Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.495
Peer-review started: February 20, 2021
First decision: March 15, 2021
Revised: March 15, 2021
Accepted: May 8, 2021
Article in press: May 8, 2021
Published online: June 15, 2021
Patients with familial adenomatous polyposis (FAP), an autosomal dominant hereditary colorectal cancer syndrome, have a lifetime risk of developing cancer of nearly 100%. Recent studies have pointed out that the gut microbiota could play a crucial role in the development of colorectal adenomas and the consequent progression to colorectal cancer. Some gut bacteria, such as Fusobacterium nucleatum, Escherichia coli, Clostridium difficile, Peptostreptococcus, and enterotoxigenic Bacteroides fragilis, could be implicated in colorectal carcinogenesis through different mechanisms, including the maintenance of a chronic inflammatory state, production of bioactive tumorigenic metabolites, and DNA damage. Studies using the adenomatous polyposis coliMin/+ mouse model, which resembles FAP in most respects, have shown that specific changes in the intestinal microbial community could influence a multistep progression, the intestinal “adenoma-carcinoma sequence”, which involves mucosal barrier injury, low-grade inflammation, activation of the Wnt pathway. Therefore, modulation of gut microbiota might represent a novel therapeutic target for patients with FAP. Administration of probiotics, prebiotics, antibiotics, and nonsteroidal anti-inflammatory drugs could potentially prevent the progression of the adenoma-carcinoma sequence in FAP. The aim of this review was to summarize the best available knowledge on the role of gut microbiota in colorectal carcinogenesis in patients with FAP.
Core Tip: A number of studies have demonstrated that gut microbiota dysbiosis could be a key factor in colorectal carcinogenesis. The adenomatous polyposis coli (APC)Min/+ mouse model has been extensively used to study the underlying mechanisms of colorectal carcinogenesis in familial adenomatous polyposis. Interventions aimed at improving dysbiosis by administration of probiotics, prebiotics, or antibiotics could decrease colorectal cancer development in APC mutation carriers.
- Citation: Biondi A, Basile F, Vacante M. Familial adenomatous polyposis and changes in the gut microbiota: New insights into colorectal cancer carcinogenesis. World J Gastrointest Oncol 2021; 13(6): 495-508
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/495.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.495
Familial adenomatous polyposis (FAP) is an autosomal dominant hereditary colorectal cancer (CRC) syndrome characterized by the development of numerous (i.e. tens to thousands) colorectal adenomas[1,2]. A mutation in the adenomatous polyposis coli (APC) gene, found on chromosome 5q21, is responsible for FAP[3]. The incidence of FAP is around 1/8300, and the onset is commonly in the second or third decade of life. The risk of CRC is nearly 100% by the time patients with FAP reach the age of 40-50 years[4,5]. Such patients have an increased risk of desmoid tumors and gastric, duodenal, biliary duct, and thyroid cancers[6]. Extraintestinal manifestations of FAP may include osteomas, dental abnormalities such as unerupted or supernumerary teeth, congenital absence of one or more teeth, odontomas, and dentigerous cysts; and congenital hypertrophy of the retinal pigment epithelium[7,8]. Prophylactic colectomy is generally performed by age 40 in patients with FAP, and is the gold standard treatment to reduce the risk of developing CRC[9]. Nonetheless, colectomy is associated with postoperative morbidity and does not reduce the risk of developing extraintestinal manifestations of FAP[10]. Endoscopic surveillance of patients with FAP and their family members has decreased the occurrence of CRC at the time of FAP diagnosis by 55% and has also increased overall survival[4,11].
Recent studies have shown that the gut microbiota could play an important role in the development of colorectal adenomas and the consequent progression to CRC[12]. Indeed, gut bacteria such as Fusobacterium nucleatum, Escherichia coli, Clostridium difficile, Peptostreptococcus, and enterotoxigenic Bacteroides fragilis, could be responsible for colorectal carcinogenesis through a number of mechanisms, including the maintenance of a chronic inflammatory state, production of bioactive tumorigenic metabolites, and DNA damage[13-15]. A number of studies investigated the interac
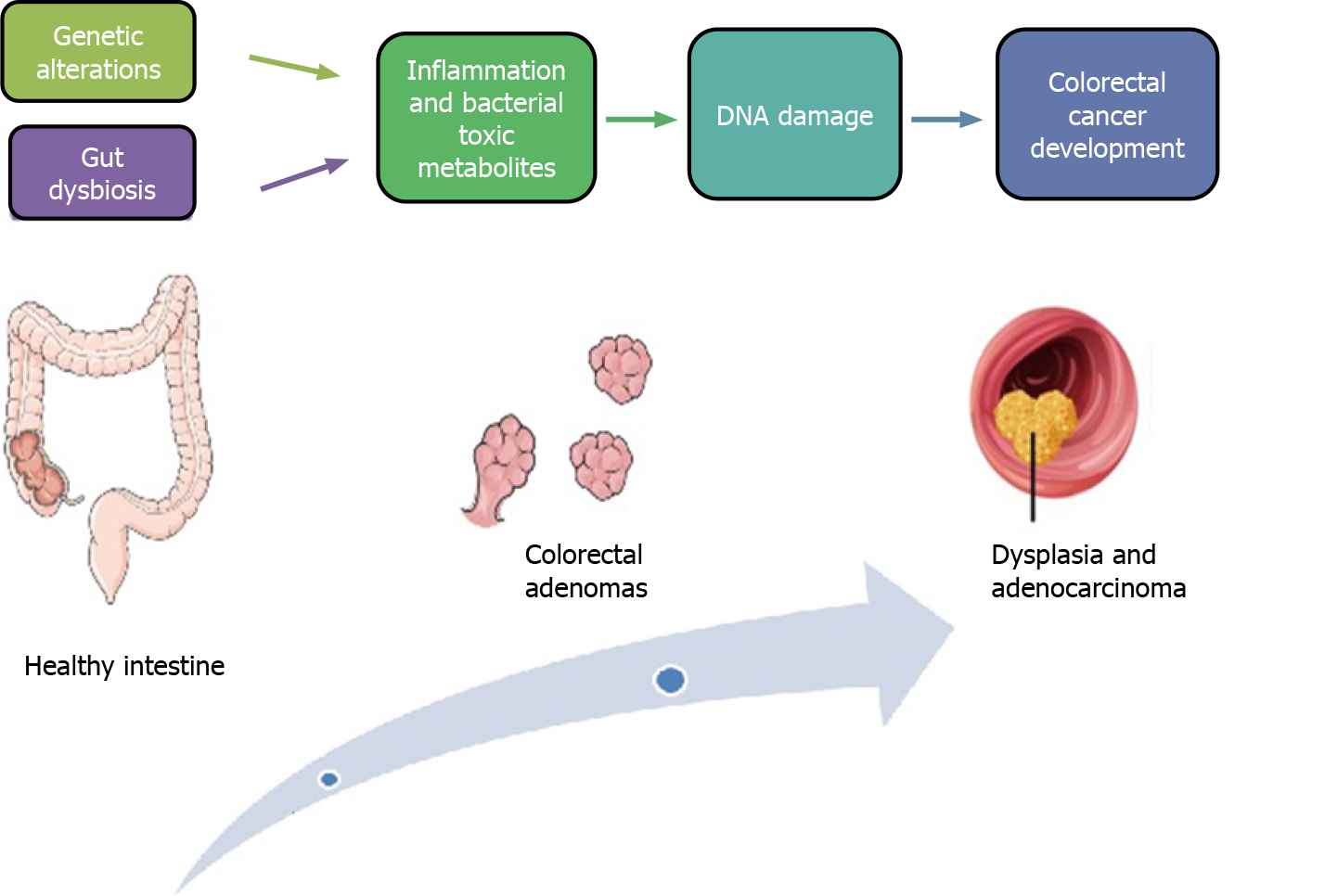
The classic colorectal carcinogenesis model described by Fearon and Vogelstein[19] includes development of most CRCs from a minimum of five or more genetic alterations, while adenomas require fewer alterations. It has been hypothesized that inactivating mutations of the APC gene could represent the initial step of the “adenoma-carcinoma sequence” (Figure 1). The APC gene is a fundamental compo
Laboratory mouse models have proven to be valuable in the study of CRC[25]. The Min (multiple intestinal neoplasia) is the first key CRC mouse model and is induced by treatment with ethylnitrosourea[26]. Adult APCMin/+ mice develop multiple intestinal polyps and anemia and usually die at a young age because of intestinal blockage and bleeding from the larger polyps[27]. Other mouse models have also been reported, such as conditional APC mutant alleles[28]. The APCMin/+ mouse model shares numerous phenotypic and genetic similarities with FAP. However, patients with FAP develop adenomas mainly in the colon, while adenomas in APCMin/+ mice are mainly located in the small intestine and have benign characteristics. Also, desmoid tumors and epidermoid cysts are rarely seen in mouse models compared with patients with FAP[29]. Nonetheless, the APCMin/+ mouse represents an outstanding experimental model for investigating genetic features and therapeutic responses of CRC in humans.
Interplay between the gut microbiota and genetic characteristics could be responsible for the genetic pattern of the adenoma-carcinoma sequence. It has been hypothesized that bacterial drivers could initiate the development of precancerous lesions and the subsequent accumulation of gene mutations[30,31]. Different gut bacteria, such as E. coli, Enterococcus faecalis, Streptococcus gallolyticus and B. fragilis have been shown to promote carcinogenesis through genotoxic effects[32]. Some E. coli strains, mainly B2 and D, strongly express virulence genes, such as those encoding toxins and effectors that could promote carcinogenesis (e.g., colibactin, cytotoxic necrotizing factors, cytolethal distending toxins, and cycle-inhibiting factor)[33,34]. Colibactin could be responsible for DNA alkylation on adenine residues, thus favoring double-strand breaks[35]. A recent study showed that expression of colibactin-producing polyketide synthase (pks+) in E. coli could was associated with the occurrence of a specific mutational signature in human gut organoids. The same mutational signature was detected in 5876 human cancer genomes in two independent study cohorts, especially in CRC[36]. Also, pks+ E. coli could be responsible for aneuploidy and abnormal cellular division, an effect promoted by the mutagen colibactin[37]. Such effects of pks+ E. coli were mainly observed in APCMin/+ mice that lacked the autophagy gene Atg16 L1, and consequently were not able to recruit the DNA repair protein RAD51, thus accumulating DNA double-strand breaks and developing tumors[38]. Enterococcus faecalis was shown to promote DNA damage by induction of inflammation and oxidative stress resulting from the release of reactive oxygen species and reactive nitrogen species[39]. Fragilysin (also known as BST), is a toxic virulence factor released by enterotoxigenic B. fragilis (ETBF) that can induce DNA damage in vivo[40]. Colonization by sulfidogenic bacteria, such as F. nucleatum, has been associated with genomic or chromosomal instability and CRC development associated with the genotoxic effects of hydrogen sulfide (H2S)[41,42]. A prior state of dysbiosis could enhance these specific bacterial genotoxic effects[31].
There is extensive evidence of an association between infectious agents and develop
| Ref. | Bacterial strain | Mechanism of carcinogenesis |
| Kostic et al[18], 2013 | F. nucleatum | Infiltration of CD11+ myeloid-derived immune cells |
| Tomkovich et al[49], 2017 | F. nucleatum and pks+ E. coli | Mediated by inflammation, with colibactin-producing E. coli but not with F. nucleatum (FadA+ or Fap2+) |
| Yang et al[50], 2017 | F. nucleatum | Regulation of miR-21 via TLR4/MYD88/NF-κB pathway |
| Wu et al[51], 2018 | F. nucleatum | TLR4/p-PAK1/p-β-catenin S675 pathway |
| Chen et al[52], 2018 | F. nucleatum | Induction of M2 macrophage polarization via TLR4. Activation of the IL-6/p-STAT3/c-MYC signaling pathway |
| Rubinstein et al[53], 2019 | F. nucleatum | FadA adhesin upregulates Annexin A1 expression through E-cadherin |
| Dejea et al[54], 2018 | Mono- or co-colonization of ETBF and pks+ E. coli | Upregulation of IL-17 and DNA damage |
| Chung et al[55], 2018 | ETBF | Pathway involving activation of IL-17R, NF-κB, Stat3, and CXCL1 |
| Goodwin et al[56], 2011 | ETBF | Production of spermine oxidase, reactive oxygen species and DNA damage |
| He et al[57], 2019 | Campylobacter jejuni | DNA damage due to cytolethal distending toxin |
| Li et al[15], 2019 | Mixed strains from fecal samples of CRC patients after antibiotic cocktails | Wnt/β-catenin and cyclin D1 pathway |
Changes in the gut microbiota, can stimulate the c-Jun/JNK and STAT3 signaling pathways, thus promoting, in combination with anemia, tumor growth in APCMin/+ mice[58]. A study carried out in APCMin/+ mice by Son et al[17] reported that mutation of the APC gene modified colonic-microbial interactions prior to polyposis. Indeed, changes in the gut microbiota, characterized by an increased relative growth of Bacteroidetes spp. identified in association with intestinal tumors, has been shown to precede the development of microscopically evident intestinal tumors in 6-wk-old APCMin/+ mice. A recent study by Dejea et al[54] detected colonic biofilms mainly composed of E. coli and B. fragilis in patients with FAP. Genes for colibactin (clbB) and B. fragilis toxin (bft) were highly expressed in the colonic mucosa of patients with FAP compared with healthy subjects. Co-colonization with E. coli and ETBF led to an increase in interleukin-17 (IL-17) and DNA damage in colonic epithelium of tumor-prone mice, compared with mice with either bacterial strain alone. As ETBF and pks+ E. coli frequently colonize young children, it has been suggested that constant co-colonization in the colon mucosa from a young age could play a role in the patho
Commensal and pathogenic bacteria were found to promote CRC development after colonizing normal colonic mucosa and promoting sustained local inflammation, and by releasing genotoxic compounds against colonic epithelial cells to induce their tumorigenic transformation[63]. Conversely, a balanced population of microbiota prevented development of CRC by producing bacterial metabolites that reduced inflammation[64]. Chronic inflammation is associated with the development of various tumors, including CRC. Inflammation of the colonic mucosa may enhance carcino
A number of studies demonstrated that the gut microbiota was responsible for the production of various bioactive food elements and micronutrients, such as essential vitamins, and the fermentation of dietary fibers and complex carbohydrates, producing short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate[72-74]. The role of butyrate in colorectal carcinogenesis is controversial[75]. In fact, in APCMin/+; Msh2-/- mice that were also deficient for the DNA mismatch repair gene MutS homolog 2, Belcheva et al[76] found that microbial metabolism of carbohydrates into SCFAs, such as butyrate, enhanced the proliferation of tumor-initiated epithelial cells, thus promoting carcinogenesis. In their study, the growth of SCFA-producing bacteria, such as Clostridiaceae, Ruminococcaceae, and Lachnospiraceae, was inhibited by antibiotic therapy or a low-carbohydrate diet, and in turn the number of polyps detected in APCMin/+; Msh2-/- mice was also reduced. On the other hand, many studies have described antineoplastic effects SCFAs, such as the suppression of inflammation, stimulation of apoptosis, and inhibition of cancer cell progression[77]. Nonetheless, further investigation is needed for clarifying the role of butyrate in CRC protection or promotion. Other bacterial metabolites, such as H2S, secondary bile acids, and nitric oxide, have been shown to contribute to progression of adenomatous colon polyps to CRC by affecting host metabolism and immunity[78].
A growing number of clinical trials have reported an association between gut bacteria and their metabolites and progression of CRC through various mechanisms[79,80]. However, the role of the gut microbiota in the progression and development of CRC is intricate and still not entirely understood, especially in patients with FAP. Currently, only a few clinical trials are recruiting subjects with FAP to determine whether modifying the gut microbiota might influence CRC development[81]. The Memorial Sloan Kettering Cancer Center in New York (United States), is conducting a clinical trial (Clinicaltrials.gov ID: NCT02371135) enrolling patients with Lynch syndrome or other hereditary colonic polyposis syndromes, in order to assess the role of the gut bacteria in CRC development. Investigators collect fecal samples, colon biopsies, and questionnaire responses on diet and lifestyle[82]. A phase 2, randomized, double-blind, placebo-controlled study sponsored by the Tel Aviv Sourasky Medical Center (Israel) is evaluating the efficacy of curcumin supplementation on polyp number and size in patients with FAP (Clinicaltrials.gov ID: NCT03061591)[83].
It has been suggested that interventions directed at improving gut dysbiosis in APCMin/+ mice, for instance through probiotics, prebiotics, some antibiotics, and nonsteroidal anti-inflammatory drugs (NSAIDs), can inhibit the progression of the adenoma-carcinoma sequence, thus reducing the development of CRC[84-86].
The ileoanal pouch is the surgical procedure of choice for patients with the classical phenotype of FAP[87]. Many studies have shown that the gut microbiota play a key role in the development of pouchitis, as supported by clinical evidence of the benefits of antibiotic therapy[88,89]. Metronidazole, ciprofloxacin, or a combination of both, is usually the initial approach, and it is often effective in chronic pouchitis[90]. A meta-analysis of 21 studies showed that antibiotics induced a significant remission rate (74%) in patients with chronic pouchitis (95% confidence interval: 56-93; P < 0.001), whereas the remission rate after administration of biologics was 53% (95% confidence interval: 30-76; P < 0.001). Conversely, steroids, bismuth, tacrolimus, and an elemental diet did not result in a significant remission, which was achieved by fecal microbiota transplantation[88]. Probiotics have been shown to be effective in the prevention of pouchitis[91]. Indeed, Shen et al[92] showed that administration of a probiotic treatment (Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, and Bifidobacterium bifidus) prevented pouchitis, decreased the Modified Pouch Disease Activity Index score, and reduced fecal pyruvate kinase and calprotectin in FAP patients after restorative proctocolectomy[93].
Gut microbiota composition and function are considerably modulated by diet[14]. An association between the intake of nondigestible fibers, such as prebiotics, and an abundance of beneficial bacteria in the gut, including Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcaceae, and Roseburia has been widely reported. Indeed administration of both probiotics and prebiotics has shown beneficial effects in prevention and reduction of the prevalence of adenomatous colon polyps[94,95]. A metagenomic study by Ni et al[96] reported a preventive effect of Lactobacillus rhamnosus GG (LGG) on polyp formation in APCMin/+ mice. The results showed that LGG had beneficial effects and reduced polyp development in mice by preserving gut microbial functionality. A study by Urbanska et al[97] reported similar results using an orally delivered probiotic formulation that reduced overall intestinal inflammation and the number of polyps in the small intestine of APCMin/+ mice after administration of microencapsulated live Lactobacillus acidophilus cells.
There is evidence that antibiotic treatment can modify the gut microbiota physiological processes and functions[98]. Some studies showed that shifts in the composition of the intestinal community caused by antibiotics were associated with development of polyps and progression to CRC. Other studies reported a possible protective effect on carcinogenesis[99-101]. A nested case-control study by Dik et al[102] reported a significant dose-dependent association between administration of penicillin and quinolone antibiotics and increased risk of CRC development. Another nested case-control study by Boursi et al[103] carried out in a large population-based database in the United Kingdom, showed similar results, and concluded that past exposure to several courses of penicillin was associated with a slight increase in CRC risk. A recent study found that long-term treatment of APCMin/+ mice with an antibiotic cocktail composed of vancomycin, neomycin, and streptomycin resulted in gut inflammation with polyposis and cancer progression, perhaps caused by specific changes of the gut microbiota and thinning of the protective mucus layer[104]. On the contrary, Belcheva et al[76] observed a decreased number of polyps in both the small and large intestine of C57BL/6 APCMin/+; Msh2-/- mice treated with ampicillin, metronidazole, neomycin, and vancomycin. The gut microbiota in APCMin/+; Msh2-/- mice might affect the develop
A number of epidemiological studies have shown an association between diet, inflammation, and cancer, including CRC[106-109]. So far, there is a lack of preventive dietary recommendations for FAP patients. A nonrandomized prospective pilot study carried out on FAP patients showed that a low-inflammatory diet based on the Mediterranean diet pattern decreased gastrointestinal markers of inflammation, such as C-reactive protein and pro-inflammatory cytokines, through a modulation of the gut microbiota composition[110]. Combination treatment with curcumin and quercetin has been reported to reduce the development of adenomas in FAP. This beneficial effect might be a result of their antioxidative, anti-inflammatory, and antiproliferative properties and the maintenance of a diverse gut microbial community[111-113]. Black raspberry powder supplementation in FAP patients significantly decreased the burden of rectal polyps and reduced staining of the mucosal proliferation marker Ki-67, compared with placebo[114]. The results could have a response to beneficial effects of the anthocyanin and fiber content of the raspberries on the diversity and composition of the gut microbiota[115,116]. Administration of berberine, an alkaloid that can be isolated from many plants including barberry (Berberis vulgaris), significantly reduced the development of CRC and restored the gut microbiota community in APCMin/+ mice fed a high fat diet[117].
There is evidence that the combination of anti-inflammatory drugs and regular endoscopic surveillance can decrease the risk of new adenomas in the rectal stump of FAP patients[118-120]. Administration of NSAIDs and omega-3 essential fatty acids reduced recurrence[121]. Even though long-term therapy with NSAIDs has been shown to increase gastrointestinal and cardiological risk, the use of omega-3 supple
The APCMin/+ mouse model has been widely used to study the underlying mechanisms of colorectal carcinogenesis in FAP. Several studies demonstrated that gut microbiota dysbiosis as a key factor in colorectal carcinogenesis. Indeed, the intestinal microbial community played an important role in the multistep process of the intestinal adenoma-carcinoma sequence, and changes in the gut microbiota were found to be responsible for mucosal barrier injury, low-grade inflammation, activation of the Wnt pathway, and subsequent progression of adenomas. Recent evidence suggests that the modulation of gut microbiota could be a novel therapeutic target in FAP patients. Administration of probiotics, prebiotics, antibiotics, and NSAIDs can prevent the progression of the adenoma-carcinoma sequence in FAP. However, further study of the role of the gut microbiota in the malignant transformation of colorectal adenoma and how microbe-targeted therapies might be useful in preventing CRC development in FAP is needed.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caba O, Vieth M S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Kemp Bohan PM, Mankaney G, Vreeland TJ, Chick RC, Hale DF, Cindass JL, Hickerson AT, Ensley DC, Sohn V, Clifton GT, Peoples GE, Burke CA. Chemoprevention in familial adenomatous polyposis: past, present and future. Fam Cancer. 2021;20:23-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Jung I, Gurzu S, Turdean GS. Current status of familial gastrointestinal polyposis syndromes. World J Gastrointest Oncol. 2015;7:347-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet. 2015;8:95-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE, Ilyas M, Kaur A, Lalloo F, Latchford A, Rutter MD, Tomlinson I, Thomas HJW, Hill J; Hereditary CRC guidelines eDelphi consensus group. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020;69:411-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 5. | GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 6. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 7. | Wang XP, Fan J. Molecular genetics of supernumerary tooth formation. Genesis. 2011;49:261-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Byrne RM, Tsikitis VL. Colorectal polyposis and inherited colorectal cancer syndromes. Ann Gastroenterol. 2018;31:24-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Herzig D, Hardiman K, Weiser M, You N, Paquette I, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes. Dis Colon Rectum. 2017;60:881-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, Lai J, Guzman MA. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch Pathol Lab Med. 2019;143:1382-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Bülow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 171] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 12. | Vacante M, Ciuni R, Basile F, Biondi A. Gut Microbiota and Colorectal Cancer Development: A Closer Look to the Adenoma-Carcinoma Sequence. Biomedicines. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 14. | Pop OL, Vodnar DC, Diaconeasa Z, Istrati M, Bințințan A, Bințințan VV, Suharoschi R, Gabbianelli R. An Overview of Gut Microbiota and Colon Diseases with a Focus on Adenomatous Colon Polyps. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Li L, Li X, Zhong W, Yang M, Xu M, Sun Y, Ma J, Liu T, Song X, Dong W, Liu X, Chen Y, Liu Y, Abla Z, Liu W, Wang B, Jiang K, Cao H. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apcmin/+ mice. EBioMedicine. 2019;48:301-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Liang S, Mao Y, Liao M, Xu Y, Chen Y, Huang X, Wei C, Wu C, Wang Q, Pan X, Tang W. Gut microbiome associated with APC gene mutation in patients with intestinal adenomatous polyps. Int J Biol Sci. 2020;16:135-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Son JS, Khair S, Pettet DW 3rd, Ouyang N, Tian X, Zhang Y, Zhu W, Mackenzie GG, Robertson CE, Ir D, Frank DN, Rigas B, Li E. Altered Interactions between the Gut Microbiome and Colonic Mucosa Precede Polyposis in APCMin/+ Mice. PLoS One. 2015;10:e0127985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1659] [Cited by in F6Publishing: 1581] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 19. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7708] [Article Influence: 226.7] [Reference Citation Analysis (1)] |
| 20. | Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10:101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 386] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 21. | Valli A, Rodriguez M, Moutsianas L, Fischer R, Fedele V, Huang HL, Van Stiphout R, Jones D, Mccarthy M, Vinaxia M, Igarashi K, Sato M, Soga T, Buffa F, Mccullagh J, Yanes O, Harris A, Kessler B. Hypoxia induces a lipogenic cancer cell phenotype via HIF1α-dependent and -independent pathways. Oncotarget. 2015;6:1920-1941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Newton IP, Kenneth NS, Appleton PL, Näthke I, Rocha S. Adenomatous polyposis coli and hypoxia-inducible factor-1{alpha} have an antagonistic connection. Mol Biol Cell. 2010;21:3630-3638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Liu W, Zhang R, Shu R, Yu J, Li H, Long H, Jin S, Li S, Hu Q, Yao F, Zhou C, Huang Q, Hu X, Chen M, Hu W, Wang Q, Fang S, Wu Q. Study of the Relationship between Microbiome and Colorectal Cancer Susceptibility Using 16SrRNA Sequencing. Biomed Res Int. 2020;2020:7828392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Valli A, Morotti M, Zois CE, Albers PK, Soga T, Feldinger K, Fischer R, Frejno M, McIntyre A, Bridges E, Haider S, Buffa FM, Baban D, Rodriguez M, Yanes O, Whittington HJ, Lake HA, Zervou S, Lygate CA, Kessler BM, Harris AL. Adaptation to HIF1α Deletion in Hypoxic Cancer Cells by Upregulation of GLUT14 and Creatine Metabolism. Mol Cancer Res. 2019;17:1531-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Bürtin F, Mullins CS, Linnebacher M. Mouse models of colorectal cancer: Past, present and future perspectives. World J Gastroenterol. 2020;26:1394-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 71] [Cited by in F6Publishing: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 26. | McIntyre RE, Buczacki SJ, Arends MJ, Adams DJ. Mouse models of colorectal cancer as preclinical models. Bioessays. 2015;37:909-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Irving AA, Yoshimi K, Hart ML, Parker T, Clipson L, Ford MR, Kuramoto T, Dove WF, Amos-Landgraf JM. The utility of Apc-mutant rats in modeling human colon cancer. Dis Model Mech. 2014;7:1215-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Zeineldin M, Neufeld KL. More than two decades of Apc modeling in rodents. Biochim Biophys Acta. 2013;1836:80-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Young M, Ordonez L, Clarke AR. What are the best routes to effectively model human colorectal cancer? Mol Oncol. 2013;7:178-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, Delchier JC. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease? Therap Adv Gastroenterol. 2013;6:215-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. 2014;16:406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 32. | Alhinai EA, Walton GE, Commane DM. The Role of the Gut Microbiota in Colorectal Cancer Causation. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 33. | Khan AA, Khan Z, Malik A, Kalam MA, Cash P, Ashraf MT, Alshamsan A. Colorectal cancer-inflammatory bowel disease nexus and felony of Escherichia coli. Life Sci. 2017;180:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Bleich RM, Arthur JC. Revealing a microbial carcinogen. Science. 2019;363:689-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, Balskus EP. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019;363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 36. | Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB, Paganelli FL, Geurts MH, Beumer J, Mizutani T, Miao Y, van der Linden R, van der Elst S; Genomics England Research Consortium; Garcia KC, Top J, Willems RJL, Giannakis M, Bonnet R, Quirke P, Meyerson M, Cuppen E, van Boxtel R, Clevers H. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature. 2020;580:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 515] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 37. | Cougnoux A, Delmas J, Gibold L, Faïs T, Romagnoli C, Robin F, Cuevas-Ramos G, Oswald E, Darfeuille-Michaud A, Prati F, Dalmasso G, Bonnet R. Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut. 2016;65:278-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Lucas C, Salesse L, Hoang MHT, Bonnet M, Sauvanet P, Larabi A, Godfraind C, Gagnière J, Pezet D, Rosenstiel P, Barnich N, Bonnet R, Dalmasso G, Nguyen HTT. Autophagy of Intestinal Epithelial Cells Inhibits Colorectal Carcinogenesis Induced by Colibactin-Producing Escherichia coli in ApcMin/+ Mice. Gastroenterology. 2020;158:1373-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Irrazabal T, Thakur BK, Kang M, Malaise Y, Streutker C, Wong EOY, Copeland J, Gryfe R, Guttman DS, Navarre WW, Martin A. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nat Commun. 2020;11:1802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Lv Y, Ye T, Wang HP, Zhao JY, Chen WJ, Wang X, Shen CX, Wu YB, Cai YK. Suppression of colorectal tumorigenesis by recombinant Bacteroides fragilis enterotoxin-2 in vivo. World J Gastroenterol. 2017;23:603-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Dahmus JD, Kotler DL, Kastenberg DM, Kistler CA. The gut microbiome and colorectal cancer: a review of bacterial pathogenesis. J Gastrointest Oncol. 2018;9:769-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 43. | van Elsland D, Neefjes J. Bacterial infections and cancer. EMBO Rep. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67:326-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 45. | Richard ML, Liguori G, Lamas B, Brandi G, da Costa G, Hoffmann TW, Pierluigi Di Simone M, Calabrese C, Poggioli G, Langella P, Campieri M, Sokol H. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 2018;9:131-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 114] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 46. | Yu LC, Wei SC, Ni YH. Impact of microbiota in colorectal carcinogenesis: lessons from experimental models. Intest Res. 2018;16:346-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Alto NM, Orth K. Subversion of cell signaling by pathogens. Cold Spring Harb Perspect Biol. 2012;4:a006114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Lahiani A, Yavin E, Lazarovici P. The Molecular Basis of Toxins' Interactions with Intracellular Signaling via Discrete Portals. Toxins (Basel). 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, Mohamadzadeh M, Liu X, Martin P, Wang GP, Oswald E, Fodor AA, Jobin C. Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 2017;77:2620-2632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 50. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 568] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 51. | Wu Y, Wu J, Chen T, Li Q, Peng W, Li H, Tang X, Fu X. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis in Mice via a Toll-Like Receptor 4/p21-Activated Kinase 1 Cascade. Dig Dis Sci. 2018;63:1210-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Chen T, Li Q, Wu J, Wu Y, Peng W, Li H, Wang J, Tang X, Peng Y, Fu X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol Immunother. 2018;67:1635-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 53. | Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D, Dalerba P, Wang TC, Han YW. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 54. | Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 627] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 55. | Chung L, Thiele Orberg E, Geis AL, Chan JL, Fu K, DeStefano Shields CE, Dejea CM, Fathi P, Chen J, Finard BB, Tam AJ, McAllister F, Fan H, Wu X, Ganguly S, Lebid A, Metz P, Van Meerbeke SW, Huso DL, Wick EC, Pardoll DM, Wan F, Wu S, Sears CL, Housseau F. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe. 2018;23:203-214.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 56. | Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA Jr. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci USA. 2011;108:15354-15359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 57. | He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, Jobin C. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68:289-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 58. | Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Kärre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 59. | Nalbantoglu I, Blanc V, Davidson NO. Characterization of Colorectal Cancer Development in Apc (min/+) Mice. Methods Mol Biol. 2016;1422:309-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1113] [Cited by in F6Publishing: 1191] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 61. | Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad AR, Gan CM, Housseau F, Sears CL. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 62. | Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 63. | Dai Z, Zhang J, Wu Q, Chen J, Liu J, Wang L, Chen C, Xu J, Zhang H, Shi C, Li Z, Fang H, Lin C, Tang D, Wang D. The role of microbiota in the development of colorectal cancer. Int J Cancer. 2019;145:2032-2041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 64. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2488] [Cited by in F6Publishing: 2823] [Article Influence: 282.3] [Reference Citation Analysis (0)] |
| 65. | Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 164] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 66. | McClellan JL, Davis JM, Steiner JL, Day SD, Steck SE, Carmichael MD, Murphy EA. Intestinal inflammatory cytokine response in relation to tumorigenesis in the Apc(Min/+) mouse. Cytokine. 2012;57:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Guo P, Tian Z, Kong X, Yang L, Shan X, Dong B, Ding X, Jing X, Jiang C, Jiang N, Yu Y. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J Exp Clin Cancer Res. 2020;39:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1511] [Cited by in F6Publishing: 1353] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 69. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1374] [Cited by in F6Publishing: 1481] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 70. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 957] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 71. | Mager LF, Wasmer MH, Rau TT, Krebs P. Cytokine-Induced Modulation of Colorectal Cancer. Front Oncol. 2016;6:96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 72. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 993] [Cited by in F6Publishing: 1253] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 73. | Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 950] [Cited by in F6Publishing: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 74. | Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 982] [Cited by in F6Publishing: 823] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 75. | Bultman SJ, Jobin C. Microbial-derived butyrate: an oncometabolite or tumor-suppressive metabolite? Cell Host Microbe. 2014;16:143-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, Moriyama EH, Copeland JK, Surendra A, Kumar S, Green B, Geddes K, Pezo RC, Navarre WW, Milosevic M, Wilson BC, Girardin SE, Wolever TMS, Edelmann W, Guttman DS, Philpott DJ, Martin A. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell. 2014;158:288-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 323] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 77. | Gill PA, van Zelm MC, Muir JG, Gibson PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 78. | O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 79. | Zitvogel L, Daillère R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017;15:465-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 80. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1596] [Cited by in F6Publishing: 1664] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 81. | Leavitt J, Saleh N. The Microbiome and Colorectal Cancer: Current Clinical Trials. Oncology (Williston Park). 2019;33:78. [PubMed] [Cited in This Article: ] |
| 82. | Stadler Z. Memorial Sloan Kettering Cancer Center. Metagenomic Evaluation of the Gut Microbiome in Patients With Lynch Syndrome and Other Hereditary Colonic Polyposis Syndromes. [accessed 2021 Feb 5]. In: ClinicalTrials.gov [Internet]. New York (NY): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02371135 ClinicalTrials.gov Identifier: NCT02371135. [Cited in This Article: ] |
| 83. | Kariv R. Turmeric Supplementation on Polyp Number and Size in Patients With Familial Adenomatous Polyposis. [accessed 2021 Feb 5]. In: ClinicalTrials.gov [Internet]. Tel Aviv: U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03061591 ClinicalTrials.gov Identifier: NCT03061591. [Cited in This Article: ] |
| 84. | Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39:4925-4943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 85. | Dutta D, Lim SH. Bidirectional interaction between intestinal microbiome and cancer: opportunities for therapeutic interventions. Biomark Res. 2020;8:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Perillo F, Amoroso C, Strati F, Giuffrè MR, Díaz-Basabe A, Lattanzi G, Facciotti F. Gut Microbiota Manipulation as a Tool for Colorectal Cancer Management: Recent Advances in Its Use for Therapeutic Purposes. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Möslein G. Surgical considerations in FAP-related pouch surgery: Could we do better? Fam Cancer. 2016;15:457-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Segal JP, Ding NS, Worley G, Mclaughlin S, Preston S, Faiz OD, Clark SK, Hart AL. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017;45:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 89. | Batista D, Raffals L. Role of intestinal bacteria in the pathogenesis of pouchitis. Inflamm Bowel Dis. 2014;20:1481-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Gionchetti P, Calafiore A, Riso D, Liguori G, Calabrese C, Vitali G, Laureti S, Poggioli G, Campieri M, Rizzello F. The role of antibiotics and probiotics in pouchitis. Ann Gastroenterol. 2012;25:100-105. [PubMed] [Cited in This Article: ] |
| 91. | Kousgaard SJ, Michaelsen TY, Nielsen HL, Kirk KF, Albertsen M, Thorlacius-Ussing O. The Microbiota Profile in Inflamed and Non-Inflamed Ileal Pouch-Anal Anastomosis. Microorganisms. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Shen B, Achkar JP, Connor JT, Ormsby AH, Remzi FH, Bevins CL, Brzezinski A, Bambrick ML, Fazio VW, Lashner BA. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003;46:748-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 93. | Tomasz B, Zoran S, Jarosław W, Ryszard M, Marcin G, Robert B, Piotr K, Lukasz K, Jacek P, Piotr G, Przemysław P, Michał D. Long-term use of probiotics Lactobacillus and Bifidobacterium has a prophylactic effect on the occurrence and severity of pouchitis: a randomized prospective study. Biomed Res Int. 2014;2014:208064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Drago L. Probiotics and Colon Cancer. Microorganisms. 2019;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 95. | Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9:854-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Ni Y, Wong VH, Tai WC, Li J, Wong WY, Lee MM, Fong FL, El-Nezami H, Panagiotou G. A metagenomic study of the preventive effect of Lactobacillus rhamnosus GG on intestinal polyp formation in ApcMin/+ mice. J Appl Microbiol. 2017;122:770-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Urbanska AM, Bhathena J, Cherif S, Prakash S. Orally delivered microencapsulated probiotic formulation favorably impacts polyp formation in APC (Min/+) model of intestinal carcinogenesis. Artif Cells Nanomed Biotechnol. 2016;44:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Kennedy EA, King KY, Baldridge MT. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front Physiol. 2018;9:1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 99. | Hale VL, Chen J, Johnson S, Harrington SC, Yab TC, Smyrk TC, Nelson H, Boardman LA, Druliner BR, Levin TR, Rex DK, Ahnen DJ, Lance P, Ahlquist DA, Chia N. Shifts in the Fecal Microbiota Associated with Adenomatous Polyps. Cancer Epidemiol Biomarkers Prev. 2017;26:85-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 100. | Xu L, Surathu A, Raplee I, Chockalingam A, Stewart S, Walker L, Sacks L, Patel V, Li Z, Rouse R. The effect of antibiotics on the gut microbiome: a metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genomics. 2020;21:263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 102. | Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig Dis Sci. 2016;61:255-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 103. | Boursi B, Haynes K, Mamtani R, Yang YX. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015;24:534-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Kaur K, Saxena A, Debnath I, O'Brien JL, Ajami NJ, Auchtung TA, Petrosino JF, Sougiannis AJ, Depaep S, Chumanevich A, Gummadidala PM, Omebeyinje MH, Banerjee S, Chatzistamou I, Chakraborty P, Fayad R, Berger FG, Carson JA, Chanda A. Antibiotic-mediated bacteriome depletion in ApcMin/+ mice is associated with reduction in mucus-producing goblet cells and increased colorectal cancer progression. Cancer Med. 2018;7:2003-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 105. | Leystra AA, Clapper ML. Gut Microbiota Influences Experimental Outcomes in Mouse Models of Colorectal Cancer. Genes (Basel). 2019;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MA. The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 107. | Adjibade M, Andreeva VA, Lemogne C, Touvier M, Shivappa N, Hébert JR, Wirth MD, Hercberg S, Galan P, Julia C, Assmann KE, Kesse-Guyot E. The Inflammatory Potential of the Diet Is Associated with Depressive Symptoms in Different Subgroups of the General Population. J Nutr. 2017;147:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Ryu I, Kwon M, Sohn C, Shivappa N, Hébert JR, Na W, Kim MK. The Association between Dietary Inflammatory Index (DII) and Cancer Risk in Korea: A Prospective Cohort Study within the KoGES-HEXA Study. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Bodén S, Myte R, Wennberg M, Harlid S, Johansson I, Shivappa N, Hébert JR, Van Guelpen B, Nilsson LM. The inflammatory potential of diet in determining cancer risk; A prospective investigation of two dietary pattern scores. PLoS One. 2019;14:e0214551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 110. | Pasanisi P, Gariboldi M, Verderio P, Signoroni S, Mancini A, Rivoltini L, Milione M, Masci E, Ciniselli CM, Bruno E, Macciotta A, Belfiore A, Ricci MT, Gargano G, Morelli D, Apolone G, Vitellaro M. A Pilot Low-Inflammatory Dietary Intervention to Reduce Inflammation and Improve Quality of Life in Patients With Familial Adenomatous Polyposis: Protocol Description and Preliminary Results. Integr Cancer Ther. 2019;18:1534735419846400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 112. | Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr, Montgomery EA, Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, Umar A, Rodriguez LM, Giardiello FM. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology. 2018;155:668-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 113. | McFadden RM, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso JG, Jobin C, Ghishan FK, Kiela PR. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm Bowel Dis. 2015;21:2483-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 114. | Wang LS, Burke CA, Hasson H, Kuo CT, Molmenti CL, Seguin C, Liu P, Huang TH, Frankel WL, Stoner GD. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev Res (Phila). 2014;7:666-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 115. | Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, Wang LS. Black Raspberries and Their Anthocyanin and Fiber Fractions Alter the Composition and Diversity of Gut Microbiota in F-344 Rats. Nutr Cancer. 2017;69:943-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 116. | Kresty LA, Fromkes JJ, Frankel WL, Hammond CD, Seeram NP, Baird M, Stoner GD. A phase I pilot study evaluating the beneficial effects of black raspberries in patients with Barrett's esophagus. Oncotarget. 2018;9:35356-35372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Wang H, Guan L, Li J, Lai M, Wen X. The Effects of Berberine on the Gut Microbiota in Apc min/+ Mice Fed with a High Fat Diet. Molecules. 2018;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 119. | Tajika M, Niwa Y, Bhatia V, Tanaka T, Ishihara M, Yamao K. Risk of ileal pouch neoplasms in patients with familial adenomatous polyposis. World J Gastroenterol. 2013;19:6774-6783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Engel C, Frayling I, Friedl W, Hes FJ, Hodgson S, Järvinen H, Mecklin JP, Møller P, Myrhøi T, Nagengast FM, Parc Y, Phillips R, Clark SK, de Leon MP, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen J. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 435] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 121. | West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 122. | Song M, Lee IM, Manson JE, Buring JE, Dushkes R, Gordon D, Walter J, Wu K, Chan AT, Ogino S, Fuchs CS, Meyerhardt JA, Giovannucci EL; VITAL Research Group. Effect of Supplementation With Marine ω-3 Fatty Acid on Risk of Colorectal Adenomas and Serrated Polyps in the US General Population: A Prespecified Ancillary Study of a Randomized Clinical Trial. JAMA Oncol. 2020;6:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Maseda D, Ricciotti E. NSAID-Gut Microbiota Interactions. Front Pharmacol. 2020;11:1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 124. | Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;22:178.e1-178.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 125. | Montrose DC, Zhou XK, McNally EM, Sue E, Yantiss RK, Gross SS, Leve ND, Karoly ED, Suen CS, Ling L, Benezra R, Pamer EG, Dannenberg AJ. Celecoxib Alters the Intestinal Microbiota and Metabolome in Association with Reducing Polyp Burden. Cancer Prev Res (Phila). 2016;9:721-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Zhao R, Coker OO, Wu J, Zhou Y, Zhao L, Nakatsu G, Bian X, Wei H, Chan AWH, Sung JJY, Chan FKL, El-Omar E, Yu J. Aspirin Reduces Colorectal Tumor Development in Mice and Gut Microbes Reduce its Bioavailability and Chemopreventive Effects. Gastroenterology. 2020;159:969-983.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |