Published online Apr 15, 2021. doi: 10.4251/wjgo.v13.i4.279
Peer-review started: November 8, 2020
First decision: December 17, 2020
Revised: December 31, 2020
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: April 15, 2021
Major societies provide differing guidance on management of Barrett’s esophagus (BE), making standardization challenging.
To evaluate the preferred diagnosis and management practices of BE among Asian endoscopists.
Endoscopists from across Asia were invited to participate in an online questionnaire comprising eleven questions regarding diagnosis, surveillance and management of BE.
Five hundred sixty-nine of 1016 (56.0%) respondents completed the survey, with most respondents from Japan (n = 310, 54.5%) and China (n = 129, 22.7%). Overall, the preferred endoscopic landmark of the esophagogastric junction was squamo-columnar junction (42.0%). Distal palisade vessels was preferred in Japan (59.0% vs 10.0%, P < 0.001) while outside Japan, squamo-columnar junction was preferred (59.5% vs 27.4%, P < 0.001). Only 16.3% of respondents used Prague C and M criteria all the time. It was never used by 46.1% of Japanese, whereas 84.2% outside Japan, endoscopists used it to varying extents (P < 0.001). Most Asian endoscopists (70.8%) would survey long-segment BE without dysplasia every two years. Adherence to Seattle protocol was poor with only 6.3% always performing it. 73.2% of Japanese never did it, compared to 19.3% outside Japan (P < 0.001). The most preferred (74.0%) treatment of non-dysplastic BE was proton pump inhibitor only when the patient was symptomatic or had esophagitis. For BE with low-grade dysplasia, 6-monthly surveillance was preferred in 61.9% within Japan vs 47.9% outside Japan (P < 0.001).
Diagnosis and management of BE varied within Asia, with stark contrast between Japan and outside Japan. Most Asian endoscopists chose squamo-columnar junction to be the landmark for esophagogastric junction, which is incorrect. Most also did not consistently use Prague criteria, and Seattle protocol. Lack of standardization, education and research are possible reasons.
Core Tip: Presently, not all guidelines agree on the management of Barrett's esophagus (BE). It is against this background that the Asian Barrett's Consortium conducted this multinational survey, which involved 569 endoscopists from 13 countries/regions, and we found that management of BE varied, with stark contrast between participants from Japan and the rest of Asia-Pacific. Most endoscopists chose squamo-columnar junction to be the landmark for esophagogastric junction, which is incorrect. Most also did not consistently use Prague criteria, and Seattle protocol. We believe that these findings will shape our future efforts to standardize the management approach of this condition.
- Citation: Kew GS, Soh AYS, Lee YY, Gotoda T, Li YQ, Zhang Y, Chan YH, Siah KTH, Tong D, Law SYK, Ruszkiewicz A, Tseng PH, Lee YC, Chang CY, Quach DT, Kusano C, Bhatia S, Wu JCY, Singh R, Sharma P, Ho KY. Multinational survey on the preferred approach to management of Barrett’s esophagus in the Asia-Pacific region. World J Gastrointest Oncol 2021; 13(4): 279-294
- URL: https://www.wjgnet.com/1948-5204/full/v13/i4/279.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i4.279
Barrett’s esophagus (BE), a premalignant condition where esophageal squamous epithelium is replaced by columnar epithelium, with biopsy confirmation of intestinal metaplasia (IM), is increasingly prevalent in Asia, from an estimated prevalence of 0.8% in the 1990s, to 2.2% for 2010-2014[1,2]. The Asian Barrett's Consortium (ABC) was founded in 2008 to develop strategies in tackling challenges faced in the management of BE in the Asian region. The group previously reported that there was substantial variability in the published prevalence of BE in Asia, noting that these studies used different methodologies, enrolled populations, endoscopic practices and histo-pathological criteria[3,4]. Additionally, guidelines from major societies worldwide provided differing definitions and viewpoints, further complicating standardization of approaches to management of BE in different countries. Ultimately, as a premalignant condition, the clinical implication of BE lies in its risk of esophageal adenocarcinoma (EAC).
Presently, guidelines on BE do not all agree on various aspects of the diagnosis of BE. For example, several western guidelines recommend the proximal end of the gastric longitudinal folds to be the endoscopic landmark of the esophagogastric junction, whereas the Japan Esophageal Society recommends the lower margin of palisading small vessels as the endoscopic landmark of choice[5]. The minimal length of columnar-lined epithelium for the diagnosis of BE also differs between guidelines. Histologically, there has been much controversy regarding the requirement of IM for the diagnosis of BE. The British, Japanese and Asia-Pacific guidelines do not require the presence of IM for its diagnosis[5-7].
The endoscopic management of BE among different Asian countries was first examined in 2011 in a study of 56 completed questionnaires across six East Asian countries (China, Korea, Japan, Thailand, Indonesia and Philippines)[8]. However, the study was limited by the small study sample size, and that it only involved countries in the East Asian region. In another study performed in the United States, it was found that practice patterns for endoscopic imaging and management of BE also varied among practicing gastroenterologists[9].
It is against this background that the ABC conducted this large scale study to evaluate the preferred diagnosis and management practices of Asian endoscopists on BE, and determine if there was a difference in the current practices and perceptions among Asian endoscopists in their management of BE. The findings will shape our future efforts to standardize the management approach of this condition.
Participants, who were gastrointestinal endoscopists, of both genders, from countries across Asia, were recruited consequentially from July 2018 to July 2019. They were first identified via regional experts and professional societies and subsequently invited to participate in the study via email. Study information and a link to survey were provided in the email. After giving consent by clicking on the first page of the link, the participants would complete the survey online. Participants were excluded if their responses were incomplete, or they were non-clinicians, and non-practicing endoscopists.
As no individually identifiable information was obtained during the questionnaire, confidentiality and anonymity were maintained. Ethics approval was obtained for this study, No. DSRB 2018/00863.
A working subgroup within the ABC, comprising five experts with extensive experience and publications in BE, first drafted the questionnaire. After several rounds of review by key members of the ABC, the 32 questions in the initial drafted were eventually truncated and modified to 11 questions in the final version. The final version (Supplementary Appendix 1) underwent content and face validation by all 18 members of the consortium before it was approved for use. There were two sections to the survey questionnaire; seven questions were dedicated to the section on “preferred diagnosis and surveillance practice” and four questions to the section on “preferred management approach”. The questions were uploaded into my survey, an online survey platform hosted by the National University of Singapore (Verint Systems Inc., New York, United States). The three online pages of questionnaire existed in two languages i.e. English and Chinese (Supplementary Appendix 2).
In brief, the first page of the questionnaire introduced the participants to ABC and the rationale for conducting the survey, and obtain implied consent from the participants. The second page collected basic demographic information including age, gender, physician or surgeon, location of practice, years of endoscopic experience, and percentage of time spent performing GI endoscopy. The third page consisted of the eleven multiple-choice questions. Responses were considered complete only if participants submitted valid responses for all questions in all three pages. No personally identifiable information would be collected.
Analysis was performed with SPSS version 25 (SPSS Inc., Chicago, United States) with statistical significance set at P < 0.05. Descriptive statistics for categorical variables were presented as n (%) and mean (SD) for numerical variables. Differences in the responses across regions were compared using Chi-square or Fisher-exact tests.
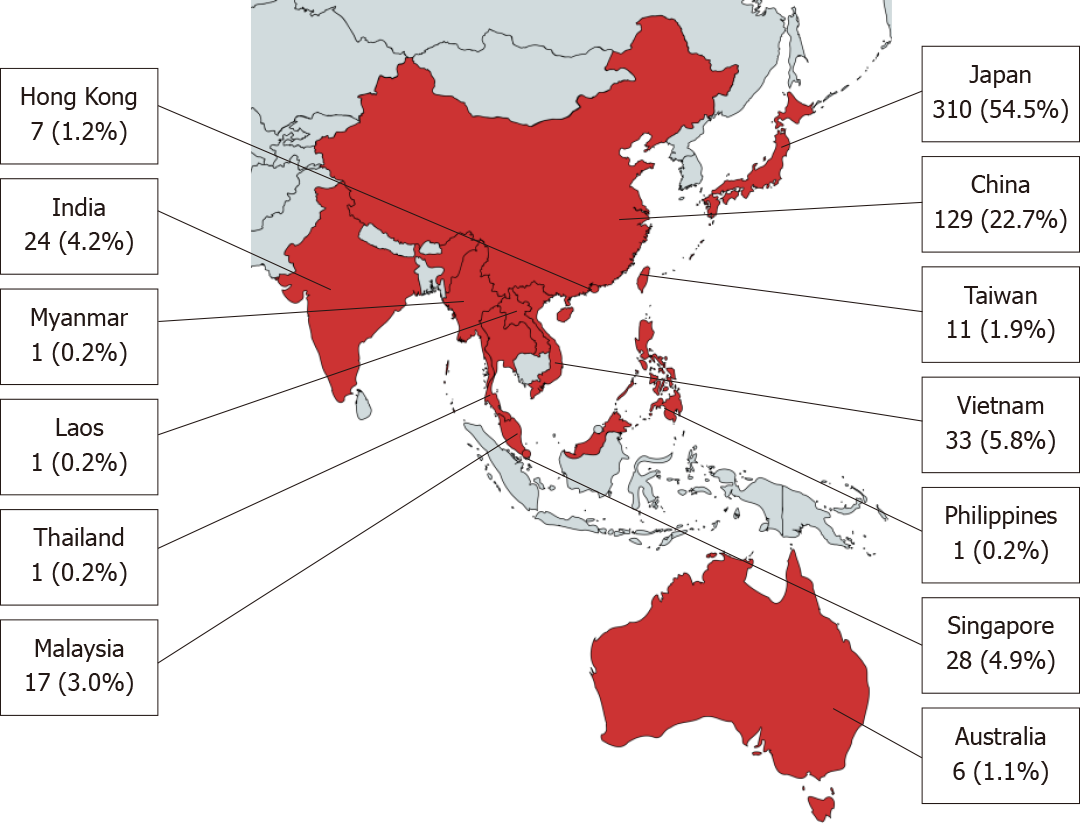
Of 1016 endoscopists invited to participate in the survey, 569 completed the questionnaire in full, giving a response rate of 56.0%. Overall, endoscopists from 13 countries participated in the survey, with the most respondents coming from Japan (n = 310, 54.5%), followed by China (n = 129, 22.7%), and Vietnam (n = 33, 5.8%) (Figure 1). The median age of the respondents was 38 years [Interquartile range (IQR): 33-46] and 443 (77.9%) were male respondents (Table 1). A total of 514 (90.3%) were physicians and the remaining 55 (9.7%) were surgeons. Almost half or 271 (47.6%) respondents were of academic background, 160 (28.1%) in private setting, and 138 (24.3%) from both settings. The median number of years of endoscopic practice was 10 years (IQR: 5-18). The number of respondents who spent at least 20% of their time performing endoscopy was 449 (78.9%).
