Published online Sep 16, 2022. doi: 10.4253/wjge.v14.i9.536
Peer-review started: January 5, 2022
First decision: June 16, 2022
Revised: June 28, 2022
Accepted: August 7, 2022
Article in press: August 7, 2022
Published online: September 16, 2022
The treatment for ampullary cancer is pancreatoduodenectomy or local ampu
To determine the necessity of and an appropriate method for investigating hilar biliary invasion of ampullary cancer.
Among 43 ampullary cancer patients, 34 underwent endoscopic treatment (n = 9) or surgery (n = 25). The use of imaging findings (thickening and enhancement of the bile duct wall on contrast-enhanced computed tomography, irregularity on endoscopic retrograde cholangiography, thickening of the entire bile duct wall on intraductal ultrasonography (IDUS), and partial thickening of the bile duct wall on IDUS) and biliary biopsy results for diagnosing hilar biliary invasion of ampullary cancer was compared.
Hilar invasion was not observed in every patient. Among the patients who did not undergo biliary stent insertion, the combination of partial thickening of the bile duct wall on IDUS and biliary biopsy results showed the highest accuracy (100%) for diagnosing hilar biliary invasion. However, each imaging method and biliary biopsy yielded some false-positive results.
Although some false-positive results were obtained with each method, the combination of partial thickening of the bile duct wall on IDUS and biliary biopsy results was useful for diagnosing hilar biliary invasion of ampullary cancer. However, hilar invasion of ampullary cancer is rare; there
Core Tip: The standard treatment for ampullary cancer is surgical resection. However, the necessity of and appropriate diagnostic method for assessing hilar invasion is unknown. In this study, the use of contrast-enhanced computed tomography, endoscopic retrograde cholangiography, intraductal ultrasonography (IDUS), and biliary biopsy for diagnosing hilar invasion of ampullary cancer was compared. Although false positives were observed for each method, the combination of partial thickening of the bile duct wall on IDUS and biliary biopsy results was efficient for accurately diagnosing hilar invasion of ampullary cancer. On the other hand, hilar invasion of ampullary cancer is rare; thus, hilar biliary investigation might be unnecessary.
- Citation: Takagi T, Sugimoto M, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Nakamura J, Takasumi M, Hashimoto M, Kato T, Kobashi R, Yanagita T, Hashimoto Y, Marubashi S, Hikichi T, Ohira H. Screening for hilar biliary invasion in ampullary cancer patients. World J Gastrointest Endosc 2022; 14(9): 536-546
- URL: https://www.wjgnet.com/1948-5190/full/v14/i9/536.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i9.536
The standard treatment for ampullary cancer is pancreatoduodenectomy. In addition, local surgical resection of the ampulla or endoscopic ampullectomy has been recently performed for ampullary cancer that does not invade the sphincter of Oddi[1-6]. To perform these treatments, an accurate assessment of the extent of biliary invasion is important. Although ampullary lesions show ductal invasion[7-9], hilar biliary invasion by ampullary lesions has not been reported. When a tumor advances to the hilar biliary duct, the extent of resection is modified accordingly.
The efficacy of contrast-enhanced computed tomography (CECT), endoscopic retrograde cholangiography (ERC), and intraductal ultrasonography (IDUS) for diagnosing the horizontal progression of bile duct cancer has been reported[10-15]. The diagnostic accuracy of CECT for lateral extension of hilar biliary cancer has been reported to be 71%-96%[13,14,16-23]. In addition, ERC following IDUS has been reported to be useful for diagnosing lateral extension of biliary ductal cancer[24-27]. The diagnostic accuracy of mapping biopsy for lateral extension of biliary ductal cancer has been reported to be 73.0%-89.0%[28-31]. However, whether these methods are effective for investigating hilar invasion in ampullary cancer patients is unknown. In this study, we aimed to reveal the best method for diagnosing hilar invasion in ampullary cancer patients.
This retrospective study aimed to identify an appropriate screening method for hilar biliary invasion of ampullary cancer. This study was approved by the Institutional Review Board of Fukushima Medical University (approval number: 2453).
This study enrolled 43 ampullary cancer patients who were treated at Fukushima Medical University between September 2009 and December 2020. Among them, 34 patients underwent resection by endoscopic treatment (n = 9) or surgery (n = 25) (Table 1). Endoscopic ampullectomy was performed when invasion into the muscular layer or bile and pancreatic ducts was not observed by ERC or IDUS. It was not necessary to obtain informed consent from the patients because this study was retrospective in design and used previously anonymized clinical data. All the patients agreed to the clinical examination and treatment by providing written consent; in the case of participants under 18 years of age, consent was obtained from a parent and/or legal guardian. The details of the study can be found on the homepage of Fukushima Medical University. All methods were carried out in accordance with relevant guidelines and regulations.
| Parameter | |
| Total patients, n | 43 |
| Unresectable or treated in other hospitals, n | 9 |
| Underwent resection, n | 34 |
| Age, yr (mean ± standard deviation) | 68.0 ± 11.1 |
| Sex, n (male/female) | 20/14 |
| UICC stage 8th edition, n | |
| I | 16 |
| II | 8 |
| III | 10 |
| Patients already having biliary stents, n | 4 |
| Treatment, n | |
| Endoscopic ampullectomy | 9 |
| Surgery | 25 |
| Hilar biliary invasion, n | 0 |
| Local recurrence, n | 0 |
The final diagnosis of hilar biliary invasion was determined according to histological diagnosis and the nonexistence of local recurrence during follow-up for more than six months. When the horizontal margin of the resected specimen was negative, hilar invasion was considered negative.
