Published online Jan 16, 2010. doi: 10.4253/wjge.v2.i1.29
Revised: September 1, 2009
Accepted: September 8, 2009
Published online: January 16, 2010
Gastric outlet obstruction (GOO) includes obstruction in the antropyloric area or in the bulbar or post bulbar duodenal segments. Though malignancy remains the most common cause of GOO in adults, a significant number of patients have benign disease. The latter include peptic ulcer disease, caustic ingestion, post-operative anastomotic state and inflammatory causes like Crohn’s disease and tuberculosis. Peptic ulcer remains the most common benign cause of GOO. Management of benign GOO revolves around confirmation of the etiology, removing the offending agent Helicobacter pylori (H. pylori), non-steroidal anti-inflammatory drugs, etc. and definitive therapy. Traditionally, surgery has been the standard mode of treatment for benign GOO. However, after the advent of through-the-scope balloon dilators, endoscopic balloon dilation (EBD) has emerged as an effective alternative to surgery in selected groups of patients. So far, this form of therapy has been shown to be effective in caustic-induced GOO with short segment cicatrization and ulcer related GOO. In the latter, EBD must be combined with eradication of H. pylori. Dilation is preferably done with wire-guided balloon catheters of incremental diameter with the aim to reach the end-point of 15 mm. While it is recommended that fluoroscopic control be used for EBD, this is not used by most endoscopists. Frequency of dilation has varied from once a week to once in three weeks. Complications are uncommon with perforation occurring more often with balloons larger than 15 mm. Attempts to augment efficacy of EBD include intralesional steroids and endoscopic incision.
- Citation: Kochhar R. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1): 29-35
- URL: https://www.wjgnet.com/1948-5190/full/v2/i1/29.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i1.29
Gastric outlet obstruction (GOO) includes obstruction in the antropyloric area or in the bulbar or post bulbar duodenal segments. Though malignancy remains a common cause of GOO in adults[1,2], a significant number of patients with GOO have benign causes. Among the latter are peptic ulcer disease, caustic ingestion, post-operative anastomotic state and inflammatory causes such as Crohn’s disease and tuberculosis. Less often, chronic pancreatitis, annular pancreas and non-steroidal anti-inflammatory drug-included strictures result in GOO (Table 1). Peptic ulcer disease is the most common cause of benign GOO. After the association between Helicobacter pylori (H. pylori) and peptic ulcer was recognized, less than 5% patients with complicated duodenal ulcer disease and less than 1%-2% with complicated gastric ulcer disease have developed this complication[3,4]. Patients with ulcer related GOO often have a long history of symptoms due to ulcer disease[5]. In a study carried out in the United States, only 14% of 85 patients with ulcer-related GOO had acute disease and obstruction was the initial manifestation of the disease[6]. It has been estimated that > 95% of cases of obstructing duodenal ulcer disease have the obstruction in the duodenal bulb, and the rest were in the postbulbar region[4]. Caustic ingestion is another important cause of benign GOO. Both acid ingestion and alkali ingestion can cause antral/pyloric scarring resulting in GOO[7,8]. About one third of patients with ingestion of strong caustics end up having GOO. In a study of 41 cases of acid ingestion, it was reported that 44.4% developed GOO[7], while in another study on alkali ingestion, 36.8% of 31 patients developed GOO[8].
| Peptic ulcer disease |
| Caustic ingestion |
| Benign tumors |
| Adenoma |
| Lipoma |
| Stromal tumors |
| Carcinoid |
| Inflammatory causes |
| Acute pancreatitis |
| Chronic pancreatitis |
| Crohn's disease |
| Cholecystitis |
| Eosinophilic gastroenteritis |
| Inflammatory polyp |
| NSAID induced strictures/rings |
| Iatrogenic |
| Post surgical scarring or anastomotic stricture |
| Postvagotomy |
| Endoscopic mucosal resection |
| Other causes |
| Tuberculosis, CMV, Cryptosporidium, |
| Annular pancreas |
| Adult hypertrophic pyloric stenosis |
| Duplication cyst |
| Amyloidosis |
| Bouveret's syndrome |
| Bezoar |
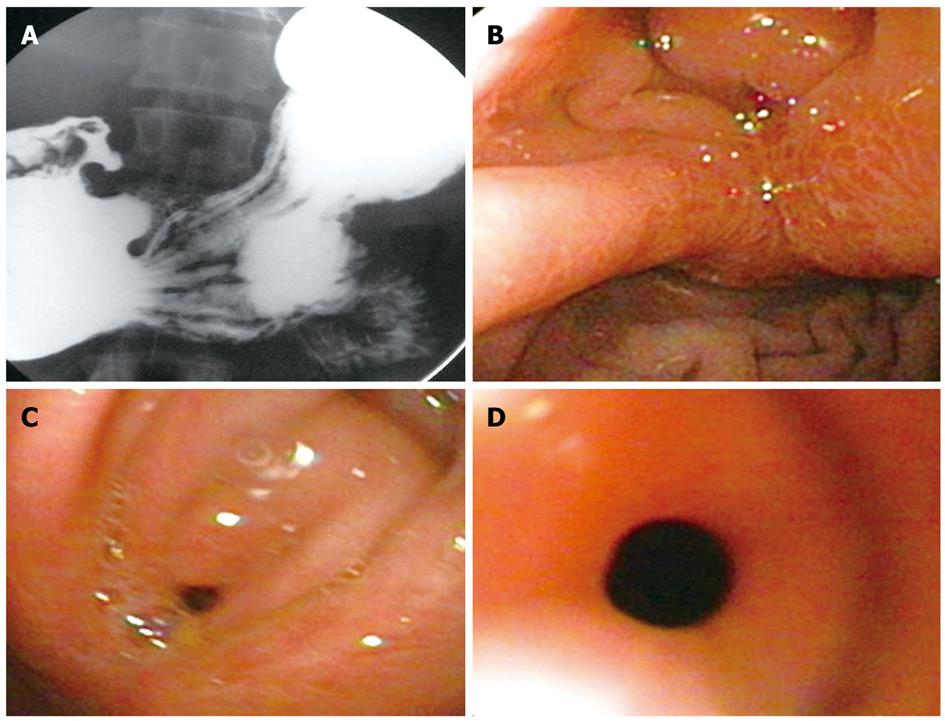
Unless the cause of GOO is obvious from the antecedent history, such as caustic ingestion, prior surgery, or known peptic ulcer disease, one must exclude malignancy in all adult patients by endoscopy and biopsy. A CT scan is very helpful in evaluating mural thickening, lymphnode enlargement and status of pancreas, biliary tract and retroperitoneum[4]. Linitus plastica can often be difficult to diagnose[4]. Barium studies are helpful in delineating the site of obstruction as well its extent (Figure 1).
All patients with symptoms of persistent GOO should be admitted to the hospital. Mild cases may be managed on an out-patient basis, however, those with fluid and electrolyte imbalance should be hospitalized. The principles of fluid and electrolyte resuscitation are: (1) intravenous volume resuscitation with normal saline; (2) replacement of potassium losses; and (3) periodic measurements of electrolytes and anterial pH[4].
Nasogastric decompensation should be instituted in all patients on hospitalization. It is especially useful in relieving pain in patients who have edema and spasm due to active ulceration. It also facilitates endoscopic examination at a later stage. Quite often, after 48-72 h of decompression and institution of proton pump inhibitors, patients feel better with subsidence of edema and spasm. Some of them will not require further treatment, while others will require a definitive treatment. Depending upon the likelihood of malignancy, a CT scan should be ordered first followed by endoscopy.
If the GOO is irreversible, or is caused by fibrotic scarring, rather than edema and spasm, it requires a definitive treatment. Until the advent of endoscopic balloon dilation (EBD), surgery was the only treatment for these patients. In the past, approximately 80%-90% of all patients with ulcer-related GOO had surgery[6]. In one study, about 60% of patients with ulcer-related GOO required surgery in the first hospitalization and 20% on subsequent hospitalization[9]. For caustic-induced GOO, surgery was the only option available as well[10]. However, recent data suggest that EBD is an effective alternative to surgery in a majority of patients with ulcer-related and caustic induced GOO[11-19]. Of course, patients with a possibility of malignancy would not be candidates for EBD. In inflammatory conditions like Crohn’s disease or infection like tuberculosis causing GOO, specific treatment for the antecedent disease is mandatory and may obviate the need for surgery or EBD.
The initial experience with EBD in patients with GOO was with fluoroscopic guided balloon catheters. However, with the advent of through-the-scope (TTS) balloon dilating catheters, EBD has become the first line of therapy in a majority of patients with benign GOO[20]. Benjamin et al[21,22] were the first workers to report the use of EBD in two studies. They combined endoscopic placement of guidewire with fluoroscopically-guided balloon dilation to treat GOO. Of the 7 patients in these two reports, only one required surgery[22]. Subsequently, a number of reports appeared highlighting the safety and efficacy of the procedure[11-20]. The use of wire-guided TTS balloons has further facilitated the procedure.
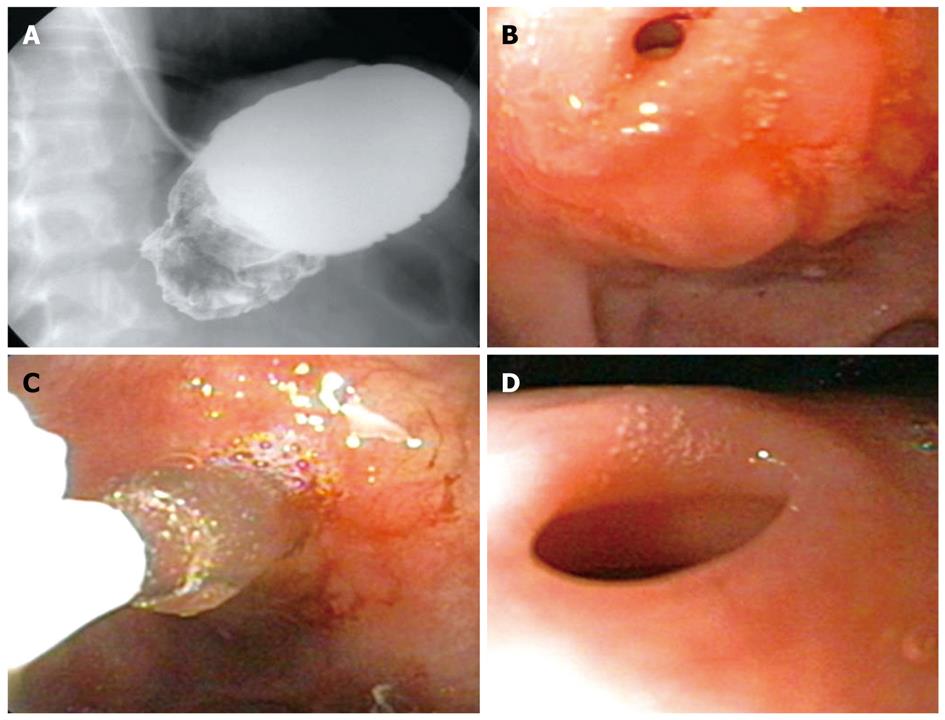
The currently used pyloric balloon dilators are available from a number of manufacturers. The balloons are available in lengths of 5.5-8.0 cm and are inflated using a hydrostatic device that is attached to a pressure gauge. Two types of balloons are currently available, one which can be dilated to a single diameter (Olympus SWIFT Balloon Dilators, Microvasive Rigiflex balloon) and the other ones that can be dilated to pre-fixed increasing diameters depending on the pressure with which they are inflated, e.g. CRE® dilators from Boston Scientific Inc (Figure 2) and Quantum TTC® Balloon Dilators from Wilson Cook.
The balloons are available in sizes of inflated diameters of 6 mm to 20 mm. The CRE and Quantum TTC® Balloon Dilators balloons have the advantage that the same balloon can be dilated to different diameters; e.g. from 10 mm to 12 mm, or 15 mm to 18 mm, making the procedure simple. The pyloric balloon dilators have an inner lumen with a flexible radio-opaque guide-wire in it, which can be used to negotiate tight strictures, and the balloon can be rail-roaded over the wire for optimal positioning.
Only localized stricture of the stomach should be chosen. The site of gastric cicatrisation is not important. CT scan to assess antral wall thickness may be a good modality to identify the “right patients” and to exclude malignancy. Endosonography may also emerge as a useful adjunct in this regard, especially in helping direct intralesional steroid injections.
Prior to the procedure, the patient should be kept fasting for 8-12 h. A gastric decompression should be carried on using a wide bore Ryles’ tube in patients who have gastric residue. Aspiration of gastric contents should be done to ensure a clearer view and to prevent regurgitation of contents into the air-passages. The patient’s diet should be restricted to liquids only, in those with severe stenosis. The patient should be taken into confidence about the need for the procedure, the surgical alternatives and possible complications, and an informed consent should be obtained. Patients are given pharyngeal anesthesia and conscious sedation administered along with hyoscine butylbromide injection prior to the procedure.
An endoscopic examination is done to visualize the narrowed segment and to look for the presence of active ulcer disease. A wire-guided balloon is preferred over a non-wire guided balloon, as it allows greater maneuverability and aids passage across a stenosed or displaced pylorus. Using an over-the-wire TTS balloon, the guide wire is pushed out of the balloon catheter tip and advanced through the stricture segment. Fluoroscopy is helpful to guide the placement of balloons in tight, tortuous and long strictures.
If the antral narrowing is associated with shortening of the lesser curvature of the stomach as well, it may be difficult to negotiate the lumen of the antrum because the plane of the antrum is at a right angle to the plane of the body. Since the capacity of the stomach is reduced in most such patients, the endoscope cannot be positioned along the long-route. It is best, in such instances, to insufflate as little air as possible and approach the narrowed segment from a distance with the balloon. However, it must be ensured that the endoscope is close to the narrowed segment when the balloon is inflated, otherwise the shaft of the balloon catheter can get kinked and the balloon can slip out of the narrowed segment. Another useful trick is to give a curve to the tip of the balloon catheter to gain access into the narrowed segment.
The balloon is negotiated across the stricture so as to ensure the centre of the stricture lies at the centre of the balloon. This can be ensured by using contrast mixed saline to inflate the balloon while using fluoroscopic assistance. The balloon is then inflated to a pre-determined pressure using an inflation device. During inflation, care has to be taken that the balloon does not slip in or slip out of the stricture. Dilation of the balloons or a single balloon with incremental diameters can be used in the same session. The choice of the diameter of the balloon is made depending on the endoscopist’s subjective assessment of the luminal diameter. During the balloon inflation, the patient is monitored for pain and endoscopic observation made for bleeding or any tear. Post-procedure the patient is monitored for signs and symptoms of perforation and bleeding for 4-6 h. In patients with suspected perforation, a contrast study should be carried out immediately using water soluble contrast medium.
Stable patients are allowed a liquid diet on the same day. The procedure is repeated every 1-2 wk until the end point of 15-18 mm lumen is achieved (see below). Once the end point has been reached, patients should be followed up for recurrence of symptoms and repeat endoscopy should be performed to look for persistence or re-appearance of ulceration. In patients with H. pylori related peptic ulcer, eradication of H. pylori should be confirmed.
As stated previously, it is best to wait for 8 wk after caustic ingestion to allow for natural healing. There are no studies that show whether balloon dilation can be carried out in the sub-acute phase.
Although different workers have dilated at 1-3 wk intervals, we advocate weekly dilation to facilitate: (a) reaching the end point of 15 mm, in a short period of time; and (b) maintaining nutritional status of the patient. Once adequate nutrition is ensured, the interval between dilations can be varied, taking into account the social circumstances; e.g. the distance the patient travels, etc.
Since patients are likely to have symptoms of GOO, it is important to maintain nutrition during the period of dilation. Total parenteral nutrition is most effective, but affordability may pose a problem. We advocate naso-jejunal tube feeding after acute corrosive injury, ≥ Gr 11b endoscopically until reepithelization is complete, which generally takes 6-8 wk before the patient is started on balloon dilation. If successful dilation can be carried out to 8-10 mm, an homogenized liquid diet can be allowed orally, ensuring adequate calorie and protein intake. As the stricture opens up, semisolids may be allowed. A good indicator of the adequacy of response to dilation is the residue seen before each dilation.
The results of EBD for peptic-GOO have been variable because not all studies have taken into account compounding factors, such as the presence of H. pylori, use of NSAIDs, and compliance with proton pump inhibitor drug intake. Immediate relief with EBD has been universally found in most studies, but the long term response has varied from a dismal 16%[19] to 100%[15]. Studies that looked for and eradicated H. pylori have reported a good long term response in 70%-80% of patients over 9 to 98 mo of follow up[12,13,18,23,24].
Boylan et al[12] found that young age, long duration of symptoms needing more than one session of EBD and continuous use of NSAIDs were associated with adverse outcomes and predicted the need for multiple dilations and surgery. DiSario et al[24] reported a longer length of the narrowed segment was a poor prognostic sign. Most of the studies have not commented upon the long-term use of proton pump inhibitors making comparisons between studies difficult.
Only a few studies have addressed the issue of H. pylori in these patients. Boylan et al[12] reported results on 40 patients with peptic-GOO in which 28 had recurrent symptoms, of whom 12 required surgery. H. pylori infection was detected in 9 patients and all achieved eradication, with only 1 requiring surgery. Among the 31 patients who were negative for H. pylori or were not investigated for it, 11 required surgery. More recently, Lam et al[25] compared the outcomes of 14 H. pylori positive patients with 11 who were negative for H. pylori. The response rate of EBD was 78.6% in the former and 45.4% in the latter. They also reported that, during a follow up of 24 mo, eradication of H. pylori combined with EBD was associated with 21% cases of ulcer complications such as bleeding or obstruction, as compared to 55% in the H. pylori negative group. Kochhar et al[15] reported a 100% response rate in 11 patients, all of whom had successful eradication of H. pylori after 1-3 sessions of EBD. A study by Cherian et al[17] also reported similar results. They subjected 21 patients to EBD for GOO (H. pylori = 12, NSAID-related = 3, both H. pylori and NSAID-related = 5 and others = 3). After eradicating H. pylori and removing the other offending cause, they achieved success in all their patients, with 17 of them requiring maintenance acid suppressive therapy.
The data quoted above shows that more than three-fourths of patients with peptic-GOO can be successfully treated with EBD provided H. pylori is eradicated, offending NSAIDs are stopped and maintenance acid suppressant therapy is given. The available literature, therefore, suggests that long term proton pump inhibitor usage may be required to prevent recurrences. However, prospective studies are needed to answer this question definitely.
Surgery has been the mainstay of treatment for caustic induced GOO, but recent reports suggest that EBD can be an effective alternative form of therapy in a select group of these patients[15,26]. However, compared to peptic-GOO cases, these patients have more recurrences and require a larger number of EBD sessions. Solt et al[13] studied 17 patients with caustic-GOO out of a total of 72 and only one-third had a long-term response with EBD.
Kochhar et al[15] reported that 8 of their 23 patients with GOO had a history of caustic ingestion, and they required 2-9 sessions of EBD, as compared to 1-3 sessions for peptic-GOO cases. In another recent study on 41 patients, they showed that caustic-induced GOO could be treated with EBD in 39 of these patients, with no recurrences over 18-58 mo of follow up[26]. The 39 patients required a mean of 5-8 sessions (range 2-13 sessions) to reach the end point of 15 mm. Only 2 patients failed to respond, one who had a perforation and another who had recurrent pain during each dilation. Only one patient had a supplementary procedure in the form of electrosurgical incision to augment the effect of EBD. However, they excluded patients with active ulceration and did not include patients with stricture length more than 2.5 cm.
Chronic pancreatitis associated GOO is reported to be more resistant to EBD, and in one study all 4 such patients required surgery. Tuberculosis was incriminated as a cause of GOO by Misra and Dwivedi[14] and they reported successful EBD in that patient. Anastomotic strictures following gastric resection have been treated with EBD by a few workers. Kozarek et al[18] had 7 such patients, out of a total of 23 patients with GOO. Only 4 of the 7 patients with anastomotic strictures had good long-term responses.
GOO associated with use of NSAID use has also been treated with EBD by a few workers. Cherian et al[17] have emphasized the fact that stopping NSAIDs is essential to successful long-term outcomes in these patients.
Patients with Crohn’s disease causing GOO have also been subjected to EBD, with variable results. Murthy[27] reported one case of obstructive Crohn’s disease who underwent repeated sessions of EBD, but failed to respond. Solt et al[13] also treated one such patient who did not respond and required surgery. Kim et al[28], however, had a satisfactory response in one patient with postbulbar Crohn’s disease, in whom they used fluoroscopically guided balloon dilation.
Anastomotic strictures following vertical band gastroplasty or gastric bypass surgery for morbid obesity have also been successfully dilated with balloon catheters endoscopically. In one such study, EBD was successful in 68% of the 40 patients studied[29]. In a retrospective analysis, Solt et al[13] found good responses in all 18 patients with postoperative strictures and 6 patients with postvagotomy functional stenosis. Fluoroscopically guided balloon dilation has also been equally successful with response rates as high as 94%[30].
A number of workers have used supplementary techniques to augment the effect of EBD. These include intralesional steroid injections and endoscopic incision of the strictures segment.
We have reported use of intralesional steroids injection in patients with caustic GOO augmented the effect of balloon dilation[31]. Two of these patients had failed to show satisfactory responses to balloon dilation while the third was given steroids right at the time of first dilation. All three patients responded with (1, 2 and 2) sessions of steroid injections. The idea of using steroids is to facilitate dilation and reduce recurrence. Intralesional steroid injections have been shown to inhibit stricture formation by interfering with collagen synthesis, fibrosis and chronic scarring[32]. It has been suggested that triamcinolone presents the cross linking of collagen that results in scar contracture; so if the scar is stretched and steroid injected into it, presumably the contracture will not occur[33]. Steroids also decrease the fibrotic healing that appears after dilation[32]. Gandhi et al[34] observed that with corticosteroid injections and dilation, longer corrosive esophageal strictures become shorter with time and thus more amenable to nonsurgical treatment. We have shown that intralesional steroid injections reduce the need for dilation in caustic esophageal strictures, and even longer strictures (> 3 cm) can be managed successfully[35]. There is only one other report of use of intralesional steroids in pyloric stenosis apart from ours, in which Lee et al[36] successfully treated two cases (one peptic, another post-pyloroplasty) with steroids and balloon dilations.
Baron et al[37] used electrosurgical incision using a standard sphincterotomy to successfully treat a patient with pyloric stenosis resistant to EBD. Hagiwara et al[38] used needle-knife radial incisions electrosurgically at gastroscopy combined with EBD in patients with refractory anastomotic pyloric stenosis. We have also used this technique in one of the patients with caustic-induced GOO, who had become pregnant while on a dilation programme[26].
There is as yet no consensus on the issue of end point of balloon dilation. Kozarek et al[18] felt that the effective balloon size required to dilate pyloric stenosis appears to be 41 Fr, as it achieves immediate relief in 80%. Most workers[12,15-17,23,26] have used 15 mm balloons as the end point while some have dilated to 10 mm or 12 mm only[13,18]. Rarely, balloons of 16 mm, 18 mm and 20 mm are used[11,13,18]. Most workers inflate the balloon for 30 s to 60 s and repeat once again before withdrawing[17]. While some workers-have used a single session of dilation, others use repeat dilations at variable intervals. All these observations are on pyloric stenosis caused by peptic ulceration or anastomotic strictures. We have been more cautious than others; in our patients with peptic and caustic induced GOO, we dilate in step-wise manner, from 8 mm to 10 mm in the first few sessions, 10 mm to 12 mm in the next few sessions and 12 mm to 15 mm subsequently[15,26].
Hydrostatic balloon dilation is generally a safe procedure. Kozarek et al[18] encountered one perforation out of 23 cases with peptic ulcer related stenosis. The balloon diameter used was not mentioned. Lau et al[11] reported 4 perforations in 54 patients. Two of their 16 patients undergoing dilation with a 16 mm balloon had perforation while 2 of the 3 undergoing 20 mm dilation perforated, with none of the 10 undergoing 18 mm dilation having any complication. It thus seems that increasing the balloon diameter beyond 15 mm is more likely to be associated with perforation. Arterial bleeding has been rarely reported, though self limiting minor bleeding is not uncommon[28]. Pain during EBD is not uncommon, but is often self limiting. In a recent study, 19.5% of patients with caustic-GOO had self-limiting pain during the procedure[26].
Peer reviewer: Tian-Le Ma, MD, Department of Gastroenterology, Rui Jin Hospital Affiliated to Medical College of Shanghai Jiao Tong University, Shanghai 200040, China
S- Editor Zhang HN L- Editor Lutze M E- Editor Ma WH
| 1. | Khullar SK, DiSario JA. Gastric outlet obstruction. Gastrointest Endosc Clin N Am. 1996;6:585-603. [Cited in This Article: ] |
| 2. | Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg. 1990;77:1023-1024. [Cited in This Article: ] |
| 3. | Paimela H, Tuompo PK, Peräkyl T, Saario I, Höckerstedt K, Kivilaakso E. Peptic ulcer surgery during the H2-receptor antagonist era: a population-based epidemiological study of ulcer surgery in Helsinki from 1972 to 1987. Br J Surg. 1991;78:28-31. [Cited in This Article: ] |
| 4. | Ferzoco SJ, Soybel DI. Gastric outlet obstruction, perforation and other complications of gastroduodenal ulcer. Therapy of digestive disorders. 2nd ed. New Delhi: Elsevier Inc 2007; 357-375. [Cited in This Article: ] |
| 5. | Kreel L, Ellis H. Pyloric stenosis in adults: A clinical and radiological study of 100 consecutive patients. Gut. 1965;6:253-261. [Cited in This Article: ] |
| 6. | Weiland D, Dunn DH, Humphrey EW, Schwartz ML. Gastric outlet obstruction in peptic ulcer disease: an indication for surgery. Am J Surg. 1982;143:90-93. [Cited in This Article: ] |
| 7. | Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702-707. [Cited in This Article: ] |
| 8. | Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of strong corrosive alkalis: spectrum of injury to upper gastrointestinal tract and natural history. Am J Gastroenterol. 1992;87:337-341. [Cited in This Article: ] |
| 9. | Jaffin BW, Kaye MD. The prognosis of gastric outlet obstruction. Ann Surg. 1985;201:176-179. [Cited in This Article: ] |
| 10. | Chaudhary A, Puri AS, Dhar P, Reddy P, Sachdev A, Lahoti D, Kumar N, Broor SL. Elective surgery for corrosive-induced gastric injury. World J Surg. 1996;20:703-706; discussion 706. [Cited in This Article: ] |
| 11. | Lau JY, Chung SC, Sung JJ, Chan AC, Ng EK, Suen RC, Li AK. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc. 1996;43:98-101. [Cited in This Article: ] |
| 12. | Boylan JJ, Gradzka MI. Long-term results of endoscopic balloon dilatation for gastric outlet obstruction. Dig Dis Sci. 1999;44:1883-1886. [Cited in This Article: ] |
| 13. | Solt J, Bajor J, Szabó M, Horváth OP. Long-term results of balloon catheter dilation for benign gastric outlet stenosis. Endoscopy. 2003;35:490-495. [Cited in This Article: ] |
| 14. | Misra SP, Dwivedi M. Long-term follow-up of patients undergoing ballon dilation for benign pyloric stenoses. Endoscopy. 1996;28:552-554. [Cited in This Article: ] |
| 15. | Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol. 2004;19:418-422. [Cited in This Article: ] |
| 16. | Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol. 1996;91:987-990. [Cited in This Article: ] |
| 17. | Cherian PT, Cherian S, Singh P. Long-term follow-up of patients with gastric outlet obstruction related to peptic ulcer disease treated with endoscopic balloon dilatation and drug therapy. Gastrointest Endosc. 2007;66:491-497. [Cited in This Article: ] |
| 18. | Kozarek RA, Botoman VA, Patterson DJ. Long-term follow-up in patients who have undergone balloon dilation for gastric outlet obstruction. Gastrointest Endosc. 1990;36:558-561. [Cited in This Article: ] |
| 19. | Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc. 1995;41:15-17. [Cited in This Article: ] |
| 20. | Yusuf TE, Brugge WR. Endoscopic therapy of benign pyloric stenosis and gastric outlet obstruction. Curr Opin Gastroenterol. 2006;22:570-573. [Cited in This Article: ] |
| 21. | Benjamin SB, Cattau EL, Glass RL. Balloon dilation of the pylorus: therapy for gastric outlet obstruction. Gastrointest Endosc. 1982;28:253-254. [Cited in This Article: ] |
| 22. | Benjamin SB, Glass RL, Cattau EL Jr, Miller WB. Preliminary experience with balloon dilation of the pylorus. Gastrointest Endosc. 1984;30:93-95. [Cited in This Article: ] |
| 23. | Griffin SM, Chung SC, Leung JW, Li AK. Peptic pyloric stenosis treated by endoscopic balloon dilatation. Br J Surg. 1989;76:1147-1148. [Cited in This Article: ] |
| 24. | DiSario JA, Fennerty MB, Tietze CC, Hutson WR, Burt RW. Endoscopic balloon dilation for ulcer-induced gastric outlet obstruction. Am J Gastroenterol. 1994;89:868-871. [Cited in This Article: ] |
| 25. | Lam YH, Lau JY, Fung TM, Ng EK, Wong SK, Sung JJ, Chung SS. Endoscopic balloon dilation for benign gastric outlet obstruction with or without Helicobacter pylori infection. Gastrointest Endosc. 2004;60:229-233. [Cited in This Article: ] |
| 26. | Kochhar R, Dutta U, Sethy PK, Singh G, Sinha SK, Nagi B, Wig JD, Singh K. Endoscopic balloon dilation in caustic-induced chronic gastric outlet obstruction. Gastrointest Endosc. 2009;69:800-805. [Cited in This Article: ] |
| 27. | Murthy UK. Repeated hydrostatic balloon dilation in obstructive gastroduodenal Crohn’s disease. Gastrointest Endosc. 1991;37:484-485. [Cited in This Article: ] |
| 28. | Kim JH, Shin JH, Di ZH, Ko GY, Yoon HK, Sung KB, Song HY. Benign duodenal strictures: treatment by means of fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005;16:543-548. [Cited in This Article: ] |
| 29. | Sataloff DM, Lieber CP, Seinige UL. Strictures following gastric stapling for morbid obesity. Results of endoscopic dilatation. Am Surg. 1990;56:167-174. [Cited in This Article: ] |
| 30. | Kim JH, Shin JH, Bae JI, Di ZH, Lim JO, Kim TH, Ko GY, Yoon HK, Sung KB, Song HY. Gastric outlet obstruction caused by benign anastomotic stricture: treatment by fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005;16:699-704. [Cited in This Article: ] |
| 31. | Kochhar R, Sriram PV, Ray JD, Kumar S, Nagi B, Singh K. Intralesional steroid injections for corrosive induced pyloric stenosis. Endoscopy. 1998;30:734-736. [Cited in This Article: ] |
| 32. | Ashcraft KW, Holder TM. The experimental treatment of esophageal strictures by intralesional steroid injections. J Thorac Cardiovasc Surg. 1969;58:685-693. [Cited in This Article: ] |
| 33. | Ketchum LD, Smith J, Robinson DW, Masters FW. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1966;38:209-218. [Cited in This Article: ] |
| 34. | Gandhi RP, Cooper A, Barlow BA. Successful management of esophageal strictures without resection or replacement. J Pediatr Surg. 1989;24:745-749; discussion 749-750. [Cited in This Article: ] |
| 35. | Kochhar R, Ray JD, Sriram PV, Kumar S, Singh K. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509-513. [Cited in This Article: ] |
| 36. | Lee M, Kubik CM, Polhamus CD, Brady CE 3rd, Kadakia SC. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc. 1995;41:598-601. [Cited in This Article: ] |
| 37. | Baron B, Gross KR. Successful dilation of pyloric stricture resistant to balloon dilation with electrocautery using a sphincterotome. J Clin Gastroenterol. 1996;23:239-241. [Cited in This Article: ] |
| 38. | Hagiwara A, Sonoyama Y, Togawa T, Yamasaki J, Sakakura C, Yamagishi H. Combined use of electrosurgical incisions and balloon dilatation for the treatment of refractory postoperative pyloric stenosis. Gastrointest Endosc. 2001;53:504-508. [Cited in This Article: ] |