INTRODUCTION
Molecular imaging is a technique that detects molecular changes in diseased cells within the mucosa. This discipline has great potential to improve medicine via detection of diseases in the early stages, identification of the extent of disease, selection of disease- and patient-specific treatments, application of directed or targeted therapy, and measurement of molecularly-specific effects of treatment[1].
Recent developmentsin optics and digital imaging technology, and new diagnostic methods combined with state-of-the-art technology have been introduced in gastrointestinal endoscopy. Various methods such as narrow-band imaging, autofluorescence imaging (AFI), Raman spectroscopy, confocal endomicroscopy, endoscopic optical spectroscopy, and magnifying endoscopyhave been developed and are under investigation. Some of these methods have already been widely used in clinical practice[2]. These endoscopic detection methods have enabled endoscopists to collect real-time in vivo histological images or “virtual biopsies” of the gastrointestinal (GI) mucosa during endoscopy. Although early diagnosis of premalignant GI lesions is very important, many studies have shown thatthe miss rate for GI lesions has not been decreased[3].
The application of molecular imaging to endoscopy for the diagnosis and treatment of GI cancer is aimed at diagnosing cancer by analyzing lesion characteristics based on molecular biological changes rather than lesion morphology, thereby increasing the efficiency of endoscopic screening and surveillance. An important advantage of performing targeted imaging of the GI mucosa is the opportunity to apply exogenous probes. Recently, several different classes of probe technology have been developed to perform targeted imaging. Such probes include antibodies, antibody fragments, peptides, nanoparticles, and activatable probes. Molecular targets for targeted imaging include proteolytic enzymes, extracellular matrix targets, cell-surface receptors, tyrosine kinases, and apoptosis markers[4].
This report aims to evaluate the current data regarding the utility of targeted imaging technology in gastroenterology and its potential future impact, particularly in the early detection of GI neoplasia.
MOLECULAR PROBES (OPTICAL CONTRAST AGENTS)
Optical contrast agents can be classified into endogenous fluorophores and exogenously administered contrast agents. Autofluorescence is the emission of a longer wavelength of light from tissue after it is excited by short-wavelength light. Fluorescence by emission is induced when endogenous tissue fluorophores (collagen, nicotinamide, adenine dinucleotide, flavin, or porphyrins) become excited[5]. Endoscopic AFI produces real-time pseudocolor images by detecting natural tissue fluorescence. Abnormal autofluorescence patterns in neoplastic tissues have been attributed to an increased nuclear-to-cytoplasmic ratio, loss of collagen, and neovascularization[6]. AFI has the advantage of not requiring the use of a contrast agent. However, as many of the autofluorescence alterations are not specific for neoplasia, autofluorescent imaging has disadvantages, such as low specificity and a high false-positive rate. Oh kawa et al[7] tested the diagnostic performance of AFI for detecting early gastric cancer. They showed that AFI was highly sensitive (sensitivity, 96.4%) but not very specific (specificity, 49.1%), as 50.9% of lesions identified as abnormal by fluorescence were benign. Although other studies have demonstrated the potential of AFI to target premalignant lesions and early cancer, the important limitation of high false-positive rates should be resolved[8].
Fluorescence imaging using exogenous probes obtains more effective images than AFI. Recent advances in molecular imaging using biomarker-targeted exogenous probes have demonstrated enhanced sensitivity and specificity for in vivo tumor imaging[1]. Exogenous probes targeting tumors include smart activatable probes, antibody fragments, peptides, and nanoparticle probes[9].Weissleder et al[10] first introduced a smart activatable probe, which was a synthetic graft copolymer consisting of poly-L-lysine sterically protected by multiple methoxy polyethylene glycol side chains to which multiple fluorochromes were attached. Smart activatable probes have their fluorescent emission effectively inhibited in the native state by fluorescence resonance energy transfer caused by the proximity of the fluorochromes to one another,but they become brightly fluorescent in areas of disease. Due to the high signal-to-background ratio, fluorescence intensity is relatively strong in the target tissue, which allows for a more accurate diagnosis. The specific target is increased protease expressed in neoplastic lesions, which cleaves lysine–lysine bonds resulting in a 15- to 30-fold enhanced signal intensity. In particular, cathepsin B is a major contributor to cleavage and activation in vivo. A previous study using a protease activatable probe demonstrated that this probe improved detection of adenomatous polyps in the small bowel of an animal model after resection and flushing[11].
Antibody probes bind to antigenic targets expressed on the cell surface in a specific manner, thereby optimizing the signal-to-background ratio. Antibodies have already been widely used to detect tumors, and the fluorescent probe-labeling method has been well established[12]. Additionally, novel treatment regimens using monoclonal antibodies have been developed to target specific molecules that play pathogenic roles in disease progression. Typical antibodies used for cancer treatment include cetuximab and panitumumab (monoclonal antibodies against epidermal growthfactor receptor) or bevacizumab (monoclonal antibody against vascular endothelial growth factor)[9]. Molecular imaging using antibody probes has a high potential to assist in the selection of patients who are likely to benefit from such tailored therapy and in the monitoring of responses to therapy. Most antibody probes have immunogenic properties and cause anallergic reaction. This type of response is frequently observed after systemic application. As antibody probes have a longer half-life, systemic application may induce the accumulation of antibody probes, causing the generation of a nonspecific background signal. Furthermore, due to the large molecular weight and size of antibody probes, it takes them longer to reach the target structure, which is disadvantageous for systemic application. F(ab′)2, Fab′, and scFV (single-chain variable) fragments lack the Fc domain and the complement-activating region, which may reduce immunogenicity[13]. Moreover, compared with an entire monoclonal antibody, antibody fragments are smaller and, therefore, able to more effectively penetrate tumor cells and accumulate. Fab′ and scFV fragments have only one binding domain, which reduces their binding ability; however, multivalency is increased by attaching several fragments to the surface of carriers or by engineering bivalent or multivalent fragments[14].
Peptides have several advantages because they consist of only a few amino acids that are highly specific and have high affinity, rapid binding kinetics, and shorter blood-clearance times. Furthermore, they have low immunogenic properties. Peptides with specific amino acid sequences that can preferentially bind to dysplastic or neoplastic tissues can be identified using the phage display technique[15]. This technique uses recombinant DNA technology to generate a library of clones that preferentially bind to the cell surface.
Several types of nanoparticles including magnetic iron oxide (IO), gold, quantum dots, and polymer-based nanoparticles have been developed recently for oncologic applications[16]. The surface of nanoparticles are usually coated with significantly stronger fluorophores for fluorescence imaging[9]. Additionally, nanoparticles can be loaded with targeted ligands, such as small molecules, peptides, antibodies, or aptamers. Nanoparticles must be fully characterized for toxicity, biodistribution, and pharmacokinetics to be highly specific and sensitive for molecular imaging.
Characteristics of exogenous probes that are promising for use in GI endoscopy include biocompatibility, affinity binding, deep tissue penetration, rapid kinetics, and low immunogenicity[12]. With regard to the administration route, the advantages of systemic application include a much more homogenous delivery of the imaging agent and a greater repeatability of agent concentration for serial studies. However, systemic administration produces more side effects than topical application. Topical application results in a much lower systemic concentration of the imaging agent, decreasing safety concerns and producing fewer regulatory hurdles to human translation. When probes are applied topically during or immediately before the endoscopic imaging procedure, specific binding to the targets must occur within several minutes, and a region of interest must be detected quickly.
MOLECULAR IMAGING INSTRUMENTS
Molecular imaging endoscopy requires high resolution to observe the large surface area of the GI mucosa and subsequently localize molecular changes in tumors. Optical spectroscopic and/or imaging techniques offer the potential for detecting the very earliest mucosal changes at the microstructural, biochemical and molecular levels. Several optical techniques currently under investigation for the endoscopic detection of precancerous GI lesions includes fluorescence spectroscopy and imaging, Raman spectroscopy, light-scattering spectroscopy (LSS), optical coherence tomography (OCT), and confocal fluorescence endomicroscopy[17].
AFI visualizes lesions including neoplasms not detectable by conventional white-light endoscopy due to differences in tissue fluorescence intensity. During AFI, normal tissue is pseudocolored green and blood vessels are dark green, whereas the hypertrophic fundic mucosa of the stomach and dysplastic or neoplastic areas appear magenta[8]. New AFI systems have a xenon light source (XCLV-260HP; Olympus, Tokyo, Japan) with a rotary red/green/blue band-pass filter. With this light source, the mucosa is sequentially illuminated with red, green, and blue light at a frequency of 20 cycles/s. The high-resolution videoendoscope (XCF-Q240FAI, Olympus) has two separate monochromatic charge-coupled devices (CCD), one for white-light endoscopy and one for AFI. The white-light mode can be switched to the autofluorescence mode by pressing a small button on the control head, and the switch is completed in 3 s[18].In the AFI mode, blue-spectrum light (395-475 nm) is delivered to excite AF, together with light in the green (540-560 nm) and red (600-620 nm) spectra. The AFI-CCD has a barrier filter that allows detection of all light with wavelengths from 490 nm to 625 nm, thereby eliminating blue excitation light. The sequentially detected images from AF along with the green reflectance, and red reflectance are integrated by the imaging processor into one AF image. AFI does not require the administration of fluorescence probes. Thus, it can be applied for cancer screening tests. The sensitivity for premalignant GI lesions increases when AFI is combined with high definition white-light imaging and narrow-band imaging to provide endoscopic trimodal imaging[19]. Endoscopic trimodal imaging has been proposed as an alternative to overcome the problems of AFI. Endoscopes with a widefield of view that can detect induced fluorescence during targeted endoscopic imaging have not yet been evaluated in larger clinical trials.
Raman spectroscopy is a form of image enhancement based on the principle that incident light (with wavelengths in the near-infrared region of the spectrum) can induce tissue biomolecules to vibrate and rotate. When light interacts with tissue molecules, it can be absorbed or scattered. Almost all of the scattered light is of the same wavelength as the incident light (elastic scattering)[20]. However, a small fraction of light undergoes so-called Raman (inelastic) scattering, in which slight shifts in energy and wavelength relative to the incident light occur because of energy exchange within a molecular structure. Raman spectroscopy can detect tissue changes at the molecular level, yielding unique “spectral fingerprints” of tissues as they become abnormal. Molckovsky et al[21] reported the first in vivo study using a fiber-optic probe via the accessory channel of the colonoscope. This study resulted in impressive accuracy of diagnosing hyperplastic (n = 9) and adenomatous (n = 10) polyps (100% sensitivity, 89% specificity, 95% overall accuracy).
LSS is based on white-light (400 nm to 700 nm) reflectance, whereby photons incident on tissue are backscattered without a change in their wavelength, providing microstructural information about the tissue. LSS measurements are performed with fiber-optic probes placed on the tissue surface via the accessory channel of the endoscope. Analysis of the intensity and wavelength of light reflected from the tissue surface provides an estimate of the size and degree of crowding of epithelial cell nuclei[17]. Recent preliminary work has suggested that LSS can be useful to identify even earlier subcellular changes associated with cancer progression[22]. In this study, a new generation of light scattering technology has detected submicron-size architectural changes in an endoscopically normal rectum. These changes were associated with the presence of neoplasia located elsewhere in the colon.
Confocal microscopy is based on tissue illumination with a low-power laser. The reflected light from the tissue is refocused onto the detector by the same lens, meaning that only returning light refocused through a pinhole is detected[23]. This process provides high-resolution images from a thin section within otherwise optically thick tissue. With technical developments, a miniaturized confocal laser scanner has been integrated into the distal tip of a flexible white-light endoscope for clinical use. Confocal endomicroscopy (Pentax EC-3870 CIFK; Pentax, Tokyo, Japan) enables confocal microscopy in addition to standard videoendoscopy[24]. The diameters of both the distal tip and the insertion tube are 12.8 mm. The distal tip contains an air- and water-jet nozzle, two light guides, a water-jet channel used to apply contrast agent, and a 2.8 mm working channel. The system uses a 488-nm excitation wavelength laser and enables the detection of 205 nm to 585 nm wavelength fluorescence.Confocal images are collected at a scan rate of approximately one frame/s, ata maximum resolution of 1024 × 1024 pixels. The optical slice thickness is 7 μm (axial resolution), and the lateral resolution is 0.7 μm.The range of the z-axis is 0 to 250 μm below the surface layer. Screen images approximate a 1000-fold magnification of the tissue in vivo. Confocal images can be generated simultaneously with endoscopic images. A slightly different approach is used for flexible probe-based confocal microscopy. Probe-based confocal laser endomicroscopy (pCLE; Cellvizio-GI; Mauna Kea Technologies, Paris, France) has been developed recently and has the advantage that a miniprobe can be easily passed through the working channel of a standard endoscope[25]. Probes generate dynamic (12 frames/s) images with a scanning field of 30 000 pixels. In addition to faster acquisition, the advantages include greater versatility of pCLE probes, which can be used in conjunction with virtually any endoscope,cholangioscope, bronchoscope, or ureteroscope, and for ad hoc usage, such as when a lesion is detected with a normal endoscope[26]. However, pCLE has a slightly lower resolution (approximately 1 μm compared with 0.7 μm for the Pentax confocal endomicroscope) and a smaller field of view (240-600 μm).
In the past few years, newly developed procedures and technologies have improved endoscopic recognition of GI neoplasms. Narrow band imaging (NBI) (with which Olympus scopes are equipped), the contrast enhancement system (i-scan) (associated with Pentax scopes) and multiband imaging (MBI) (with which theFujinon scope is equipped) are used in combination with magnification and high resolution endoscopy[27]. These imaging techniques can improve visualization of the vascular network and surface texture of the mucosa and can facilitate endoscopic diagnoses. NBI uses rotating filters in front of light sources to narrow the bandwidth of the projected light, and increases the blue spectrum intensity of the light used. This shorter wavelength is more readily absorbed by hemoglobin and has shallow penetration into only the superficial layer, thereby enhancing the visualization of superficial capillaries. The advantages of NBI include enhanced mucosal contrast at the push of a button and ease of neoplastic and non-neoplastic lesion differentiation. However, NBI results in poorer illumination of the background and a learning curve effect is observed, even for experienced endoscopists[28].
To date, AFI, NBI and CLE have been compared separately with conventional endoscopy. Trials should be extended to investigate different patient groups, as the optimal endoscopic modality may vary. AFI or NBI may be the examination of choice for general screening and CLE may be used for ulcerative colitis surveillance. Further large randomized controlled trials are needed to determine which modality would be the most suitable for various patient subpopulations.
PRECLINICAL AND CLINICAL STUDIES IN GI ENDOSCOPY
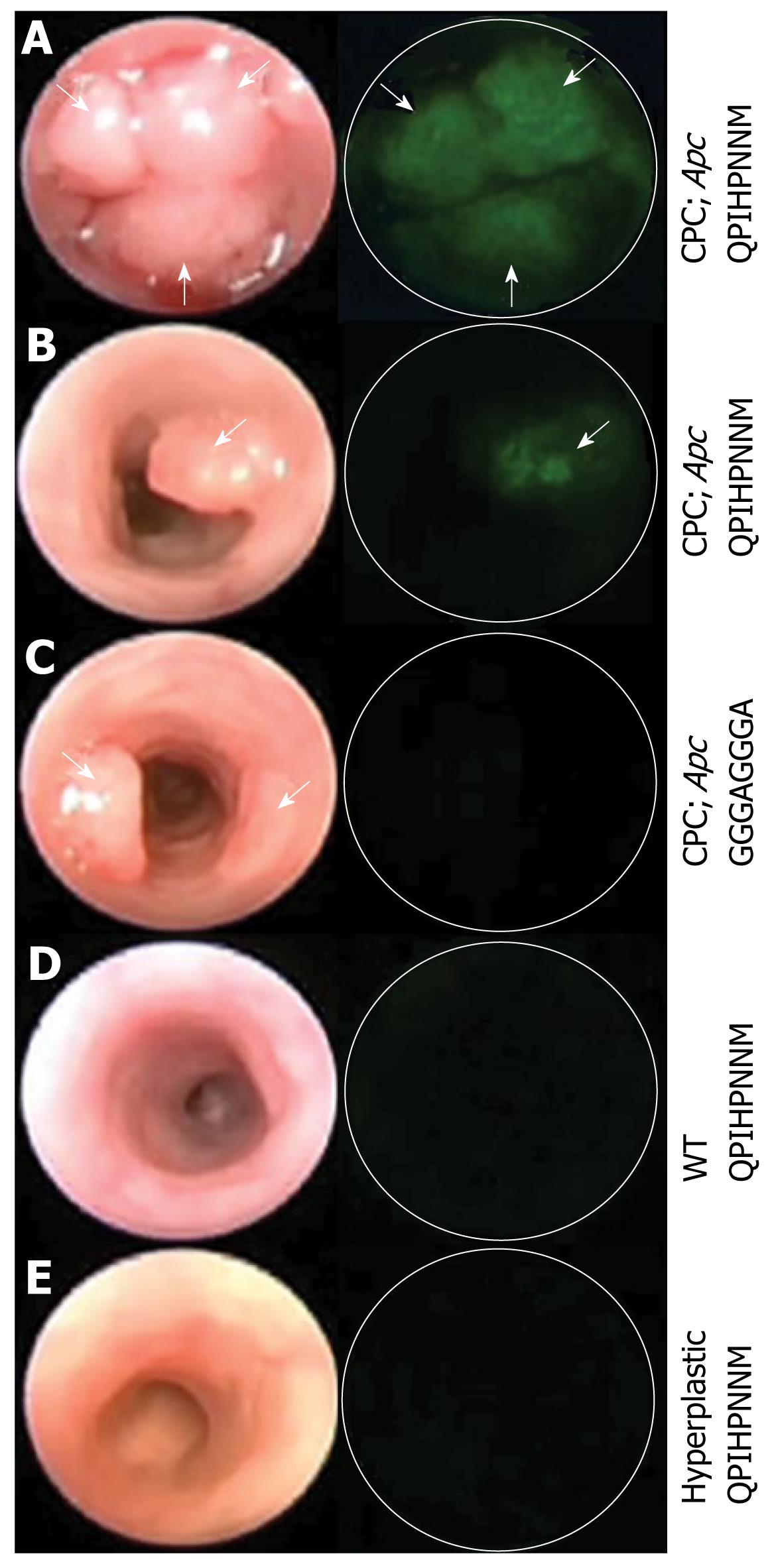
In vivo molecular imaging has been applied to GI endoscopy in various preclinical and clinical trials. Keller et al[29] performed fluorescence endoscopy with a fluorescent dye-labeled monoclonal antibody against carcinoembryonic antigen in patients with colonic polypoid lesions. Fluorescence probes were topically applied during a colonoscopy. A conventional endoscope was used, and its optical range was improved via two narrow-band filters. Specific fluorescence signals were present in most carcinomas and some adenomas. This study provided important information for further trials. Further advances were achieved in subsequent animal studies. Wang’s group[12] performed in vivo molecular imaging using topically applied fluorescence-labeled peptides to target the detection of high-grade dysplasia in Barrett’s esophagus. An affinity peptide with the ASYNYDA sequence was selected using phage display techniques. Fluorescent images using a wide-field endoscope sensitive to fluorescence revealed a region of high-grade dysplasia that was confirmed by histology. In a more recent study by the same group, peptides, which preferentially bind to adenomas in the CPC; Apc mouse model, a genetically engineered mouse that produces adenomatous polyps, were selected using an in vivo phage display[30]. In vivo binding was demonstrated using a fluorescein label with a wide-field endoscope (Figure 1).
Figure 1 Images from wide-field endoscopy videos after topical application of fluorescence-labeled peptides.
The left and right columns represent frames from white light and fluorescence endoscopy, respectively. A: Multiple adenomas; B: Single adenoma in a CPC; Apc mouse. The fluorescently labeled target peptide shows positive binding to multiple adenomas and a single adenoma; C: The control peptide shows minimal binding to a single adenoma; D:Control mouse lacking Cre recombinase transgene; E:The hyperplastic epithelium in a mutant K-ras mouse model. The target peptide also shows minimal binding to control mouse and hyperplastic epithelium. White arrows identify adenomas. Reproduced from Miller et al[30].
Recently, near-infrared (NIR) fluorescence probes suitable for in vivo imaging have been developed. Several proteases are overexpressed in a number of cancers[31,32].NIR imaging techniques combined with an NIR optical molecular probe activated by protease shows high specificity and sensitivity for tumor detection. In an animal study, an NIR probe specific for the enzyme cathepsin B, a protease upregulated in colorectal neoplasia, was administered intravenously[11]. The cathepsin B-activated molecular beacon demonstrated a high specificity for detecting small adenomatous polyps in ApcMin/+ mice. Recently, a minimally invasive imaging catheter has been developed to simultaneously and independently emit white light and NIR fluorescence[33]. When a protease-activatable probe was administered to an orthotopic colon cancer mouse model, microcatheter imaging demonstrated tumors with a higher target-to-background ratio[34]. Another study demonstrated that capsule endoscopy can be combined with molecular imaging[35]. NIR fluorescent signals of different intensities were detected after mouse models with adenomas were injected intravenously with a cathepsin B-activated probe, and the dissected intestine was imaged with capsule endoscopy under white or NIR fluorescent light.
Currently, targeted imaging techniques using Raman spectroscopy for clinical applications are being developed. This methodology includes the use of an accessory Raman endoscope in conjunction with topically applied tumor targeting Raman nanoparticles. Zavaleta et al[36] utilized surface-enhanced Raman scattering (SERS) nanoparticles as tumor targeting contrast agents. SERS is a plasmonic effect in which small molecules absorbed onto a nanoroughened noble metal surface, like gold, experience a dramatic increase in the incident electromagnetic field, resulting in a 2- to 4-fold higher Raman effect. In this study, intrarectally injected mice had localized uptake in the colon with minimal uptake in other organs. The benefit of SERS Raman nanoparticles as molecular imaging agents is the ability to conjugate them with specific tumor-targeting ligands, such as tumor-specific peptides, and then topically apply them to the tissue of interest to increase targeting efficiency while decreasing systemic toxicity.
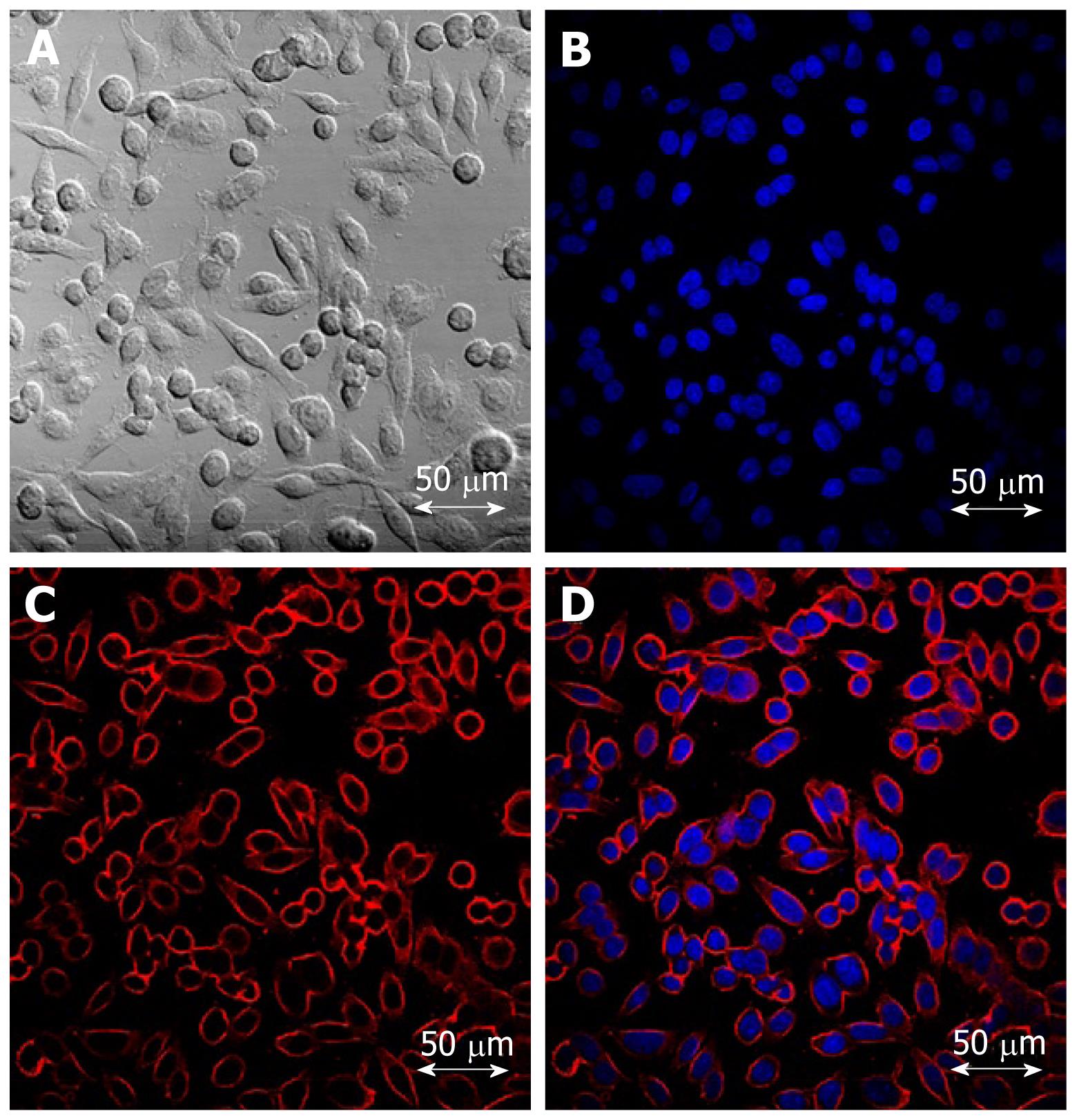
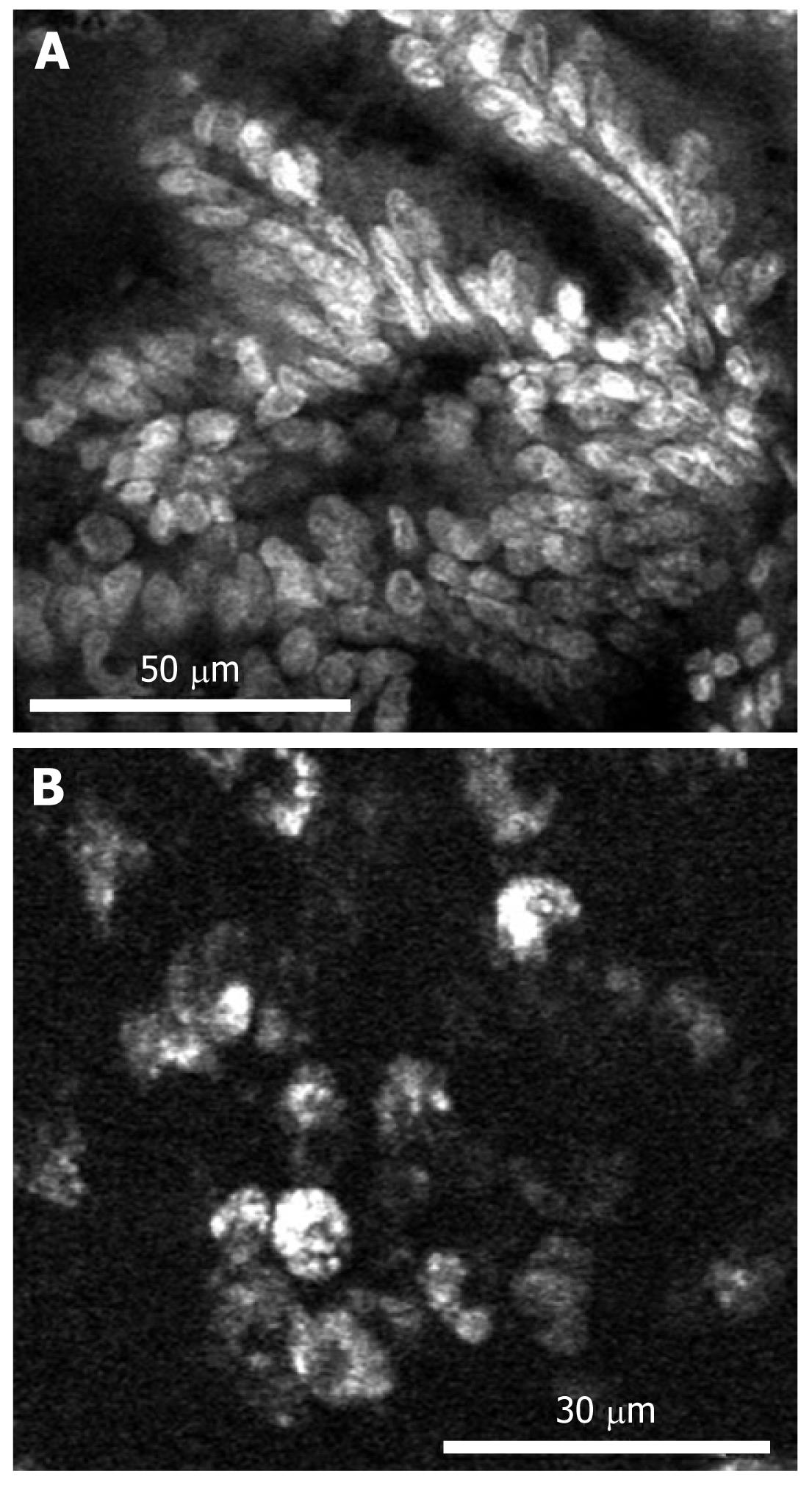
Since confocal laser endomicroscopy was introduced, several studies have reported molecular imaging with CLE. In the first study, Hsiung et al[15] developed a specific heptapeptide sequence (VRPMPLQ), which was screened using a phage display, that preferentially bound to human colorectal neoplasms. This peptide was conjugated with fluorescein and topically applied to the colonic mucosa of patients undergoing colonoscopy. Then an image was obtained using probe-based confocal laser endomicroscopy delivered through the working channel of a standard colonoscope. The fluorescein-conjugated peptide bound more strongly to dysplastic colonocytes than to adjacent normal cell sand had a sensitivity of 81% and a specificity of 82%. In a second trial, differentiation of tumor cells based on their epidermal growth factor receptor (EGFR)-expression patterns was achieved in a mouse model of human colorectal cancer xenografts[37]. After injecting a fluorescently-labeled whole antibody targeting EGFR, confocal laser endomicroscopy accurately identified EGFR expression. Further more, a CLE analysis of EGFR expression in ex vivo human tissue specimens allowed neoplastic tissue to be distinguished from non-neoplastic tissues after topical administration of labeled antibodies. We have developed a silica-coated IO nanoparticle that includes fluorescent materials. For whole-body molecular imaging, these nanoparticles were conjugated with cetuximab, a humanized chimeric anti-EGFR monoclonal antibody, that can specifically target colon cancer cells expressing EGFR on their cell membranes (Figure 2)[38]. After intravenously injecting a mouse model with human colorectal cancer xenografts, magnetic resonance imaging demonstrated significant changes in T2-weighted signals. Further studies are needed to apply our targeted nanoparticles to confocal endomicroscopy. In another trial, molecular imaging in surgical specimens from patients and in the APC min mouse model, a colorectal cancer xenograft model, was achieved after applying a fluorescein-labeled antibody against vascular endothelial growth factor (VEGF)[39]. CLE enabled the cytoplasmic distribution of VEGF to be displayed in most APC min ouse and xenograft tumors. Additionally, the VEGF-specific signal was significantly higher in malignant specimens than in samples from healthy mucosa (Figure 3). This study showed that CLE can contribute to the early detection of at-risk lesions and potentially predict responses to anti-VEGF-targeted treatment.
Figure 2 Confocal laser scanning microscopy images of colon cancer cells treated with cetuximab-conjugated magnetofluorescent nanoparticles.
A: A bright field image; B: Nuclear staining with DAPI; C: RITC (Rhodamine B isothiocyanate) fluorescent image; D: Overlay of B and C. Cellular uptake was so significant that the outer cell membranes of HCT116 cells can be clearly delineated by the images of the particles.
Figure 3 Imaging of vascular endothelial growth factor in the biopsy specimen of human colorectal adenocarcinoma.
A: Nonspecific nuclear and cellular staining using acriflavine; B: VEGF-specific staining using AF488-labeled antibodies. The antibody accumulates in the cytoplasm of the tumor cells, but not the nuclei. Reproduced with permission from Foersch et al[39].
FUTURE DIRECTION AND OPPORTUNITIES
The current screening method for premalignant GI lesions and cancers uses standard white-light endoscopy to detect morphological changes and lesions in the mucosa. Subsequent histopathological analysis of biopsy specimens is the gold standard for final diagnosis and treatment. Random biopsy sampling is commonly used for cancer screening and surveillance of diseases such as Barrett’s esophagus, gastric intestinal metaplasia, and inflammatory bowel diseases. However, a large mucosal area is at risk for developing cancer, and a nonrepresentative biopsy may miss lesions. Recent advances in molecular imaging have substantially influenced the endoscopic diagnosis of various GI diseases and our understanding of disease pathogenesis.
The essential elements to successfully apply molecular imaging to GI endoscopy are the identification of biomarkers for molecular targets and the development of appropriate molecular probes, application routes, adequate ligand targeting, and a high-resolution endoscope with wide-field view capable of visualizing the fluorescent signal. In the future, multimodality devices incorporating a wide field and high-resolution microscopic morphological imaging could further enhance the imaging-plane depth. For example, two-photon fluorescence endomicroscopy could show higher resolution and deeper penetration compared to single photon devices. The approach of first detecting suspicious lesions using whole-body molecular imaging and then characterizing the lesions by targeted endoscopic imaging might improve early disease diagnosis and evaluate response to therapy. In addition to these requirements, the safety and pharmacokinetics of the molecular probes must be investigated.
Recently, a variety of methods have been developed for computer-aided detection of GI neoplasms to overcome the limitations of conventional structural imaging tools, which include magnetic resonance imaging (MRI) and computed tomography (CT). Computed tomographic colonography (CTC) is an emerging technique for the detection of colorectal neoplasms, which has the potential to become an effective screening procedure for examining the entire colon[40]. However, problems such as a long interpretation time and the high variability of diagnostic accuracy among reviewers need to be addressed. Computer-aided detection (CAD) for CTC is attractive because it has the potential to circumvent these obstacles. Several approaches have been developed for the detection of polyps in CTC, including the use of the surface curvature with a rule-based filter, a volumetric shape index, and the extent of curvature[41]. Additionally, several methods have been proposed for computer-aided evaluation of GI images or video. Iakovidis et al[42] have developed a novel intelligent system for automatic detection of colonic and gastric adenomas in endoscopic videos, which uses color–texture image features and incorporates non-linear Support Vector Machines (SVMs) to achieve improved detection accuracy. Computer-aided evaluation is useful in automatically classifying NBI magnifying colonoscopicimages[43]. These approaches could be used in combination with molecular imaging tools.Advances in molecular imaging techniques will provide better patient-management strategies and make treatment more personalized.
Peer reviewers: Zhiwei Huang, PhD, Assistant Professor, Department of Bioengineering, Faculty of Engineering, National University of Singapore, 9 Engineering Drive 1, 117576, Singapore; Dimitris K Iakovidis, PhD, Assistant Professor, Technological Educational Institute of Lamia, 3rd km Old National Road Lamias-Athinas, Lamia GR 35100, Greece
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC