Published online Aug 16, 2013. doi: 10.4253/wjge.v5.i8.407
Revised: June 12, 2013
Accepted: July 18, 2013
Published online: August 16, 2013
Malignant peritoneal mesothelioma is a rare aggressive tumor of the peritoneum. An increasing number of malignant mesothelioma cases have been reported in recent years. We report here a very rare case of malignant peritoneal mesothelioma with both umbilical hernia and umbilical metastasis which is also called Sister Mary Joseph’s nodule. We performed laparoscopy which showed specific laparoscopic findings, and the pathological findings of the biopsy specimen led to the diagnosis. This case was associated with umbilical hernia which could be induced by massive ascites. A newly developed abdominal hernia should be noted as a primary symptom of malignant peritoneal mesothelioma, as shown in the present case.
Core tip: Malignant peritoneal mesothelioma is a rare aggressive tumor of the peritoneum. We performed laparoscopy which showed specific laparoscopic findings, and the pathological findings of the biopsy specimen led to the diagnosis. This case was associated with umbilical hernia and umbilical metastasis, which is also called as Sister Mary Joseph’s nodule.
- Citation: Tsuruya K, Matsushima M, Nakajima T, Fujisawa M, Shirakura K, Igarashi M, Koike J, Suzuki T, Mine T. Malignant peritoneal mesothelioma presenting umbilical hernia and Sister Mary Joseph’s nodule. World J Gastrointest Endosc 2013; 5(8): 407-411
- URL: https://www.wjgnet.com/1948-5190/full/v5/i8/407.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i8.407
Malignant mesothelioma is arising from the mesothelium lining of a body cavity and is associated with asbestos exposure. An increasing number of malignant mesothelioma cases have been reported in recent years. In malignant peritoneal mesothelioma, abdominal hernias are a common complication, probably due to massive ascites accompanying the disease. Most are inguinal hernias but the development of an umbilical hernia is very rare. Umbilical metastasis, which is also called Sister Mary Joseph’s nodule, is a rare complication of malignant peritoneal mesothelioma. We are reporting a very rare case of malignant peritoneal mesothelioma with both umbilical hernia and umbilical metastasis.
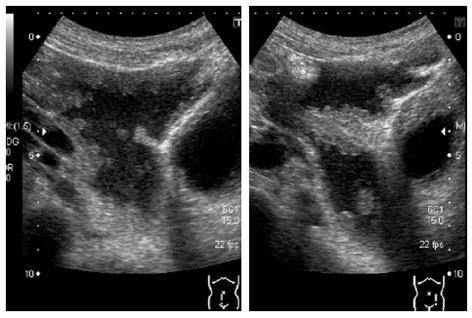
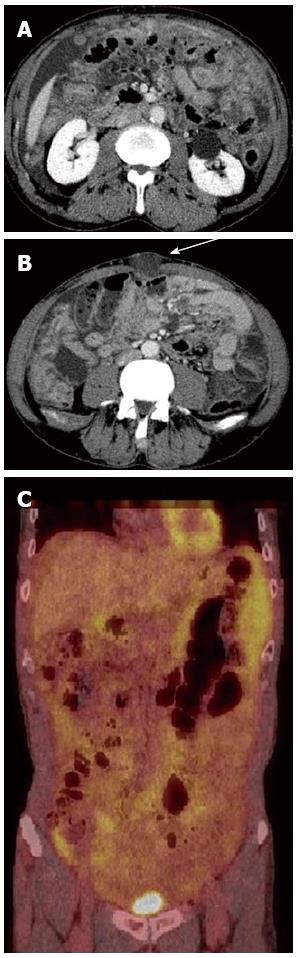
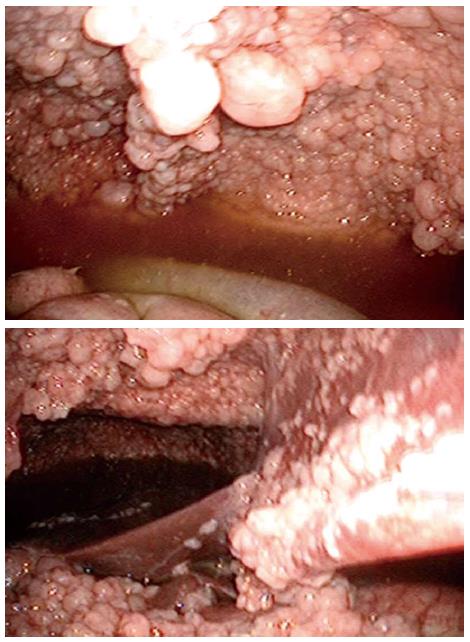
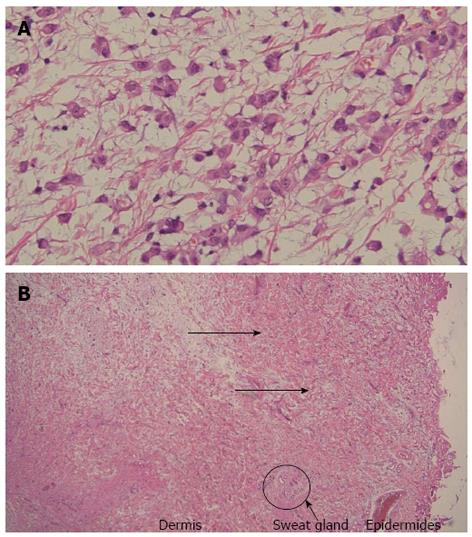
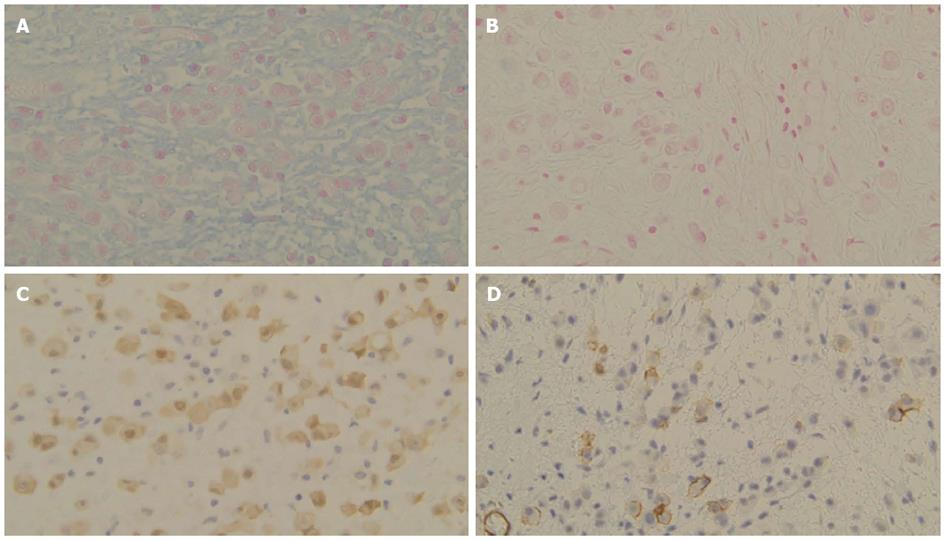
A 64-year-old man visited our hospital for further examination of a moderate amount of ascites and multiple peritoneal masses observed during an annual health check. There was nothing noteworthy in his medical history and family history. He had no clear history of asbestos exposure. Physical examination demonstrated moderate abdominal distension with fluctuation and umbilical hernia. Bowel sounds were reduced and there was no abdominal pain or tenderness. Laboratory investigations revealed hypoalbuminemia (35 mg/L), a high level of C-reactive protein (3.5 mg/L), and levels of CEA and CA19-9 were within the normal range. No neoplastic lesions were observed by upper and lower gastrointestinal endoscopy. Abdominal ultrasound showed moderate ascites, irregular thickening of the mesentery and the peritoneum, and numerous nodules present (Figure 1). Contrast enhanced computed tomography showed a moderate amount of ascites and enhanced multiple nodules on the thickened peritoneum and mesenterium (Figure 2). By 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), mild accumulation of FDG was observed over the entire abdominal region, but no localized strong accumulation suggesting this was the primary lesion (Figure 2C). Aspirated ascites was cloudy and a muddy yellow color with increased cell populations present (7660/μL), most of which were histiocytes (93%) with some mesothelial cells (6%). Protein concentration of the ascites reached levels as high as 41 mg/L, indicating that the ascites was exudative. The hyaluronic acid concentration of the ascites was very high (436000 ng/L). Cytology indicated atypical mesothelial cells. These data strongly suggested malignant peritoneal mesothelioma and therefore, laparoscopy was performed. Laparoscopic observation showed multiple whitish nodules on the peritoneum and the mesenterium including falciform ligament of the liver with a slightly cloudy yellow ascites (Figure 3). Biopsy of the nodular lesions was performed. Additionally, Umbilical hernioplasty was performed owing to avoidance of the risk for incarceration and the pathological assessment of the umbilical region was also done. According to the histopathological findings of the nodular lesion, hematoxylin and eosin (HE) staining showed the infiltration of many tumor cells having acidophilic cytoplasm with large nuclei (Figure 4A). A mucus-like substance in the tumor background was positive by alcian blue staining, and was negative after hyaluronidase treatment, which suggested the presence of accumulated hyaluronic acid. Immunohistochemical stainings of calretinin, D2-40, and CK5/6, which are markers for mesothelial cells, were all positive, while Ber-EP4 and MOC31, which are markers for adenocarcinomas, were negative (Figure 5). Therefore, the final diagnosis was epithelioid type of malignant peritoneal mesothelioma. Umbilical hernia was repaired by surgery and the infiltration of tumor cells into the dermis was observed in the resected specimen of the umbilical region (Figure 4B).
The patient transferred to another hospital of his own will. According to the response mail from the hospital, he received a radical operation in which the peritoneal nodules were removed as much as possible, however, he got debilitated after the surgery which did not permit further chemotherapy. He died 6 mo after the diagnosis due to the progression of the malignancy.
Malignant mesothelioma originates in the pleura, peritoneum, pericardium and tunica vaginalis[1], where a lining of mesothelial cells is present. The main causes of mesothelioma are known to include exposure to asbestos[2] and erionite (natural mineral fiber). In Japan, a large amount of asbestos was used for buildings during the high-growth period (1950-1990). It is believed that the incubation period is approximately 30-40 years and it is estimated that the number of mesothelioma cases will therefore reach a peak between 2020-2030.
It has been reported that 85.5% malignant mesothelioma occurs in the pleura, 13.2% in the peritoneum, 0.8% in the pericardium, and 0.5% in the testicular tunica vaginalis[3]. According to the histological classification, malignant mesothelioma can be classified as 3 types: epithelioid type, sarcomatoid type, and biphasic type. The frequencies of the 3 types of pleural mesothelioma, epithelioid, sarcomatoid, biphasic type, and unknown classification were reported to be 53.6%, 23.3%, 18.3%, and 4.8%, respectively[3]. In Japan, the frequencies of peritoneal mesothelioma including epithelioid, sarcomatoid, biphasic type, and unknown classification were reported to be 71.6%, 11.6%, 12.6%, and 4.2%, respectively[3]. In the United States, Yan et al[4] reported that there were no sarcomatoid type cases, and that the incidence of epithelioid and biphasic type were 92% and 8%, respectively. In both reports, fewer sarcomatoid type and increased epithelioid type cases were observed in malignant peritoneal mesothelioma, compared with pleural mesothelioma.
The clinical symptoms associated with malignant peritoneal mesothelioma include abdominal distention, abdominal pain, abdominal mass, a loss of appetite, weight loss, fever and diarrhea, but there are no disease specific symptoms[5]. Abdominal hernia was reported to occur in 6%-12% of patients with malignant peritoneal mesothelioma[6,7], but the majority of these were in the inguinal region. According to a report by Acherman et al[6], the primary symptoms in 51 cases of malignant peritoneal mesothelioma included 17 cases (33%) of abdominal pain, 5 cases (10%) of abdominal pain and distention, 16 cases (31%) of abdominal swelling, and 6 cases (12%) with the new onset of hernias. Among these, 5 of the hernias occurred in the inguinal region while one occurred in the umbilical region. Thus, although umbilical hernia is rare, a newly developed abdominal hernia should be noted as a primary symptom of malignant peritoneal mesothelioma.
Moreover, the present case was associated with umbilical invasion of the tumor. Metastasis of malignant tumors to the umbilicus is also known as Sister Mary Joseph’s nodule[8]. The vascular system around the umbilicus forms an arteriovenous loop and lymphoid vessel network, which continues to the round ligament of the liver. Regarding the metastasis pathway to the umbilicus, lymphogenous and hematogenous metastasis, and direct invasion from the surrounding tumor or along the round ligament of the liver have been reported[9-11]. In the present case, laparoscopy revealed numerous thick nodules growing on both the parietal peritoneum and the round ligament of the liver next to the umbilical region. Thus, direct invasion by either or both of the above pathways was most likely. Boyde et al[12] reported 4 cases (3.4%) of umbilical metastasis in 89 cases of malignant peritoneal mesothelioma. In all 4 cases, the histological types were epithelioid type and the present case was similar. The umbilicus is one of the weakest regions in the abdominal wall, where the umbilical cord was previously penetrated and aponeurosis and subcutaneous fat are lacking. It is thought that umbilical hernia occurs in adults when the abdominal pressure increases due to pregnancy, obesity, large abdominal tumor, ascites, or peritoneal dialysis[13,14]. In the present case, we observed umbilical metastasis, which might have weakened the surrounding connective tissue, and caused high intraabdominal pressure due to massive ascites. Both factors might contribute to the development of the umbilical hernia.
In summary, we experienced an extremely rare case of malignant peritoneal mesothelioma associated with umbilical metastasis and umbilical hernia. The incidence of malignant peritoneal mesothelioma is expected to increase in the near future. Although it is rare, a newly developed abdominal hernia should be noted as a primary symptom of malignant peritoneal mesothelioma, as shown in the present case.
P- Reviewer Hussain A S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Raptopoulos V. Peritoneal mesothelioma. Crit Rev Diagn Imaging. 1985;24:293-328. [PubMed] [Cited in This Article: ] |
| 2. | Newhouse ML, Thompson H. Mesothelioma of pleura and peritoneum following exposure to asbestos in the London area. Br J Ind Med. 1965;22:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 50] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Gemba K, Fujimoto N, Kato K, Aoe K, Takeshima Y, Inai K, Kishimoto T. National survey of malignant mesothelioma and asbestos exposure in Japan. Cancer Sci. 2012;103:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Yan TD, Popa E, Brun EA, Cerruto CA, Sugarbaker PH. Sex difference in diffuse malignant peritoneal mesothelioma. Br J Surg. 2006;93:1536-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Mirarabshahii P, Pillai K, Chua TC, Pourgholami MH, Morris DL. Diffuse malignant peritoneal mesothelioma--an update on treatment. Cancer Treat Rev. 2012;38:605-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Acherman YI, Welch LS, Bromley CM, Sugarbaker PH. Clinical presentation of peritoneal mesothelioma. Tumori. 2003;89:269-273. [PubMed] [Cited in This Article: ] |
| 7. | Mirabella F. [Peritoneal mesothelioma and abdominal hernias]. Minerva Med. 1996;87:21-24. [PubMed] [Cited in This Article: ] |
| 8. | Powell FC, Cooper AJ, Massa MC, Goellner JR, Su WP. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 172] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Steck WD, Helwig EB. Tumors of the umbilicus. Cancer. 1965;18:907-915. [PubMed] [Cited in This Article: ] |
| 10. | Dubreuil A, Dompmartin A, Barjot P, Louvet S, Leroy D. Umbilical metastasis or Sister Mary Joseph’s nodule. Int J Dermatol. 1998;37:7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Heatley MK. Sister Mary Joseph’s nodule in malignant mesothelioma. Histopathology. 2004;45:299-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Boyde AM, Attanoos RL. Sister Mary Joseph’s nodule in malignant peritoneal mesothelioma. Histopathology. 2003;43:303-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Arroyo A, García P, Pérez F, Andreu J, Candela F, Calpena R. Randomized clinical trial comparing suture and mesh repair of umbilical hernia in adults. Br J Surg. 2001;88:1321-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Cherney DZ, Siccion Z, Chu M, Bargman JM. Natural history and outcome of incarcerated abdominal hernias in peritoneal dialysis patients. Adv Perit Dial. 2004;20:86-89. [PubMed] [Cited in This Article: ] |