| Respondent demographics | Study cohort (n = 569) | Percentage (%) |
| Age, years (median, IQR) | 38 (33-46) | - |
| Gender | ||
| Male | 443 | 77.9 |
| Female | 126 | 22.1 |
| Specialty | ||
| Physician | 514 | 90.3 |
| Surgeon | 55 | 9.7 |
| Place of practice | ||
| Australia | 6 | 1.1 |
| China | 129 | 22.7 |
| Hong Kong | 7 | 1.2 |
| India | 24 | 4.2 |
| Japan | 310 | 54.5 |
| Laos | 1 | 0.2 |
| Malaysia | 17 | 3.0 |
| Myanmar | 1 | 0.2 |
| Philippines | 1 | 0.2 |
| Singapore | 28 | 4.9 |
| Taiwan | 11 | 1.9 |
| Thailand | 1 | 0.2 |
| Vietnam | 33 | 5.8 |
| Type of practice | ||
| Private institution | 160 | 28.1 |
| Academic institution | 271 | 47.6 |
| Both | 138 | 24.3 |
| Years of endoscopic practice (median, IQR) | 10 (5-18) | - |
| Percentage of time performing endoscopy | ||
| < 20% | 120 | 21.1 |
| 20%-40% | 189 | 33.2 |
| 40%-60% | 150 | 26.4 |
| 60%-80% | 72 | 12.7 |
| > 80% | 38 | 6.7 |
Based on the overall surveyed population, the squamo-columnar junction was the preferred endoscopic landmark of the esophagogastric junction in 42.0% of all respondents, followed by distal margin of palisade vessels in 36.7%. Only 19.5% preferred to use the proximal margin of gastric folds as their landmark (Table 2). About half of respondents (48.3%) used any length of columnar lined epithelium in the esophagus as their preferred endoscopic definition of BE. Only 16.3% of respondents used the Prague C and M criteria all the time in their assessment of BE, while 32.3% never did. When asked about the comfort level of endoscopic assessment of BE, only 13.0% rated that they are 100% comfortable with their endoscopic diagnosis of BE, while 45.2% were at least 70% comfortable with it. Any columnar tissue was the most preferred (37.1%) histological definition of BE, followed by specialized IM, and gastric metaplasia at 25.7%, and 17.4% respectively, while 19.9% endoscopists did not require any histological confirmation.
| Question | Option | Results |
| Q1. What is your preferred endoscopic landmark of the esophagogastric junction? | Squamo-columnar Junction (Z-line) | 42.0% |
| Proximal margin of gastric folds | 19.5% | |
| Distal margin of palisade vessels | 36.7% | |
| Diaphragmatic pinch | 1.8% | |
| Q2. What is your preferred endoscopic definition of Barrett’s esophagus? | Length of columnar lined epithelium ≥ 2 cm | 29.0% |
| Length of columnar lined epithelium ≥ 1 cm | 22.7% | |
| Any length of columnar lined epithelium in the esophagus | 48.3% | |
| Q3. How often do you use the Prague C and M criteria in your assessment of Barrett’s esophagus? | All the time | 16.3% |
| > 70% of the time | 9.5% | |
| 30%-70% of the time | 11.8% | |
| < 30% of the time | 30.1% | |
| Never | 32.3% | |
| Q4. How comfortable are you with endoscopic assessment (white-light with or without advanced imaging technology) in the diagnosis of Barrett’s esophagus? | 100% comfortable | 13.0% |
| > 70% comfortable | 45.2% | |
| 30%-70% comfortable | 28.8% | |
| < 30% comfortable | 10.5% | |
| Not at all | 2.5% | |
| Q5. What is your preferred histologic definition of Barrett’s esophagus? | Any columnar tissue | 37.1% |
| Specialized intestinal metaplasia | 25.7% | |
| Gastric metaplasia | 17.4% | |
| No histological confirmation required | 19.9% | |
| Q6. In your practice, how regular do you survey your long-segment Barrett’s esophagus without dysplasia? | Every 2 yr | 70.8% |
| Every 3 yr | 13.0% | |
| Every 5 yr | 3.0% | |
| None at all | 13.2% | |
| Q7. How often do you follow the Seattle protocol (i.e. four-quadrant biopsies every 2 cm) in your biopsies of Barrett’s esophagus during surveillance endoscopy? | All the time | 6.3% |
| > 70% of the time | 6.0% | |
| 30%-70% of the time | 9.1% | |
| < 30% of the time | 29.9% | |
| Never | 48.7% | |
| Q8. What is your preferred treatment of Barrett’s esophagus without dysplasia? | Lifelong PPI | 21.3% |
| PPI only when patient has symptoms of gastroesophageal reflux or evidence of esophagitis | 74.0% | |
| Radiofrequency ablation | 2.3% | |
| Anti-reflux procedure (e.g., fundoplication) | 2.5% | |
| Q9. For Barrett’s esophagus patients whose biopsies showed indefinite for dysplasia, your preferred approach is: | Confirm with second pathologist and repeat endoscopy after a course of PPI | 44.8% |
| Surveillance 6-monthly | 30.2% | |
| Surveillance yearly | 24.1% | |
| Surveillance 3-5 yearly | 0.9% | |
| Q10. For Barrett’s esophagus patients without a lesion but whose biopsies showed low grade dysplasia, your preferred approach is: | Surveillance 6-monthly | 55.5% |
| Surveillance yearly | 21.3% | |
| Surveillance 3-5 yearly | 1.8% | |
| Ablative therapy, e.g., radiofrequency, cryotherapy, argon plasma coagulation | 9.5% | |
| Endoscopic mucosal resection | 3.9% | |
| Endoscopic submucosal dissection | 8.1% | |
| Q11. For Barrett’s esophagus patients without a lesion but whose biopsies showed high grade dysplasia, your preferred treatment is: | Endoscopic mucosal resection | 17.0% |
| Endoscopic submucosal dissection | 68.2% | |
| Ablative therapy, e.g., radiofrequency, cryotherapy, argon plasma coagulation | 11.2% | |
| Surgery, e.g., esophagectomy | 3.5% |
Regarding surveillance of BE, most (70.8%) would survey long-segment BE without dysplasia every two years. Only 6.3% of respondents would adhere to the Seattle protocol in their biopsies all the time during surveillance endoscopy. Almost half (48.7%) never followed it at all, while another 29.9% would adhere only less than 30% of the time.
The preferred treatment of BE without dysplasia was to prescribe proton pump inhibitor (PPI) only when the patient has symptoms of gastroesophageal reflux disease (GERD) or evidence of esophagitis (74.0%). For BE with biopsies that showed indefinite for dysplasia, most (44.8%) would confirm with a second pathologist and repeat endoscopy after a course of PPI, while 30.2% would perform surveillance 6-monthly. For BE who had low grade dysplasia without a lesion, the most preferred option was surveillance 6-monthly in 55.5%, while 21.3% would perform yearly surveillance. For high grade dysplasia (HGD) without a lesion, endoscopic submu-cosal dissection was the preferred treatment (68.2%).
Results were further evaluated post hoc by comparing responses by countries/regions to investigate if practices were influenced by regional differences. Approximately half of all responses came from Japan, giving us the opportunity to analyse responses between Japan and the rest of Asia combined, as presented in Table 3. As practices might also be influenced by differences in awareness of the guidelines, responses were also analysed by comparing academic vs non-academic endoscopists, as presented in Table 4. For the purpose of the analysis, we define academic endoscopists (n = 409) as those who practiced only in academic centers (n = 271), and those who practiced in both academic and private centers (n = 138). Non-academic endoscopists (n = 160) are defined as those who practiced solely in the private sector.
| Question | Option | Japan | Rest of Asia | P value |
| Q1. What is your preferred endoscopic landmark of the esophagogastric junction? | Squamo-columnar Junction (Z-line) | 27.4% | 59.5% | < 0.001 |
| Proximal margin of gastric folds | 12.6% | 27.8% | ||
| Distal margin of palisade vessels | 59.0% | 10.0% | ||
| Diaphragmatic pinch | 1.0% | 2.7% | ||
| Q2. What is your preferred endoscopic definition of Barrett’s esophagus? | Length of columnar lined epithelium ≥ 2 cm | 23.2% | 35.9% | < 0.001 |
| Length of columnar lined epithelium ≥ 1 cm | 12.6% | 34.7% | ||
| Any length of columnar lined epithelium in the esophagus | 64.2% | 29.3% | ||
| Q3. How often do you use the Prague C and M criteria in your assessment of Barrett’s esophagus? | All the time | 11.3% | 22.4% | < 0.001 |
| > 70% of the time | 4.5% | 15.4% | ||
| 30%-70% of the time | 8.7% | 15.4% | ||
| < 30% of the time | 29.4% | 30.9% | ||
| Never | 46.1% | 15.8% | ||
| Q4. How comfortable are you with endoscopic assessment (white-light with or without advanced imaging technology) in the diagnosis of Barrett’s esophagus? | 100% comfortable | 17.1% | 8.1% | < 0.001 |
| > 70% comfortable | 51.6% | 37.5% | ||
| 30%-70% comfortable | 24.2% | 34.4% | ||
| < 30% comfortable | 6.5% | 15.4% | ||
| Not at all | 0.6% | 4.6% | ||
| Q5. What is your preferred histologic definition of Barrett’s esophagus? | Any columnar tissue | 35.2% | 39.4% | < 0.001 |
| Specialized intestinal metaplasia | 16.8% | 36.3% | ||
| Gastric metaplasia | 16.1% | 18.9% | ||
| No histological confirmation required | 31.9% | 5.4% | ||
| Q6. In your practice, how regular do you survey your long-segment Barrett’s esophagus without dysplasia? | Every 2 yr | 82.3% | 57.1% | < 0.001 |
| Every 3 yr | 4.8% | 22.8% | ||
| Every 5 yr | 1.6% | 4.6% | ||
| None at all | 11.3% | 15.4% | ||
| Q7. How often do you follow the Seattle protocol (i.e. four-quadrant biopsies every 2 cm) in your biopsies of Barrett’s esophagus during surveillance endoscopy? | All the time | 2.6% | 10.8% | < 0.001 |
| > 70% of the time | 4.2% | 8.1% | ||
| 30%-70% of the time | 2.3% | 17.4% | ||
| < 30% of the time | 17.7% | 44.4% | ||
| Never | 73.2% | 19.3% | ||
| Q8. What is your preferred treatment of Barrett’s esophagus without dysplasia? | Lifelong PPI | 15.8% | 27.8% | < 0.001 |
| PPI only when patient has symptoms of gastroesophageal reflux or evidence of esophagitis | 81.9% | 64.5% | ||
| Radiofrequency Ablation | 1.0% | 3.9% | ||
| Anti-reflux procedure (e.g. fundoplication) | 1.3% | 3.9% | ||
| Q9. For Barrett’s esophagus patients whose biopsies showed indefinite for dysplasia, your preferred approach is: | Confirm with second pathologist and repeat endoscopy after a course of PPI | 32.6% | 59.5% | < 0.001 |
| Surveillance 6-monthly | 37.7% | 21.2% | ||
| Surveillance yearly | 29.0% | 18.1% | ||
| Surveillance 3-5 yearly | 0.6% | 1.2% | ||
| Q10. For Barrett’s esophagus patients without a lesion but whose biopsies showed low grade dysplasia, your preferred approach is: | Surveillance 6-monthly | 61.9% | 47.9% | < 0.001 |
| Surveillance yearly | 21.9% | 20.5% | ||
| Surveillance 3-5 yearly | 1.0% | 2.7% | ||
| Ablative therapy, e.gv., radiofrequency, cryotherapy, argon plasma coagulation | 1.0% | 19.7% | ||
| Endoscopic mucosal resection | 1.6% | 6.6% | ||
| Endoscopic submucosal dissection | 12.6% | 2.7% | ||
| Q11. For Barrett’s esophagus patients without a lesion but whose biopsies showed high grade dysplasia, your preferred treatment is: | Endoscopic mucosal resection | 12.6% | 22.4% | < 0.001 |
| Endoscopic submucosal dissection | 83.5% | 49.8% | ||
| Ablative therapy, e.g., radiofrequency, cryotherapy, argon plasma coagulation | 2.6% | 21.6% | ||
| Surgery, e.g., esophagectomy | 1.3% | 6.2% |
| Question | Option | Academic | Non-academic | P value |
| Q1. What is your preferred endoscopic landmark of the esophagogastric junction? | Squamo-columnar Junction (Z-line) | 37.4% | 53.8% | 0.005 |
| Proximal margin of gastric folds | 21.0% | 15.6% | ||
| Distal margin of palisade vessels | 39.9% | 28.8% | ||
| Diaphragmatic pinch | 1.7% | 1.9% | ||
| Q2. What is your preferred endoscopic definition of Barrett’s esophagus? | Length of columnar lined epithelium ≥ 2 cm | 26.4% | 35.6% | 0.094 |
| Length of columnar lined epithelium ≥ 1 cm | 23.5% | 20.6% | ||
| Any length of columnar lined epithelium in the esophagus | 50.1% | 43.8% | ||
| Q3. How often do you use the Prague C and M criteria in your assessment of Barrett’s esophagus? | All the time | 19.3% | 8.8% | 0.004 |
| > 70% of the time | 11.0% | 5.6% | ||
| 30%-70% of the time | 11.0% | 13.8% | ||
| < 30% of the time | 28.4% | 34.4% | ||
| Never | 30.3% | 37.5% | ||
| Q4. How comfortable are you with endoscopic assessment (white-light with or without advanced imaging technology) in the diagnosis of Barrett’s esophagus? | 100% comfortable | 13.9% | 10.6% | 0.043 |
| > 70% comfortable | 46.5% | 41.9% | ||
| 30%-70% comfortable | 28.4% | 30.0% | ||
| < 30% comfortable | 8.3% | 16.3% | ||
| Not at all | 2.9% | 1.3% | ||
| Q5. What is your preferred histologic definition of Barrett’s esophagus? | Any columnar tissue | 35.2% | 41.9% | 0.093 |
| Specialized intestinal metaplasia | 28.4% | 18.8% | ||
| Gastric metaplasia | 16.4% | 20.0% | ||
| No histological confirmation required | 20.0% | 19.4% | ||
| Q6. In your practice, how regular do you survey your long-segment Barrett’s esophagus without dysplasia? | Every 2 yr | 69.9% | 73.1% | 0.001 |
| Every 3 yr | 16.1% | 5.0% | ||
| Every 5 yr | 2.7% | 3.8% | ||
| None at all | 11.2% | 18.1% | ||
| Q7. How often do you follow the Seattle protocol (i.e. four-quadrant biopsies every 2 cm) in your biopsies of Barrett’s esophagus during surveillance endoscopy? | All the time | 6.6% | 5.6% | 0.281 |
| > 70% of the time | 6.6% | 4.4% | ||
| 30%-70% of the time | 9.8% | 7.5% | ||
| < 30% of the time | 27.4% | 36.3% | ||
| Never | 49.6% | 46.3% | ||
| Q8. What is your preferred treatment of Barrett’s esophagus without dysplasia? | Lifelong PPI | 23.2% | 16.3% | 0.091 |
| PPI only when patient has symptoms of gastroesophageal reflux or evidence of esophagitis | 73.1% | 76.3% | ||
| Radiofrequency Ablation | 1.7% | 3.8% | ||
| Anti-reflux procedure (e.g. fundoplication) | 2.0% | 3.8% | ||
| Q9. For Barrett’s esophagus patients whose biopsies showed indefinite for dysplasia, your preferred approach is: | Confirm with second pathologist and repeat endoscopy after a course of PPI | 45.2% | 43.8% | 0.973 |
| Surveillance 6-monthly | 30.1% | 30.6% | ||
| Surveillance yearly | 23.7% | 25.0% | ||
| Surveillance 3-5 yearly | 1.0% | 0.6% | ||
| Q10. For Barrett’s esophagus patients without a lesion but whose biopsies showed low grade dysplasia, your preferred approach is? | Surveillance 6-monthly | 56.7% | 52.5% | 0.956 |
| Surveillance yearly | 20.3% | 23.8% | ||
| Surveillance 3-5 yearly | 1.7% | 1.9% | ||
| Ablative therapy, e.g., radiofrequency, cryotherapy, argon plasma coagulation | 9.3% | 10.0% | ||
| Endoscopic mucosal resection | 3.9% | 3.8% | ||
| Endoscopic submucosal dissection | 8.1% | 8.1% | ||
| Q11. For Barrett’s esophagus patients without a lesion but whose biopsies showed high grade dysplasia, your preferred treatment is? | Endoscopic mucosal resection | 19.1% | 11.9% | 0.037 |
| Endoscopic submucosal dissection | 67.2% | 70.6% | ||
| Ablative therapy, e.g., radiofrequency, cryotherapy, argon plasma coagulation | 11.2% | 11.3% | ||
| Surgery, e.g., esophagectomy | 2.4% | 6.3% |
Distal palisade vessels was the preferred landmark to define esophagogastric junction among 59.0% respondents from Japan vs 10.0% of the respondents from the rest of Asia (P < 0.001), whilst squamo-columnar junction was the preferred landmark in 59.5% of the respondents from outside Japan vs 27.4% respondents from within Japan (P < 0.001). Any length of columnar-lined epithelium was the preferred endoscopic definition of BE in 64.2% of Japanese compared to 34.7%, and 35.9% of the respondents from outside Japan who preferred a minimum length of 1 cm, and 2 cm, respectively (P < 0.001). There were 46.1% respondents from Japan who did not use the Prague C and M criteria, whereas outside Japan, 84.2% of respondents did so to varying extents (P < 0.001). Regarding histology, in Japan, 31.9% of respondents felt that no histological confirmation was required and 35.2% required only columnar tissue, but outside Japan, 39.4% preferred only columnar tissue while 36.3% required specialized IM to diagnose BE (P < 0.001).
The preferred landmark of the esophagogastric junction was the distal margin of palisade vessels among the academic endoscopists (39.9%), and squamo-columnar junction among the non-academic endoscopists (53.8%) (P = 0.005). Academic endoscopists were two times more likely to use the Prague C and M all the time (19.3% vs 8.8%, P = 0.004) compared to their non-academic counterparts. However, there was no difference in the endoscopic definition of BE as both the academic (50.1%) and non-academic endoscopists (43.8%) agreed with the definition being any length of columnar lined epithelium in the esophagus (P = 0.094). Neither was there any difference in the preferred histological definition of BE of any columnar tissue among academic (35.2%), and non-academic endoscopists (41.9%) (P = 0.093). On the other hand, academic endoscopists were more comfortable with the endoscopic assessment of BE, with 13.9% and 46.5% of academic endoscopists being 100% and > 70% comfortable respectively, compared to 10.6% and 41.9% of non-academic endoscopists being 100% and > 70% comfortable respectively (P = 0.043).
More respondents within Japan (82.3%) performed two-yearly surveillance endoscopy for long segment non-dysplastic BE than those outside Japan (57.1%) (P < 0.001). The Seattle protocol for biopsies was never performed among 73.2% of Japanese respondents, whereas outside Japan 80.7% of respondents used the Seattle protocol for biopsies to various extent (P < 0.001).
For long-segment BE without dysplasia, both academic (69.9%) and non-academic endoscopists (73.1%) were equally agreeable to perform two-yearly surveillance endoscopy. However, the Seattle protocol was never performed in as many of the academic endoscopists (49.6%) as their non-academic counterparts (46.3%) (P = 0.281).
For non-dysplastic BE, 81.9% of Japanese endoscopists preferred to treat it with PPI only if there was concurrent presence of esophagitis or when the patient was symptomatic. In contrast, 64.5% of respondents from outside Japan would do the same (P < 0.001). For histologic finding that was indefinite for dysplasia, a third (32.6%) of Japanese would confirm it with a second pathologist and another third (37.7%) would repeat the endoscopy 6-monthly, but outside Japan, 59.5% of respondents would confirm it with a second pathologist (P < 0.001). For low grade dysplasia without a lesion, 6-monthly surveillance was preferred in 61.9% of respondents from Japan vs 47.9% of respondents from outside Japan (P < 0.001). For HGD without lesion, endoscopic submucosal dissection was the preferred option for 83.5% of Japanese vs 49.8% from outside Japan (P < 0.001).
There was no difference in the management of the various aspects of BE and its related neoplasia between academic and non-academic endoscopists. For BE without dysplasia, most of the academic (73.1%) and non-academic endoscopists (76.3%) were equally agreeable to prescribe PPI only if the patient was symptomatic for GERD or if esophagitis was present (P = 0.091). For biopsies showing indefinite for dysplasia, 45.2% of academic endoscopists and 43.8% of non-academic endoscopists would confirm with a second pathologist and repeat endoscopy after a course of PPI (P = 0.973). For biopsies of BE without a lesion showing low grade dysplasia, most academic (56.7%) and non-academic endoscopists (52.5%) would prefer 6-monthly surveillance (P = 0.956). For biopsies showing HGD, 67.2% of the academic endoscopists, and 70.6% of the private endoscopists would prefer endoscopic submucosal dissection for treatment (P = 0.037).
The most striking finding from our study was the misidentification of the squamo-columnar junction as the esophagogastric junction by most endoscopists outside Japan (59.5%). Within Japan, more than half of the endoscopists (59.0%) correctly pointed out that the distal margin of the lower esophageal palisade vessels represents the anatomical esophagogastric junction. These were consistent with the Japanese Esophageal Society (JES) guidelines, which recommend the lower margin of the palisading small vessels as the endoscopic landmark and, if those are unclear, the oral margin of the longitudinal folds of the greater curvature of the stomach may be used instead[5]. There were also more non-academic endoscopists (53.8%) compared to academic endoscopists (37.4%) who preferred the squamo-columnar junction as the esophagogastric junction. The use of squamo-columnar junction as the esopha-gogastric junction is incorrect as the squamo-columnar junction frequently displaces proximally in patients with hiatus hernia, ulcers, and columnar lined epithelium[10]. Hence, the squamo-columnar junction is not stable and should not be used as the landmark for esophagogastric junction. The finding, which has not been previously reported, to our knowledge, suggests that there is a need to change this perception among a significant proportion of endoscopists in Asia.
The differences in management of BE was first examined in a study by Ishimura et al[8] back in 2011, however the study was limited by its small sample size of 56 participants and involved countries in East Asia only[8]. Our current study of 569 participants across many more countries served to provide greater insight on some of these differences. It is clear from our study that endoscopists across the Asia Pacific region differed in their preferences on the diagnosis, surveillance, and management of BE, and particularly so when comparing endoscopists who practiced in Japan compared to other regions, as well as among academic and non-academic endoscopists.
The differing practices are not surprising given that various international guidelines were non-uniform in their recommendations for the diagnosis and management of BE. For example, the American guidelines recommend the diagnosis of BE when the salmon-colored mucosa extends into the esophagus by ≥ 1 cm proximal to the esophagogastric junction with biopsy confirmation of IM[11], whereas the British guidelines define BE as metaplastic columnar epithelium, confirmed histologically, replacing squamous epithelium by ≥ 1 cm above the esophagogastric junction and IM is no longer a prerequisite[6]. On the other hand, the JES defines BE as the presence of columnar epithelium continuous from the stomach with or without IM, with at least one of the following: Esophageal duct glands, squamous islands and double-layer muscularis mucosae[5].
Accurate assessment of BE on endoscopy is important given the risk of malignancy with BE[12]. The Prague C and M classification, introduced in 2006, describes the assessment of BE based on circumferential and maximal extent, as well as the endoscopic landmarks[13]. In our study, 46.1% of endoscopists in Japan reported that they had never used it, compared to 15.8% of endoscopists outside of Japan. In a study performed in Japan comparing the Japanese criteria to the Prague C and M classification, the investigators found a significantly higher esophagogastric junction identification rate and endoscopic BE diagnosis, and concluded that the Japanese criteria may be more suited to the Japanese population[14]. Non-academic endoscopists were also found to be less likely to use the Prague C and M, compared to academic endoscopists, and we postulate that this might be because academic endoscopists were more familiar with the Prague C and M classification.
In terms of histological diagnosis, there was also a difference in opinions among endoscopists in Japan compared to rest of Asia. The Japanese endoscopists mostly preferred either the presence of any columnar tissue or the absence of any histological confirmation, whereas in the rest of Asia, endoscopists mostly preferred either the presence of any columnar tissue or specialized IM. Currently, all major guidelines[5,6] required histological confirmation of columnar epithelium, and the American guidelines even included the need for histological finding of IM for the diagnosis of BE to be made[11]. The risk for neoplastic progression is thought to be higher in patients with coexisting IM[15]. However, endoscopic and histopathological correlation of BE had been shown to be poor, and previous studies had shown that most patients who had an initial endoscopic diagnosis of BE did not have BE confirmed on histology on subsequent endoscopy[16,17].
The Seattle protocol, proposed by Levine et al[18] in 2000, is a systemic assessment of the esophagus, and involves targeted biopsy of suspicious lesions, as well as biopsies of macroscopically normal BE segment in each quadrant at 1-2 cm intervals starting from the esophagogastric junction. In our survey, the overall adherence to the Seattle protocol is poor, with only 6.3% of all endoscopists performing it all the time. 73.2% of endoscopists in Japan had never used Seattle protocol in their practice, while 63.7% of endoscopists outside Japan adhered to it less than 30% of the time. Our results depicting poor adherence suggest that the Seattle protocol is not popular amongst Asian endoscopists in general. One possible reason is short segment BE is the predominant form of BE in Asia[19], and endoscopists may perform narrow band imaging (NBI) during endoscopic assessment to identify sites of dysplasia instead, which is less laborious and time-consuming. Moreover, in a randomized cross-over trial done by Sharma et al[19], the team had shown that NBI targeted biopsies had the same IM detection rate as high-definition white light examination with Seattle protocol, while requiring fewer biopsies. NBI targeted biopsies had also detected more areas with dysplasia[19]. However, many endoscopists are not trained in the effective use of NBI. The underutilisation of the Seattle protocol and hence lack of histological confirmation leading to possibly reduced confidence on diagnosis may have contributed to the more intensive endoscopic surveillance as seen in many countries in Asia. This new finding warrants further study as it has implications in terms of cost-effectiveness.
The role of PPI in reducing risk of EAC or HGD in patients with BE is unclear, and there are conflicting evidence in the literature and recommendations from interna
In patients with BE with low grade dysplasia, guidelines on its management differed as well, with the British guidelines suggesting that patients be surveyed endoscopically at 6-monthly intervals[6], while the American guidelines recommended that endoscopic therapy to be considered as the preferred treatment modality, although endoscopic surveillance every 12 mo is an acceptable alternative[11]. As a result, opinions varied across countries as well, with significant differences between Japan and rest of Asia. What was consistent though, was that both guidelines agreed that the diagnosis of low grade dysplasia should be confirmed by additional pathologists.
The differences in diagnosis and treatment approaches of BE could be explained by the relatively low prevalence of BE, particularly in countries outside Japan in Asia[23]. As a result, endoscopists do not manage enough BE, and may not be familiar with the diagnosis and management of BE. Western guidelines[6,11] do not recognize BE of less than 1 cm, whereas in Asia, the Japanese defined BE as presence of any columnar lined epithelium[5]. In Asia, it may be necessary to use a different BE diagnosis, rather than adopting Western standards as they are.
Some limitations faced in our study include the fact that the languages used in the survey were limited to English and Chinese, which could have affected the participation rate. We also appreciate that many technical terms may not have equivalent translations in other languages and could have possibly been interpreted differently, and perhaps adding endoscopic images could have helped. In addition, the survey sample may not be representative of the major opinions in the various countries, especially in areas such as Myanmar, Laos, Thailand, Philippines, Australia and Hong Kong, where there were less than 10 participants. However, this should not have a major impact on the observation that there was a difference in the practice pattern in Asia. Participation was voluntary, which might have resulted in selection bias in our study, but we have managed to achieve a response rate of > 50%, which is considered as acceptable in most surveys.
Moving forward, based on the results of our study, the ABC has identified three main aspects to be addressed in future efforts, which are the lack of standardization, education and research. To address the lack of standardization, ABC as well as other affiliated Asian societies have embarked on an international consensus led by regional experts to standardize diagnostic landmarks, surveillance intervals, and treatment approaches in Asia, which may be different from Western countries. More can also be done to educate Asian endoscopists, emphasizing on the importance of protocols, particularly those that have significantly influenced clinical outcomes. Educational efforts can be led by regional Asian experts to improve understanding and adherence, while pictures and diagrams can be used to effectively overcome language barriers. On the research front, collaborative research among colleagues within the Asia-pacific region is important. ABC plans to conduct further studies to identify and address the limitations, such as finding out the reasons for non-adherence to certain protocols in Asia, and then addressing them accordingly. More outcome-based research in Asia should be encouraged, which will also help to educate and convince fellow endoscopists within the region. Prospective follow-up studies on the recommen-dations by Asian guidelines can help provide evidence to back-up these recommendations, further reinforcing these guidelines to fellow Asian endoscopists.
In conclusion, the diagnosis, surveillance and management of BE appear to vary widely within Asia, with stark contrast between endoscopists who practiced within Japan and outside Japan. Most Asian endoscopists also chose squamo-columnar junction to be the landmark for esophagogastric junction, which is incorrect. There was inconsistent use of Prague criteria, and Seattle protocol by most Asian endoscopists. The lack of standardization, education and research are possible causes for the observed differences.
Barrett’s esophagus (BE), a premalignant condition, is associated with increased risk of esophageal adenocarcinoma. However, major societies provide differing guidance on management of BE, making standardization challenging.
The Asian Barrett’s Consortium was founded in 2008 to develop strategies in tackling challenges faced in the management of BE in the Asia. The group previously reported that there was substantial variability in the published prevalence of BE in Asia, noting that these studies used different methodologies, enrolled populations, endoscopic practices and histopathological criteria. To further our efforts to improve our management of BE, we wanted to understand the varying management practices of endoscopists from various countries in Asia.
The study aimed to evaluate the preferred diagnosis and management practices of Asian endoscopists on BE. The findings will shape our future efforts to standardize the management approach of this condition.
An online survey comprising 11 questions, was distributed to gastrointestinal endoscopists from countries across Asia from July 2018 to July 2019. The survey questions were categorized to “preferred diagnosis and surveillance practice” and “preferred management approach”.
The study found that 42.0% of all endoscopists incorrectly used the squamo-columnar junction to identify the esophagogastric junction. Prague C and M criteria was seldomly used by endoscopists, and adherence to Seattle protocol was poor with only 6.3% always performing it. There were also differences in diagnosis and management of BE when comparing endoscopists within Japan and outside Japan.
Diagnosis and management of BE varied within Asia, with stark contrast between Japan and outside Japan. Lack of standardization, education and research are possible reasons to account for such differences.
Further research is required to identify reasons for non-adherence to certain protocols in the management of BE, and how we could attempt to standardize diagnosis and management of BE.
We would like to thank the Japanese Society of Gastroenterology and the Asian Pacific Association of Gastroenterology for the survey dissemination.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ono S, Zaninotto G S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Shiota S, Singh S, Anshasi A, El-Serag HB. Prevalence of Barrett's Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015;13:1907-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Chang CY, Cook MB, Lee YC, Lin JT, Ando T, Bhatia S, Chow WH, El-Omar EM, Goto H, Li YQ, McColl K, Reddy N, Rhee PL, Sharma P, Sung JJ, Ghoshal U, Wong JY, Wu JC, Zhang J, Ho KY; Asian Barrett's Consortium. Current status of Barrett's esophagus research in Asia. J Gastroenterol Hepatol. 2011;26:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Soh YSA, Lee YY, Gotoda T, Sharma P, Ho KY; Asian Barrett's Consortium. Challenges to diagnostic standardization of Barrett's esophagus in Asia. Dig Endosc. 2019;31:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 606] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 6. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J; British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 858] [Cited by in F6Publishing: 808] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 7. | Fock KM, Talley N, Goh KL, Sugano K, Katelaris P, Holtmann G, Pandolfino JE, Sharma P, Ang TL, Hongo M, Wu J, Chen M, Choi MG, Law NM, Sheu BS, Zhang J, Ho KY, Sollano J, Rani AA, Kositchaiwat C, Bhatia S. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett's oesophagus. Gut. 2016;65:1402-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Ishimura N, Amano Y, Sollano JD, Zhu Q, Kachintorn U, Rani AA, Hahm KB, Takahashi S, Arakawa T, Joh T, Matsumoto T, Naito Y, Suzuki H, Ueno F, Fukudo S, Fujiwara Y, Kamiya T, Uchiyama K, Kinoshita Y; IGICS Study Group. Questionnaire-based survey conducted in 2011 concerning endoscopic management of Barrett's esophagus in East Asian countries. Digestion. 2012;86:136-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Singh M, Gupta N, Gaddam S, Balasubramanian G, Wani S, Sinh P, Aghaie K, Higbee AD, Rastogi A, Kanakadandi V, Bansal A, Sharma P. Practice patterns among U.S. gastroenterologists regarding endoscopic management of Barrett's esophagus. Gastrointest Endosc. 2013;78:689-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Huang Q. Definition of the esophagogastric junction: a critical mini review. Arch Pathol Lab Med. 2011;135:384-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 990] [Cited by in F6Publishing: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 12. | Gopal DV, Lieberman DA, Magaret N, Fennerty MB, Sampliner RE, Garewal HS, Falk GW, Faigel DO. Risk factors for dysplasia in patients with Barrett's esophagus (BE): results from a multicenter consortium. Dig Dis Sci. 2003;48:1537-1541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 731] [Cited by in F6Publishing: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 14. | Kinjo T, Kusano C, Oda I, Gotoda T. Prague C&M and Japanese criteria: shades of Barrett's esophagus endoscopic diagnosis. J Gastroenterol. 2010;45:1039-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | Khandwalla HE, Graham DY, Kramer JR, Ramsey DJ, Duong N, Green LK, El-Serag HB. Barrett's esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what's next? Am J Gastroenterol. 2014;109:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Meining A, Ott R, Becker I, Hahn S, Mühlen J, Werner M, Höfler H, Classen M, Heldwein W, Rösch T. The Munich Barrett follow up study: suspicion of Barrett's oesophagus based on either endoscopy or histology only--what is the clinical significance? Gut. 2004;53:1402-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Levine DS, Blount PL, Rudolph RE, Reid BJ. Safety of a systematic endoscopic biopsy protocol in patients with Barrett's esophagus. Am J Gastroenterol. 2000;95:1152-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, Hall M, Mathur SC, Wani SB, Hoffman B, Gaddam S, Fockens P, Bergman JJ. Standard endoscopy with random biopsies vs narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 20. | Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Jankowski JAZ, de Caestecker J, Love SB, Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks C, Attwood S, Harrison R, Barr H, Moayyedi P; AspECT Trial Team. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392:400-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 22. | Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett's oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther. 2014;39:984-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Lee HS, Jeon SW. Barrett esophagus in Asia: same disease with different pattern. Clin Endosc. 2014;47:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |