Published online Jan 16, 2014. doi: 10.4253/wjge.v6.i1.13
Revised: November 11, 2013
Accepted: December 9, 2013
Published online: January 16, 2014
AIM: To evaluate the safety and technical success of endoscopic radiofrequency ablation (RFA) for palliative treatment of malignant hilar bile duct obstruction.
METHODS: In this study, a recently CE and FDA-approved endoscopic RFA catheter was first tested in an ex vivo pig liver model to study the effect of electrosurgical variables on the extent of the area of induced necrosis. Subsequently, a retrospective analysis was conducted of all patients treated with endoscopic RFA for malignant biliary obstruction at our center between February 2012 and April 2013. All patients received an additional plastic stent implantation into the biliary tree following RFA.
RESULTS: In the pig model, ablation time of 60-90 seconds using the bipolar soft coagulation mode at 8-10 watts with an effect of 8 was found to be the most feasible setting. Twelve patients (5 females, 7 males; mean age, 70 years) underwent 19 endoscopic RFA (range, 1-5) sessions. Deployment of RFA was successful in all patients. Systemic chemotherapy was administered in four patients. We observed biliary bleeding 4-6 wk after the intervention in three cases and two of these patients died: in one patient, spontaneous hemobilia occurred, whereas bleeding started during stent extraction in the other. In the third patient, bleeding was stopped by insertion of a non-covered self-expanding metal stent. Another three patients developed cholangitis during follow-up. Seven patients died during follow-up and median survival was 6.4 mo (95%CI: 0.05-12.7) from the time of the first RFA.
CONCLUSION: Endoscopic RFA is an easy to perform and technically highly successful procedure. However, hemobilia possibly associated with RFA occurred in three of our patients. Therefore, larger prospective studies are needed to further evaluate the safety and efficacy of this promising new method.
Core tip: Radiofrequency ablation (RFA) is a promising tool for the treatment of patients with perihilar and intrahepatic bile duct cancer. While RFA is easy to perform and technical success rates are high, the outcome of patients remains unclear. Therefore, the long-term efficacy of this treatment approach needs to be studied in randomized trials.
- Citation: Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, Trojan J, Albert JG. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc 2014; 6(1): 13-19
- URL: https://www.wjgnet.com/1948-5190/full/v6/i1/13.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i1.13
Hilar cholangiocarcinoma (CCA) accounts for about 70% of biliary tumors[1] and is not amenable to curative surgical resection in more than two thirds of cases at the time of diagnosis[1]. Palliative chemotherapy may increase median survival when a combination of chemotherapeutic agents is used[2]. In addition, endoscopic insertion of plastic endoprostheses or self-expanding metal stents (SEMS) plays an important role in the palliative treatment of biliary tract cancer[3,4].
Randomized controlled trials have indicated that endoscopic local ablation of intraductal CCAs by use of photodynamic therapy (PDT) significantly improves survival in non-resectable tumors[5,6] and these findings are also supported by non-randomized studies[7-10]. However, PDT is complex and expensive, requiring highly specialized equipment. Recently, ablation of intraductal tumors has been simplified by the introduction of a radiofrequency ablation (RFA) probe, the Habib EndoHBP probe (EMcision UK, London, United Kingdom) that is inserted through the working channel of a side-viewing endoscope during endoscopic retrograde cholangiopancreatography (ERCP) into the extra- and/or intra-hepatic biliary tract[11].
To date, few data exist on the clinical applicability of this new device. We therefore performed a retrospective analysis of all consecutive patients treated with endoscopic RFA for malignant biliary obstruction at our center, with a special emphasis on technical success rates, safety and patient survival.
Before the start of RFA treatments in patients, we performed an experimental pre-clinical study in an ex-vivo pig liver model to investigate the effect of electrosurgical variables on the extent of the area of RFA-induced necrosis. All procedures were performed using a RFA probe for bipolar cautery intended for use in endoscopic surgical procedures as described below (Habib EndoHPB; EMcision UK, London, United Kingdom; Figure 1).
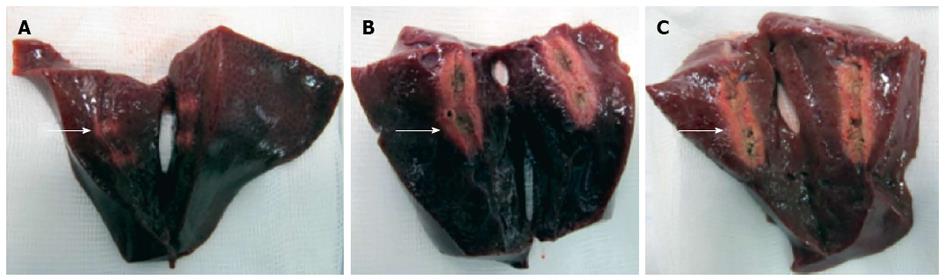
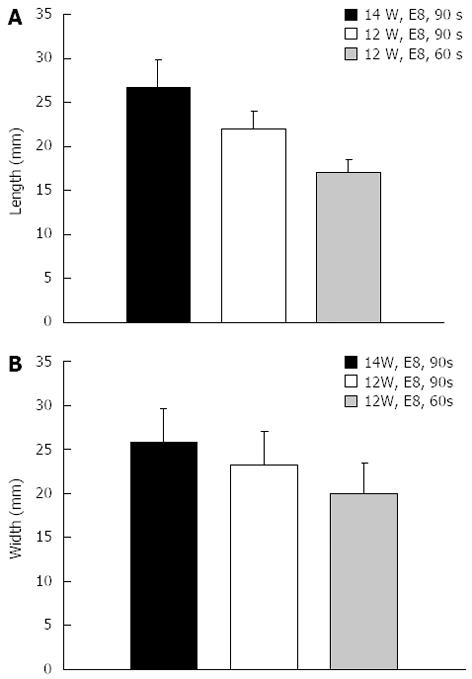
In total, five consecutive freshly resected livers from adult pigs were obtained. All experiments were started within eight hours after the pigs had been euthanized and were performed at room temperature. The probe was advanced into the center of the liver over a guidewire and radiofrequency ablation was performed using different variations of power (watts), mode (effect) and ablation time. Ablations were performed with each technical setting three times in a row and the probe was inserted at least three centimeters apart from other ablation sites. Immediately after each application, the liver was cut along the guidewire with a scalpel to identify the ablation extent. The maximum diameter and length of the ablated area was measured separately for each application (Figure 2).
RFA was performed in patients with unresectable malignant biliary duct obstruction after the most feasible (8 to 10 watts, effect 8, 60-90 s) probe settings for optimal radiofrequency ablation had been identified in the pre-clinical study at our center starting in February 2012. All patients treated with endoscopic RFA for malignancy of an intrahepatic or perihilar bile duct were eligible for inclusion in this study and the study protocol was approved by the ethics committee of the Medical School of the University of Frankfurt, Germany. None of the patients had been eligible for curative surgery or had undergone a previous explorative laparotomy due to locally advanced disease or comorbidities. However, all patients reported symptoms such as painless jaundice or weight loss. Histological diagnoses were obtained by percutaneous needle biopsy or intraductal endoscopically operated biopsy during ERCP. All endoscopic RFA procedures were ascertained in an interdisciplinary conference of consultant physicians from the departments of surgery, gastroenterology and medical oncology and treatment recommendations were given in consent.
All endoscopic procedures (ERCP) were performed by an experienced pancreatobiliary endoscopist under standard operating conditions with a commercially available duodenoscope (TJF-160 VR or TJF Q180V, Olympus medical, Tokyo, Japan). Previously inserted plastic endoprostheses were removed before cholangiography, which were then used to confirm biliary length, diameter and localization of the tumor stenosis.
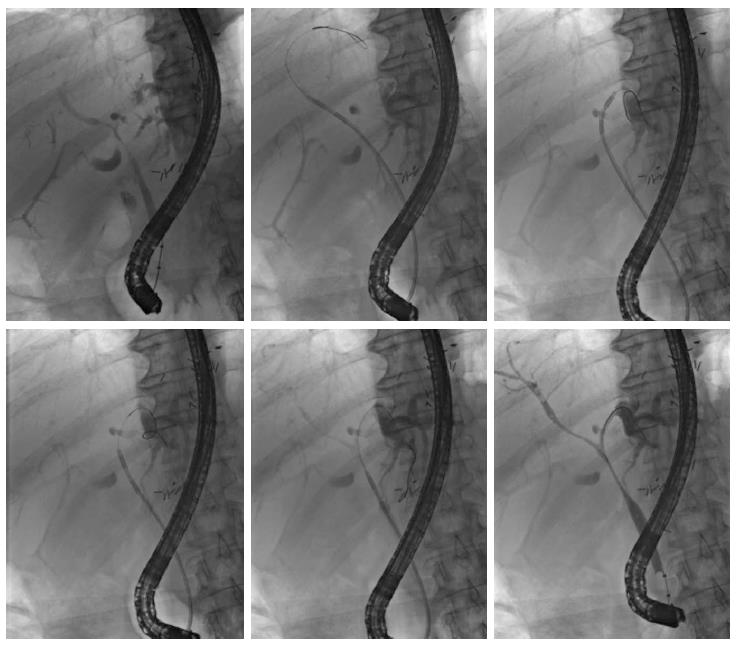
For endoscopic RFA, a recently FDA-approved and CE-certified[12] catheter was used. This probe (Habib EndoHPB; EMcision UK, London, United Kingdom) features two ring electrodes at the tip, lying 8 mm apart from each other (Figure 1). The catheter measures 8 French (2.6 mm) in diameter and 1.8 m in length. The RFA catheter can be connected to a bipolar electrosurgical generator to produce a cylindrical necrosis around the ring electrodes. The extent of the necrotic area depends on the mode of the electrosurgical generator, the power and ablation time. For the present study, the VIO 200D generator (Erbe Elektromedizin, Tübingen, Germany) with the “bipolar soft coagulation” mode, effect 8, 8 to 10 watts, for 90 s for treatment of the patients. Power was applied with 8 watts for the left or right intrahepatic biliary ducts and 10 watts for the subhilar section of the common hepatic or common bile duct, respectively. The RFA catheter was placed under fluoroscopic visualization of the biliary system after having visualized the tumor stenosis by injecting contrast medium (Iomeprol, Imeron® 300M, Bracco Imaging Deutschland, Konstanz, Germany) into the bile duct system via a standard ERCP probe. Positioning of the RFA catheter was performed exactly within the tumor stricture by using a guidewire. In cases of a stenosis more than 15 mm in length, repeated applications of RFA were carefully applied without overlapping the treated segments (1-4 applications per intervention). Thus, the RFA catheter was positioned into the right and/or the left intrahepatic bile ducts and we aimed to treat all segments involved in each specific tumor-dependent setting (Figure 1). After RFA treatment, plastic endoprostheses (Gastrosoft; Optimed, Ettlingen, Germany) were inserted according to standard protocols. The technical success of RFA was defined as positioning the RFA catheter at the region of interest and applying coagulation current as intended with consecutive successful insertion of an endoprosthesis.
The clinical part of this study is a retrospective cohort study. Data were analyzed from patients who underwent endoscopic RFA between February 2012 and April 2013 at our study center. The primary endpoint was the technical feasibility of endoscopic RFA. Secondary outcome measures included peri-interventional complications and overall survival.
Descriptive statistics are shown as mean ± SD or median and range, as appropriate. Survival was assessed using Kaplan-Meier statistics. All analyses were performed using the SPSS statistics software package for Mac (Version 20.0; IBM, Somers, NY, United States).
In the ex-vivo pig liver model, significant differences in length and diameter of RFA-induced necrosis with variation of the electrosurgical parameters time, power and effect were observed. With an ablation time of 60-90 s using the bipolar soft coagulation mode, at 10 watts, effect 8 (equivalent to the recommendation of the manufacturer), a mean necrotic area of 22 mm × 9 mm (length × diameter) could be induced. Power seemed to have a more pronounced effect on tissue destruction when compared to time or mode. A power of 7 watts or less did not seem to produce a significant necrosis. Applying power in the range of 8-10 watts seemed to be most appropriate for intraductal biliary use and higher power was associated with deep tissue destruction (Figures 2 and 3).
In total, 19 RFA treatment cycles were performed in twelve patients (5 females, 7 males) with mean age of 70 years (median, 75; range, 33-85); Table 1, Figure 4.
| Patient | Patient gender/age | Tumor location | No. of RFA treatment cycles | Follow-up (mo) | Outcome |
| 1 | F/78 | CCA Bismuth IV | 2 | 6.4 | Dead |
| 2 | F/73 | Intrahepatic CCA | 1 | 0.3 | Dead |
| 3 | M/72 | CCA Bismuth IV | 5 | 19.8 | Alive |
| 4 | M/85 | CCA Bismuth IV | 2 | 6.2 | Dead |
| 5 | M/81 | CCA Bismuth IV | 1 | 1.1 | Dead |
| 6 | M/33 | Gastric carcinoma | 1 | Lost to follow up | - |
| 7 | F/77 | Gallbladder cancer | 1 | 6.6 | Dead |
| 8 | F/78 | CCA Bismuth IV | 1 | 1.3 | Dead |
| 9 | M/47 | CCA Bismuth IV | 1 | 14.1 | Alive |
| 10 | F/78 | CCA Bismuth IV | 1 | 1.2 | Dead |
| 11 | M/61 | Gallbladder cancer | 1 | 4.0 | Alive |
| 12 | M/72 | Intrahepatic CCA | 1 | 2.9 | Alive |
All patients presented with malignant bile duct obstruction of the hepatic hilus (Klatskin like tumors). Final diagnosis included intrahepatic CCA in 2 patients, Bismuth stage IV in 8 patients, carcinoma of the gall bladder in two patients and metastases of gastric small cell carcinoma in one patient. Patients underwent either one (n = 9), two (n = 2) or five (n = 1) intraductal RFA applications that were considered technically successful during all applications. The ablations were applied to the left (n = 6), right (n = 9) or the main bile duct (n = 5) and RFA applications within one intervention ranged from 1 to 4 according to stricture length and/or bilateral vs unilateral tumor stenosis. Four of the patients who had been diagnosed with CCA were also treated with systemic chemotherapy (cisplatin plus gemcitabine).
We observed biliary bleeding 4 to 6 wk after the intervention in three patients and two of these patients died of hemorrhagic shock. While one of these patients developed spontaneous hemobilia, bleeding started during stent extraction in the other patients that was successfully stopped in one patient by insertion of a non-covered self-expanding metal stent (SEMS). None of the three patients had undergone chemotherapy concomitantly to endoscopic treatment. Of the remaining patients, four patients developed recurrent cholangitis during follow-up that could be successfully managed with stent exchange and antibiotic therapy.
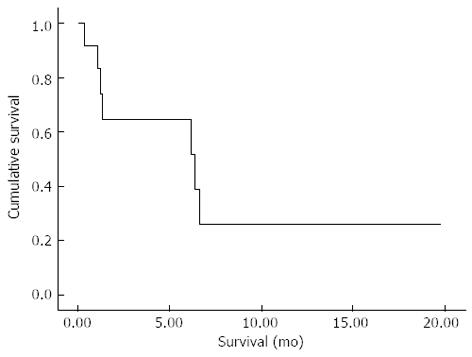
From the time of the first RFA in each patient, the 30 and 90 d mortality of the entire cohort was 8.3% and 50%, respectively; Figure 5. The extrapolated median survival from the first RFA and the time of diagnosis were 6.4 (95%CI: 0.05-12.7) mo and 8.5 (95%CI: 4.6-12.4) mo, respectively.
Successful stenting of the biliary tree with prior or additive photodynamic therapy has been demonstrated to show the longest overall survival and has been referred to as the “gold standard” for endoscopic treatment of malignant biliary obstruction[5,13]. However, the management of patients treated with PDT is expensive and time consuming and more feasible endoscopic options with equal survival benefit are warranted. Endoscopically applicable RFA represents a novel expansion of a method that is well known from its percutaneous applications and which has shown promising results in recently published case series. However, the safety of endoscopic RFA for biliary malignancy has not yet been clearly defined. Clinical data on intrahepatic treatment of CCA are scarce and many of the published studies included mostly extrahepatic tumors (Table 2). We here report our own experience on performing endoscopic RFA in 12 patients with malignant bile duct obstruction (mostly Klatskin Bismuth IV) due to hilar tumors of different etiologies. In our study, the technical applicability of RFA procedures was found to be excellent, successful in all patients, and this is in line with previously published studies. Indeed, in the largest study published so far, the technical success rate was reported to be 95%[12].
| Ref. | Year | n | Localization of the tumor | Complications |
| Figueroa-Barojas et al[15] | 2011 | 8 | Intra- and extra-hepatic | Pain: 4 |
| Pancreatitis and Cholecystitis: 1 | ||||
| Steel et al[12] | 2011 | 21 | Extrahepatic | Empyema of the gallbladder: 1 |
| Dolak et al[16] | 2012 | 43 | Intrahepatic | Hemobilia: 2 |
| Liver infarction: 1 | ||||
| Empyema of the gallbladder: 1 | ||||
| Cholangitis: 1 | ||||
| Mizandari et al[17] | 2012 | 39 | Intra- and extrahepatic | Pain: 15 |
| Own experience | 2013 | 12 | Intrahepatic | Hemobilia: 3 (2 deaths) |
Despite this, we did observe three cases of hemobilia that occurred 4-6 wk after RFA application. Two out of these three patients died from the consequences of hemorrhagic shock, while bleeding was successfully stopped in one patient by immediate SEMS insertion into the bleeding bile duct.
Although bleeding occurred several weeks after the RFA procedure in all three patients, a possible direct relationship to RFA may be assumed. In the two patients in whom bleeding occurred during stent extraction, deep necrosis induced by RFA may have been discarded from the perihilar tissue when necrotic material was removed while extracting the plastic endoprosthesis, thereby resulting in injury of a major blood vessel. Another possible explanation could be a strong necrotic effect induced by RFA that may have led to an increased angiogenic response within the tumor causing the recruitment of new vessel branches within the treated tissue. However, either of these hypotheses requires confirmation by analysis of immunohistochemical staining and biochemical processing of the treated tissues. Possible preemptive strategies to avoid biliary bleeding complications could include pre-interventional investigation with intraductal ultrasound (IDUS) to rule out large blood vessels in the vicinity of the ablation site. For the prevention of late bleeding complications, insertion of a SEMS directly after the RFA procedure seems to be feasible[12]. Severe complications associated with endoscopic RFA treatment have also been reported from most other published studies. For example, Dolak et al[14] reported that severe bleeding occurred in two of their patients and liver infarction in another patient, while Steel et al[12] reported that two of their patients required percutaneous gallbladder drainage for empyema.
Another secondary outcome measure of our study was overall survival following RFA therapy, which was shown to be 6.4 mo. In the above-mentioned Austrian multicenter study, the overall survival following RFA application was 10.6 mo. However, this was a multicenter cohort, involving 58 patients in total[14]. Other reported outcome measures included the increase of the diameter of tumor strictures[12] or stent patency at follow-up[12,14].
Taken together, our study shows that endoscopic RFA for malignant bile obstruction is a technically feasible and easy-to-apply procedure. However, based on the current experience, RFA should not be applied outside of study protocols given the risk of potentially fatal bleeding. Thus, randomized studies comparing PDT plus stenting vs RFA plus stenting, both with or without chemotherapy, are clearly desired.
Treatment with curative intent may be offered only for a minority of patients with cholangiocarcinoma. We evaluated the technical feasibility and safety of endoscopic radiofrequency ablation (RFA) for palliative treatment of malignant biliary obstruction.
A new endoscopic RFA probe (Habib EndoHPB, EMcision United Kingdom, London, United Kingdom) has recently been CE and FDA-approved, thereby offering a new palliative treatment option for the therapy of malignant biliary strictures. The catheter can be positioned and applied during endoscopic retrograde cholangiopancreatography using a specific guidewire.
This study demonstrates that endoscopic RFA is easy to perform and a technically highly successful tool for the endoscopic treatment of biliary malignancies. However, severe bleeding occurred in three of our patients that may have been directly associated with RFA although it occurred several weeks after the respective RFA applications.
This study evaluates a new therapeutic approach in the palliative treatment of patients with malignant biliary obstruction.
The authors showed in their retrospective study that endoscopic RFA is technically highly feasible for the treatment of malignant biliary strictures. However, severe complications such as biliary bleeding may occur and larger, prospective studies are warranted.
P- Reviewers: Anthony YBT, Konstantinos T, Wehrmann T S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [Cited in This Article: ] |
| 2. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [Cited in This Article: ] |
| 3. | Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189-200. [Cited in This Article: ] |
| 4. | Demols A, Maréchal R, Devière J, Van Laethem JL. The multidisciplinary management of gastrointestinal cancer. Biliary tract cancers: from pathogenesis to endoscopic treatment. Best Pract Res Clin Gastroenterol. 2007;21:1015-1029 Available]. [Cited in This Article: ] |
| 5. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. [Cited in This Article: ] |
| 6. | Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426-2430. [Cited in This Article: ] |
| 7. | Kahaleh M, Mishra R, Shami VM, Northup PG, Berg CL, Bashlor P, Jones P, Ellen K, Weiss GR, Brenin CM. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290-297. [Cited in This Article: ] |
| 8. | Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J, Hauss J. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230-239. [Cited in This Article: ] |
| 9. | Dechene A, Hilgard P, Maldonado-Lopez EJ, Riemann JF, Gerken G, Zoepf T. Dechene A, Hilgard P, Maldonado-Lopez EJ, Riemann JF, Gerken G, Zoepf T. Survival Difference in Patients with Photodynamic Therapy of Nonresectable Bile Duct Cancer Using Different Hematoporphyrins. Gastrointest Endosc. 2007;65:AB227. [Cited in This Article: ] |
| 10. | Prasad GA, Wang KK, Baron TH, Buttar NS, Wongkeesong LM, Roberts LR, LeRoy AJ, Lutzke LS, Borkenhagen LS. Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma. Clin Gastroenterol Hepatol. 2007;5:743-748. [Cited in This Article: ] |
| 11. | Itoi T, Isayama H, Sofuni A, Itokawa F, Tamura M, Watanabe Y, Moriyasu F, Kahaleh M, Habib N, Nagao T. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci. 2012;19:543-547. [Cited in This Article: ] |
| 12. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [Cited in This Article: ] |
| 13. | Lee TY, Cheon YK, Shim CS, Cho YD. Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J Gastroenterol. 2012;18:5589-5594. [Cited in This Article: ] |
| 14. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C, Wolkersdörfer G, Madl C, Berr F, Trauner M, Püspök A; for the Austrian Biliary RFA Study Group. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2013;Epub ahead of print. [Cited in This Article: ] |
| 15. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [Cited in This Article: ] |
| 16. | Dolak W, Tribl B, Schwaighofer H, Vogel W, Plieschnegger W, Siebert F, Hellmich B, Holzäpfel A, Wasilewski M, Gschwantler M. Endoscopic radiofrequency ablation for malignant biliary obstruction: results of 43 procedures at 9 austrian referral centers. Endoscopy. 2012;44:A14. [Cited in This Article: ] |
| 17. | Mizandari M, Pai M, Xi F, Valek V, Tomas A, Quaretti P, Golfieri R, Mosconi C, Guokun A, Kyriakides C. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013;36:814-819. [Cited in This Article: ] |