Copyright
©The Author(s) 2019.
World J Gastrointest Oncol. May 15, 2019; 11(5): 424-435
Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.424
Published online May 15, 2019. doi: 10.4251/wjgo.v11.i5.424
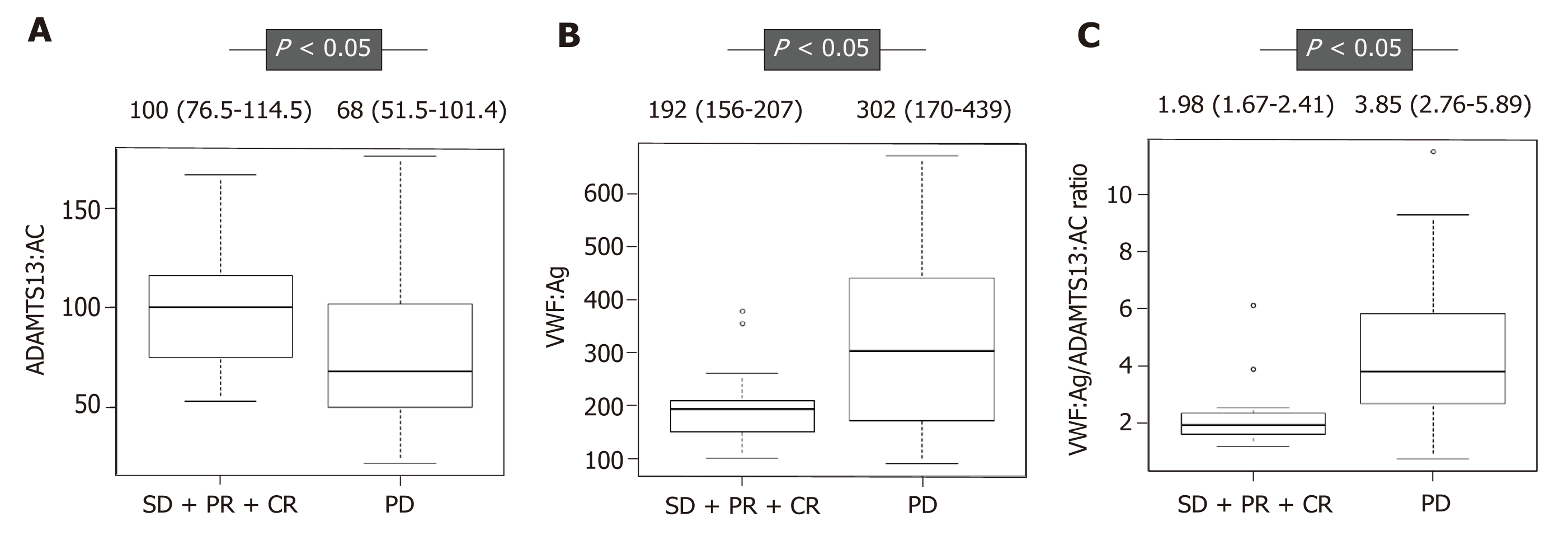
Figure 1 Plasma ADAMTS13:AC and VWF:Ag levels in patients with hepatocellular carcinoma receiving sorafenib treatment.
A: ADAMTS13:AC level was significantly higher in patients with hepatocellular carcinoma receiving sorafenib treatment who achieved stable disease (SD), partial response (PR), and complete response (CR) than those who achieved progressive disease (PD) (P < 0.05); B, C: In contrast, VWF:Ag, and the VWF:Ag/ADAMTS13:AC ratio levels were significantly lower in those with SD, PR, and CR than those with PD (P < 0.05, P < 0.05). ADAMTS13: A disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13; ADAMTS13:AC: ADAMTS13 activity; VWF: Von Willebrand factor; VWF:Ag: VWF antigen; VWF:Ag/ADAMTS13:AC ratio: The ratio of VWF:Ag to ADAMTS13:AC; SD: Stable disease; PR: Partial response; CR: Complete response; PD: Progressive disease.
- Citation: Takaya H, Namisaki T, Shimozato N, Kaji K, Kitade M, Moriya K, Sato S, Kawaratani H, Akahane T, Matsumoto M, Yoshiji H. ADAMTS13 and von Willebrand factor are useful biomarkers for sorafenib treatment efficiency in patients with hepatocellular carcinoma. World J Gastrointest Oncol 2019; 11(5): 424-435
- URL: https://www.wjgnet.com/1948-5204/full/v11/i5/424.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i5.424