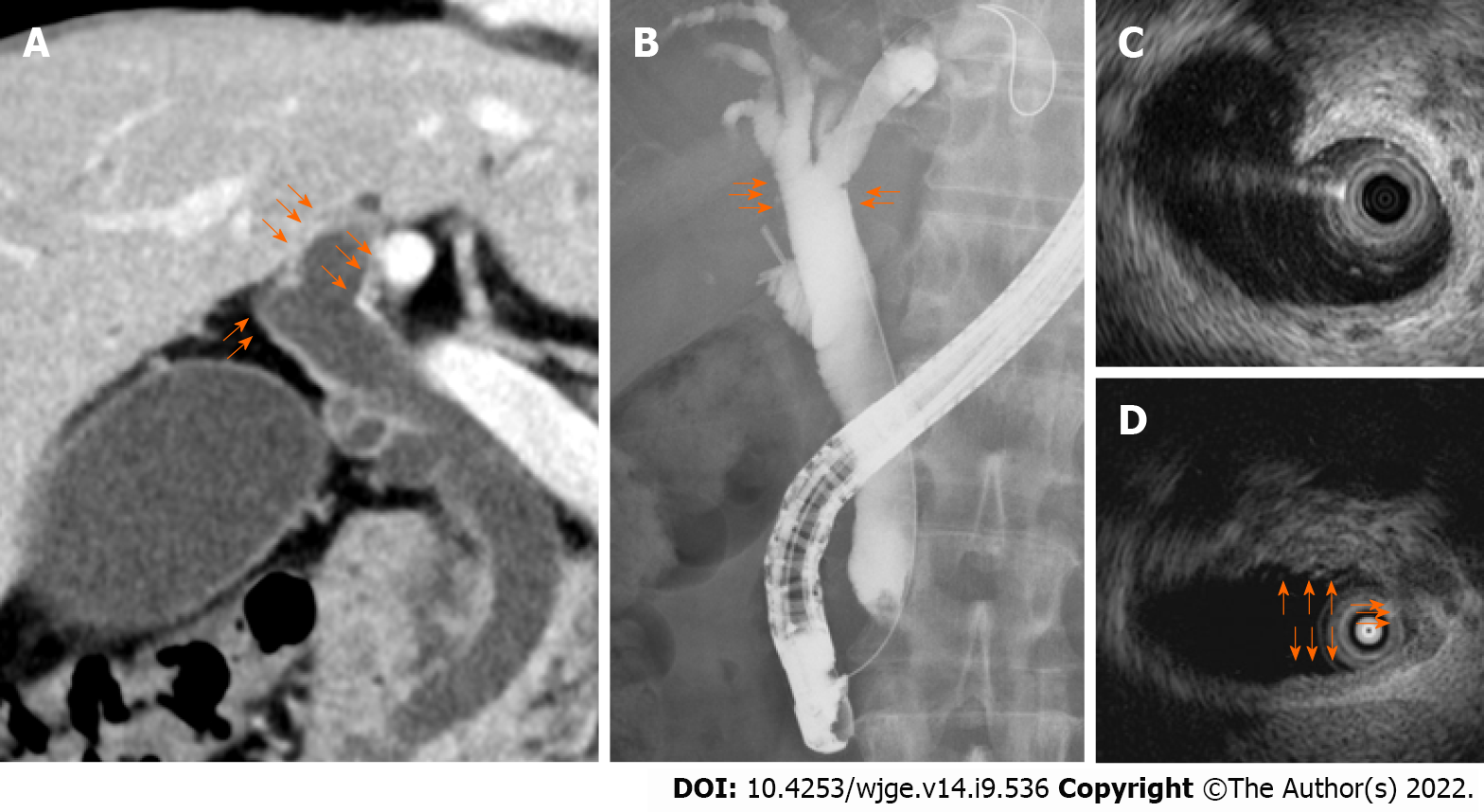
Useful methods for diagnosing hilar invasion were investigated in 34 ampullary cancer patients who underwent endoscopic therapy or surgery. The assessed imaging findings of hilar biliary invasion were thickening and enhancement of the bile duct wall on CECT (Figure 1A), irregularity on ERC (Figure 1B), thickening of the entire bile duct wall on IDUS (Figure 1C), and partial thickening of the bile duct wall on IDUS (Figure 1D). The usefulness of hilar biliary biopsy was also considered. Thickening of the bile duct wall on IDUS was defined as a diameter of the bile duct wall greater than 2 mm.
All imaging findings were evaluated by more than two pancreaticobiliary disease specialists. Endoscopic retrograde cholangiopancreatography (ERCP) was performed as follows. With the patient in a prone position, a duodenoscope was inserted after sufficient sedation was achieved with midazolam. When the duodenoscope reached the Vater papilla, biliary cannulation was initiated. Tumor pro
JF260 V, JF240, and TJF240 duodenoscopes (Olympus, Tokyo, Japan) were used. An MTW ERCP tapered catheter (MTW Endoskopie, Wesel, Germany) and Tandem XL cannula (Boston Scientific Japan, Tokyo, Japan) were used as the ERC catheters. Endo Jaw FB231K (Olympus) or Radial JawTM 4 Biopsy Forceps (Boston Scientific Japan) were used for biliary biopsy.
Post-ERC pancreatitis (PEP) and adverse events were diagnosed according to Cotton’s criteria[32]. PEP was defined as an elevated serum amylase level more than three times the normal upper limit with abdominal pain for more than 24 h after ERC. In addition, all PEP patients were confirmed to have peripancreatic inflammation by CECT. The severity of PEP was categorized as follows: mild: extended hospitalization for 2-3 d; moderate: extended hospitalization for 4-10 d; and severe: Extended hospitalization for more than 10 d, hemorrhagic pancreatitis, and pseudocysts that required intervention. The severity of bleeding was categorized as follows: Mild: Clinical evidence of bleeding, hemoglobin decrease < 3 g/dL, and no need for transfusion; moderate: Transfusion (4 units or less) and no angiographic intervention or surgery; and severe: Transfusion (5 units or more) or intervention (angiographic or surgical).
The imaging findings and biliary biopsy results were compared with respect to their ability to diagnose hilar invasion of ampullary cancer by Fisher’s exact test. The Bonferroni method and Holm method were used to adjust for multiple comparisons. EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan) was used for statistical analysis. A P value < 0.05 was considered indicative of a sig
The patient characteristics and treatment results are shown in Table 1. The mean age of the patients was 68.0 ± 11.1 years. There were 20 male patients and 14 female patients. The numbers of the different lesion stages were as follows: I: 16; II: 8; and III: 10. Disease stage was classified according to the Union for International Cancer Control classification 8th edition[33]. Four patients had already undergone biliary stent insertion in other hospitals. No histological hilar biliary invasion or local recurrence was observed in any patient.
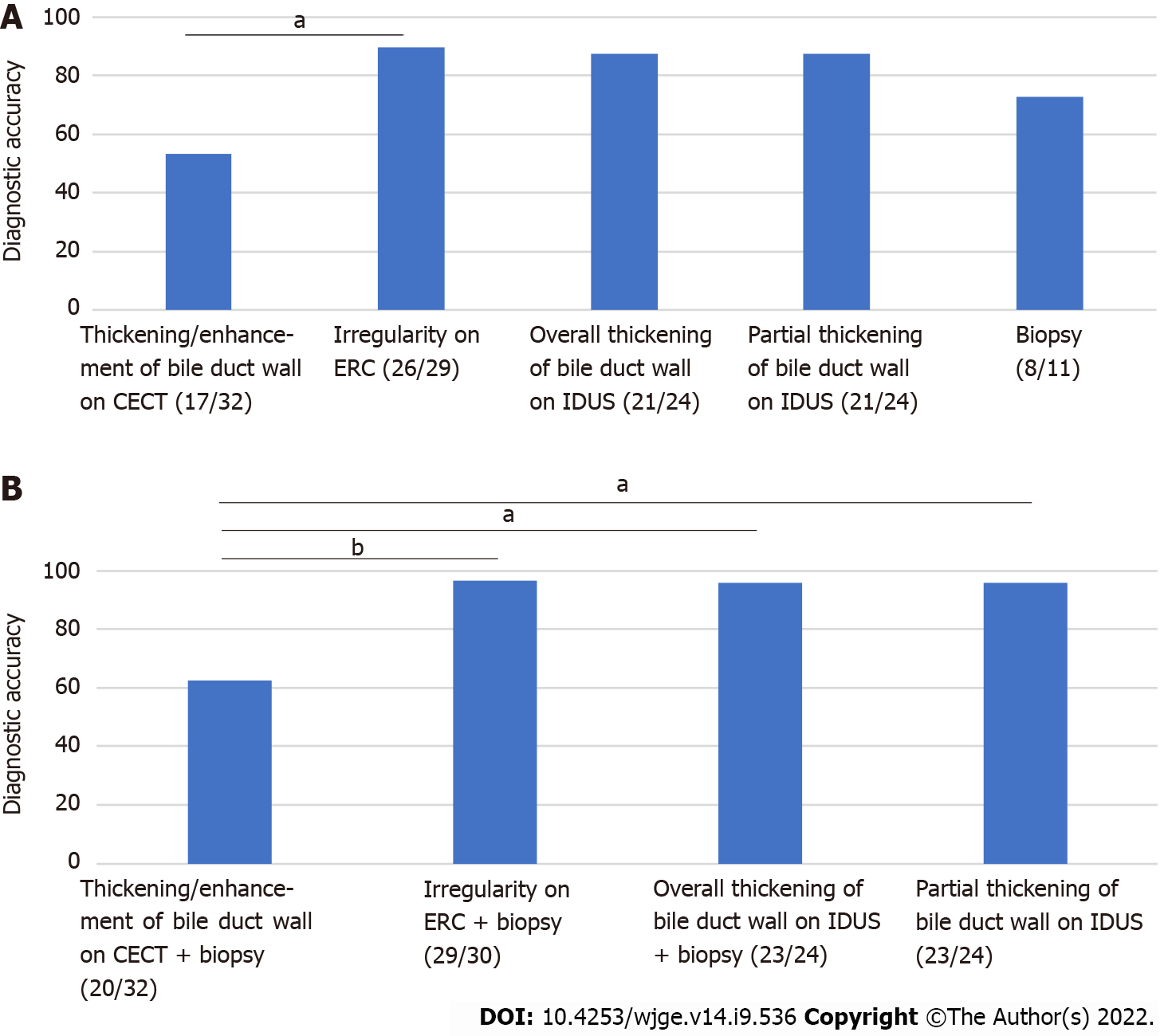
Among the methods explored for diagnosing hilar biliary invasion of ampullary cancer, hilar biliary irregularity on ERC showed the highest diagnostic accuracy (thickening and enhancement of the bile duct wall on CECT: 53.1% (17/32); irregularity on ERC: 89.7% (26/29); thickening of the entire bile duct wall on IDUS: 87.5% (21/24); partial thickening of the bile duct wall on IDUS 87.5% (21/24), biliary biopsy results 72.7% (8/11), P value < 0.01) (Figure 2A). The diagnostic accuracy of irregularity on ERC for hilar invasion of ampullary cancer was significantly higher than that of thickening and enhancement of the bile duct wall on CECT (P value = 0.02).
Comparisons of the various combinations [imaging findings and biliary biopsy results) for diagnosing hilar biliary invasion revealed that the diagnostic accuracies of irregularity on ERC + biliary biopsy results (96.7% (29/30)], thickening of the entire bile duct wall on IDUS + biliary biopsy results [95.8% (23/24)], and partial thickening of the bile duct wall on IDUS + biliary biopsy results [95.8% (23/24)] were significantly higher than that of thickening and enhancement of the bile duct wall on CECT + biliary biopsy results [62.5% (20/32), P value < 0.01, = 0.02, and = 0.02, respectively] (Figure 2B).
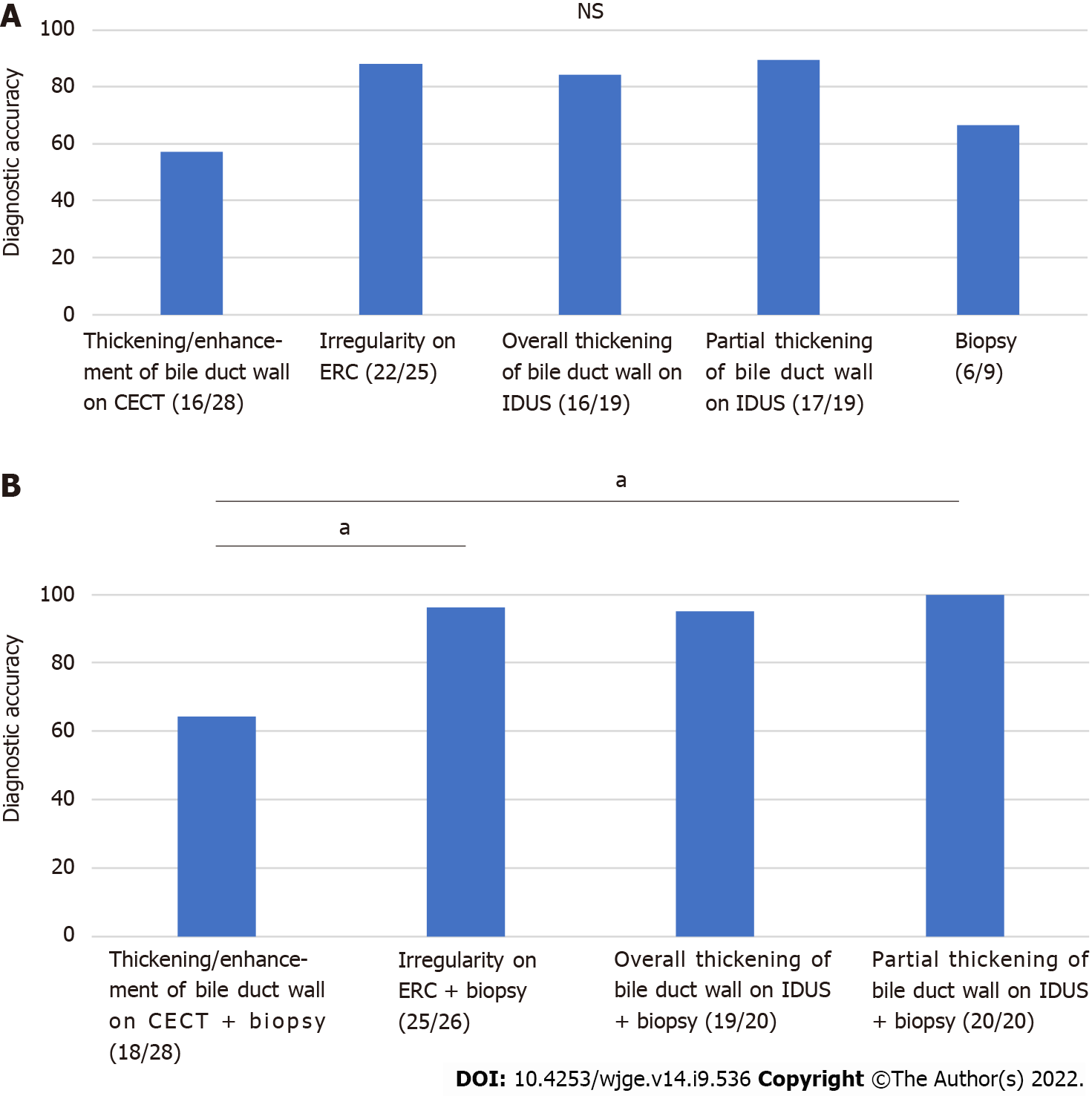
Partial thickening of the bile duct wall on IDUS showed the highest diagnostic accuracy among the explored methods (thickening and enhancement of the bile duct wall on CECT: 57.1% (16/28); irregularity on ERC: 88.0% (22/25); thickening of the entire bile duct wall on IDUS: 84.2% (16/19); partial thickening of the bile duct wall on IDUS 89.5% (17/19); biliary biopsy: 66.7% (6/9); P value < 0.035 but no significant differences in pairwise comparisons) (Figure 3A).
Among the investigated combinations (imaging findings and biliary biopsy results) for diagnosing hilar biliary invasion of ampullary cancer, the combination of partial thickening of the bile duct on IDUS and biliary biopsy results showed the highest diagnostic accuracy (thickening and enhancement of the bile duct wall on CECT + hilar biliary biopsy results: 64.3% (18/28); irregularity on ERC + biliary biopsy results: 96.2% (25/26); thickening of the entire bile duct wall on IDUS + biliary biopsy results: 95.0% (19/20); partial thickening of the bile duct wall on IDUS + biliary biopsy results: 100% (20/20); P value < 0.01) (Figure 3B). The combination of irregularity on ERC and biliary biopsy results and the combination of partial thickening of the bile duct wall on IDUS and biliary biopsy results each had a significantly higher diagnostic accuracy for hilar biliary invasion of ampullary cancer than the combination of thickening and enhancement of the bile duct wall on CECT and biliary biopsy results (P value = 0.027, 0.017).
The adverse events are listed in Table 2. Postendoscopic ampullectomy bleeding occurred in two patients. Both patients improved with endoscopic hemostasis and transfusion. PEP occurred in three patients, all of whom improved with conservative treatment.
| Adverse event | n |
| Post-endoscopic ampullectomy bleeding | |
| Mild | 0 |
| Moderate | 2 |
| Severe | 0 |
| Post-ERC pancreatitis | |
| Mild | 0 |
| Moderate | 3 |
| Severe | 0 |
In this study, we investigated appropriate methods for diagnosing hilar biliary invasion of ampullary cancer. Hilar biliary invasion was not observed in all ampullary cancer patients. Although some false-positive results were obtained with each method, the diagnostic accuracy of the combination of partial thickening of the bile duct wall on IDUS and hilar biliary biopsy results for hilar biliary invasion was 100% for patients without biliary stents. On the other hand, thickening and enhancement of the hilar bile duct wall on CECT was not effective for diagnosing this condition.
Ampullary cancer occasionally develops concurrently with upstream biliary ductal cancer[34,35]. However, as described in the introduction, hilar biliary invasion of resectable ampullary cancer has rarely been reported. In fact, hilar invasion of ampullary cancer was not observed in this study. In past reports that have described the results of treatment or surgery for ampullary cancer, pancreaticobiliary type, lymph node metastasis, advanced T stage, and large tumors were identified as risk factors for poor prognosis[36-41]. Hilar biliary invasion was not listed as a risk factor in these reports. Taking the risk of PEP into consideration, it is possible that investigation of hilar biliary invasion in ampullary cancer is not necessary.
Thickening of the bile duct wall on CECT has been reported in cholestasis caused by several diseases (for example, cholangitis, common bile duct stones, pancreatitis and malignant biliary stricture)[42]. In a past systematic review and meta-analysis, the diagnostic accuracy of computed tomography (CT) for assessing the extent of bile duct invasion was 64%-96%[13]. In this study, the diagnostic accuracy of CECT for assessing hilar biliary invasion of ampullary cancer was lower than that reported in a previous meta-analysis. Regarding the CECT findings of ampullary cancer, papillary bulging and organ invasion have been identified as predictive factors of tumor recurrence or poor survival[43]. However, hilar bile duct wall thickness was not mentioned in the associated study. Thickening and enhancement of the hilar bile duct wall on CECT was not useful. It is thought that ampullary cancer exists at the exit of the bile duct and that the tumors more often close the biliary duct than other biliary diseases. This closure leads to thickening of the hilar bile duct wall; however, in this study, ampullary cancer did not invade the hilar bile duct.
The diagnostic accuracy of IDUS was higher among those patients without biliary stents. Biliary drainage can cause thickening of the bile duct wall, and IDUS should be performed before biliary drainage. Thickening on the cancerous portion of the bile duct wall has been reported to be heterogeneous and partially protruded[24-27,44]. In this study, partial thickening of the bile duct wall on IDUS showed the best accuracy among the investigated methods for diagnosing hilar invasion of ampullary cancer in patients without a biliary stent. Naitoh et al[45] reported that bile duct wall thickening in the nonstricture region was unremarkable in bile duct cancer patients. However, false-positive cases (diameter of the hilar bile duct wall from 2-3.3 mm) were observed in this study. Therefore, the evaluation of the nonstricture portion on IDUS in patients with ampullary cancer is not believed to be equivalent to that in patients with common bile duct cancer. Therefore, the detection of partial thickening of the bile duct wall should be combined with other methods.
False-positive hilar biliary biopsy results were found in three cases. Although this number is low, such results might influence the operative method. Therefore, false positives in hilar biliary biopsy should be avoided. Regarding the reason for these false positives, it is highly likely that biopsy forceps contact the ampullary cancer. The efficacy of cholangioscopy in diagnosing biliary lesions has been reported[46-56]. However, passing the ampullary cancer is difficult with cholangioscopy. To avoid contact of the biopsy forceps with the tumor and to improve the diagnostic accuracy of hilar biliary biopsy for ampullary cancer patients, biliary biopsy with a catheter that introduces biopsy forceps could be useful[30,31]. When biliary biopsy with a catheter is unavailable, the combination of biliary biopsy and IDUS should be considered.
This study has some limitations. First, this was a retrospective study performed at a single institution. A multicenter prospective study is needed to verify the results of this study. Second, a few patients underwent all examinations (CECT, ERC, IDUS, and biliary biopsy). In future studies, a higher number of cases would be desirable. Third, as described above, ampullary cancer patients with hilar biliary invasion were not included in this study. To improve the false-negative rate, a study involving cases of hilar biliary invasion is needed.
Although false-positive results were obtained with each method, the combination of partial thickening of the bile duct on IDUS and biliary biopsy results was useful for diagnosing hilar biliary invasion of ampullary cancer. In addition, it is recommended that hilar biliary biopsy be performed through a catheter to avoid contamination from the cancer. However, hilar invasion of ampullary cancer is rare, and the risk of PEP from hilar investigation exists. Therefore, hilar investigation might be unnecessary for ampullary cancer patients.
The standard treatment for ampullary cancer is pancreaticoduodenectomy or focal ampullectomy. Before resection, it is important to accurately diagnose the biliary invasion of ampullary cancer. However, the method that accurately evaluates hilar invasion of ampullary cancer is unknown.
Several methods [contrast-enhanced computed tomography (CECT), endoscopic retrograde cholangiography (ERC), intraductal ultrasonography (IDUS), biliary biopsy] can be used to diagnose the range of ampullary cancer invasion. However, detailed data of these methods for diagnosing the biliary invasion range of ampullary cancer have not been previously reported. Therefore, presurgical examination is not established in ampullary cancer patients.
To reveal the necessity of hilar investigation in ampullary cancer and a useful method for diagnosing whether ampullary cancer invades the hilar biliary duct.
Diagnosability was compared between CECT, ERC, IDUS, and biliary biopsy in ampullary cancer patients who underwent pancreaticoduodenectomy or focal ampullectomy.
The combination of biliary biopsy results and partial thickening of the bile duct wall on IDUS was efficient for diagnosing hilar invasion of ampullary cancer.
Although false positives were observed for each method, hilar invasion was appropriately diagnosed based on the combination of biliary biopsy results and partial thickening of the bile duct wall on IDUS. However, hilar biliary invasion is rare in ampullary cancer. Therefore, hilar investigation might be unnecessary for ampullary cancer patients.
The results of this study contribute to the establishment of a systematic method for diagnosing hilar invasion and selecting treatments for ampullary cancer patients.
We thank all the staff at the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of Fukushima Medical University Hospital. We also thank American Journal Experts for providing English language editing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Costache RS, Romania; Dhaliwal A, United States; Li HL, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Goldberg M, Zamir O, Hadary A, Nissan S. Wide local excision as an alternative treatment for periampullary carcinoma. Am J Gastroenterol. 1987;82:1169-1171. [PubMed] [Cited in This Article: ] |
| 3. | Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006;63:292-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Knox RA, Kingston RD. Carcinoma of the ampulla of Vater. Br J Surg. 1986;73:72-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Sharp KW, Brandes JL. Local resection of tumors of the ampulla of Vater. Am Surg. 1990;56:214-217. [PubMed] [Cited in This Article: ] |
| 6. | Tarazi RY, Hermann RE, Vogt DP, Hoerr SO, Esselstyn CB Jr, Cooperman AM, Steiger E, Grundfest S. Results of surgical treatment of periampullary tumors: a thirty-five-year experience. Surgery. 1986;100:716-723. [PubMed] [Cited in This Article: ] |
| 7. | Irani S, Arai A, Ayub K, Biehl T, Brandabur JJ, Dorer R, Gluck M, Jiranek G, Patterson D, Schembre D, Traverso LW, Kozarek RA. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Klein A, Tutticci N, Bourke MJ. Endoscopic resection of advanced and laterally spreading duodenal papillary tumors. Dig Endosc. 2016;28:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | van der Wiel SE, Poley JW, Koch AD, Bruno MJ. Endoscopic resection of advanced ampullary adenomas: a single-center 14-year retrospective cohort study. Surg Endosc. 2019;33:1180-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee. Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fisher LR, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan K, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Shergill AK, Dominitz JA, Cash BD. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, Yamato H, Kudo T, Tanaka E, Hirano S, Kondo S, Asaka M. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Kawashima H, Itoh A, Ohno E, Itoh Y, Ebata T, Nagino M, Goto H, Hirooka Y. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg. 2013;257:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Senda Y, Nishio H, Oda K, Yokoyama Y, Ebata T, Igami T, Sugiura T, Shimoyama Y, Nimura Y, Nagino M. Value of multidetector row CT in the assessment of longitudinal extension of cholangiocarcinoma: correlation between MDCT and microscopic findings. World J Surg. 2009;33:1459-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Tamada K, Ushio J, Sugano K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol. 2011;2:203-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, Choi BI. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Cho ES, Park MS, Yu JS, Kim MJ, Kim KW. Biliary ductal involvement of hilar cholangiocarcinoma: multidetector computed tomography versus magnetic resonance cholangiography. J Comput Assist Tomogr. 2007;31:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Endo I, Shimada H, Sugita M, Fujii Y, Morioka D, Takeda K, Sugae S, Tanaka K, Togo S, Bourquain H, Peitgen HO. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007;142:666-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Unno M, Okumoto T, Katayose Y, Rikiyama T, Sato A, Motoi F, Oikawa M, Egawa S, Ishibashi T. Preoperative assessment of hilar cholangiocarcinoma by multidetector row computed tomography. J Hepatobiliary Pancreat Surg. 2007;14:434-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Watadani T, Akahane M, Yoshikawa T, Ohtomo K. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med. 2008;26:402-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chen HW, Lai EC, Pan AZ, Chen T, Liao S, Lau WY. Preoperative assessment and staging of hilar cholangiocarcinoma with 16-multidetector computed tomography cholangiography and angiography. Hepatogastroenterology. 2009;56:578-583. [PubMed] [Cited in This Article: ] |
| 22. | Akamatsu N, Sugawara Y, Osada H, Okada T, Itoyama S, Komagome M, Shin N, Cho N, Ishida T, Ozawa F, Hashimoto D. Diagnostic accuracy of multidetector-row computed tomography for hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:731-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Kim HM, Park JY, Kim KS, Park MS, Kim MJ, Park YN, Bang S, Song SY, Chung JB, Park SW. Intraductal ultrasonography combined with percutaneous transhepatic cholangioscopy for the preoperative evaluation of longitudinal tumor extent in hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:286-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Moon SH, Kim MH. The role of endoscopy in the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2012;76:645-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Tabata T, Kamisawa T, Hara S, Kuruma S, Chiba K, Kuwata G, Fujiwara T, Egashira H, Koizumi K, Fujiwara J, Arakawa T, Momma K, Kurata M, Honda G, Tsuruta K, Itoi T. Differentiating immunoglobulin g4-related sclerosing cholangitis from hilar cholangiocarcinoma. Gut Liver. 2013;7:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Kuwatani M, Kawakami H, Zen Y, Kawakubo K, Kudo T, Abe Y, Kubo K, Sakamoto N. Difference from bile duct cancer and relationship between bile duct wall thickness and serum IgG/IgG4 levels in IgG4-related sclerosing cholangitis. Hepatogastroenterology. 2014;61:1852-1856. [PubMed] [Cited in This Article: ] |
| 27. | Naitoh I, Zen Y, Nakazawa T, Ando T, Hayashi K, Okumura F, Miyabe K, Yoshida M, Nojiri S, Kanematsu T, Ohara H, Joh T. Small bile duct involvement in IgG4-related sclerosing cholangitis: liver biopsy and cholangiography correlation. J Gastroenterol. 2011;46:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ito K, Sakamoto Y, Isayama H, Nakai Y, Watadani T, Tanaka M, Ushiku T, Akamatsu N, Kaneko J, Arita J, Hasegawa K, Kokudo N. The Impact of MDCT and Endoscopic Transpapillary Mapping Biopsy to Predict Longitudinal Spread of Extrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2018;22:1528-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Yao S, Taura K, Okuda Y, Kodama Y, Uza N, Gouda N, Minamiguchi S, Okajima H, Kaido T, Uemoto S. Effect of mapping biopsy on surgical management of cholangiocarcinoma. J Surg Oncol. 2018;118:997-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Okada H, Uza N, Matsumori T, Matsumoto S, Muramoto Y, Ota S, Nakamura T, Yoshida H, Hirano T, Kuwada T, Marui S, Sogabe Y, Morita T, Kakiuchi N, Mima A, Ueda T, Nishikawa Y, Tsuda M, Maruno T, Shiokawa M, Takahashi K, Taura K, Minamiguchi S, Kodama Y, Seno H. A novel technique for mapping biopsy of bile duct cancer. Endoscopy. 2021;53:647-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Hijioka S, Hara K, Mizuno N, Imaoka H, Mekky MA, Nagashio Y, Sekine M, Tajika M, Tanaka T, Ishihara M, Hosoda W, Yatabe Y, Shimizu Y, Niwa Y, Yamao K. A novel technique for endoscopic transpapillary "mapping biopsy specimens" of superficial intraductal spread of bile duct carcinoma (with videos). Gastrointest Endosc. 2014;79:1020-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1890] [Cited by in F6Publishing: 1934] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 33. | Brierley JD, Gospodarowicz MK, Wittekind C. TNM-Classification of Malignant Tumours. 8th ed. New Jersey: Wiley-Blackwell, 2017. [Cited in This Article: ] |
| 34. | Nishihara K, Tsuneyoshi M, Shimura H, Yasunami Y. Three synchronous carcinomas of the papilla of Vater, common bile duct and pancreas. Pathol Int. 1994;44:325-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Hirono S, Tani M, Terasawa H, Kawai M, Ina S, Uchiyama K, Nakamura Y, Kakudo K, Yamaue H. A collision tumor composed of cancers of the bile duct and ampulla of Vater--immunohistochemical analysis of a rare entity of double cancer. Hepatogastroenterology. 2008;55:861-864. [PubMed] [Cited in This Article: ] |
| 36. | Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Park HM, Park SJ, Han SS, Hong SK, Hong EK, Kim SW. Very early recurrence following pancreaticoduodenectomy in patients with ampullary cancer. Medicine (Baltimore). 2019;98:e17711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 38. | Zimmermann C, Wolk S, Aust DE, Meier F, Saeger HD, Ehehalt F, Weitz J, Welsch T, Distler M. The pathohistological subtype strongly predicts survival in patients with ampullary carcinoma. Sci Rep. 2019;9:12676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Moekotte AL, van Roessel S, Malleo G, Rajak R, Ecker BL, Fontana M, Han HS, Rabie M, Roberts KJ, Khalil K, White SA, Robinson S, Halimi A, Zarantonello L, Fusai GK, Gradinariu G, Alseidi A, Bonds M, Dreyer S, Jamieson NB, Mowbray N, Al-Sarireh B, Mavroeidis VK, Soonawalla Z, Napoli N, Boggi U, Kent TS, Fisher WE, Tang CN, Bolm L, House MG, Dillhoff ME, Behrman SW, Nakamura M, Ball CG, Berger AC, Christein JD, Zureikat AH, Salem RR, Vollmer CM, Salvia R, Besselink MG, Abu Hilal M; International Study Group on Ampullary Cancer (ISGACA) Collaborators, Aljarrah R, Barrows C, Cagigas MN, Lai ECH, Wellner U, Aversa J, Dickson PV, Ohtsuka T, Dixon E, Zheng R, Kowalski S, Freedman-Weiss M. Development and external validation of a prediction model for survival in patients with resected ampullary adenocarcinoma. Eur J Surg Oncol. 2020;46:1717-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Vilhordo DW, Gregório C, Valentini DF Jr, Edelweiss MIA, Uchoa DM, Osvaldt AB. Prognostic Factors of Long-term Survival Following Radical Resection for Ampullary Carcinoma. J Gastrointest Cancer. 2021;52:872-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Nappo G, Galvanin J, Gentile D, Capretti G, Pulvirenti A, Bozzarelli S, Rimassa L, Spaggiari P, Carrara S, Petitti T, Gavazzi F, Zerbi A. Long-term outcomes after pancreatoduodenectomy for ampullary cancer: The influence of the histological subtypes and comparison with the other periampullary neoplasms. Pancreatology. 2021;21:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Schulte SJ, Baron RL, Teefey SA, Rohrmann CA Jr, Freeny PC, Shuman WP, Foster MA. CT of the extrahepatic bile ducts: wall thickness and contrast enhancement in normal and abnormal ducts. AJR Am J Roentgenol. 1990;154:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Yoen H, Kim JH, Hur BY, Ahn SJ, Jeon SK, Choi SY, Lee KB, Han JK. Prediction of tumor recurrence and poor survival of ampullary adenocarcinoma using preoperative clinical and CT findings. Eur Radiol. 2021;31:2433-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Kamisawa T, Okazaki K. Role of endoscopic retrograde cholangiography in autoimmune pancreatitis. Pancreatology. 2016;16:798-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Takahashi S, Joh T. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Siddiqui AA, Mehendiratta V, Jackson W, Loren DE, Kowalski TE, Eloubeidi MA. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol. 2012;10:466-71; quiz e48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Manta R, Frazzoni M, Conigliaro R, Maccio L, Melotti G, Dabizzi E, Bertani H, Manno M, Castellani D, Villanacci V, Bassotti G. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013;27:1569-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Nishikawa T, Tsuyuguchi T, Sakai Y, Sugiyama H, Miyazaki M, Yokosuka O. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointest Endosc. 2013;77:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608-14.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 51. | Tanaka R, Itoi T, Honjo M, Tsuchiya T, Kurihara T, Tsuji S, Tonozuka R, Kamada K, Sofuni A, Mukai S. New digital cholangiopancreatoscopy for diagnosis and therapy of pancreaticobiliary diseases (with videos). J Hepatobiliary Pancreat Sci. 2016;23:220-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? Gastrointest Endosc. 2016;84:681-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Figueroa Marrero A, Chavarría-Herbozo CM, de la Serna Higuera C, Pérez-Miranda M. Long-standing indeterminate biliary stricture with iterative negative tissue sampling revealed as cholangiocarcinoma under SpyGlassTM cholangiocoscopy. Rev Esp Enferm Dig. 2017;109:220-221. [PubMed] [Cited in This Article: ] |
| 54. | Lee YN, Moon JH, Choi HJ, Lee TH, Choi MH, Cha SW, Cho YD, Park SH. Direct peroral cholangioscopy for diagnosis of bile duct lesions using an I-SCAN ultraslim endoscope: a pilot study. Endoscopy. 2017;49:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Pereira P, Peixoto A, Andrade P, Macedo G. Peroral cholangiopancreatoscopy with the SpyGlass® system: what do we know 10 years later. J Gastrointestin Liver Dis. 2017;26:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Onoyama T, Takeda Y, Kawata S, Kurumi H, Koda H, Yamashita T, Hamamoto W, Sakamoto Y, Matsumoto K, Isomoto H. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann Transl Med. 2020;8:1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |