene expression and immune checkpoint markers (Table 3). Comparable to the chemotherapy studies, only two immunotherapy studies were designed or extended to include the assessment of anticancer effects.
| Immunomodulator | Compound name | Brand name | Ref. |
| IFN-α | [192,226,228,229] | ||
| RIG-I/NOD2 agonist | SB 9200 | Inarigivir | [227] |
| TLR7 agonist | GS-96201 | Vesatolimod | [173] |
| APR002 | [225] | ||
| RG7854 | [38] | ||
| TLR8 agonist | GS-9688 | Selgantolimod | [174] |
| TLR9 agonist | CpG-ODN | [234] | |
| TLR9-dependent and -independent pathways | AIC649 | [235] | |
| JVRS-1002 | [175] | ||
| Viral gene expression inhibitor | RG7834 | [226] | |
| Immune checkpoint inhibitor | Anti-PD-L1 | [236] |
Short-term, oral administration of the small molecule GS-9620 targeting toll-like receptor (TLR) 7 induced durable antiviral efficacy in woodchucks treated with different doses and dosing frequencies[173]. In the group with the greatest antiviral effect, animals at an age of 12-14 mo received the agonist every other day for approximately 4 wk, initially at 5 mg/kg and then at 2.5 mg/kg after a treatment interruption for 9-10 d due to liver enzyme increase and thrombocytopenia that both reversed during the dose holiday. Treatment in this group induced a rapid reduction in serum WHV DNA of 6.2 Log10 that was accompanied by declines in intrahepatic WHV cccDNA and undetectable serum WHsAg. Suppressed WHV replication was sustained in all woodchucks during the 31-week follow-up period, and a subset of animals also seroconverted to antibodies against WHsAg during this time. At the end of the study in week 35, all animals were found to be HCC-free during postmortem examination. When combining all woodchucks enrolled in the various treatment groups of this study, and by including only animals that completed treatment and experienced sustained viral suppression, TLR7 agonism reduced the HCC incidence from 71% in placebo-treated control woodchucks to 8% in GS-9620-treated animals. The antiviral and anticancer effects were attributed to the activation of an immune response based on the induction CD8+ T-cells, NK-cells and B-cells, and the production of type I and II interferons in the liver. A follow-up study further indicated that GS-9620 not only targets TLR7 but also TLR8 when administered at high doses[174], possibly explaining the most superior antiviral effect observed so far in the woodchuck animal model with a single agent during short duration treatment.
Intravenous administration of JVRS-100, a complex of non-coding plasmid DNA and cationic liposomes, every second week for 12 wk at two separate doses to woodchucks with liver tumors at an age of 2 years resulted in antiviral and anticancer effects[175]. Since the high serum loads of viral DNA and antigens typically present during chronic WHV infection mediated immune suppression, and thus resistance to treatment, only animals with rather low levels of viremia and antigenemia were enrolled in the study. Serum WHV DNA declined by 0.9 Log10 during JVRS-100 treatment and during the 12-week follow-up period, especially in animals that received the higher dose, but the antiviral effect was transient and less pronounced for WHsAg. Although treatment did not induce a regression of preexisting liver tumors, the higher dose prevented the formation of new tumors for 6 mo. These effects were associated with the activation of immune responses that involved CD4+ and CD8+ T-cells and T helper cell type I (Th1) cytokines, such as IFN-α, tumor necrosis factor-α (TNF-α), and interleukin (IL) 2 and 12 in liver and blood, and that apparently blocked the conversion of virus-induced chronic liver disease into HCC.
Overall, these studies demonstrated that long-term treatment with nucleos(t)ide analogs primarily delays but sometimes prevents liver tumor development in woodchucks. Since these studies established a correlation between suppressed viral replication and reduced liver disease progression, early initiation and prolonged duration of conventional antiviral treatment appear most critical for the prevention of hepatitis virus-induced HCC. Since the applied treatment regimens resulted in less cellular damage and liver injury, they most likely deferred the transformation of altered hepatocytes into liver tumors. Short-term immunomodulation, either rather broad or more targeted, mediated lasting protection against formation of new liver tumors or HCC onset. In two studies, immunomodulation was associated with improved or newly elicited humoral and cellular immune responses to viral antigens that were reduced by treatment, and thus could no longer act as endogenous tolerogens.
Since liver tumors obtain their nutrient blood supply from the hepatic artery[176], hepatic artery infusion- (HAI) supported chemotherapy has been applied for the treatment of both primary and metastatic liver cancers in patients and shown to be an effective treatment for unresectable advanced HCC[177]. Effectiveness of this intervention relates to the concentration of chemotherapeutics in HCCs by direct delivery to the tumors, with limited systemic exposure in the liver[178]. In one woodchuck study, HAI ports were placed in the gastroduodenal artery and infused with a curaxin-based experimental anticancer drug, once per week for 3 wk at a dose of 17 mg/kg[179]. Curaxin targets a histone chaperon expressed at high levels in cancer[180] and activates the p53 tumor suppressor gene, while it simultaneously suppresses inhibition of NF-κB[181]. Tumor growth in woodchucks was suppressed after repeated treatment and the anticancer effect was associated with increases in intratumoral T-cell infiltration and tumor cell apoptosis.
Woodchucks were also applied for testing the preventive effect of long-term, oral treatment with sorafenib[182]. Sorafenib, a small molecule receptor inhibitor of several surface tyrosine kinases, is a standard first-line therapy approved for the treatment of human HCC. Although this drug has both proapoptotic and antiangiogenic properties, the treatment benefit of sorafenib is modest, as only a 3-mo improvement in the overall survival is achieved and its indication is restricted to patients with well-preserved liver function[183]. The underlying mechanism of sorafenib-mediated anticancer activity has not been fully elucidated. Sorafenib was administered daily to woodchucks at two separate doses (i.e., 2.5 mg/kg and 5 mg/kg) using a 5-d-on and 2-d-off schedule until tumor development was observed. Although all animals presented with liver tumors independent of the sorafenib dose applied, the lower dose was associated with smaller initial tumor volumes and delayed tumor growth that was associated with an increase in intratumoral CD3+ T-cell infiltration. An effect of sorafenib on chronic WHV infection was not noted. Interestingly, short-term, oral, daily sorafenib administration for 90 d was unable to reciprocate the anticancer effect obtained during long-term treatment. The study concluded that sorafenib has immunomodulatory activity that is dependent on the dose and treatment duration. Caution, however, is warranted when applying higher doses of sorafenib, because of its immunosuppressive function that relates to an increased activity of nuclear factor of activated T-cells 1 (NFAT1) and results in the in vitro inhibition of T-cell proliferation and in an increase in programmed cell death protein 1 (PD-1) expression of CD8+ T-cells, as demonstrated in woodchucks.
Woodchucks with liver tumors were further used to evaluate different ablation techniques for human HCC. One study demonstrated the feasibility of tumor excision, percutaneous alcohol ablation followed by tumorectomy, and laser photocoagulation in this animal model[86]. Extended survival for up to 16-18 mo was achieved with the first two modalities, but multiple tumor recurrence distant from the resection area occurred ultimately in all animals. A second study investigated the effect of a saline-linked dissecting sealer on the remaining tumor beds (i.e., in situ margins) after initial removal of neoplasms by tumorectomy[184]. Surface application of this device induced a heat zone area of up to 5 mm in depth, inside which residual tumor cells, if present, were efficiently destroyed, suggesting that this approach could be beneficial in reducing marginal recurrence after tumor resection. A third study tested radiofrequency ablation (RFA) using a low energy protocol and a 1.0 cm probe that produced a consistent burn area within liver tumors, as determined by necrosis of tumor cells, but was unable to fully ablate larger lesions[185]. A final study assessed the effectiveness of passive scattering proton beams with high dose fractionation[186]. Three fractionations were applied every other day within one week to the hepatic neoplasm. A partial regression of the treated liver tumor was noticed at week 3 post-treatment, which continued until the nodule disappeared at week 9, as also confirmed during postmortem evaluation one week later. The study concluded that a delayed but complete imaging response to proton beam treatment of HCC was achieved in woodchucks without visible gastrointestinal toxicity.
Gene therapeutic strategies based on the induction of apoptosis, antiangiogenesis, or anticancer immune response were assessed in woodchucks for the treatment of human HCC. In one study, an adenoviral vector encoding for the thymidine kinase (TK) of herpes simplex virus under the control of the ubiquitous cytomegalovirus promoter for conferring sensitivity to ganciclovir (GCV) treatment was administered to liver tumors either directly or indirectly via the hepatic artery[187]. Transduction of tumor cells and subsequent drug administration resulted in an anticancer effect in two woodchucks that was mediated by GCV-induced apoptosis; however, a third animal died due to acute liver failure that was attributed to the transduction of adjacent, nonneoplastic hepatocytes. Although tumor regression was not achieved, necrotic areas were present in tumors one week after treatment. The study emphasized the need to make vector transduction more specific to liver tumor cells by controlling TK expression with HCC-specific promoters, such as the AFP promoter.
Two other studies tested the anticancer activity mediated by the cytokine IL-12. In the first study, murine IL-12 was expressed from a replication-competent Semliki Forest virus (SFV) vector[188]. Use of this vector has the advantage that the antitumor effect mediated by the cytokine is enhanced via the induction of apoptosis in tumor cells that replicate SFV. A single, intratumoral injection of the vector at increasing doses during laparotomy produced a dose-dependent tumor regression that was 80% with the highest dose. Correlating with the temporary IL-12 expression, partial tumor remission was transient and neoplasms began to regrow between 6 and 14 wk after treatment. In addition, all animals experienced a temporary reduction in serum viremia and/or antigenemia. The anticancer and antiviral effects were associated with augmented T-cell responses to tumor and viral antigens, as well as increased expression of CD4 and CD8 markers and IFN-γ and TNF-α in peripheral blood mononuclear cells. In the second study, a single dose of an adenoviral vector encoding for murine IL-12 and the costimulatory B7.1/CD80 molecule for activating T-cells was injected into liver tumors during laparotomy or under MRI guidance[189]. Transduction of tumor cells resulted in a tumor regression of 80% on average, with one animal experiencing an almost complete tumor elimination within 7 wk. Regression was associated with the induction of an anticancer immune response, as demonstrated by a massive infiltration of CD4+ and CD8+ T-cells into tumors and an increase in intratumoral IFN-γ production. The long-term anticancer effect could not be evaluated, as almost all animals were euthanized two weeks after treatment.
A final study investigated the anticancer effect mediated by antiangiogenic proteins and cytokines in woodchucks[190]. Single dose treatment via the hepatic artery with an adenoviral vector encoding for human pigment epithelium-derived factor (PEDF) and endostatin in combination with an adenoviral vector for the expression of woodchuck granulocyte-macrophage colony-stimulating factor (GM-CSF) and murine IL-12 induced a tumor regression of 90%. The partial tumor remission obtained by combination treatment was superior to the 56% and 76% reduction in tumor volume that was achieved by treatment with antiangiogenic proteins or cytokines alone, respectively. An antiviral effect was not noted during the study and serum viremia and antigenemia remained unchanged in all animals. The tumor regression induced by combination treatment was attributed to several factors, including increased infiltration of CD3+ T-cells into tumors, high intratumoral levels of NK-cells, apoptosis of tumor cells, reductions in tumor vasculature (i.e., reduced microvessel density), and declines in immune checkpoint markers [i.e., PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)] most likely present on regulatory or immunotolerant T-cells within tumors. Since animals were only followed for two weeks after treatment, the durability of the anticancer effect could not be evaluated.
Overall, these studies established that chemotherapy, radiotherapy, and gene therapy of liver tumors are effective means for the treatment of hepatitis virus-induced HCC in woodchucks. Since some studies established a correlation between anticancer immune response and partial tumor remission, approaches which are based on immunomodulation or checkpoint inhibition for inducing functional cure of chronic HBV infection, appear promising and should further be evaluated in woodchucks for treatment of human HCC.
WHV-infected, immunocompetent woodchucks are used to model chronic HBV infection and HCC in humans. Over the past four decades, woodchucks have been applied in the investigation of mechanisms involved in viral immunopathogenesis and hepatocarcinogenesis, in the development of new contrast agents to enhance the detection of hepatic neoplasms by various imaging techniques, in the improvement of tumor ablation strategies based on transarterial embolization and radiotherapy, and in the evaluation of therapeutic interventions directed against the severe outcome of hepatitis virus-induced liver disease. Although the latter was only assessed in a limited number of studies, in which liver tumors were targeted by indirect and direct means, the continued application of woodchucks will support not only the many efforts to cure chronic HBV infection by new antivirals and immunomodulators, but also to treat the associated disease sequelae. Future studies can take advantage of the recently identified woodchuck transcriptome[79,191,192] and genome[193] for generating all needed protein-based markers and assays, as well as of the translational value of woodchucks in predicting therapeutic efficacy against chronic HBV infection in patients[174,192,194]. Thus, chronic WHV carrier woodchucks progressing to HCC within a reasonable time will greatly aid the development and evaluation of the safety and efficacy of new anticancer prophylaxis or therapy in a relevant animal model. Increased testing of anticancer approaches in the woodchuck animal model will ultimately improve the chances for prevention and therapy of HBV-induced HCC.
In memory of Dr. Bud Tennant of Cornell University. We gratefully acknowledge Drs. John Gerin and Paul Cote of Georgetown University and Diana Berard and Dr. Rajen Koshy of the National Institute of Allergy and Infectious Diseases for encouragement and intellectual support.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: National Cancer Institute, No. 1-R43-CA133993-01; National Institute of Allergy and Infectious Diseases, No. N01-AI-05399; and National Institute of Allergy and Infectious Diseases, No. HHSN272201000011I.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K S-Editor: Gong ZM L-Editor: A P-Editor: Li JH
| 1. | Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Tan A, Koh S, Bertoletti A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb Perspect Med. 2015;5:a021428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 3. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 410] [Article Influence: 68.3] [Reference Citation Analysis (1)] |
| 4. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors; Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 302] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 5. | WHO. Hepatitis B. [accessed January 23, 2021] Available from: https://wwwwhoint/en/news-room/fact-sheets/detail/hepatitis-b. [Cited in This Article: ] |
| 6. | Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96:e5904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 312] [Article Influence: 62.4] [Reference Citation Analysis (1)] |
| 8. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2290] [Cited by in F6Publishing: 2247] [Article Influence: 374.5] [Reference Citation Analysis (0)] |
| 9. | Alter H, Block T, Brown N, Brownstein A, Brosgart C, Chang KM, Chen PJ, Chisari FV, Cohen C, El-Serag H, Feld J, Gish R, Glenn J, Greten T, Guo H, Guo JT, Hoshida Y, Hu J, Kowdley KV, Li W, Liang J, Locarnini S, Lok AS, Mason W, McMahon B, Mehta A, Perrillo R, Revill P, Rice CM, Rinaudo J, Schinazi R, Seeger C, Shetty K, Tavis J, Zoulim F. A research agenda for curing chronic hepatitis B virus infection. Hepatology. 2018;67:1127-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. J Hepatol. 2017;67:847-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 155] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 11. | Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237-257. [PubMed] [Cited in This Article: ] |
| 12. | Paul SB, Manjunatha YC, Acharya SK. Palliative treatment in advanced hepatocellular carcinoma: has it made any difference? Trop Gastroenterol. 2009;30:125-134. [PubMed] [Cited in This Article: ] |
| 13. | Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Daher S, Massarwa M, Benson AA, Khoury T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J Clin Transl Hepatol. 2018;6:69-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 15. | Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT. Management and Treatment of Hepatocellular Carcinoma with Immunotherapy: A Review of Current and Future Options. J Clin Transl Hepatol. 2020;8:168-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Saffo S, Taddei TH. Systemic Management for Advanced Hepatocellular Carcinoma: A Review of the Molecular Pathways of Carcinogenesis, Current and Emerging Therapies, and Novel Treatment Strategies. Dig Dis Sci. 2019;64:1016-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lu LC, Cheng AL, Poon RT. Recent advances in the prevention of hepatocellular carcinoma recurrence. Semin Liver Dis. 2014;34:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene. 2010;29:2309-2324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19780] [Article Influence: 2197.8] [Reference Citation Analysis (17)] |
| 20. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264-1273. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2337] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 21. | Nam SW, Jung JJ, Bae SH, Choi JY, Yoon SK, Cho SH, Han JY, Han NI, Yang JM, Lee YS. Clinical outcomes of delayed clearance of serum HBsAG in patients with chronic HBV infection. Korean J Intern Med. 2007;22:73-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Bellezza CA, Sexton S, Curtin LI, Concannon PW, Baldwin BH, Graham LA, Hornbuckle WE, Roth L, Tennant BC. The laboratory woodchuck (Marmota monax). In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT (eds). American College of Laboratory Animal Medicine, Laboratory Animal Medicine. 3rd ed. Cambridge (MA): Academic Press, 2015: 351-386. [DOI] [Cited in This Article: ] |
| 23. | Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533-4537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 483] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981;1:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 149] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Galibert F, Chen TN, Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982;41:51-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 244] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Girones R, Cote PJ, Hornbuckle WE, Tennant BC, Gerin JL, Purcell RH, Miller RH. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci USA. 1989;86:1846-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Mason WS. Animal models and the molecular biology of hepadnavirus infection. Cold Spring Harb Perspect Med. 2015;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 556] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 29. | Gust ID, Burrell CJ, Coulepis AG, Robinson WS, Zuckerman AJ. Taxonomic classification of human hepatitis B virus. Intervirology. 1986;25:14-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 127] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Popper H, Roth L, Purcell RH, Tennant BC, Gerin JL. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 202] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Snyder RL, Tyler G, Summers J. Chronic hepatitis and hepatocellular carcinoma associated with woodchuck hepatitis virus. Am J Pathol. 1982;107:422-425. [PubMed] [Cited in This Article: ] |
| 33. | Cote PJ, Korba BE, Miller RH, Jacob JR, Baldwin BH, Hornbuckle WE, Purcell RH, Tennant BC, Gerin JL. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000;31:190-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Roggendorf M, Kosinska AD, Liu J, Lu M. The Woodchuck, a Nonprimate Model for Immunopathogenesis and Therapeutic Immunomodulation in Chronic Hepatitis B Virus Infection. Cold Spring Harb Perspect Med. 2015;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Tennant BC, Gerin JL. The woodchuck model of hepatitis B virus infection. ILAR J. 2001;42:89-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Cote PJ, Korba BE, Tennant BC, Gerin JL. Immunopathogenesis and immunomodulation of woodchuck hepatitis virus infection. In: Hollinger FB, Lemon SM, Margolis HS (eds). Viral hepatitis and liver disease. Baltimore (MD): Lippincott Williams & Wilkins, 1991: 483-486. [Cited in This Article: ] |
| 37. | Tennant BC, Toshkov IA, Peek SF, Jacob JR, Menne S, Hornbuckle WE, Schinazi RD, Korba BE, Cote PJ, Gerin JL. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology. 2004;127:S283-S293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Suslov A, Wieland S, Menne S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr Opin Virol. 2018;30:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Kosinska AD, Liu J, Lu M, Roggendorf M. Therapeutic vaccination and immunomodulation in the treatment of chronic hepatitis B: preclinical studies in the woodchuck. Med Microbiol Immunol. 2015;204:103-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Michalak TI. Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B. Front Immunol. 2020;11:853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Cote PJ, Toshkov I, Bellezza C, Ascenzi M, Roneker C, Ann Graham L, Baldwin BH, Gaye K, Nakamura I, Korba BE, Tennant BC, Gerin JL. Temporal pathogenesis of experimental neonatal woodchuck hepatitis virus infection: increased initial viral load and decreased severity of acute hepatitis during the development of chronic viral infection. Hepatology. 2000;32:807-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Wong DC, Shih JW, Purcell RH, Gerin JL, London WT. Natural and experimental infection of woodchucks with woodchuck hepatitis virus, as measured by new, specific assays for woodchuck surface antigen and antibody. J Clin Microbiol. 1982;15:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Millman I, Southam L, Halbherr T, Simmons H, Kang CM. Woodchuck hepatitis virus: experimental infection and natural occurrence. Hepatology. 1984;4:817-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Tyler GV, Snyder RL, Summers J. Experimental infection of the woodchuck (Marmota monax monax) with woodchuck hepatitis virus. Lab Invest. 1986;55:51-55. [PubMed] [Cited in This Article: ] |
| 45. | Kajino K, Jilbert AR, Saputelli J, Aldrich CE, Cullen J, Mason WS. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792-5803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Guy CS, Mulrooney-Cousins PM, Churchill ND, Michalak TI. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J Virol. 2008;82:8579-8591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669-6674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 48. | Suresh M, Czerwinski S, Murreddu MG, Kallakury BV, Ramesh A, Gudima SO, Menne S. Innate and adaptive immunity associated with resolution of acute woodchuck hepatitis virus infection in adult woodchucks. PLoS Pathog. 2019;15:e1008248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Hüser N, Durantel D, Liang TJ, Münk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 694] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 50. | Wieland SF, Eustaquio A, Whitten-Bauer C, Boyd B, Chisari FV. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc Natl Acad Sci USA. 2005;102:9913-9917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, Chou WM, Wettengel JM, Malo A, Bohne F, Hoffmann D, Eyer F, Thimme R, Falk CS, Thasler WE, Heikenwalder M, Protzer U. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 52. | Xu C, Guo H, Pan XB, Mao R, Yu W, Xu X, Wei L, Chang J, Block TM, Guo JT. Interferons accelerate decay of replication-competent nucleocapsids of hepatitis B virus. J Virol. 2010;84:9332-9340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Liu Y, Nie H, Mao R, Mitra B, Cai D, Yan R, Guo JT, Block TM, Mechti N, Guo H. Interferon-inducible ribonuclease ISG20 inhibits hepatitis B virus replication through directly binding to the epsilon stem-loop structure of viral RNA. PLoS Pathog. 2017;13:e1006296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 54. | Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74:4165-4173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Wieland SF, Spangenberg HC, Thimme R, Purcell RH, Chisari FV. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc Natl Acad Sci USA. 2004;101:2129-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 56. | Anderson AL, Banks KE, Pontoglio M, Yaniv M, McLachlan A. Alpha/beta interferon differentially modulates the clearance of cytoplasmic encapsidated replication intermediates and nuclear covalently closed circular hepatitis B virus (HBV) DNA from the livers of hepatocyte nuclear factor 1alpha-null HBV transgenic mice. J Virol. 2005;79:11045-11052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Hong X, Luckenbaugh L, Perlman D, Revill PA, Wieland SF, Menne S, Hu J. Characterization and Application of Precore/Core-Related Antigens in Animal Models of Hepatitis B Virus Infection. Hepatology. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Hodgson PD, Michalak TI. Augmented hepatic interferon gamma expression and T-cell influx characterize acute hepatitis progressing to recovery and residual lifelong virus persistence in experimental adult woodchuck hepatitis virus infection. Hepatology. 2001;34:1049-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Guo JT, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, Barrasa MI, Mason WS, Seeger C. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74:1495-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Hodgson PD, Grant MD, Michalak TI. Perforin and Fas/Fas ligand-mediated cytotoxicity in acute and chronic woodchuck viral hepatitis. Clin Exp Immunol. 1999;118:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Korba BE, Cote PJ, Wells FV, Baldwin B, Popper H, Purcell RH, Tennant BC, Gerin JL. Natural history of woodchuck hepatitis virus infections during the course of experimental viral infection: molecular virologic features of the liver and lymphoid tissues. J Virol. 1989;63:1360-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Korba BE, Cote PJ, Gerin JL. Mitogen-induced replication of woodchuck hepatitis virus in cultured peripheral blood lymphocytes. Science. 1988;241:1213-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Coffin CS, Michalak TI. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J Clin Invest. 1999;104:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Michalak TI, Pardoe IU, Coffin CS, Churchill ND, Freake DS, Smith P, Trelegan CL. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Mason WS, Jilbert AR, Summers J. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci USA. 2005;102:1139-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Summers J, Jilbert AR, Yang W, Aldrich CE, Saputelli J, Litwin S, Toll E, Mason WS. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci USA. 2003;100:11652-11659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Chauhan R, Churchill ND, Mulrooney-Cousins PM, Michalak TI. Initial sites of hepadnavirus integration into host genome in human hepatocytes and in the woodchuck model of hepatitis B-associated hepatocellular carcinoma. Oncogenesis. 2017;6:e317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Tennant BC. Animal models of hepadnavirus-associated hepatocellular carcinoma. Clin Liver Dis. 2001;5:43-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Hong X, Luckenbaugh L, Mendenhall M, Walsh R, Cabuang L, Soppe S, Revill PA, Burdette D, Feierbach B, Delaney W, Hu J. Characterization of Hepatitis B Precore/Core-Related Antigens. J Virol. 2021;95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Ponzetto A, Forzani B. Animal models of hepatocellular carcinoma: hepadnavirus-induced liver cancer in woodchucks. Ital J Gastroenterol. 1991;23:491-493. [PubMed] [Cited in This Article: ] |
| 71. | Seeger C, Baldwin B, Hornbuckle WE, Yeager AE, Tennant BC, Cote P, Ferrell L, Ganem D, Varmus HE. Woodchuck hepatitis virus is a more efficient oncogenic agent than ground squirrel hepatitis virus in a common host. J Virol. 1991;65:1673-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Korba BE, Wells FV, Baldwin B, Cote PJ, Tennant BC, Popper H, Gerin JL. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989;9:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Cote PJ, Gerin JL. The woodchuck as a model of hepadnavirus infection, pathogenesis and therapy. Forum Trends Exp Clin Med. 1996;6:131-159. [Cited in This Article: ] |
| 74. | Tennant BC, Gerin JL. The woodchuck model of hepatitis B virus infection. In: Arias IM, Boyer J, Fausto N, Jakoby WB, Schachter D, Shafritz DA (eds). The liver: Biology and pathology. 3rd ed. New York (NY): Raven Press, 1994: 1455-1466. [Cited in This Article: ] |
| 75. | Nakamura I, Nupp JT, Cowlen M, Hall WC, Tennant BC, Casey JL, Gerin JL, Cote PJ. Pathogenesis of experimental neonatal woodchuck hepatitis virus infection: chronicity as an outcome of infection is associated with a diminished acute hepatitis that is temporally deficient for the expression of interferon gamma and tumor necrosis factor-alpha messenger RNAs. Hepatology. 2001;33:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Menne S, Jacob JR, Tennant BC, Gerin JL, Cote PJ. Role of type 1 versus type 2 immune responses in liver during the onset of chronic woodchuck hepatitis virus infection. Hepatology. 2003;37:771-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 78. | Ferrari C. HBV and the immune response. Liver Int. 2015;35 Suppl 1:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 126] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 79. | Fletcher SP, Chin DJ, Ji Y, Iniguez AL, Taillon B, Swinney DC, Ravindran P, Cheng DT, Bitter H, Lopatin U, Ma H, Klumpp K, Menne S. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology. 2012;56:820-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 80. | Michalak TI, Hodgson PD, Churchill ND. Posttranscriptional inhibition of class I major histocompatibility complex presentation on hepatocytes and lymphoid cells in chronic woodchuck hepatitis virus infection. J Virol. 2000;74:4483-4494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Guy CS, Wang J, Michalak TI. Hepatocytes as cytotoxic effector cells can induce cell death by CD95 ligand-mediated pathway. Hepatology. 2006;43:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Guy CS, Rankin SL, Wang J, Michalak TI. Hepatocytes can induce death of contacted cells via perforin-dependent mechanism. Hepatology. 2008;47:1691-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Zhang E, Zhang X, Liu J, Wang B, Tian Y, Kosinska AD, Ma Z, Xu Y, Dittmer U, Roggendorf M, Yang D, Lu M. The expression of PD-1 ligands and their involvement in regulation of T cell functions in acute and chronic woodchuck hepatitis virus infection. PLoS One. 2011;6:e26196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Sitia G, Isogawa M, Kakimi K, Wieland SF, Chisari FV, Guidotti LG. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13717-13722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Zhang JY, Zou ZS, Huang A, Zhang Z, Fu JL, Xu XS, Chen LM, Li BS, Wang FS. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS One. 2011;6:e17484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 86. | Gouillat C, Manganas D, Zoulim F, Vitrey D, Saguier G, Guillaud M, Ain JF, Duque-Campos R, Jamard C, Praves M, Trepo C. Woodchuck hepatitis virus-induced carcinoma as a relevant natural model for therapy of human hepatoma. J Hepatol. 1997;26:1324-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Popper H, Shih JW, Gerin JL, Wong DC, Hoyer BH, London WT, Sly DL, Purcell RH. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. Hepatology. 1981;1:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Mi LJ, Patil J, Hornbuckle WE, Cote PJ, Gerin JL, Tennant BC, Paronetto F. DNA ploidy analysis of hepatic preneoplastic and neoplastic lesions in woodchucks experimentally infected with woodchuck hepatitis virus. Hepatology. 1994;20:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 89. | Fourel G, Trepo C, Bougueleret L, Henglein B, Ponzetto A, Tiollais P, Buendia MA. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990;347:294-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 204] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Kaneko S, Oshima T, Kodama K, Aoyama S, Yoshikawa H, Unoura M, Fukuoka K, Matsushita F, Morimoto H, Kobayashi K. Stable integration of woodchuck hepatitis virus DNA in transplanted tumors and established tissue culture cells derived from a woodchuck primary hepatocellular carcinoma. Cancer Res. 1986;46:3608-3613. [PubMed] [Cited in This Article: ] |
| 91. | Fuchs K, Heberger C, Weimer T, Roggendorf M. Characterization of woodchuck hepatitis virus DNA and RNA in the hepatocellular carcinomas of woodchucks. Hepatology. 1989;10:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Popper H, Shafritz DA, Hoofnagle JH. Relation of the hepatitis B virus carrier state to hepatocellular carcinoma. Hepatology. 1987;7:764-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 93. | Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 502] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 94. | Roth L, King JM, Hornbuckle WE, Harvey HJ, Tennant BC. Chronic hepatitis and hepatocellular carcinoma associated with persistent woodchuck hepatitis virus infection. Vet Pathol. 1985;22:338-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Nada T, Moriyasu F, Kono Y, Suginoshita Y, Matsumura T, Kobayashi K, Nakamura T, Chiba T. Sonographic detection of tumor blood flow using a new contrast agent in woodchuck hepatomas. J Ultrasound Med. 1997;16:485-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, Kanwal F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 97. | Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MM, Dubinsky TJ, Kolokythas O, Lalwani N. Hepatocellular carcinoma in the noncirrhotic liver. AJR Am J Roentgenol. 2014;203:W34-W47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Abe K, Kurata T, Shikata T, Tennant BC. Enzyme-altered liver cell foci in woodchucks infected with woodchuck hepatitis virus. Jpn J Cancer Res. 1988;79:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Toshkov I, Hacker HJ, Roggendorf M, Bannasch P. Phenotypic patterns of preneoplastic and neoplastic hepatic lesions in woodchucks infected with woodchuck hepatitis virus. J Cancer Res Clin Oncol. 1990;116:581-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Radaeva S, Li Y, Hacker HJ, Burger V, Kopp-Schneider A, Bannasch P. Hepadnaviral hepatocarcinogenesis: in situ visualization of viral antigens, cytoplasmic compartmentation, enzymic patterns, and cellular proliferation in preneoplastic hepatocellular lineages in woodchucks. J Hepatol. 2000;33:580-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Tu T, Mason WS, Clouston AD, Shackel NA, McCaughan GW, Yeh MM, Schiff ER, Ruszkiewicz AR, Chen JW, Harley HA, Stroeher UH, Jilbert AR. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J Viral Hepat. 2015;22:737-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 102. | Mason WS, Liu C, Aldrich CE, Litwin S, Yeh MM. Clonal expansion of normal-appearing human hepatocytes during chronic hepatitis B virus infection. J Virol. 2010;84:8308-8315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Cullen JM, Linzey DW, Gebhard DH. Nuclear ploidy of normal and neoplastic hepatocytes from woodchuck hepatitis virus-infected and uninfected woodchucks. Hepatology. 1994;19:1072-1078. [PubMed] [Cited in This Article: ] |
| 104. | Jacob JR, Sterczer A, Toshkov IA, Yeager AE, Korba BE, Cote PJ, Buendia MA, Gerin JL, Tennant BC. Integration of woodchuck hepatitis and N-myc rearrangement determine size and histologic grade of hepatic tumors. Hepatology. 2004;39:1008-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 652] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 106. | Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-7392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 753] [Cited by in F6Publishing: 851] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 107. | Marion PL, Van Davelaar MJ, Knight SS, Salazar FH, Garcia G, Popper H, Robinson WS. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci USA. 1986;83:4543-4546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Jayant K, Habib N, Huang KW, Warwick J, Arasaradnam R. Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma. Diagnostics (Basel). 2020;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 286] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Terradillos O, Billet O, Renard CA, Levy R, Molina T, Briand P, Buendia MA. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene. 1997;14:395-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 225] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Madden CR, Finegold MJ, Slagle BL. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851-3858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Feitelson MA, Lee J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007;252:157-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 113. | Fowler MJ, Greenfield C, Chu CM, Karayiannis P, Dunk A, Lok AS, Lai CL, Yeoh EK, Monjardino JP, Wankya BM. Integration of HBV-DNA may not be a prerequisite for the maintenance of the state of malignant transformation. An analysis of 110 liver biopsies. J Hepatol. 1986;2:218-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A, Kennedy PT. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology 2016; 151: 986-998. e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 115. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 571] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 116. | Chauhan R, Michalak TI. Kinetics of DNA damage repair response accompanying initial hepadnavirus-host genomic integration in woodchuck hepatitis virus infection of hepatocyte. Cancer Genet. 2020;244:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Freitas N, Lukash T, Gunewardena S, Chappell B, Slagle BL, Gudima SO. Relative Abundance of Integrant-Derived Viral RNAs in Infected Tissues Harvested from Chronic Hepatitis B Virus Carriers. J Virol. 2018;92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Freitas N, Cunha C, Menne S, Gudima SO. Envelope proteins derived from naturally integrated hepatitis B virus DNA support assembly and release of infectious hepatitis delta virus particles. J Virol. 2014;88:5742-5754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Wei Y, Fourel G, Ponzetto A, Silvestro M, Tiollais P, Buendia MA. Hepadnavirus integration: mechanisms of activation of the N-myc2 retrotransposon in woodchuck liver tumors. J Virol. 1992;66:5265-5276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Hansen LJ, Tennant BC, Seeger C, Ganem D. Differential activation of myc gene family members in hepatic carcinogenesis by closely related hepatitis B viruses. Mol Cell Biol. 1993;13:659-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Bruni R, Conti I, Villano U, Giuseppetti R, Palmieri G, Rapicetta M. Lack of WHV integration nearby N-myc2 and in the downstream b3n and win loci in a considerable fraction of liver tumors with activated N-myc2 from naturally infected wild woodchucks. Virology. 2006;345:258-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Bruni R, D'Ugo E, Villano U, Fourel G, Buendia MA, Rapicetta M. The win locus involved in activation of the distal N-myc2 gene upon WHV integration in woodchuck liver tumors harbors S/MAR elements. Virology. 2004;329:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Yang D, Faris R, Hixson D, Affigne S, Rogler CE. Insulin-like growth factor II blocks apoptosis of N-myc2-expressing woodchuck liver epithelial cells. J Virol. 1996;70:6260-6268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Ueda K, Ganem D. Apoptosis is induced by N-myc expression in hepatocytes, a frequent event in hepadnavirus oncogenesis, and is blocked by insulin-like growth factor II. J Virol. 1996;70:1375-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Renard CA, Fourel G, Bralet MP, Degott C, De La Coste A, Perret C, Tiollais P, Buendia MA. Hepatocellular carcinoma in WHV/N-myc2 transgenic mice: oncogenic mutations of beta-catenin and synergistic effect of p53 null alleles. Oncogene. 2000;19:2678-2686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K, Kaneko S. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009;49:1098-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 127. | Connolly E, Melegari M, Landgraf P, Tchaikovskaya T, Tennant BC, Slagle BL, Rogler LE, Zavolan M, Tuschl T, Rogler CE. Elevated expression of the miR-17-92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am J Pathol. 2008;173:856-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 128. | Chen HS, Kaneko S, Girones R, Anderson RW, Hornbuckle WE, Tennant BC, Cote PJ, Gerin JL, Purcell RH, Miller RH. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J Virol. 1993;67:1218-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 300] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 129. | Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011;46:974-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 130. | Wang WH, Studach LL, Andrisani OM. Proteins ZNF198 and SUZ12 are down-regulated in hepatitis B virus (HBV) X protein-mediated hepatocyte transformation and in HBV replication. Hepatology. 2011;53:1137-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Studach LL, Menne S, Cairo S, Buendia MA, Hullinger RL, Lefrançois L, Merle P, Andrisani OM. Subset of Suz12/PRC2 target genes is activated during hepatitis B virus replication and liver carcinogenesis associated with HBV X protein. Hepatology. 2012;56:1240-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, Seo DD, Jang MK, Yu E, Kim KW. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Li YT, Liu CJ, Su TH, Cheng HR, Jeng YM, Lin HL, Wang CC, Kao JH, Chen PJ, Chen DS, Wu HL. Characterization of metastatic tumor antigen 1 and its interaction with hepatitis B virus X protein in NF-κB signaling and tumor progression in a woodchuck hepatocellular carcinoma model. Oncotarget. 2016;7:47173-47185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Tseng PL, Tai MH, Huang CC, Wang CC, Lin JW, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, Changchien CS, Hu TH. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Finn RS, Zhu AX. Targeting angiogenesis in hepatocellular carcinoma: focus on VEGF and bevacizumab. Expert Rev Anticancer Ther. 2009;9:503-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 136. | Huang H, Salavaggione O, Rivera L, Mukherjee S, Brekken R, Tennant B, Iyer R, Adjei A. Woodchuck VEGF (wVEGF) characteristics: Model for angiogenesis and human hepatocellular carcinoma directed therapies. Arch Biochem Biophys. 2019;661:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 137. | Altadill A, Rodríguez M, González LO, Junquera S, Corte MD, González-Dieguez ML, Linares A, Barbón E, Fresno-Forcelledo M, Rodrigo L, Vizoso FJ. Liver expression of matrix metalloproteases and their inhibitors in hepatocellular carcinoma. Dig Liver Dis. 2009;41:740-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 138. | Ochoa-Callejero L, Toshkov I, Menne S, Martínez A. Expression of matrix metalloproteinases and their inhibitors in the woodchuck model of hepatocellular carcinoma. J Med Virol. 2013;85:1127-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Tao K, Qian N, Tang Y, Ti Z, Song W, Cao D, Dou K. Increased expression of a disintegrin and metalloprotease-9 in hepatocellular carcinoma: implications for tumor progression and prognosis. Jpn J Clin Oncol. 2010;40:645-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 226] [Cited by in F6Publishing: 230] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 141. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1665] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 142. | Rizzetto M. Hepatitis D Virus: Introduction and Epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 143. | Taylor JM. Virology of hepatitis D virus. Semin Liver Dis. 2012;32:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 144. | Freitas N, Salisse J, Cunha C, Toshkov I, Menne S, Gudima SO. Hepatitis delta virus infects the cells of hepadnavirus-induced hepatocellular carcinoma in woodchucks. Hepatology. 2012;56:76-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 145. | Shiga J, Ohnishi S, Imawari M, Yamamoto K, Koshimizu K, Sasaki N. Development and growth pattern of small hepatocellular carcinomas in woodchucks--analysis of an animal model of human hepatocellular carcinoma by ultrasonography. Jikken Dobutsu. 1991;40:545-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 146. | Lisi D, Kondili LA, Ramieri MT, Giuseppetti R, Bruni R, Della Rocca C, De Santis A, Rapicetta M. Ultrasonography in the study of hepatocellular carcinoma in woodchucks chronically infected with WHV. Lab Anim. 2003;37:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | McKenzie EJ, Jackson M, Sun J, Volotovskyy V, Gruwel ML. Monitoring the development of hepatocellular carcinoma in woodchucks using 31P-MRS. MAGMA. 2005;18:201-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 148. | McKenzie EJ, Jackson M, Turner A, Gregorash L, Harapiak L. Chronic care and monitoring of woodchucks (Marmota monax) during repeated magnetic resonance imaging of the liver. J Am Assoc Lab Anim Sci. 2006;45:26-30. [PubMed] [Cited in This Article: ] |
| 149. | Hornbuckle WE, Graham ES, Roth L, Baldwin BH, Wickenden C, Tennant BC. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax). Lab Anim Sci. 1985;35:376-381. [PubMed] [Cited in This Article: ] |
| 150. | Cote PJ, Gerin JL, Tennant BC. Alpha-fetoprotein in the woodchuck model of hepadnavirus infection and disease: normal physiological patterns and responses to woodchuck hepatitis virus infection and hepatocellular carcinoma. Cancer Res. 1990;50:7843-7851. [PubMed] [Cited in This Article: ] |
| 151. | Dahmen U, Radtke A, Schröder T, Chi H, Madrahimov N, Lu M, Schenk A, Peitgen KH, Dirsch O. Median liver lobe of woodchuck as a model to study hepatic outflow obstruction: a pilot study. Liver Int. 2008;28:1236-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 152. | Mikhail AS, Mauda-Havakuk M, Partanen A, Karanian JW, Pritchard WF, Wood BJ. Liver-specific 3D sectioning molds for correlating in vivo CT and MRI with tumor histopathology in woodchucks (Marmota monax). PLoS One. 2020;15:e0230794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 153. | Nachabé R, Hendriks BH, Schierling R, Hales J, Racadio JM, Rottenberg S, Ruers TJ, Babic D. Real-Time In Vivo Characterization of Primary Liver Tumors With Diffuse Optical Spectroscopy During Percutaneous Needle Interventions: Feasibility Study in Woodchucks. Invest Radiol. 2015;50:443-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Aravalli RN, Golzarian J, Cressman EN. Animal models of cancer in interventional radiology. Eur Radiol. 2009;19:1049-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 155. | Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114-118. [PubMed] [Cited in This Article: ] |
| 156. | Rose SC, Halstead GD, Narsinh KH. Pressure-Directed Embolization of Hepatic Arteries in a Porcine Model Using a Temporary Occlusion Balloon Microcatheter: Proof of Concept. Cardiovasc Intervent Radiol. 2017;40:1769-1776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 157. | Kim AY, Yacoub JH, Field DH, Park BU, Kallakury B, Korolowicz KE, Menne S. Suitability of the woodchuck HCC as a preclinical model for evaluation of intra-arterial therapies. Animal Model Exp Med. 2020;3:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 158. | Wilkins LR, Stone JR, Mata J, Hawrylack A, Kubicka E, Brautigan DL. The Use of the Woodchuck as an Animal Model for Evaluation of Transarterial Embolization. J Vasc Interv Radiol. 2017;28:1467-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 159. | Pritchard WF, Woods DL, Esparza-Trujillo JA, Starost MF, Mauda-Havakuk M, Mikhail AS, Bakhutashvili I, Leonard S, Jones EC, Krishnasamy V, Karanian JW, Wood BJ. Transarterial Chemoembolization in a Woodchuck Model of Hepatocellular Carcinoma. J Vasc Interv Radiol 2020; 31: 812-819. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 160. | Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 846] [Cited by in F6Publishing: 967] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 161. | Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 746] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 162. | Liu LY, Ma XZ, Ouyang B, Ings DP, Marwah S, Liu J, Chen AY, Gupta R, Manuel J, Chen XC, Gage BK, Cirlan I, Khuu N, Chung S, Camat D, Cheng M, Sekhon M, Zagorovsky K, Abdou Mohamed MA, Thoeni C, Atif J, Echeverri J, Kollmann D, Fischer S, Bader GD, Chan WCW, Michalak TI, McGilvray ID, MacParland SA. Nanoparticle Uptake in a Spontaneous and Immunocompetent Woodchuck Liver Cancer Model. ACS Nano. 2020;14:4698-4715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 163. | Zoulim F. Evaluation of novel strategies to combat hepatitis B virus targetting wild-type and drug-resistant mutants in experimental models. Antivir Chem Chemother. 2001;12 Suppl 1:131-142. [PubMed] [Cited in This Article: ] |
| 164. | Peek SF, Toshkov IA, Erb HN, Schinazi RF, Korba BE, Cote PJ, Gerin JL, Tennant BC. 3'-Thiacytidine (3TC) delays development of hepatocellular carcinoma (HCC) in woodchucks with experimentally induced chronic woodchuck hepatitis virus (WHV) infection. Preliminary results of a lifetime study. Hepatology. 1997;26 Suppl:368A. [Cited in This Article: ] |
| 165. | Mason WS, Cullen J, Moraleda G, Saputelli J, Aldrich CE, Miller DS, Tennant B, Frick L, Averett D, Condreay LD, Jilbert AR. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 166. | Tatti KM, Korba BE, Stang HL, Peek S, Gerin JL, Tennant BC, Schinazi RF. Mutations in the conserved woodchuck hepatitis virus polymerase FLLA and YMDD regions conferring resistance to lamivudine. Antiviral Res. 2002;55:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 167. | Zhou T, Saputelli J, Aldrich CE, Deslauriers M, Condreay LD, Mason WS. Emergence of drug-resistant populations of woodchuck hepatitis virus in woodchucks treated with the antiviral nucleoside lamivudine. Antimicrob Agents Chemother. 1999;43:1947-1954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 168. | Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, Kuhns MC, Liang TJ, Hoofnagle JH. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 281] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 169. | Lok AS, Hussain M, Cursano C, Margotti M, Gramenzi A, Grazi GL, Jovine E, Benardi M, Andreone P. Evolution of hepatitis B virus polymerase gene mutations in hepatitis B e antigen-negative patients receiving lamivudine therapy. Hepatology. 2000;32:1145-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 170. | Liu X, Schinazi RF. Hepatitis B virus resistance to lamivudine and its clinical implications. Antivir Chem Chemother. 2002;13:143-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 171. | Colonno RJ, Genovesi EV, Medina I, Lamb L, Durham SK, Huang ML, Corey L, Littlejohn M, Locarnini S, Tennant BC, Rose B, Clark JM. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J Infect Dis. 2001;184:1236-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 172. | Korba BE, Cote PJ, Menne S, Toshkov I, Baldwin BH, Wells FV, Tennant BC, Gerin JL. Clevudine therapy with vaccine inhibits progression of chronic hepatitis and delays onset of hepatocellular carcinoma in chronic woodchuck hepatitis virus infection. Antivir Ther. 2004;9:937-952. [PubMed] [Cited in This Article: ] |
| 173. | Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GH, Tennant BC. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 174. | Daffis S, Balsitis S, Chamberlain J, Zheng J, Santos R, Rowe W, Ramakrishnan D, Pattabiraman D, Spurlock S, Chu R, Kang D, Mish M, Ramirez R, Li L, Li B, Ma S, Hung M, Voitenleitner C, Yon C, Suresh M, Menne S, Cote P, Delaney WE 4th, Mackman R, Fletcher SP. Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis B. Hepatology. 2021;73:53-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 175. | Fairman J, Liu KH, Menne S. Prevention of liver tumor formation in woodchucks with established hepatocellular carcinoma by treatment with cationic liposome-DNA complexes. BMC Cancer. 2017;17:172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 176. | BREEDIS C, YOUNG G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977. [PubMed] [Cited in This Article: ] |
| 177. | Leal JN, Kingham TP. Hepatic artery infusion chemotherapy for liver malignancy. Surg Oncol Clin N Am. 2015;24:121-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 178. | Kerr DJ, Los G. Pharmacokinetic principles of locoregional chemotherapy. Cancer Surv. 1993;17:105-122. [PubMed] [Cited in This Article: ] |
| 179. | Kim M, Powers CA, Curtin LI, Fisher DT, Sexton S, Gurova KV, Skitzki JJ, Iyer RV. A Translational Hepatic Artery Infusion (HAI) Model for Hepatocellular Carcinoma in Woodchucks. J Surg Res. 2020;251:126-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 180. | Fleyshman D, Prendergast L, Safina A, Paszkiewicz G, Commane M, Morgan K, Attwood K, Gurova K. Level of FACT defines the transcriptional landscape and aggressive phenotype of breast cancer cells. Oncotarget. 2017;8:20525-20542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 181. | Gasparian AV, Burkhart CA, Purmal AA, Brodsky L, Pal M, Saranadasa M, Bosykh DA, Commane M, Guryanova OA, Pal S, Safina A, Sviridov S, Koman IE, Veith J, Komar AA, Gudkov AV, Gurova KV. Curaxins: anticancer compounds that simultaneously suppress NF-κB and activate p53 by targeting FACT. Sci Transl Med. 2011;3:95ra74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 182. | Iyer RV, Maguire O, Kim M, Curtin LI, Sexton S, Fisher DT, Schihl SA, Fetterly G, Menne S, Minderman H. Dose-Dependent Sorafenib-Induced Immunosuppression Is Associated with Aberrant NFAT Activation and Expression of PD-1 in T Cells. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 183. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9507] [Article Influence: 594.2] [Reference Citation Analysis (1)] |
| 184. | Kianmanesh R, Ogata S, Paradis V, Sauvanet A, Belghiti J. Heat-zone effect after surface application of dissecting sealer on the "in situ margin" after tumorectomy for liver tumors. J Am Coll Surg. 2008;206:1122-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 185. | Burke CT, Cullen JM, State A, Gadi S, Wilber K, Rosenthal M, Bulysheva A, Pease A, Mauro MA, Fuchs H. Development of an animal model for radiofrequency ablation of primary, virally induced hepatocellular carcinoma in the woodchuck. J Vasc Interv Radiol 2011; 22: 1613-1618. e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 186. | Cheng CW, Machtay M, Dorth J, Sergeeva O, Xia H, Manaspon C, Wu H, Iyer R, Sexton S, Xin W, Exner AA, Lee Z. Delayed response to proton beam treatment of hepatocellular carcinoma. BJR Case Rep. 2020;6:20180125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 187. | Bilbao R, Gérolami R, Bralet MP, Qian C, Tran PL, Tennant B, Prieto J, Bréchot C. Transduction efficacy, antitumoral effect, and toxicity of adenovirus-mediated herpes simplex virus thymidine kinase/ ganciclovir therapy of hepatocellular carcinoma: the woodchuck animal model. Cancer Gene Ther. 2000;7:657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 188. | Rodriguez-Madoz JR, Liu KH, Quetglas JI, Ruiz-Guillen M, Otano I, Crettaz J, Butler SD, Bellezza CA, Dykes NL, Tennant BC, Prieto J, González-Aseguinolaza G, Smerdou C, Menne S. Semliki forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma. J Virol. 2009;83:12266-12278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Pützer BM, Stiewe T, Rödicker F, Schildgen O, Rühm S, Dirsch O, Fiedler M, Damen U, Tennant B, Scherer C, Graham FL, Roggendorf M. Large nontransplanted hepatocellular carcinoma in woodchucks: treatment with adenovirus-mediated delivery of interleukin 12/B7.1 genes. J Natl Cancer Inst. 2001;93:472-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 190. | Huang KW, Wu HL, Lin HL, Liang PC, Chen PJ, Chen SH, Lee HI, Su PY, Wu WH, Lee PH, Hwang LH, Chen DS. Combining antiangiogenic therapy with immunotherapy exerts better therapeutical effects on large tumors in a woodchuck hepatoma model. Proc Natl Acad Sci USA. 2010;107:14769-14774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 191. | Liu Y, Wang B, Wang L, Vikash V, Wang Q, Roggendorf M, Lu M, Yang D, Liu J. Transcriptome Analysis and Comparison of Marmota monax and Marmota himalayana. PLoS One. 2016;11:e0165875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 192. | Fletcher SP, Chin DJ, Gruenbaum L, Bitter H, Rasmussen E, Ravindran P, Swinney DC, Birzele F, Schmucki R, Lorenz SH, Kopetzki E, Carter J, Triyatni M, Thampi LM, Yang J, AlDeghaither D, Murreddu MG, Cote P, Menne S. Intrahepatic Transcriptional Signature Associated with Response to Interferon-α Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS Pathog. 2015;11:e1005103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 193. | Alioto TS, Cruz F, Gómez-Garrido J, Triyatni M, Gut M, Frias L, Esteve-Codina A, Menne S, Kiialainen A, Kumpesa N, Birzele F, Schmucki R, Gut IG, Spleiss O. The Genome Sequence of the Eastern Woodchuck (Marmota monax) - A Preclinical Animal Model for Chronic Hepatitis B. G3 (Bethesda). 2019;9:3943-3952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 194. | Korba BE, Cote P, Hornbuckle W, Tennant BC, Gerin JL. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology. 2000;31:1165-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 195. | Goldberg BB, Hilpert PL, Burns PN, Liu JB, Newman LM, Merton DA, Witlin LA. Hepatic tumors: signal enhancement at Doppler US after intravenous injection of a contrast agent. Radiology. 1990;177:713-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 196. | Forsberg F, Goldberg BB, Liu JB, Merton DA, Rawool NM, Shi WT. Tissue-specific US contrast agent for evaluation of hepatic and splenic parenchyma. Radiology. 1999;210:125-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 197. | Forsberg F, Liu JB, Merton DA, Rawool NM, Goldberg BB. Parenchymal enhancement and tumor visualization using a new sonographic contrast agent. J Ultrasound Med. 1995;14:949-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 198. | Forsberg F, Roy R, Merton DA, Rawool NM, Liu JB, Huang M, Kessler D, Goldberg BB. Conventional and hypobaric activation of an ultrasound contrast agent. Ultrasound Med Biol. 1998;24:1143-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 199. | Goldberg BB, Liu JB, Burns PN, Merton DA, Forsberg F. Galactose-based intravenous sonographic contrast agent: experimental studies. J Ultrasound Med. 1993;12:463-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 200. | Matsumura T, Moriyasu F, Kono Y, Chiba T. [Contrast-enhanced power Doppler imaging of the liver--preliminary animal study]. Nihon Rinsho. 1998;56:985-989. [PubMed] [Cited in This Article: ] |
| 201. | Nada T, Moriyasu F, Kono Y, Matsumura T. [Sonographic depiction of woodchuck hepatomas using intravenously injected contrast agents]. Nihon Rinsho. 1998;56:980-984. [PubMed] [Cited in This Article: ] |
| 202. | Forsberg F, Liu JB, Chiou HJ, Rawool NM, Parker L, Goldberg BB. Comparison of fundamental and wideband harmonic contrast imaging of liver tumors. Ultrasonics. 2000;38:110-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 203. | Forsberg F, Goldberg BB, Liu JB, Merton DA, Rawool NM. On the feasibility of real-time, in vivo harmonic imaging with proteinaceous microspheres. J Ultrasound Med. 1996;15:853-60; quiz 861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 204. | Kono Y, Moriyasu F, Nada T, Suginoshita Y, Matsumura T, Kobayashi K, Nakamura T, Chiba T. Gray scale second harmonic imaging of the liver: a preliminary animal study. Ultrasound Med Biol. 1997;23:719-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 205. | Kallfelz FA, Hornbuckle WE, Harvey HJ, Wallace RJ, Potkewitz LG, Roth L, Tennant BC. Scintigraphic diagnosis of primary hepatocellular carcinoma in the woodchuck (Marmota monax). Am J Vet Res. 1986;47:573-576. [PubMed] [Cited in This Article: ] |
| 206. | Salem N, Kuang Y, Wang F, Maclennan GT, Lee Z. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging. 2009;53:144-156. [PubMed] [Cited in This Article: ] |
| 207. | Salem N, Kuang Y, Corn D, Erokwu B, Kolthammer JA, Tian H, Wu C, Wang F, Wang Y, Lee Z. [(Methyl)1-(11)c]-acetate metabolism in hepatocellular carcinoma. Mol Imaging Biol. 2011;13:140-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 208. | Tenley N, Corn DJ, Yuan L, Lee Z. The effect of fasting on PET Imaging of Hepatocellular Carcinoma. J Cancer Ther. 2013;4:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 209. | Kuang Y, Salem N, Tian H, Kolthammer JA, Corn DJ, Wu C, Wang F, Wang Y, Lee Z. Imaging lipid synthesis in hepatocellular carcinoma with [methyl-11c]choline: correlation with in vivo metabolic studies. J Nucl Med. 2011;52:98-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 210. | Kolthammer JA, Corn DJ, Tenley N, Wu C, Tian H, Wang Y, Lee Z. PET imaging of hepatocellular carcinoma with 18F-fluoroethylcholine and 11C-choline. Eur J Nucl Med Mol Imaging. 2011;38:1248-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 211. | Sergeeva O, Kepe V, Zhang Y, Miller-Atkins GA, Keynon JD, Iyer R, Sexton S, Awadallah A, Xin W, Saunthararajah Y, Chan ER, Lee Z. [18F] Clofarabine for PET Imaging of Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 212. | Sergeeva O, Zhang Y, Kenyon JD, Miller-Atkins GA, Wu C, Iyer R, Sexton S, Wojtylak P, Awadallah A, Xin W, Chan ER, O'Donnel JK, Lee Z. PET imaging of hepatocellular carcinoma with anti-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in comparison with L-[S-methyl-11C]methionine. EJNMMI Res. 2019;9:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 213. | Salem N, MacLennan GT, Kuang Y, Anderson PW, Schomisch SJ, Tochkov IA, Tennant BC, Lee Z. Quantitative evaluation of 2-deoxy-2[F-18]fluoro-D-glucose-positron emission tomography imaging on the woodchuck model of hepatocellular carcinoma with histological correlation. Mol Imaging Biol. 2007;9:135-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 214. | Sergeeva O, Zhang Y, Kenyon J, Miller-Atkins G, Sergeev M, Verbus E, Iyer R, Sexton S, Kepe V, Avril N, Saunthararajah Y, Chan ER, Lee Z. Liver background uptake of [18F]FLT in PET imaging. Am J Nucl Med Mol Imaging. 2020;10:212-225. [PubMed] [Cited in This Article: ] |
| 215. | Ohtomo K, Shiga J, Sasaki Y, Itai Y. [Iron oxide-enhanced MR imaging of hepatocellular carcinoma of woodchuck]. Nihon Igaku Hoshasen Gakkai Zasshi. 1991;51:433-435. [PubMed] [Cited in This Article: ] |
| 216. | Reimer P, Weissleder R, Brady TJ, Yeager AE, Baldwin BH, Tennant BC, Wittenberg J. Experimental hepatocellular carcinoma: MR receptor imaging. Radiology. 1991;180:641-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 217. | Cullen JM, Li DH, Brown C, Eisenberg EJ, Cundy KC, Wolfe J, Toole J, Gibbs C. Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 2001;45:2740-2745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 218. | Jacob JR, Korba BE, Cote PJ, Toshkov I, Delaney WE 4th, Gerin JL, Tennant BC. Suppression of lamivudine-resistant B-domain mutants by adefovir dipivoxil in the woodchuck hepatitis virus model. Antiviral Res. 2004;63:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 219. | Menne S, Butler SD, George AL, Tochkov IA, Zhu Y, Xiong S, Gerin JL, Cote PJ, Tennant BC. Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother. 2008;52:3617-3632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 220. | Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 221. | Jacquard AC, Nassal M, Pichoud C, Ren S, Schultz U, Guerret S, Chevallier M, Werle B, Peyrol S, Jamard C, Rimsky LT, Trepo C, Zoulim F. Effect of a combination of clevudine and emtricitabine with adenovirus-mediated delivery of gamma interferon in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 2004;48:2683-2692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 222. | Peek SF, Cote PJ, Jacob JR, Toshkov IA, Hornbuckle WE, Baldwin BH, Wells FV, Chu CK, Gerin JL, Tennant BC, Korba BE. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology. 2001;33:254-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 223. | Cullen JM, Smith SL, Davis MG, Dunn SE, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff MT, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (-)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076-2082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 224. | Korba BE, Schinazi RF, Cote P, Tennant BC, Gerin JL. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 2000;44:1757-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 225. | Korolowizc KE, Li B, Huang X, Yon C, Rodrigo E, Corpuz M, Plouffe DM, Kallakury BV, Suresh M, Wu TY, Miller AT, Menne S. Liver-Targeted Toll-Like Receptor 7 Agonist Combined With Entecavir Promotes a Functional Cure in the Woodchuck Model of Hepatitis B Virus. Hepatol Commun. 2019;3:1296-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 226. | Menne S, Wildum S, Steiner G, Suresh M, Korolowicz K, Balarezo M, Yon C, Murreddu M, Hong X, Kallakury BV, Tucker R, Yang S, Young JAT, Javanbakht H. Efficacy of an Inhibitor of Hepatitis B Virus Expression in Combination With Entecavir and Interferon-α in Woodchucks Chronically Infected With Woodchuck Hepatitis Virus. Hepatol Commun. 2020;4:916-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 227. | Suresh M, Korolowicz KE, Balarezo M, Iyer RP, Padmanabhan S, Cleary D, Gimi R, Sheri A, Yon C, Kallakury BV, Tucker RD, Afdhal N, Menne S. Antiviral Efficacy and Host Immune Response Induction during Sequential Treatment with SB 9200 Followed by Entecavir in Woodchucks. PLoS One. 2017;12:e0169631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 228. | Berraondo P, Di Scala M, Korolowicz K, Thampi LM, Otano I, Suarez L, Fioravanti J, Aranda F, Ardaiz N, Yang J, Kallakury BV, Tucker RD, Vasquez M, Menne S, Prieto J, González-Aseguinolaza G. Liver-directed gene therapy of chronic hepadnavirus infection using interferon alpha tethered to apolipoprotein A-I. J Hepatol. 2015;63:329-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 229. | Korba BE, Cote P, Hornbuckle W, Schinazi R, Gangemi JD, Tennant BC, Gerin JL. Enhanced antiviral benefit of combination therapy with lamivudine and alpha interferon against WHV replication in chronic carrier woodchucks. Antivir Ther. 2000;5:95-104. [PubMed] [Cited in This Article: ] |
| 230. | Standring DN, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B, Juodawlkis A, Tennant B, Korba B, Cote P, Cretton-Scott E, Schinazi RF, Myers M, Bryant ML, Sommadossi JP. Antiviral beta-L-nucleosides specific for hepatitis B virus infection. Antivir Chem Chemother. 2001;12 Suppl 1:119-129. [PubMed] [Cited in This Article: ] |
| 231. | Bryant ML, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B, Juodawlkis A, Tennant B, Korba B, Cote P, Marion P, Cretton-Scott E, Schinazi RF, Sommadossi JP. Antiviral L-nucleosides specific for hepatitis B virus infection. Antimicrob Agents Chemother. 2001;45:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 232. | Bryant ML, Bridges EG, Placidi L, Faraj A, Loi AG, Pierra C, Dukhan D, Gosselin G, Imbach JL, Hernandez B, Juodawlkis A, Tennant B, Korba B, Cote P, Cretton-Scott E, Schinazi RF, Sommadossi JP. Anti-HBV specific beta-L-2'-deoxynucleosides. Nucleosides Nucleotides Nucleic Acids. 2001;20:597-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 233. | Ahn B, Lee BC, Kim HJ, Park S, Lee Y, Choi Y, Kim HE, Kim YW. hzVSF, a novel HBV therapeutic candidate, shows WHsAg loss in woodchuck hepatitis model and safety in phase I clinical study. J Hepatol. 2020;73 Suppl 1:S861. [DOI] [Cited in This Article: ] |
| 234. | Meng Z, Zhang X, Pei R, Zhang E, Kemper T, Vollmer J, Davis HL, Glebe D, Gerlich W, Roggendorf M, Lu M. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res. 2016;125:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 235. | Paulsen D, Weber O, Ruebsamen-Schaeff H, Tennant BC, Menne S. AIC649 Induces a Bi-Phasic Treatment Response in the Woodchuck Model of Chronic Hepatitis B. PLoS One. 2015;10:e0144383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 236. | Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, Möller I, Seiz P, Glebe D, Wang B, Yang D, Lu M, Roggendorf M. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |