Published online Jun 16, 2010. doi: 10.4253/wjge.v2.i6.203
Revised: May 25, 2010
Accepted: June 1, 2010
Published online: June 16, 2010
The gold-standard management of acute cholecystitis is cholecystectomy. Surgical intervention may be contraindicated due to permanent causes. To date, the classical approach is percutaneous cholecystostomy in patients unresponsive to medical therapy. However, with this treatment some patients may experience discomfort, complications and a decrease in their quality of life. In these cases, endoscopic ultrasound (EUS)-guided gallbladder drainage may represent an effective minimally invasive alternative. Our objective is to describe in detail this new and not well-known technique: EUS-guided cholecystenterostomy. We will describe how the patient should be prepared, what accessories are needed and how the technique is performed. We will also discuss the possible indications for this technique and will provide a brief review based on published reports and our own experience.
- Citation: Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc 2010; 2(6): 203-209
- URL: https://www.wjgnet.com/1948-5190/full/v2/i6/203.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i6.203
Acute cholecystitis is not an uncommon reason for consultation in general surgery or gastroenterology. The gold-standard management is cholecystectomy. However, surgical intervention may be contraindicated in very elderly patients, patients with high surgical risk due to significant co-morbidities or those with a poor prognosis of their basal disease. In patients with risk of sepsis and without surgical indication, a gallbladder drainage method may be lifesaving. Percutaneous cholecystostomy is the most common approach in patients who are unresponsive to medical therapy[1]. Nevertheless, the percutaneous approach with a catheter draining purulent material into a bag may produce infection of the puncture point, needs special care, is associated with cosmetic disfigurement and discomfort and usually affects the quality of life[2]. An interesting question is when the catheter should be withdrawn in patients without surgical option. Moreover, some patients with advanced hepatobiliary malignancy have a biliary metallic stent that may be a permanent gateway for infective agents into the biliary tree. EUS-guided transluminal drainage methods are speedily gaining acceptance as an effective approach in a variety of conditions such as pseudocyst drainage, abscess drainage, pancreaticogastrostomy and hepaticogastrostomy[3-10]. Therefore, in this context, EUS-guided cholecystenterostomy may be a useful alternative that might not significantly affect the quality of life of these patients[2,11,12].
In-patients are usually already receiving antibiotic treatment unlike most outpatients. In patients not receiving antibiotics, an intravenous therapy of broad-spectrum antibiotics must be started prior to the procedure as is common in patients with cholecystitis. Once patients are admitted, they must continue with intravenous antibiotic therapy for some days and then with oral antibiotics until the resolution of infection. When the gallbladder is drained, it is better to take sample for culture if possible and to treat the infection according to the antibiogram.
Patients with a severe abdominal infection usually have difficulties with gastric emptying especially if they are not on absolute diet. Therefore, in patients who are eating, a soft midday meal without vegetables and then an exclusively clear liquid diet is recommended. If there is a functional, organic gastric or intestinal subocclusion, it may be advisable to add prokinetic drugs and, in some cases, to place a nasogastric aspiration tube. The goal is to keep the gastric and duodenal lumen clean during the procedure.
Special care must be taken with patients being treated with non-steroidal anti-inflammatory, antiaggregant or anticoagulant drugs. If drainage is necessary, it is compulsory to improve coagulation status prior to the procedure.
The procedures are relatively long and must be performed under conscious-sedation or under anesthesia. A mixture of midazolam and meperidine or propofol and/or remifentanil may be used depending on the patient’s characteristics and local expertise. In any case, it is highly recommended to have at least one pulsoximetry and it is advisable to have electrocardiographic recording, capnography and, if necessary, the possibility of patient intubation.
The procedure is usually carried out in two possible positions. If the use of X-ray is not expected, it can be done in left lateral decubitus. When X-ray is used, it is better to perform the procedure in a supine position to obtain a good radiological projection. As patients are usually sedated or anesthetized it is desirable to seek the maximum stability in their posture.
The most useful endoscope to perform an EUS-guided acute cholecystitis drainage is a linear ultrasonic gastrovideoscope with large diameter working channel, forceps elevator and Doppler (usually called therapeutic echoendoscope). In some rare cases, other endoscopes such as a large channel side-viewing endoscope or different kinds of gastroscopes can be useful. In this paper we will refer to conventional therapeutic linear echoendoscopes.
Although by using the one-step system most of the drainage can be performed without radiological control, it is highly desirable to have X-ray equipment at hand. When the procedure is going to be performed using the method in several steps, it must be done with radiological control. If a cutting current is going to be used, it is necessary to have an electrosurgical unit. This unit must be able to use blended cutting and electrocoagulation current.
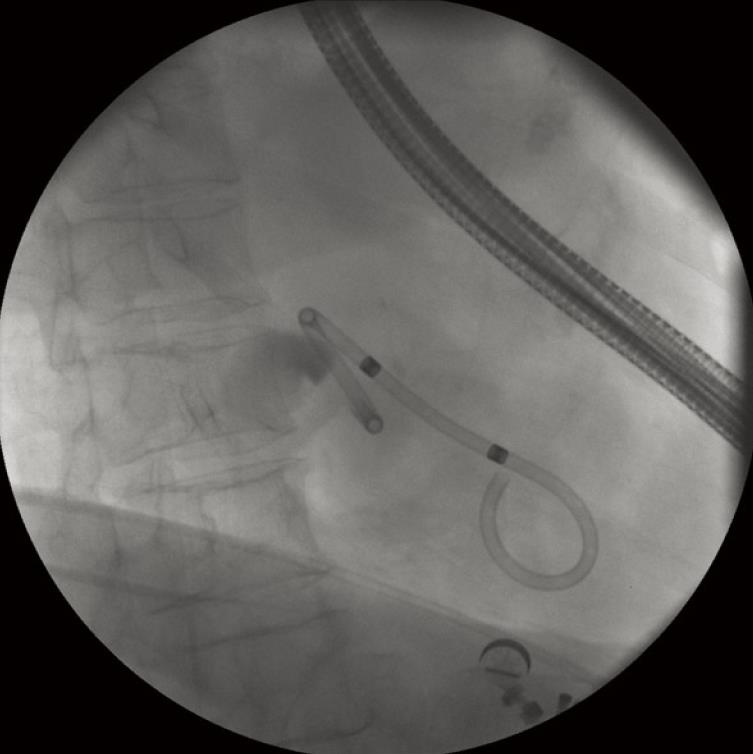
There are many approaches and many useful devices to make a perfect drainage. We will describe in detail our favorite system (one-step) (Figure 1) and more briefly the other gadgets that are also commonly used in pancreatobiliary endoscopy. The one-step system NWOA (Giovannini Needle Wire Oasis, Cook Ireland Ltd®, Limerick, Ireland) is available in two sizes: 8.5 and 10 fr. Each size is designed to place stents of 8.5 or 10 fr respectively. The 8.5-stent is a modified Cotton-Leung® (Amsterdam) Biliary Stent and the 10-stent is a modified Soehendra® Tannenbaum® Biliary Stent. This system is only adapted to straight stents. Both sizes of stents have their advantages and disadvantages: 8.5 fr stents are placed much more easily but also theoretically more easily blocked.
The system consists of four basic elements that are telescopically positioned. The external element is a plastic positioner-pusher catheter of 8.5 or 10 fr according to the stent size. Within it is a plastic dilator-introducer catheter which overhangs about 90 mm at the tip. The innermost element within the introducer catheter is a long guide wire with a movable core (to regulate the stiffness) and a metallic tip that allows electrosurgical current to be used as a needle-knife. The stent is placed on the tip of the pusher catheter and over the introducer catheter. Stents of 5 cm in length are normally used and when they are placed on the system they must be put with the tapered tip forward. This device was chiefly designed to perform drainage of pancreatic pseudocysts under endoscopic ultrasound guidance (Figure 1).
To perform the procedure in several steps the required devices to puncture the gallbladder are a normal 19-gauge endoscopic ultrasound needle and a 0.035 inch hydrophilic guide wire. An available 10 fr cystotome that is designed to electrosurgically cannulate the transgastric or transduodenal wall into a visibly bulging pancreatic fluid collection is useful too. A cystotome is a device manufactured by Cook® consisting of a 10 fr catheter with another 5 fr catheter inside it and within this second catheter a 0.038 inch needle-knife wire. The 10 fr external catheter tip has a diathermic ring to extend the initial hole to 10 fr[2]. Cystotomes of smaller caliber (Cysto-Gastro-Set of 6 and 8.5 fr) manufactured by Endo-flex® GmbH (Voerde, Düsseldorf) have recently come on to the market.
If the cystotome is not going to be used to enlarge the hole, a graduated dilation catheter[11] or a balloon dilator[12] has to be used. The stents may be straight or with pigtail ends. Within the procedure in several steps, double pigtail stents are usually preferred (Figure 2). In order to place the stent, a standard stent introducer set is usually utilised.
In some cholecystites with a dense content, lithiasis and pus, it can be useful to place a pigtail end nasobiliary catheter for continuous washing. We prefer a small caliber catheter (e.g. 5 fr) because it is more difficult for accidental looping and strangulation to occur and it is less troublesome for the patient. The outer end of the catheter must be connected to a gravity drip with a normal saline solution.
The procedure starts with a linear therapeutic echoendoscope because most patients have previously been studied with a transabdominal ultrasound, CT-scan, MRI and/or a radial endoscopic ultrasonography. In this kind of pathology it is not uncommon for the distal gastric antrum, the pylorus and/or the duodenal frame to be inflamed with stiffness and/or strictures. It is necessary to seek the more stable position in which the echoendoscope tip is positioned in front of the gallbladder. In order to do this, in some cases the long route must be taken with the tip positioned forward and upward in the prepyloric antrum. In other cases, it may be necessary to inflate the balloon inside the duodenal bulb to anchor the endoscope tip and then pull the echoendoscope slowly taking the short route. This is a very important maneuver as it will give stability and axial force to puncture. Next, the wall between the gut lumen and the gallbladder is explored, measuring its thickness and the presence of blood vessels. It is recommended that a scan of the wall with color or power Doppler is done. As far as possible, the window where the distance is the smallest and with no vessels between surfaces is chosen[2]. To achieve this, in some cases the echoendoscope is positioned in the duodenal bulb[2,11,12]. The access point usually corresponds to the gallbladder neck or body[11]. Theoretically the risk of leakage into the peritoneum decreases if there are inflammatory adhesions between surfaces. Moreover, there might be other factors related to the procedure such as the use of graduate dilators or electrosurgical current. It should be borne in mind that the graduated dilation catheter exerts an axial force that could detach the surfaces. On the other hand, the diathermic effect may help to keep the surfaces together due to the melting of tissues and the inflammatory reaction[2].
In the one-step technique, the use of 8.5 fr stents is preferred because they penetrate more easily through the stomach wall. The stent must be preloaded on the tip of the positioner-pusher catheter and over the dilator-introducer catheter. Care must be taken to place the tapered tip of the stent in a forward position to facilitate penetration. The metallic tip of the internal guide wire must protrude 1mm out of the tip of the dilator-introducer catheter to obtain the needle-knife effect. When this guide wire is correctly positioned, it is fixed by tightening the screw of the contact pin adapter. There is usually not much space between the enteral wall, the gallbladder and the hepatic surface. This might increase the risk of perforation of the contralateral wall of the gallbladder with the needle-knife tip. One trick to avoid this is to place the tapered tip of the stent immediately behind the tip of the needle-knife, to put the tip of positioner-pusher catheter next the end of the stent and to strongly fix the system with a surgical artery clamp placed near the catheter handle.
The kit has a flap positioner sleeve to lay flat the flap during stent introduction inside the working channel of the echoendoscope. However, when 8.5 fr stents are used it is not necessary to use this gadget because the flap in folded back position fits well through the large working channel. The contact pin adapter must be connected to the cable of the electrosurgical unit and the patient must have the electrosurgical patient electrode connected, preferably on the abdominal skin but not in the X-ray field. Usually 250 W of blended (cutting and electrocoagulation) flow current is used with the intention that the needle-knife incision does not slip on the intestinal surface while the fulguration creates a correct size hole.
When the tip of the one-step device appears in the ultrasound image, the best incision angle is found as perpendicular as possible to the wall between the intestinal lumen and the gallbladder. Therefore, the up/down control and the elevator forceps control are used. If the incision angle is not the correct one a false way could be caused. When the tip of the device rests on the intestinal surface in the right direction, the cutting pedal of the electrosurgical unit can be stepped on and the one-step system pushed slowly but decisively. With a little effort, the needle-knife, the dilator-introducer catheter and the tapered tip of the stent will penetrate the gallbladder. During all this time it is very important not to separate the tip of the echoendoscope from the wall because otherwise the ultrasound image would be lost and, above all, the axial force. It is usually possible to watch the gallbladder content boiling on the tip of the introducer catheter, and moreover, in some cases, to watch the stent flap inside the gallbladder.
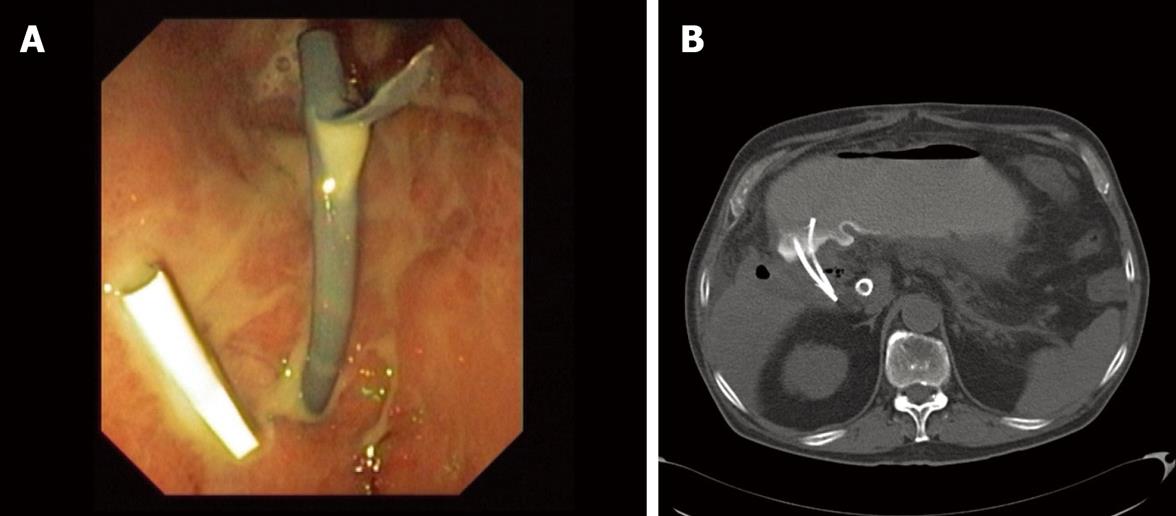
The stent is well positioned when the internal tip and flap are inside the gallbladder lumen. Only at this moment is it possible to separate very carefully and slowly the tip of the echoendoscope from the intestinal surface without losing the direction. Once this is done, the stent can be seen going through the wall in the optical image and its end with the external flap out of the wall and out of the echoendoscope channel. With the positioner-pusher catheter it is possible to control the depth of penetration. Then, the clamp that is fixing the positioner-pusher catheter to the dilator-introducer catheter can be released and then carefully pulled out until the stent is released. The output of gallbladder content through the stent hole can immediately be seen. If the output of liquid is not seen, it should be seen while aspirating with the endoscope. If despite all these maneuvers the liquid does not come out, either the stent is not well placed or there is a dense or solid content. If the liquid is clear bile, one stent may be enough. If the liquid is cloudy, purulent or with small particles of lithiasis, it is better to put more than one stent (usually two) (Figure 3A). The patient is kept under observation for a few days and then followed up as an outpatient. It is desirable to obtain a little quantity of content of the gallbladder in order to perform a culture and an antibiogram.
When there is thick pus and lithiasis it is necessary to place a nasobiliary pigtail end catheter for continuous washing. As argued above, a 5 fr catheter is usually used. This size of nasobiliary catheter has the advantage of perfectly fitting through the 8.5 fr stents. The internal guide wire of the one-step kit or a new hydrophilic guide wire of 0.035 inch and 480 cm in length is used to place it. It is necessary to place this guide wire deeply inside the gallbladder through one of the stents. Then the nasobiliary drainage is cautiously advanced over the guide wire until its pig tail tip is placed within the gallbladder.
By using a slim gastroscope the correct position and functioning of the stents and the washing catheter can be checked. If the system works adequately when saline solution is injected under pressure through the nasobiliary catheter it must come out through the unoccupied stent. Using X-ray control, the nasobiliary catheter also allows a contrast study to be performed with ease, permitting the early detection of leaks[2]. If there are no leaks, the nasobiliary catheter is connected to a gravity drip for washing with normal saline solution. A washing flow no greater than 40 mL/h to avoid leaks is used. In critical patients it is important to cautiously control the balance of liquids to avoid hydric overload. If a leak is detected, it is better to connect the nasobiliary catheter to a vacuum system to aspirate. In our opinion, while aspiration exists the patient will need to remain on absolute diet to avoid contamination of the gallbladder content through the stent.
The patient should be admitted and under continuous washing for at least one week. Then a new CT-scan or MRI is recommended to check the evolution (Figure 3B). If evolution is satisfactory, the removal of the washing catheter should be considered. Otherwise, the permeability of the stents using X-ray control and injecting radiographic liquid contrast through the nasobiliary catheter should be checked. If the stents are obstructed, a new endoscopic procedure to clean the system is needed. In the event that there is ascites, a leak between the wall of the gallbladder and the stomach is likely to be the cause. In this situation the flow of the saline solution should be stopped, an intermittent vacuum system connected to the nasobiliary catheter and oral feeding stopped. At this time, close monitoring of the patient is desirable.
The nasobiliary catheter should be withdrawn with endoscopic control to avoid removing the stent occupied by this. The stent is fixed by holding it with a grasping-forceps by the external flap and then the entire catheter with visual control of the stent is withdrawn. Finally, the permeability of all the stents is checked by aspirating with the endoscope. These patients must stay for one or two days more and then if there are no complications they must be discharged with oral antibiotic treatment. The technique described here was modeled on our established experience with endoscopic drainage of pancreatic-fluid collections. From a technical point of view, cholecystenterostomy is executed in essentially the same fashion as a cystoenterostomy is made to drain pseudocysts.
To perform the procedure in several steps availability of X-ray equipment is a practical requirement in all the steps. When a window without vessels is selected, the gallbladder is punctured with a 19-gauge endoscopic ultrasound needle as in a standard diagnostic puncture. At this moment, it may be interesting to aspirate a quantity of content of the gallbladder to decompress it and, if necessary, to perform a culture. Decompressing the gallbladder beforehand may be good to be able to inject radiographic contrast medium in order to reduce the risk of leakages. Next, if necessary, contrast is injected and some radiological imaging performed. Then, the needle lumen is washed by injecting saline solution to lubricate its interior to facilitate the advancement of the 0.035-inch hydrophilic guide wire. Now, the guide wire is pushed through the needle lumen deeply into the gallbladder lumen. By using X-ray control it must be confirmed that the guide wire is coiled in the fundus of the gallbladder to provide a suitable anchorage.
Once this is done, the EUS-needle can be exchanged for a 10 fr cystotome to enlarge the initial hole by using blended cutting current[2]. When the procedure is performed in several steps, this device is preferred instead of using different kinds of dilators because it is not necessary to separate the ultrasound probe from the gut wall while the cystotome enlarges the hole. However, the main reason is that the physical force of the dilators might detach the gallbladder whereas the melting of tissue and the inflammatory reaction of the fulguration may help to keep the surfaces together. If there is no cystotome, a graduated dilation catheter or a balloon dilator may be used[11]. But with this kind of devices the ultrasound probe contact and the axial force may be lost. When the hole is dilated and the guide wire within the cavity, the endoscope is slowly separated and a stent placed using a 10 fr standard introducer catheter. In these cases 10 fr double pig-tail end stents are usually used (Figure 3A). If it is necessary to place a new stent or a nasobiliary catheter, the procedure is started again from the beginning. To avoid this, advantage is taken of the possibility that two guide wires can be inserted simultaneously into the 10 fr cystoenterostome in parallel position[2]. This procedure takes longer than the one-step method and in our opinion it is less safe as we may lose access at any time, especially if the patient moves, because the time between the initial puncture and stent placement is too long.
Cholecystectomy must be the standard management for acute cholecystitis in the majority of patients. In patients unresponsive to conservative measures and without indication for urgent surgery, percutaneous cholecystostomy can be a bridge treatment toward elective surgery. However, there is also a group of patients that due to their clinical characteristics will never be candidates for surgery. These may mainly be very elderly patients, patients with high and permanent surgical risk or patients with an oncological disease and a limited life expectancy.
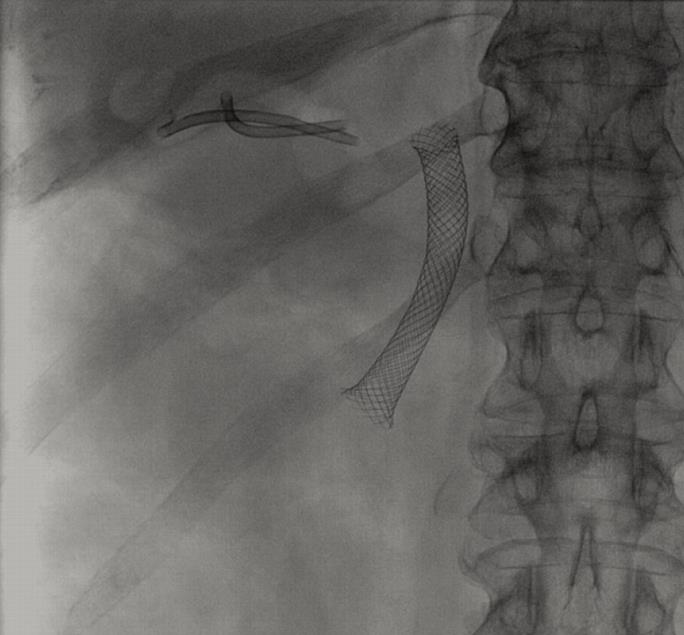
To date, percutaneous cholecystostomy is the most commonly used alternative to decompress the gallbladder. However, in some patients with important comorbidities, significant complications may occur with the percutaneous approach, including intrahepatic bleeding and sepsis[13,14]. Tube dislodgement is a frequent event, needing repeat procedures[1, 13,15]. Moreover, attempts at definitive removal of the catheter are associated with a high recurrence rate of cholecystitis[1,14,16], above all, in patients with a permanent gateway of infective agents (Figure 4). Therefore, an interesting question is when the catheter should be withdrawn in patients without surgical option and while the cause is present. From the patient´s perspective, to have a permanent catheter draining purulent material from the body to a bag may lead to a distortion of self-image and may impose significant physical restrictions[2]. Discomfort, local pain and infection of the puncture point are not infrequent. To minimize these undesirable effects maintenance of the system is required, including irrigation, dressings and bag and catheter changes. This severely impacts on the quality of life of the patients with a terminal malignancy[2]. Endoscopic transcystic drainage by retrograde route may be a valid non-surgical alternative[17] but this technique is difficult in some patients and practically impossible with the presence of previously placed metal biliary stents (Figure 4)[2]. In our opinion, this group of patients could benefit from an EUS-guided cholecystenterostomy.
We have performed four EUS-guided cholecystenterostomies on four different patients; one patient with advanced Alzheimer’s disease and three patients with advanced malignancies that involved the hepatobiliary region and biliary stents (Figure 4). All these patients had a complicated acute cholecystitis with perforation and perigallbladder abscess. The first procedure was performed with the several steps technique using a 10 fr cystotome because this is a well-contrasted technique commonly used in other drainage procedures. The other three procedures were performed with the one-step system NWOA of 8.5 fr without X-ray guidance and they took significantly shorter than the several steps technique. In the last procedure we placed three stents due to the fact that the first stent migrated inside the gallbladder. This problem was caused by the large thickness and rigidity of the wall to be passed through; the gastric peristaltic movements caused the stent to enter the gallbladder. The two following stents were correctly placed.
The technique described is based on our established experience with endoscopic drainage of pancreatic-fluid collections: i.e. more than 25 cases. We prefer the one-step technique due to several reasons. We believe that the several steps technique takes too much time from the moment the needle reaches the gallbladder until the stent is released. During this time access may be accidentally lost and the procedure needs to start again from the beginning. This is an undesirable situation that could jeopardize the procedure. To perform the procedure in several steps, X-ray guidance and several different kinds of devices are absolutely necessary. By using the one-step system, the puncture and the stent placement are a simultaneous action which brings with it great advantages. The time that passes from the puncture until stent release is minimal and reduces considerably the risk of an accidental loss of access and possible complications due to this eventuality. Additionally, as argued above, the tissue fulguration created by an electrosurgical device may result in a more avid tissue reaction with better adhesion between surfaces and superior long-term fibrotic patency of the tract than can be achieved with only physical disruption by dilation. However, this point of debate is yet to be proven in a systematic fashion[2]. In the future, our own technique will probably change due to accumulated experience, the introduction of new tricks and the development of new devices.
EUS-guided cholecystenterostomy has been performed successfully in two published series and in a brief report with a single patient with a total of 13 patients[2,11,12]. All these patients showed rapid clinical improvement and they did not experience major complications. There were two minor complications without clinical relevance: a minor intraprocedural bile leak[2] and a pneumoperitoneum[11]. In our short experience we have had no relevant complications and our patients progressed satisfactorily. To avoid complications it is important to correctly select patients. We believe that an adequate inflammatory adherence between the gallbladder and gastric wall in the window that we are using is a guarantee of success.
One important issue is when to remove the stents. Based on our experience in the drainage of pancreatic fluid collections, we believe that in most cases it is not necessary to remove them. In our small series, the patient with Alzheimer’s disease spontaneously expelled the stent after approximately two months. This patient had no new episodes of cholecystitis for more than a year of follow-up possibly due to the formation of a permanent mature fistula. The development of a fistula between the gallbladder and the intestine is one of the natural ways of spontaneous resolution of an acute cholecystitis. The other three patients, including the patient whose stent migrated into the gallbladder, still have their stents without complications related to them but it is too early to draw conclusions. Probably these patients will die due to their basal disease and the stents will have no negative impact.
EUS-guided cholecystenterostomy is technically feasible. In expert hands it seems safe, effective and relatively easy. In the cases described in the literature all the patients have progressed adequately in a short period of time without significant complications related to the procedure. We are absolutely convinced that, in patients with terminal malignancies, this procedure might offer a better quality of life than other non-surgical techniques such as percutaneous cholecystostomy.
Future developments in this area should probably include devices to fix the gallbladder to the site of the puncture or special stents that seal the surfaces to prevent leaks. This might reduce risks and extend the application of this new approach. In our view, new accessories should be developed, preferably in one-step modality, so that the procedure becomes even safer, easier and quicker.
Peer reviewer: Sheng-Lei Yan, MD, Division of Gastroenterology, Department of Internal Medicine, Chang Bing Show Chwan Memorial Hospital, No.6, Lugong Rd., Lugang Township, Changhua County 505, Taiwan, China
| 1. | Bakkaloglu H, Yanar H, Guloglu R, Taviloglu K, Tunca F, Aksoy M, Ertekin C, Poyanli A. Ultrasound guided percutaneous cholecystostomy in high-risk patients for surgical intervention. World J Gastroenterol. 2006;12:7179-7182. [Cited in This Article: ] |
| 2. | Kwan V, Eisendrath P, Antaki F, Le Moine O, Devière J. EUS-guided cholecystenterostomy: a new technique (with videos). Gastrointest Endosc. 2007;66:582-586. [Cited in This Article: ] |
| 3. | Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524-529. [Cited in This Article: ] |
| 4. | Seewald S, Ang TL, Teng KY, Groth S, Zhong Y, Richter H, Imazu H, Omar S, Polese L, Seitz U. Endoscopic ultrasound-guided drainage of abdominal abscesses and infected necrosis. Endoscopy. 2009;41:166-174. [Cited in This Article: ] |
| 5. | Seewald S, Imazu H, Omar S, Groth S, Seitz U, Brand B, Zhong Y, Sikka S, Thonke F, Soehendra N. EUS-guided drainage of hepatic abscess. Gastrointest Endosc. 2005;61:495-498. [Cited in This Article: ] |
| 6. | Varadarajulu S, Drelichman ER. EUS-guided drainage of pelvic abscess (with video). Gastrointest Endosc. 2007;66:372-376. [Cited in This Article: ] |
| 7. | Wehrmann T, Stergiou N, Vogel B, Riphaus A, Köckerling F, Frenz MB. Endoscopic debridement of paraesophageal, mediastinal abscesses: a prospective case series. Gastrointest Endosc. 2005;62:344-349. [Cited in This Article: ] |
| 8. | François E, Kahaleh M, Giovannini M, Matos C, Devière J. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128-133. [Cited in This Article: ] |
| 9. | Bories E, Pesenti C, Cai llol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287-291. [Cited in This Article: ] |
| 10. | Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-S7. [Cited in This Article: ] |
| 11. | Lee SS, Park do H, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008-1012. [Cited in This Article: ] |
| 12. | Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007;65:735-737. [Cited in This Article: ] |
| 13. | Spira RM, Nissan A, Zamir O, Cohen T, Fields SI, Freund HR. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183:62-66. [Cited in This Article: ] |
| 14. | van Overhagen H, Meyers H, Tilanus HW, Jeekel J, Laméris JS. Percutaneous cholecystectomy for patients with acute cholecystitis and an increased surgical risk. Cardiovasc Intervent Radiol. 1996;19:72-76. [Cited in This Article: ] |
| 15. | Ito K, Fujita N, Noda Y, Kobayashi G, Kimura K, Sugawara T, Horaguchi J. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. AJR. 2004;183:193-196. [Cited in This Article: ] |
| 16. | Hamy A, Visset J, Likholatnikov D, Lerat F, Gibaud H, Savigny B, Paineau J. Percutaneous cholecystostomy for acute cholecystitis in critically ill patients. Surgery. 1997;121:398-401. [Cited in This Article: ] |
| 17. | Toyota N, Takada T, Amano H, Yoshida M, Miura F, Wada K. Endoscopic naso-gallbladder drainage in the treatment of acute cholecystitis: alleviates inflammation and fixes operator's aim during early laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2006;13:80-85. [Cited in This Article: ] |