Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.1065
Peer-review started: April 22, 2020
First decision: April 26, 2020
Revised: July 16, 2020
Accepted: August 16, 2020
Article in press: August 16, 2020
Published online: September 15, 2020
Human epidermal growth factor receptor 2 (HER2) amplification is a molecular driver for a subset of colorectal cancers (CRCs) and one of the major causes of anti-epidermal growth factor receptor (EGFR) treatment failure. Compared to dual anti-HER2 treatments, which have been shown to be effective in HER2-positive metastatic CRC patients, single-agent anti-HER2 therapy is rarely used to treat CRC.
Herein, we report a case of RAS/BRAF-wild-type metastatic CRC that was identified as HER2-positive through circulating tumor DNA (ctDNA) testing by next-generation sequencing following the failure of two lines of therapy. Subsequently, the patient was given lapatinib monotherapy that led to a partial response with a progression-free survival of 7.9 mo. Moreover, serial ctDNA detection was used to monitor the efficacy of lapatinib. The aberration of HER2 copy number disappeared when radiographic assessment revealed a partial response. However, a high level of HER2 amplification was detected again at the time of disease progression. Finally, a phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation was identified at the time of tumor progression, which may explain the acquired resistance to lapatinib.
This is the first case report of HER2-positive RAS/BRAF wild-type metastatic CRC patient responding to lapatinib monotherapy. It highlights that ctDNA testing is an effective and feasible approach to evaluate the efficacy of anti-HER2 therapy.
Core Tip: While dual anti-human epidermal growth factor receptor 2 (HER2) therapies are recommend as standard treatment for HER2-positive advanced colorectal cancer, anti-HER2 monotherapy is rarely used to treat this population. This case is the first report of a HER2-positive RAS/BRAF-wild-type metastatic colorectal cancer patient achieving a partial response following lapatinib monotherapy without serious adverse events. Next generation sequencing based circulating tumor DNA testing played an essential role in both treatment decision and efficacy/resistance monitoring.
- Citation: Guan JL, Liu JH, Wang Q, Cong YW, Chen YX, Huang KF, Huang ML, Huang L. Response of human epidermal growth factor receptor 2-positive colorectal cancer to lapatinib monotherapy: A case report. World J Gastrointest Oncol 2020; 12(9): 1065-1072
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/1065.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.1065
Human epidermal growth factor receptor 2 (HER2) amplification or overexpression, which has been observed in 5% of colorectal cancer (CRC) patients[1,2], is considered a molecular driver in the development and progression of the disease[3]. It is also considered as the leading cause of anti-EGFR therapy failure[4-7].
Both HER2 Amplification for Colo-rectaL cancer Enhanced Stratification (HERACLES) and My Pathway trial have suggested that dual anti-HER2 treatments are effective in HER2-positive metastatic CRC (mCRC)[2,8]. In the HERACLES trial, 30% (8/27) of the HER2-positive mCRC patients treated with trastuzumab plus lapatinib presented an objective response; the median progression-free survival (PFS) was 21 wk[2]. My Pathway trial indicated an ORR of 38%, and the median time to progression of 5.6 mo in 13 HER2 amplified mCRC patients who received a combination of trastuzumab and petuzumab[8].
At present, single-agent anti-HER2 treatment is rarely used to treat HER2-positive mCRC; even though, a few studies (mainly preclinical studies) have suggested that patients with HER2 amplifications might benefit from anti-HER2 monotherapy[7,9,10]. Herein, we report the case of an HER2-positive and RAS/BRAF wild-type mCRC patient who responded to lapatinib monotherapy as the third-line therapy.
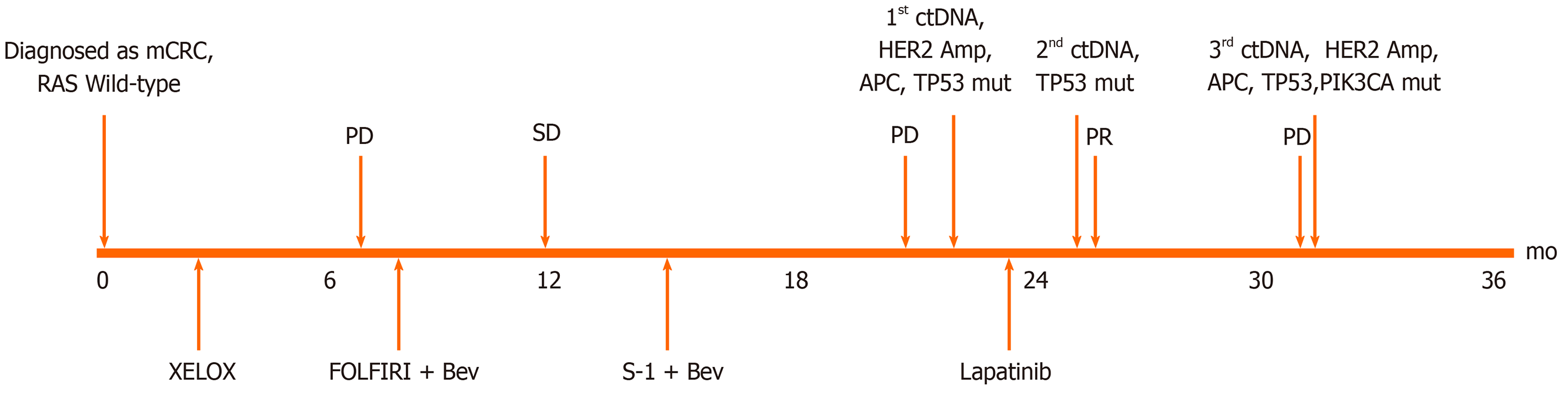
A 55-year-old man who suffered from hematochezia for 3 mo consulted our hospital in July 2016 (Figure 1).
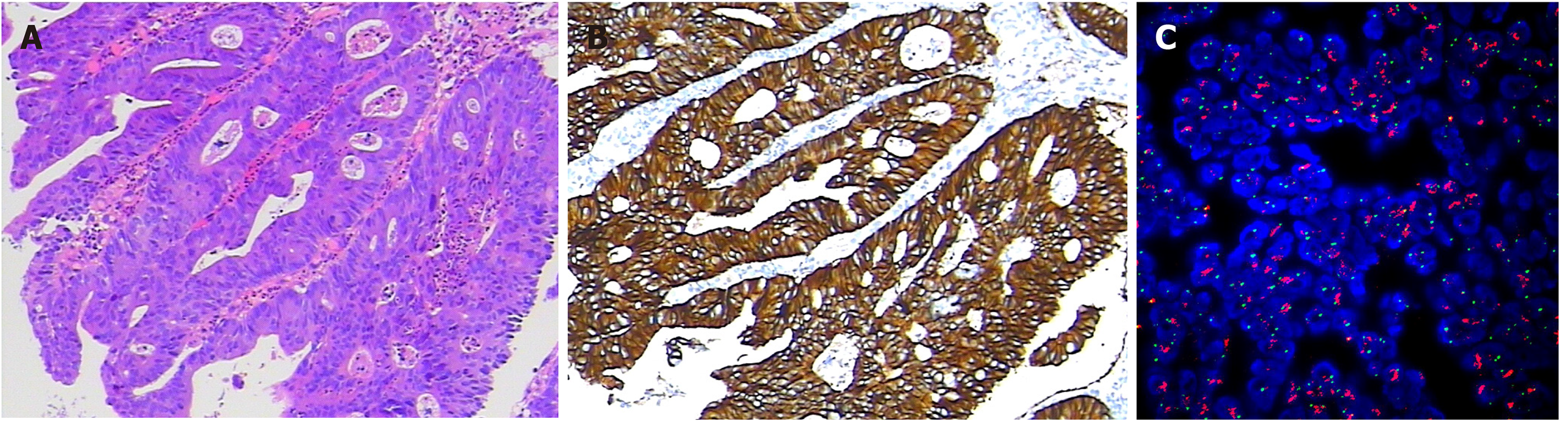
The pathological diagnosis following biopsy showed “moderately differentiated adenocarcinoma of the sigmoid colon” (Figure 2A). Abdominal enhanced computed tomography (CT) showed mesenteric lymph node metastasis and multiple low-density lesions in the liver. The capecitabine plus oxaliplatin (XELOX) scheme was recommended for the neoadjuvant therapy regimen for three cycles. However, even with therapy, the evaluation was progressive disease (PD) given that lymph node and liver lesions continued to grow; thus, surgery was excluded.
From February 2 to October 14, 2017, folinic acid, fluorouracil, and irinotecan (FOLFIRI) plus bevacizumab was implemented for 12 cycles, and the classification was stable disease (SD); bevacizumab plus S-1 was offered as the maintenance treatment. On March 30, 2018, CT indicted disease progression. Due to intestinal obstruction, the patient underwent laparoscopic intestinal adhesion lysis and transverse colostomy on May 30, 2018.
The patient had no medical history of past illness.
The patient neither smoke tobacco nor consume alcohol. There was no notable family medical history, such as cancer.
The height and weight of the patient were 160 cm and 56.8 kg, respectively. On July 15, 2018, the performance status (PS) was 1 for this patient, with a body temperature of 36.3 °C, pulse rate of 86 beats/min, and blood pressure of 152/96 mmHg.
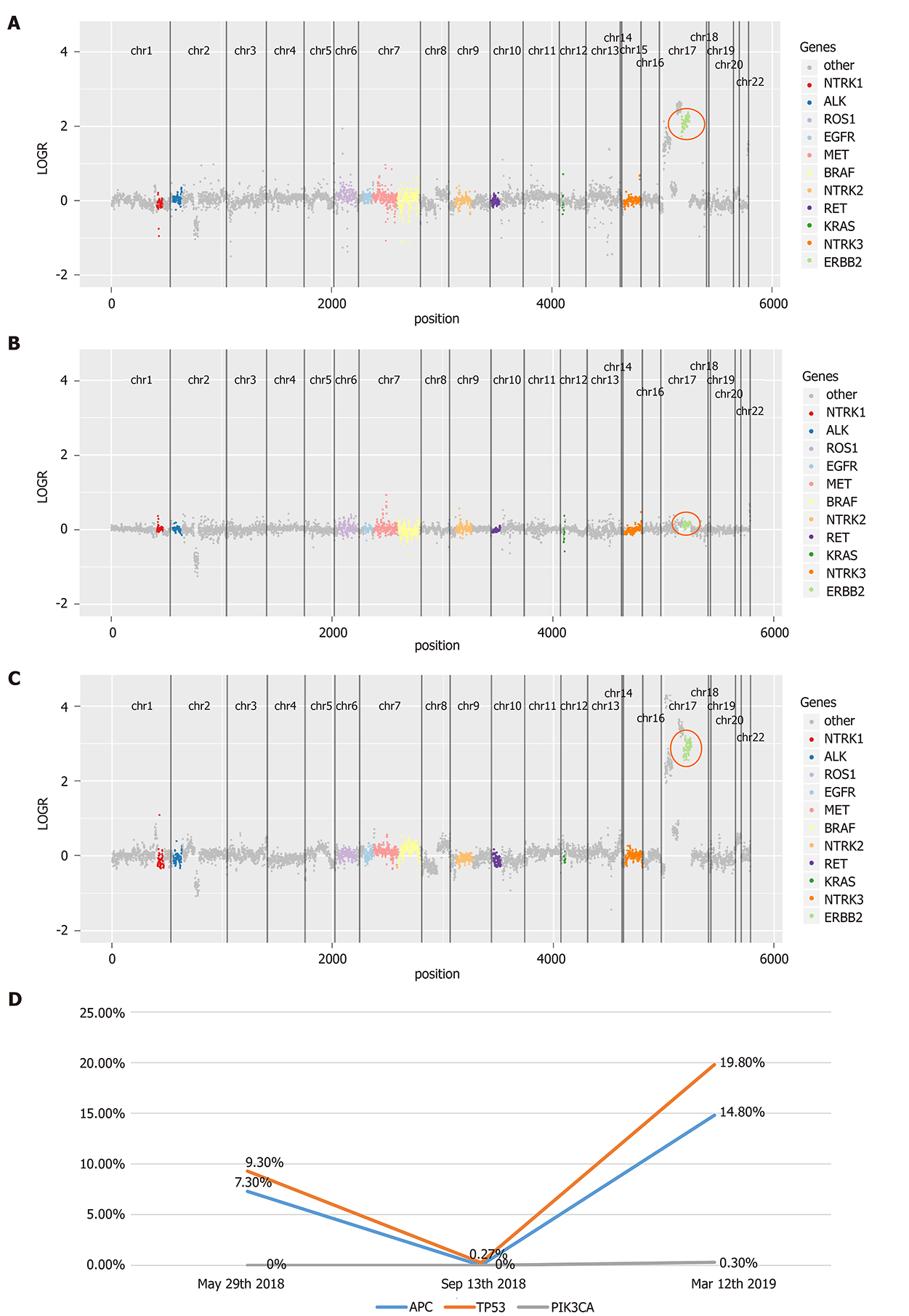
In July 2016, immunohistochemistry (IHC) and polymerase chain reaction (PCR) were performed to guide therapy at diagnosis. Kirsten rat sarcoma 2 viral oncogene homolog (KRAS), neuroblastoma RAS Viral Oncogene Homolog (NRAS), and v-raf murine sarcoma viral oncogene homolog B1 (BRAF) were wild-type, and mismatch repair proteins were proficient. On May 29, 2018, a peripheral blood sample was assessed for circulating tumor DNA (ctDNA) using next-generation sequencing (NGS) with an array of 189 genes to determine whether ras-rat sarcoma viral oncogene homolog/v-raf-murine sarcoma viral oncogene homolog (RAS/RAF) status changed during the past 2 years and EGFR monoclonal antibody could be used as the third-line therapy. The test results showed that KRAS, NRAS, and BRAF were still wild-type. Interestingly, high-level amplification of HER2 (absolute copy number: 63.67) (Figure 3A) was identified at the same time, which was reported as the resistance mechanism of anti-EGFR therapy previously[4-7]. Also, adenomatous polyposis coli (APC) and tumor protein 53 (TP53) alterations were detected, with a mutant allele frequency (MAF) of 7.3% and 9.3%, respectively. IHC for HER2 showed a score of 3+ based on the tumor tissue biopsy obtained on July 22, 2016 (Figure 2B), while fluorescence in situ hybridization (FISH) showed high-level amplification of HER2 (Figure 2C); thus, the two tests confirmed the results of ctDNA.
On March 30, 2018, CT showed that most of the liver lesions were increased compared to January 17, 2018, while new, multiple lesions were detected in the lung. Thus, the classification was PD, and the patient failed to respond to two lines of therapy.
The patient was diagnosed with CRC.
After consulting the patient, trastuzumab plus lapatinib was recommended as the third line therapy; nonetheless, due to financial reasons, the patient decided to go with lapatinib monotherapy (administered orally at a dose of 1250 mg per d for 21 d and stopped for 7 d , a 28-d treatment cycles), which was started on July 15, 2018.
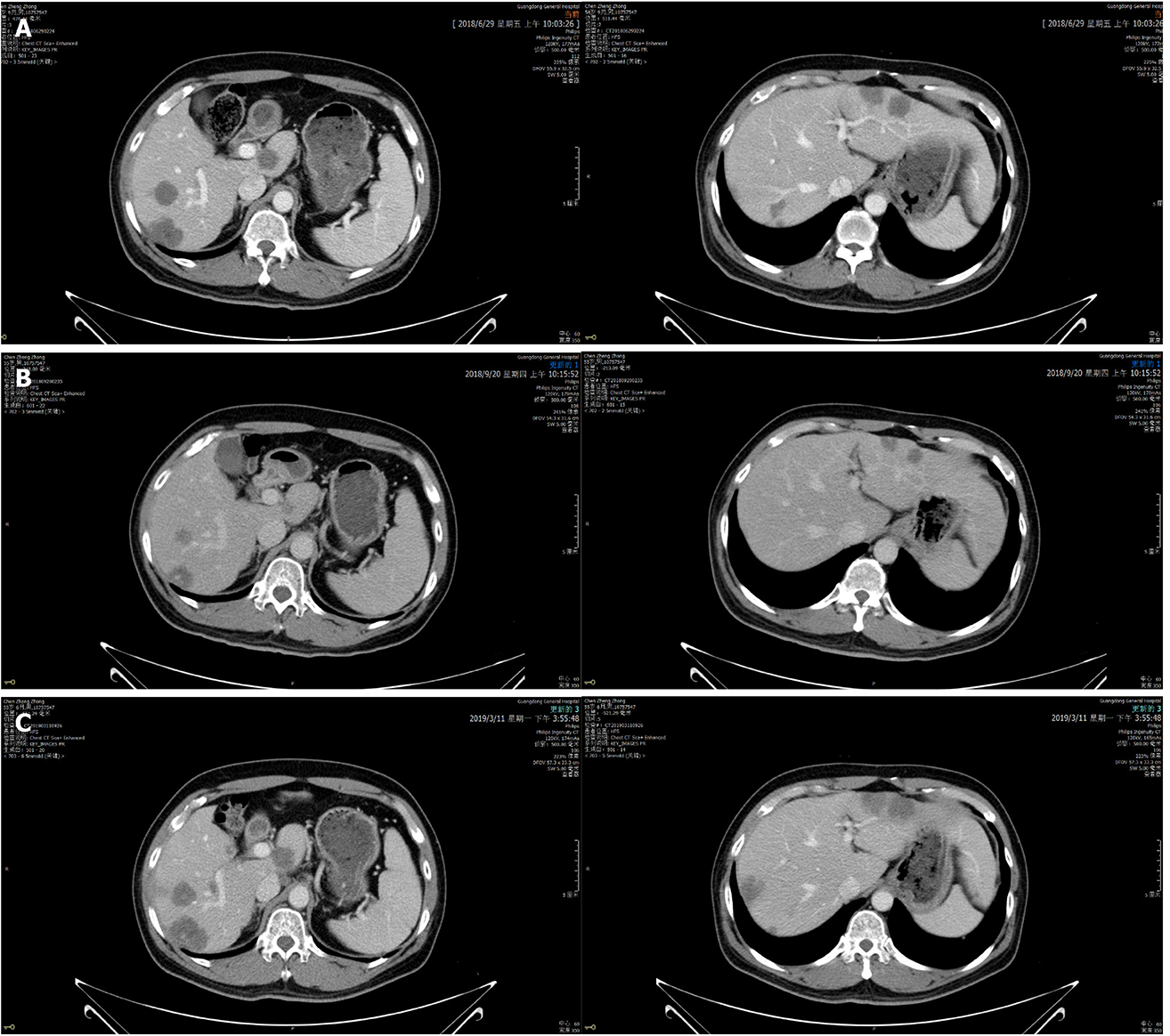
On September 20, 2018, enhanced CT showed reduced sigmoid colon wall thickening. Besides, most lesions of the liver, retroperitoneal lymph nodes, and multiple metastases in the lung were reduced (Figure 4). The comprehensive curative effect evaluation was PR. On September 13, 2018, ctDNA showed that the HER2 amplification and the APC mutation disappeared (Figure 3B), while the MAF of TP53 was reduced to 0.27% (Figure 3D). Moreover, the level of CEA changed from 59.29 to 7.93 ng/mL as a response to lapatinib treatment. In December 2018, no disease progression was observed (Figure S1).
Based on the enhanced CT on March 11, 2019, the multiple metastases in the liver and lung increased (Figure 2), while HER2 amplification reappeared (Figure 3C). The MAF of APC and TP53 was 14.8% and 19.8%, respectively. In addition, a new PIK3CA alteration with an MAF of 0.3% was detected (Figure 3D), which was demonstrated to be the resistant mechanism of anti-HER2 therapy in breast cancer[11-14]. The subsequent evaluation was PD, and the PFS of lapatinib was 7.9 mo. Strikingly, no adverse events occurred during lapatinib therapy.
HER2 amplification is the molecular driver for a small part of CRCs[1,2], which has been implicated in resistance to anti-EGFR treatment[4-7]. Bertotti et al[7] found that HER2 amplification was a potential primary resistant mechanism to EGFR monoclonal antibody in a patient-derived tumor xenograft model. Subsequently, in a clinical cohort of 97 RAS/BRAF wild-type metastasis CRC, the patients with HER2 amplification had significantly shorter PFS as compared to HER2 wild-type patients in second-line anti-EGFR therapy (2.9 mo vs 8.1 mo; hazard ratio (HR): 5.0; P < 0.0001)[4]. Moreover, results from two recent clinical trials suggested that HER2-positive advanced CRC might benefit from dual anti-HER2 treatments[2,8].
Herein, we report on a single case of HER2-positive mCRC treated with lapatinib monotherapy. An objective response was achieved, and the PFS was 7.9 mo. According to our knowledge, only one case of HER2-positive mCRC treated with single-agent trastuzumab-DM1 has been reported so far. In that patient, the disease was under control for 7 mo[9]. According to the HERACLES trial, the primary reason for selecting dual HER2-targeting therapy was based on the preclinical results of patient-derived xenografts (PDX)[7,10], which proved that pertuzumab or trastuzumab combined with lapatinib was more effective when treating HER2-positive mCRC PDX. Interestingly, lapatinib monotherapy led to tumor regression in both two PDX studies; still, its effect was weaker than that of the dual HER2-targeting, while tumors treated with pertuzumab or trastuzumab monotherapy showed a sustained increase[7,10]. Further investigation revealed that lapatinib could reduce the phosphorylation of extracellular-signal-regulated kinase (ERK) and S6 in cancer cells, which was only mildly impaired by pertuzumab or trastuzumab monotherapy[7]. These studies indicated that lapatinib monotherapy might be effective for HER2-positive mCRC, which is consistent with our observations. Yet, further investigation is needed to confirm these findings.
The potential underlying mechanism might be that lapatinib acts as a dual EGFR and HER2 inhibitor. Yonesaka K et al[5] demonstrated that inhibiting HER2 signaling in HER2-amplified colon cancer models could restore their sensitivity to EGFR monoclonal antibody in vitro and in vivo. Moreover, an HER2-positive mCRC patient who underwent anti-EGFR therapy responded well to lapatinib plus cetuximab[15]. Therefore, the inhibition of both HER2 and EGFR is another potential strategy to treat HER2-positive and RAS/BRAF wild-type mCRC. Dual anti-HER2 therapy is an expensive treatment that may cause serious adverse events; 22% of mCRC cases have been shown to have grade 3 adverse events in HERACLES trial[2]. Intriguingly, no adverse events occurred during lapatinib monotherapy. It is still unclear whether adverse events of lapatinib monotherapy would be less than those of dual therapy, indeed, we need more clinical trials to further answer this question, and lapatinib monotherapy is not recommended except clinical trials.
Because ctDNA could overcome the tumor tissue related limitations, such as heterogeneity and tissue that is hard to obtain, it is widely used in metastatic tumors, especially in patients who failed to respond to first-line standard therapy. Serial ctDNA detection had a major role in this case, mainly from three following aspects: (1) Genotyping: HER2 status of this patient was unclear at the time of diagnosis. High-level HER2 amplification was identified after two-line therapy based on ctDNA with broad molecular profiling, followed by IHC and FISH analyses. Because anti-HER2 treatment is not a standard therapy for mCRC, and HER2 overexpression might change after systemic therapy[9], ctDNA could be a suitable approach to detect HER2 status in such patients; (2) Monitoring: The MAF change due to genetic alterations between the serial ctDNA test was correlated to tumor burden, which was demonstrated using ctDNA to monitor chemotherapy or chemotherapy plus bevacizumab in mCRC[16,17]. In this case, serial ctDNA detection during treatment was used to assess the efficacy of lapatinib, which was consistent with the results of the radiographic assessment; and (3) Identifying acquired resistance mechanism: PIK3CA is the downstream pathway of HER2, which was reported as the acquired resistant mechanism to anti-HER2 treatment in metastatic breast cancer[11-14] and was first identified in CRC by Siravegna et al[18] through ctDNA monitoring. Serial ctDNA is helpful for the diagnosis and monitoring of the treatment of HER2-positive mCRC when standard therapies fail.
This is the first case report of HER2-positive RAS/BRAF wild-type metastatic CRC patient responding to lapatinib monotherapy. It highlights that ctDNA testing is an effective and feasible approach to evaluate the efficacy of anti-HER2 therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Passardi A S-Editor: Wang DM L-Editor: Wang TQ P-Editor: Li JH
| 1. | Ross JS, Fakih M, Ali SM, Elvin JA, Schrock AB, Suh J, Vergilio JA, Ramkissoon S, Severson E, Daniel S, Fabrizio D, Frampton G, Sun J, Miller VA, Stephens PJ, Gay LM. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124:1358-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 3. | Martinelli E, Troiani T, Sforza V, Martini G, Cardone C, Vitiello PP, Ciardiello D, Rachiglio AM, Normanno N, Sartore-Bianchi A, Marsoni S, Bardelli A, Siena S, Ciardiello F. Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, RAS wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open. 2018;3:e000299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, Saletti P, De Dosso S, Spitale A, Tejpar S, Kalogeras KT, Mazzucchelli L, Frattini M, Cappuzzo F. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Jänne PA. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 6. | Sartore-Bianchi A, Amatu A, Porcu L, Ghezzi S, Lonardi S, Leone F, Bergamo F, Fenocchio E, Martinelli E, Borelli B, Tosi F, Racca P, Valtorta E, Bonoldi E, Martino C, Vaghi C, Marrapese G, Ciardiello F, Zagonel V, Bardelli A, Trusolino L, Torri V, Marsoni S, Siena S. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist. 2019;24:1395-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C, Ribero D, Russolillo N, Muratore A, Massucco P, Pisacane A, Molinaro L, Valtorta E, Sartore-Bianchi A, Risio M, Capussotti L, Gambacorta M, Siena S, Medico E, Sapino A, Marsoni S, Comoglio PM, Bardelli A, Trusolino L. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 693] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 8. | Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 9. | Parikh A, Atreya C, Korn WM, Venook AP. Prolonged Response to HER2-Directed Therapy in a Patient With HER2-Amplified, Rapidly Progressive Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 2017;15:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Leto SM, Sassi F, Catalano I, Torri V, Migliardi G, Zanella ER, Throsby M, Bertotti A, Trusolino L. Sustained Inhibition of HER3 and EGFR Is Necessary to Induce Regression of HER2-Amplified Gastrointestinal Carcinomas. Clin Cancer Res. 2015;21:5519-5531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1213] [Cited by in F6Publishing: 1207] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Liu Y, Du Y, Yin W, Lu J. The predictive role of phosphatase and tensin homolog (PTEN) loss, phosphoinositol-3 (PI3) kinase (PIK3CA) mutation, and PI3K pathway activation in sensitivity to trastuzumab in HER2-positive breast cancer: a meta-analysis. Curr Med Res Opin. 2013;29:633-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Cizkova M, Susini A, Vacher S, Cizeron-Clairac G, Andrieu C, Driouch K, Fourme E, Lidereau R, Bièche I. PIK3CA mutation impact on survival in breast cancer patients and in ERα, PR and ERBB2-based subgroups. Breast Cancer Res. 2012;14:R28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B, Pierga JY, Marty M, de Cremoux P, Spyratos F, Bieche I. Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer. 2013;108:1807-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Deeken JF, Wang H, Subramaniam D, He AR, Hwang J, Marshall JL, Urso CE, Wang Y, Ramos C, Steadman K, Pishvaian MJ. A phase 1 study of cetuximab and lapatinib in patients with advanced solid tumor malignancies. Cancer. 2015;121:1645-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, Tacey M, Wong R, Singh M, Karapetis CS, Desai J, Tran B, Strausberg RL, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26:1715-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 17. | Yamauchi M, Urabe Y, Ono A, Miki D, Ochi H, Chayama K. Serial profiling of circulating tumor DNA for optimization of anti-VEGF chemotherapy in metastatic colorectal cancer patients. Int J Cancer. 2018;142:1418-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, Martino C, Lanman RB, Nagy RJ, Fairclough S, Rospo G, Corti G, Bartolini A, Arcella P, Montone M, Lodi F, Lorenzato A, Vanzati A, Valtorta E, Cappello G, Bertotti A, Lonardi S, Zagonel V, Leone F, Russo M, Balsamo A, Truini M, Di Nicolantonio F, Amatu A, Bonazzina E, Ghezzi S, Regge D, Vanzulli A, Trusolino L, Siena S, Marsoni S, Bardelli A. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell. 2018;34:148-162.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |