Published online Dec 16, 2019. doi: 10.4253/wjge.v11.i12.573
Peer-review started: May 8, 2019
First decision: August 2, 2019
Revised: August 17, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: December 16, 2019
Propofol is commonly used for sedation during endoscopic procedures. Data suggests its superiority to traditional sedatives used in endoscopy including benzodiazepines and opioids with more rapid onset of action and improved post-procedure recovery times for patients. However, Propofol requires administration by trained healthcare providers, has a narrow therapeutic index, lacks an antidote and increases risks of cardio-pulmonary complications.
To compare, through a systematic review of the literature and meta-analysis, sedation with propofol to traditional sedatives with or without propofol during endoscopic procedures.
A literature search was performed using MEDLINE, Scopus, EMBASE, the Cochrane Library, Scopus, LILACS, BVS, Cochrane Central Register of Controlled Trials, and The Cumulative Index to Nursing and Allied Health Literature databases. The last search in the literature was performed on March, 2019 with no restriction regarding the idiom or the year of publication. Only randomized clinical trials with full texts published were included. We divided sedation therapies to the following groups: (1) Propofol versus benzodiazepines and/or opiate sedatives; (2) Propofol versus Propofol with benzodiazepine and/or opioids; and (3) Propofol with adjunctive benzodiazepine and opioid versus benzodiazepine and opioid. The following outcomes were addressed: Adverse events, patient satisfaction with type of sedation, endoscopists satisfaction with sedation administered, dose of propofol administered and time to recovery post procedure. Meta-analysis was performed using RevMan5 software version 5.39.
A total of 23 clinical trials were included (n = 3854) from the initial search of 6410 articles. For Group I (Propofol vs benzodiazepine and/or opioids): The incidence of bradycardia was not statistically different between both sedation arms (RD: -0.01, 95%CI: −0.03–+0.01, I2: 22%). In 10 studies, the incidence of hypotension was not statistically difference between sedation arms (RD: 0.01, 95%CI: −0.02–+0.04, I2: 0%). Oxygen desaturation was higher in the propofol group but not statistically different between groups (RD: −0.03, 95%CI: −0.06–+0.00, I2: 25%). Patients were more satisfied with their sedation in the benzodiazepine + opioid group compared to those with monotherapy propofol sedation (MD: +0.89, 95%CI: +0.62–+1.17, I2: 39%). The recovery time after the procedure showed high heterogeneity even after outlier withdrawal, there was no statistical difference between both arms (MD: -15.15, 95%CI: −31.85–+1.56, I2: 99%). For Group II (Propofol vs propofol with benzodiazepine and/or opioids): Bradycardia had a tendency to occur in the Propofol group with benzodiazepine and/or opioid-associated (RD: -0.08, 95%CI: −0.13–−0.02, I2: 59%). There was no statistical difference in the incidence of bradycardia (RD: -0.00, 95%CI: −0.08–+0.08, I2: 85%), desaturation (RD: −0.00, 95%CI: −0.03–+0.02, I2: 44%) or recovery time (MD: -2.04, 95%CI: −6.96–+2.88, I2: 97%) between sedation arms. The total dose of propofol was higher in the propofol group with benzodiazepine and/or opiates but with high heterogeneity. (MD: 70.36, 95%CI: +53.11–+87.60, I2: 61%). For Group III (Propofol with benzodiazepine and opioid vs benzodiazepine and opioid): Bradycardia and hypotension was not statistically significant between groups (RD: -0.00, 95%CI: −0.002–+0.02, I2: 3%; RD: 0.04, 95%CI: −0.05–+0.13, I2: 77%). Desaturation was evaluated in two articles and was higher in the propofol + benzodiazepine + opioid group, but with high heterogeneity (RD: 0.15, 95%CI: 0.08–+0.22, I2: 95%).
This meta-analysis suggests that the use of propofol alone or in combination with traditional adjunctive sedatives is safe and does not result in an increase in negative outcomes in patients undergoing endoscopic procedures.
Core tip: Propofol is commonly used for sedation during endoscopic procedures with increasing data suggesting its superiority to other sedatives, however with reported concerns about possible adverse events. This systematic review and meta-analysis discusses different variants of propofol-based sedation and how they compare to alternative sedatives such as those utilizing benzodiazepines and opioids. We demonstrate that the use of propofol, alone or in conjunction with alternative sedatives, is safe and carries no particular negative outcomes when compared to the widely available combination of alternative sedatives using in endoscopy.
- Citation: Delgado AAA, de Moura DTH, Ribeiro IB, Bazarbashi AN, dos Santos MEL, Bernardo WM, de Moura EGH. Propofol vs traditional sedatives for sedation in endoscopy: A systematic review and meta-analysis. World J Gastrointest Endosc 2019; 11(12): 573-588
- URL: https://www.wjgnet.com/1948-5190/full/v11/i12/573.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i12.573
In recent years, the use of propofol for sedation during endoscopy has been shown to be safe and effective. Propofol is associated with rapid onset of action and a short time to recovery of patient's cognitive functions post procedure[1-4]. Propofol may be administered alone or in combination with other sedative agents, such as benzodiazepines or opioids[5-7].
Sedation using propofol alone can be associated with risks and complications since it requires administration of larger doses to achieve an adequate level of sedation, which in turn can lead to dose-dependent adverse events[5,8]. Additionally, the isolated use of this drug has disadvantages related to its pharmacokinetic properties. Propofol can induce deep sedation, has no antidote for reversibility, has a narrow therapeutic index and causes adverse events including cardiopulmonary compromise requiring resuscitation[9,10].
However, previous studies have shown that the use of propofol as an adjunct to traditional sedatives such as benzodiazepines and opioids with moderate patient sedation is associated with lower risk of complications, improved patient cooperation and satisfaction, and shorter time to recovery post procedure[5,11].
Several studies have compared the use of propofol alone versus its use with adjunctive sedatives[5,6,8,12-19]. However, these studies included insufficient numbers of patients to produce significant and conclusive results regarding the differences between propofol and alternative sedation. In this study, we perform a systematic review and meta-analysis comparing sedation with propofol to traditional sedatives (with or without propofol) during endoscopic procedures.
A protocol was established and documented prior to initiating the study to specify eligibility criteria and analytical methods for the studies included in this systematic review and meta-analysis in keeping with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study was documented in a protocol registered in International Prospective Register of Systematic Reviews (PROSPERO) database (CRD 42017057305)[20].
Eligibility criteria were based on population, intervention, comparison, outcomes and study design strategy. Only randomized clinical trials with full texts published were included.
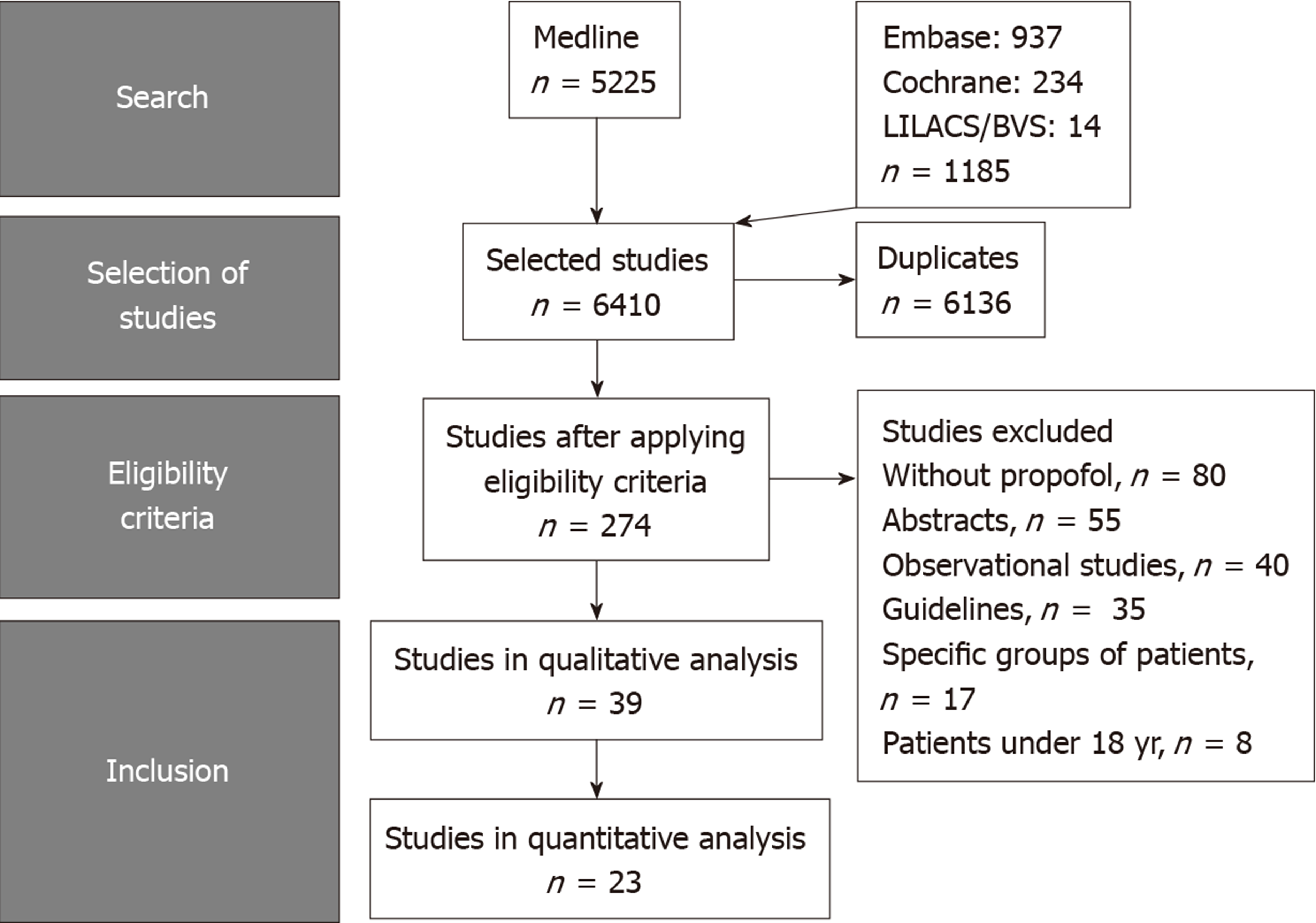
A literature search was performed using MEDLINE, Scopus, EMBASE, the Cochrane Library, Scopus, LILACS, BVS, Cochrane Central Register of Controlled Trials, and The Cumulative Index to Nursing and Allied Health Literature databases. The last search in the literature was performed on March, 2019 with no restriction regarding the idiom or the year of publication. The titles and abstracts of all potentially relevant studies were screened for eligibility. Duplicates were removed. If necessary, we accessed complementary and supplemental information in the research protocols of the studies available on the online registration platforms (for example, Clinical Trials or PROSPERO). The reference lists of studies of interest were then manually reviewed for additional articles by cross checking bibliographies. Two reviewers (Delgado AAA and Ribeiro IB) independently screened the titles and abstracts of all the articles according to predefined inclusion and exclusion criteria as described below. Any differences were resolved by mutual agreement and in consultation with the third reviewer (de Moura DTH). For the systematic review, we included studies that met all the eligibility criteria, and for the meta-analysis, those that allowed the extraction of data from text, tables or graphs. Selection process is summarized in Figure 1.
(1) Adults (over 18 years of age); (2) Undergoing endoscopic examination of the gastrointestinal tract (including esophagogastroduodenoscopy. and colonoscopy); and (3) Outpatient and elective procedures.
Studies meeting any of the following criteria were excluded: (1) Types of participants: (a) Under the age of 18 years; and (b) Group of patients with specific comorbidities (obesity, pregnant and lactating women, cardiovascular diseases, pulmonary diseases, ascites, renal failure and abdominal surgeries); (2) Types of intervention and outcomes: (a) Studies not using propofol in one of the treatment arms; (b) Studies that did not evaluate the outcomes of interest; and (c) Studies without extractable data; and (3) Intervention and control: (a) Patients receiving propofol alone or in combination with traditional combined sedative agents (benzodiazepines and opioids); and (b) Traditional combined sedatives (benzodiazepines and opioids) with or without concurrent propofol use.
The following data were collected for each trial: (1) Patient characteristics; (2) Characteristics of intervention and comparison: Type of medication, doses, form of application; and (3) Outcomes as previously described.
To simplify our analysis and data representation, we chose to subdivide the included studies into different groups according to the sedation regimens administered to the intervention and control groups (Table 1), as follows: (1) Group I - propofol vs benzodiazepine and opioid; (2) Group II - propofol vs propofol, benzodiazepine and/or opioid; and (3) Group III - propofol, benzodiazepine and opioid vs benzodiazepine and opioid.
| Author | Ref. | Country | Year | Patients (n = 3854) | Interven-tion | Interven-tion (n = 1834) | Control | Control (n = 2004) | Procedure | Jadad |
| Seifert | [19] | Germany | 2000 | 239 | PROP | 120 | PROP + MDZ | 119 | Endoscopy/ERCP | 5 |
| Sipe | [39] | USA | 2002 | 80 | PROP | 40 | MDZ + MEP | 40 | Colono-scopy | 4 |
| Vargo | [35] | USA | 2002 | 75 | PROP | 38 | MDZ + MEP | 37 | ERCP/EUS | 4 |
| Ulmer | [40] | USA | 2003 | 100 | PROP | 50 | MDZ + FTN | 50 | Colono-scopy | 4 |
| Riphaus | [41] | Germany | 2005 | 150 | PROP | 75 | MDZ + MEP | 75 | ERCP | 5 |
| VanNatta | [12] | USA | 2006 | 200 | PROP | 50 | FTN + PROP/MDZ + PROP/MDZ + FTN + PROP | 150 | Colono-scopy | 3 |
| Fanti | [42] | Italy | 2007 | 270 | PROP | 135 | PROP + MDZ | 135 | EUS | 5 |
| Dewitt | [44] | USA | 2008 | 80 | PROP | 40 | MDZ + MEP | 40 | EUS | 5 |
| Kongkam | [43] | Thailand | 2008 | 134 | PROP | 67 | MDZ + MEP | 67 | ERCP | 4 |
| Schilling | [45] | Germany | 2009 | 150 | PROP | 75 | MDZ + MEP | 75 | ERCP/EUS/Enteroscopy | 5 |
| Pascual | [26] | Cuba | 2011 | 512 | PROP | 256 | MDZ + MEP | 256 | Colono-scopy | 4 |
| Lee | [25] | Korea | 2011 | 222 | PROP + MDZ + MEP | 102 | MDZ + MEP | 104 | Endoscopy/ERCP | 5 |
| Chun | [31] | Korea | 2012 | 135 | PROP | 67 | PROP + MDZ | 68 | Stomach ESD | 3 |
| Angsuwatcharakon | [30] | Thailand | 2012 | 205 | PROP + MDZ + MEP | 103 | MDZ + MEP | 102 | ERCP | 3 |
| Lee | [29] | Korea | 2012 | 206 | PROP | 104 | PROP + MDZ + FTN | 102 | ERCP/EUS | 5 |
| Zuo | [28] | China | 2012 | 100 | PROP | 49 | MDZ + FTN | 51 | Endomicroscopy | 5 |
| Levitzky | [27] | USA | 2012 | 110 | PROP + MDZ + FTN | 55 | MDZ + FTN | 55 | Endoscopy | 3 |
| Gurbulak | [33] | Turkey | 2014 | 124 | PROP | 62 | MDZ + MEP | 62 | Colono-scopy | 5 |
| Chan | [32] | Taiwan | 2014 | 220 | PROP | 110 | PROP + MDZ + AFTN | 110 | Endoscopy + colonoscopy | 5 |
| Hsu | [36] | Taiwan | 2015 | 100 | PROP | 50 | PROP + MDZ +FTN | 50 | Endoscopy + colonoscopy | 1 |
| Haytural | [34] | Turkey | 2015 | 90 | PROP | 30 | PROP + FTN/PROP + RFTN | 60 | ERCP | 1 |
| Li | [38] | China | 2016 | 90 | PROP | 30 | PROP + FTN | 60 | Colono-scopy | 3 |
| Schroeder | [37] | USA | 2016 | 262 | PROP | 126 | MDZ + FTN | 136 | Colono-scopy | 4 |
As treatment effect size may differ due to detection, performance, selection, and bias, the methodological evaluation of the studies was performed. The adequacy of blinding, randomization, description of withdrawals and dropouts were determined by two authors working independently, using the Jadad scale[21] for the evaluation of randomized clinical trials.
This meta-analysis was performed using intention-to-treat data when possible. For all outcomes, absolute or mean risk difference was calculated. We considered statistically significant differences with 95%CI, P < 0.05, using the Mantel Hantzel tests (categorical variables) or inverse variance (continuous variables).
The effects of the treatment were expressed graphically through forest plots and the heterogeneity of the studies were evaluated by the method proposed by Higgins et al[22], denominated I2, seeking values lower than 50%, using the fixed effect model. Risk of bias amongst studies was assessed using funnel plot analysis.
For treatment effects in which there is strong heterogeneity (I2 - 50%) test with fixed effect, we exclude studies that were outside the limits of the funnel plot and the heterogeneity was reevaluated. If there was a reduction in heterogeneity (I2 < 50%), the excluded study was considered an outlier, that is, responsible for the high heterogeneity due to a publication bias and, consequently, it was not part of the final meta-analysis. If an outlier was not identified and the heterogeneity remained high, the Higgins et al[22] test was randomly selected. Review Manager 5 (RevMan 5) version 5.39 (by the Cochrane Collaboration, 2015) was the software chosen to run the meta-analysis.
Reporting bias across studies was evaluated by funnel plot graphical analysis. For each trial, the treatment effect was plotted against the measure of study precision and by Egger's test[23]. Asymmetrical funnel plot suggests the presence of reporting bias, methodological bias or true heterogeneity between smaller and larger studies.
In the presence of an asymmetrical funnel plot or high heterogeneity, (I2 ≥ 50%) a sensitivity analysis was conducted to explore how the results of the meta-analysis could change under different assumptions[24]. Heterogeneity and funnel plot analysis before and after the removal of each study from the meta-analysis were assessed to identify the studies accounting for inconsistency among trials. If heterogeneity was reduced to below 50% after the removal of the outlier, the corrected intervention effect estimate was applied and the results were interpreted with caution. If inconsistency did not decrease, it was considered true heterogeneity.
Among the 6410 articles screened from our initial search strategy, 23 randomized controlled studies were selected, including a total of 3854 patients[12,19,25-45]. Figure 1 summarizes the selection process of the studies.
Of the 3854 patients included, 1574 received propofol as the sole sedative while 2280 received midazolam, meperidine, fentanyl, remifentanil, alfentanil in combination with propofol (Table 1). There was wide geographical representation of studies included, encompassing a wide diversity of endoscopic procedures. The Jadad score was greater than 3 in 91.3% of the studies included in the meta-analysis, suggesting adequate methodological quality of these studies. All studies are available online, in full text format.
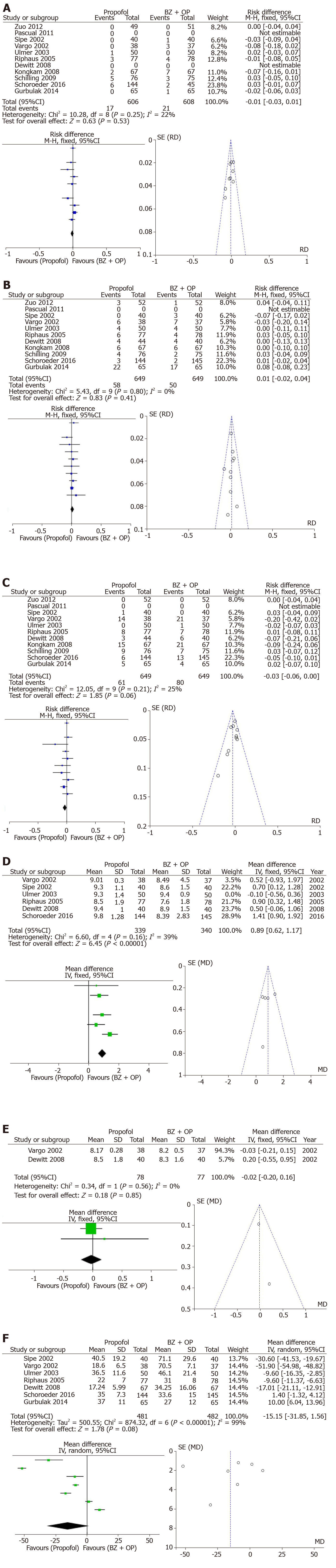
Group I: In 11 studies evaluating the incidence of bradycardia, there were no observed differences between both treatment arms (RD: -0.01, 95%CI: −0.03–+0.01, I2: 22%, Figure 2A). In 10 studies assessing hypotension, no difference existed between both arms (RD: 0.01, 95%CI: −0.02–+0.04, I2: 0%, Figure 2B). The incidence of desaturation was higher in the propofol group however this did not reach statistical significance (RD: −0.03, 95%CI: −0.06–+0.00, I2: 25%, Figure 2C). Patient satisfaction with the visual analog scale was available in 6 of the included studies with a trend towards greater satisfaction in the benzodiazepine and/or opioid group (MD: +0.89, 95%CI: +0.62–+1.17, I2: 39%, Figure 2D). Endoscopist satisfaction with sedation was evaluated in only 2 studies without differences observed between both arms (MD: −0.02, 95%CI: −0.20–+0.16, I2: 0%, Figure 2E). Recovery time after the procedure was evaluated in 7 studies and revealed high heterogeneity. Even after withdrawal of the outlier, there was no statistical difference in recovery time between both arms (MD: -15.15, 95%CI: −31.85–+1.56, I2: 99%, Figure 2F).
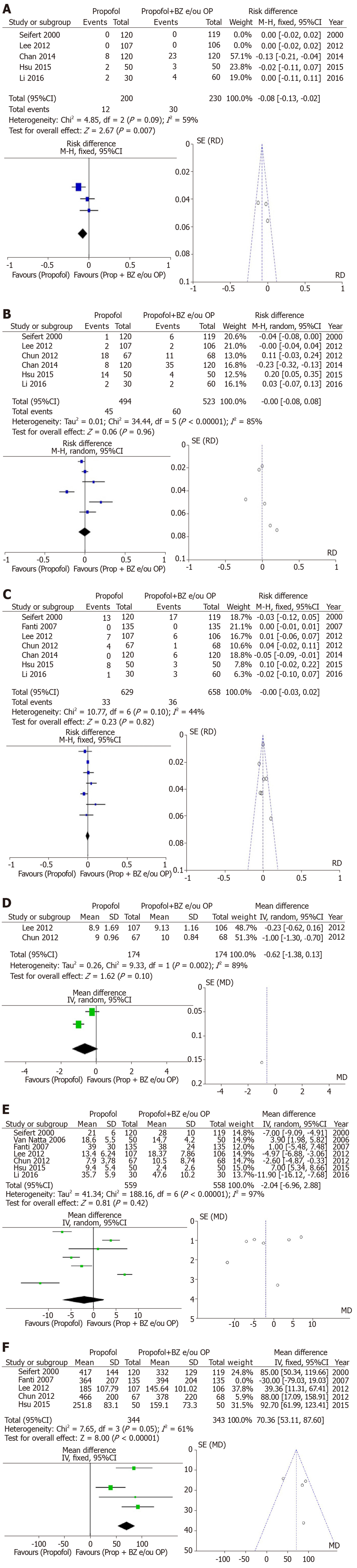
Group II: Bradycardia was assessed in 5 of the included studies and had a greater trend of occurrence in the Propofol arm with benzodiazepine and/or opioid-associated compared to the propofol alone arm (RD: -0.08, 95%CI: −0.13–−0.02, I2: 59%, Figure 3A). In 6 studies assessing hypotension, there was no difference between the both arms (RD: -0.00, 95%CI: −0.08–+0.08, I2: 85%, Figure 3B). Desaturation was evaluated in 7 studies without significant difference (RD: −0.00, 95%CI: −0.03–+0.02, I2: 44%, Figure 3C). Patient satisfaction with the visual analog scale was available in 2 of the included studies and was different between both arms (MD: -0.62, 95%CI: -1.38–+0.13, I2: 89%, Figure 3D). The recovery time after the procedure was evaluated in 7 studies and the result showed high heterogeneity without statistical difference between the groups (MD: -2.04, 95%CI: −6.96–+2.88, I2: 97%, Figure 3E). The total dose of propofol was evaluated in 5 articles and was noted to be higher in the propofol with benzodiazepine and/or opiates arm but with high heterogeneity. The funnel-plot test did not demonstrate outliers (MD: 70.36, 95%CI: +53.11–+87.60, I2: 61%, Figure 3F).
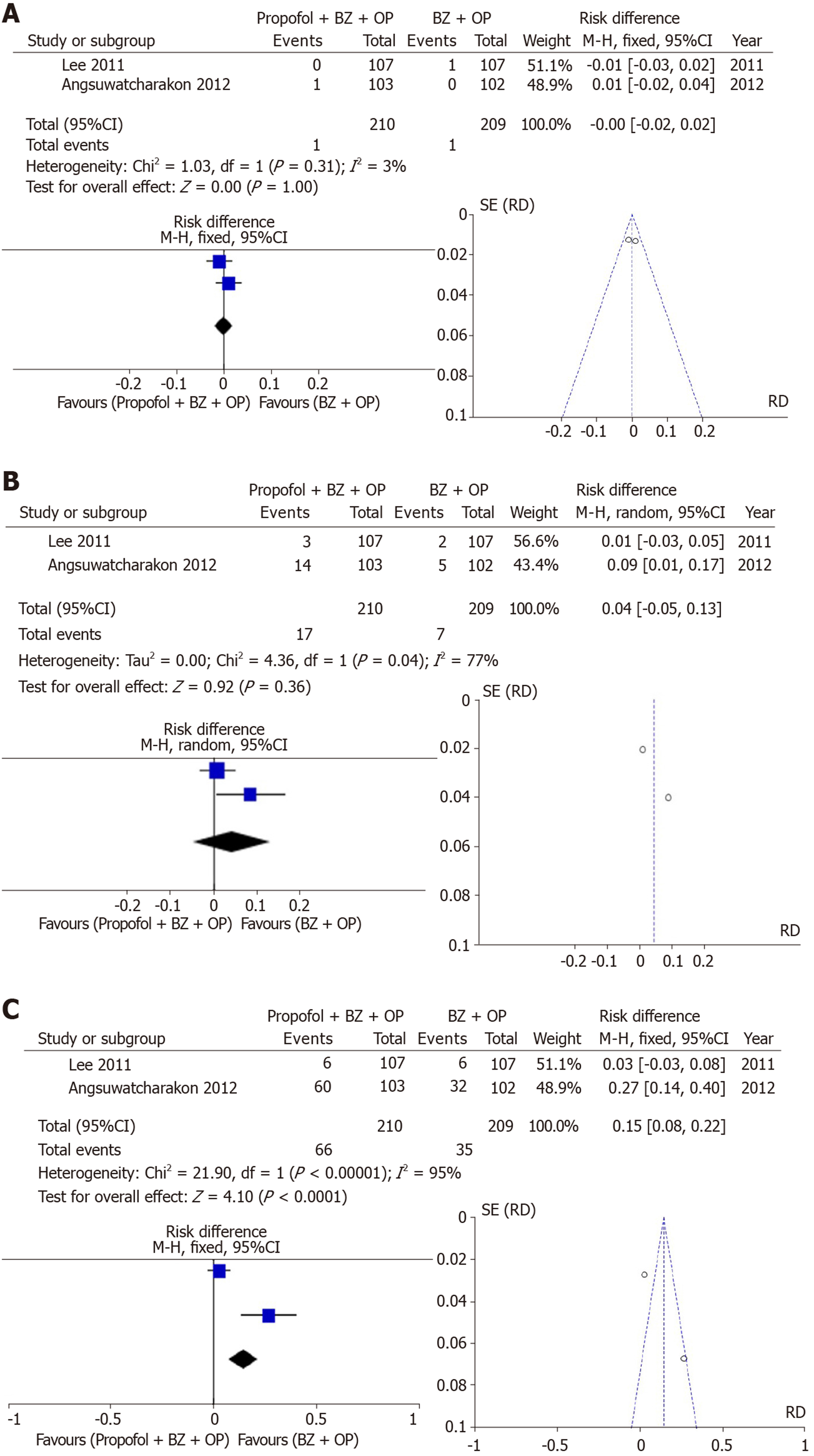
Group III: Two studies examined the incidence of bradycardia which was not different between sedation arms (RD: -0.00, 95%CI: −0.002–+0.02, I2: 3%, Figure 4A). Additionally, two studies examining the incidence of hypotension showed no statistically significant differences between sedation arms (RD: 0.04, 95% CI: −0.05–+0.13, I2: 77%, Figure 4B). Desaturation, however, evaluated in two studies was more commonly seen with propofol + benzodiazepine + opioid arm but with high heterogeneity (RD: 0.15, 95%CI: 0.08–+0.22, I2: 95%, Figure 4C).
The use of propofol in endoscopy is increasingly common[46-48] for both diagnostic and advanced therapeutic procedures[48,49,58,50-57]. Propofol has proven to be safe and affordable, yielding satisfactory and efficient sedation for patients undergoing endoscopy[59]. Propofol can be used as a single agent for anesthesia induction and maintenance, leads to rapid induction of anesthesia, carries a low half-life and is associated with fast recovery from anesthesia[46]. However, Propofol can be associated with dose-dependent complications, including the risk of major respiratory depression and several cardiovascular adverse events[54,60].
Our study adds to the plethora of literature describing the use of propofol for endoscopy sedation, but also highlights how it compares to numerous alternative and adjunctive sedatives.
After analyzing 23 clinical trials[12,19,25-45], many of good quality and adequate methodological design, including a total of 3854 patients, we note no significant differences in many outcomes measures between the sedation arms involving propofol alone or in combination with alternative sedatives.
In contrast to the last meta-analysis published[55], our study demonstrates no statistical difference in outcome measures with regards to hypotension, oxygen desaturation and post-procedure anesthetic recovery when using propofol alone or in combination with benzodiazepines and/or opioids.
There was a trend towards increased incidence of bradycardia in patients in the propofol with benzodiazepine and opioid sedation arm compared to those on propofol alone. Incidence of bradycardia did not differ between other arms in the different treatment groups. No differences existed in recovery time between all sedation arms in all treatment groups as reported in the meta-analysis by Wang et al[61].
Previous meta-analyses[2,3,61] also demonstrated significantly fewer adverse effects with propofol sedation. In our study, we observed that propofol used alone or in conjunction with other sedatives including opioids and benzodiazepines is safe and did not result in increased adverse events in patients undergoing endoscopic procedures as demonstrated by Sethi et al[55] which also included sedation for advanced endoscopic procedures such as endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, and double-balloon enteroscopy.
Due to the properties of propofol, its doses are very volatile[9,60]. Higher doses are associated with elevated cardiovascular risk and low doses are associated with several complications, especially in therapeutic procedures that cause pain. Propofol has a limited analgesic effect and higher doses are often required, when used as single agent for sedation in endoscopy. Several studies have reported that the combination of propofol with other sedative agents is a reasonable option to obtain the adequate depth of sedation, avoiding high dose-related side effects of propofol, allow for improve patient tolerance, prolong recovery time, and control of pain[5,13,15,17]. However, our study did not demonstrate an advantage to propofol alone without significant statistical differences when comparing propofol to alternative agents (with and without propofol).
In our meta-analysis, patient satisfaction with the use of a visual analog scale lead to a trend towards greater satisfaction for patients undergoing sedation with benzodiazepine and opioids when compared to propofol use. The satisfaction of the endoscopists was evaluated in a few studies included in this meta-analysis however did not reveal significant differences in endoscopists satisfaction.
There are several limitations to our analysis. Similar to prior publications and meta-analysis evaluating sedation in endoscopy, we observe high heterogeneity between the several studies aggregated in the meta-analysis, which must be taken into account in the interpretation of its results.
There are several reasons for heterogeneity present in the published literature for studies included. One reason may be due to intrinsic differences in populations examined with each study, specifically age, weight, body mass which may alter the amount of sedation required and tolerability. Additionally, the education level for various populations included may influence patient satisfaction levels. Another reason for heterogeneity is lack of uniformity between the comparisons made, the individual administering sedation (anesthesiologist, non-anesthetist physicians, certified registered nurse anesthetists), acceptable and max doses of sedatives, means of sedative administration (intermittent bolus vs continuous infusion) and criteria for re-administration of sedative if more is required. With regards to the procedures performed, diagnostic and therapeutic interventions can have differences in total procedure times, which in turn can influence the type of sedation, doses required, level of sedation and maintenance of sedation. This can certainly influence heterogeneity.
Finally, while it is important to highlight the clinical aspects of sedation use, it is imperative to understand that cost plays a significant role in the choice of sedation. While not assessed in this study, we believe cost effectiveness should be highlighted in future sedation meta-analysis.
In conclusion, this meta-analysis suggests that the use of propofol alone or in combination with traditional adjunctive sedatives is safe and does not result in an increase in negative outcomes.
Endoscopy has transformed over the past several decades to encompass significant advances and procedural innovation, with the hope to provide better care for ill patients. However, with technical advances and innovation comes increasingly prolonged and complex procedures. This change in the endoscopic platform, alongside the higher acuity of patients, has demanded a change in the approach for procedural sedation to ensure safe interventions.
The change in the sedation landscape for endoscopy over the past several decades necessitates a better understanding of sedation types and how they compare to each other for the modern practicing endocsopist.
We aimed to compare sedation with propofol, alone or in combination with adjunctive sedations, to traditional sedation in endoscopy through a systematic review of the literature and meta-analysis.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered in International Prospective Register of Systematic Reviews international database. The search was performed in the electronic databases MEDLINE (via PubMed), LILACS (via BVS) and Cochrane/Central Register of Controlled Trials. The quality of the selected papers was evaluated by Jadad score and all articles used were selected by consensus of three authors.
A total of 23 clinical trials (n = 3854), from an initial search of 6410 articles, were included. For Group I (Propofol vs benzodiazepine and/or opioids): The incidence of bradycardia, hypotension, oxygen desaturation and post procedure recovery time was not statistically different between both arms. For Group II (Propofol vs propofol with benzodiazepine and/or opioids): Bradycardia tended to occur in the propofol group with benzodiazepine and/or opioid-associated but there was no statistical difference in the incidence of bradycardia, desaturation or recovery time between sedation arms. For Group III (Propofol with benzodiazepine and opioid vs benzodiazepine and opioid): Bradycardia, desaturation and, hypotension was not statistically significant between groups.
Our findings suggest that the use of propofol alone or in combination with traditional adjunctive sedatives is safe and does not result in an increase in negative outcomes in patients undergoing endoscopic procedures.
Future studies should consider methods for standardization of sedation use to allow for less heterogeneity amongst studies and to improve analysis in future metanalyses to come. Future studies should also highlight cost effectiveness of various sedations used.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin OST, Maltz C S-Editor: Wang JL L-Editor: A E-Editor: Liu JH
| 1. | Singh H, Poluha W, Cheung M, Choptain N, Baron KI, Taback SP. Propofol for sedation during colonoscopy. Cochrane Database Syst Rev. 2008;CD006268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 3. | Bo LL, Bai Y, Bian JJ, Wen PS, Li JB, Deng XM. Propofol vs traditional sedative agents for endoscopic retrograde cholangiopancreatography: a meta-analysis. World J Gastroenterol. 2011;17:3538-3543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, Sakai P, de Moura EGH. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558-E567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Hsieh YH, Chou AL, Lai YY, Chen BS, Sia SL, Chen IC, Chang YL, Lin HJ. Propofol alone versus propofol in combination with meperidine for sedation during colonoscopy. J Clin Gastroenterol. 2009;43:753-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Padmanabhan U, Leslie K, Eer AS, Maruff P, Silbert BS. Early cognitive impairment after sedation for colonoscopy: the effect of adding midazolam and/or fentanyl to propofol. Anesth Analg. 2009;109:1448-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Zhang R, Lu Q, Wu Y. The Comparison of Midazolam and Propofol in Gastrointestinal Endoscopy: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech. 2018;28:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Paspatis GA, Manolaraki MM, Vardas E, Theodoropoulou A, Chlouverakis G. Deep sedation for endoscopic retrograde cholangiopancreatography: intravenous propofol alone versus intravenous propofol with oral midazolam premedication. Endoscopy. 2008;40:308-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Coté GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, Edmundowicz SA, Mullady DK, Jonnalagadda SS. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Lee DW, Chan AC, Sze TS, Ko CW, Poon CM, Chan KC, Sin KS, Chung SC. Patient-controlled sedation versus intravenous sedation for colonoscopy in elderly patients: a prospective randomized controlled trial. Gastrointest Endosc. 2002;56:629-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | VanNatta ME, Rex DK. Propofol alone titrated to deep sedation versus propofol in combination with opioids and/or benzodiazepines and titrated to moderate sedation for colonoscopy. Am J Gastroenterol. 2006;101:2209-2217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Disma N, Astuto M, Rizzo G, Rosano G, Naso P, Aprile G, Bonanno G, Russo A. Propofol sedation with fentanyl or midazolam during oesophagogastroduodenoscopy in children. Eur J Anaesthesiol. 2005;22:848-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Heuss LT, Hanhart A, Dell-Kuster S, Zdrnja K, Ortmann M, Beglinger C, Bucher HC, Degen L. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: a randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest Endosc. 2011;74:1207-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Moerman AT, Herregods LL, De Vos MM, Mortier EP, Struys MM. Manual versus target-controlled infusion remifentanil administration in spontaneously breathing patients. Anesth Analg. 2009;108:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Moerman AT, Struys MM, Vereecke HE, Herregods LL, De Vos MM, Mortier EP. Remifentanil used to supplement propofol does not improve quality of sedation during spontaneous respiration. J Clin Anesth. 2004;16:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Ong WC, Santosh D, Lakhtakia S, Reddy DN. A randomized controlled trial on use of propofol alone versus propofol with midazolam, ketamine, and pentazocine "sedato-analgesic cocktail" for sedation during ERCP. Endoscopy. 2007;39:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Paspatis GA, Charoniti I, Manolaraki M, Vardas E, Papanikolaou N, Anastasiadou A, Gritzali A. Synergistic sedation with oral midazolam as a premedication and intravenous propofol versus intravenous propofol alone in upper gastrointestinal endoscopies in children: a prospective, randomized study. J Pediatr Gastroenterol Nutr. 2006;43:195-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Seifert H, Schmitt TH, Gültekin T, Caspary WF, Wehrmann T. Sedation with propofol plus midazolam versus propofol alone for interventional endoscopic procedures: a prospective, randomized study. Aliment Pharmacol Ther. 2000;14:1207-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12295] [Cited by in F6Publishing: 12049] [Article Influence: 803.3] [Reference Citation Analysis (0)] |
| 21. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12275] [Cited by in F6Publishing: 12441] [Article Influence: 444.3] [Reference Citation Analysis (0)] |
| 22. | Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39087] [Cited by in F6Publishing: 41743] [Article Influence: 1987.8] [Reference Citation Analysis (1)] |
| 23. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 393] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1:247-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Lee CK, Lee SH, Chung IK, Lee TH, Park SH, Kim EO, Lee SH, Kim HS, Kim SJ. Balanced propofol sedation for therapeutic GI endoscopic procedures: a prospective, randomized study. Gastrointest Endosc. 2011;73:206-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Pascual MG, Zayas Berbes M, Sáez Baños M, Abreu Vázquez Mdel R, Martínez Leyva L. [Propofol versus midazolam and pethidine in the colonoscopy realization]. Acta Gastroenterol Latinoam. 2011;41:214-220. [PubMed] [Cited in This Article: ] |
| 27. | Levitzky BE, Lopez R, Dumot JA, Vargo JJ. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone: a randomized clinical trial. Endoscopy. 2012;44:13-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Zuo XL, Li Z, Liu XP, Li CQ, Ji R, Wang P, Zhou CJ, Liu H, Li YQ. Propofol vs midazolam plus fentanyl for upper gastrointestinal endomicroscopy: a randomized trial. World J Gastroenterol. 2012;18:1814-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Lee TH, Lee CK, Park SH, Lee SH, Chung IK, Choi HJ, Cha SW, Moon JH, Cho YD, Hwangbo Y, Kim SJ. Balanced propofol sedation versus propofol monosedation in therapeutic pancreaticobiliary endoscopic procedures. Dig Dis Sci. 2012;57:2113-2121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Angsuwatcharakon P, Rerknimitr R, Ridtitid W, Kongkam P, Poonyathawon S, Ponauthai Y, Sumdin S, Kullavanijaya P. Cocktail sedation containing propofol versus conventional sedation for ERCP: a prospective, randomized controlled study. BMC Anesthesiol. 2012;12:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Chun SY, Kim KO, Park DS, Kim SY, Park JW, Baek IH, Kim JH, Park CK. Safety and efficacy of deep sedation with propofol alone or combined with midazolam administrated by nonanesthesiologist for gastric endoscopic submucosal dissection. Gut Liver. 2012;6:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Chan WH, Chang SL, Lin CS, Chen MJ, Fan SZ. Target-controlled infusion of propofol versus intermittent bolus of a sedative cocktail regimen in deep sedation for gastrointestinal endoscopy: comparison of cardiovascular and respiratory parameters. J Dig Dis. 2014;15:18-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Gurbulak B, Uzman S, Kabul Gurbulak E, Gul YG, Toptas M, Baltali S, Anil Savas O. Cardiopulmonary safety of propofol versus midazolam/meperidine sedation for colonoscopy: a prospective, randomized, double-blinded study. Iran Red Crescent Med J. 2014;16:e19329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Haytural C, Aydınlı B, Demir B, Bozkurt E, Parlak E, Dişibeyaz S, Saraç A, Özgök A, Kazancı D. Comparison of Propofol, Propofol-Remifentanil, and Propofol-Fentanyl Administrations with Each Other Used for the Sedation of Patients to Undergo ERCP. Biomed Res Int. 2015;2015:465465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Vargo JJ, Zuccaro G, Dumot JA, Shermock KM, Morrow JB, Conwell DL, Trolli PA, Maurer WG. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology. 2002;123:8-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 36. | Hsu CD, Huang JM, Chuang YP, Wei HY, Su YC, Wu JY, Wang WM, Hsu HT, Huang HF, Lu IC, Lu DV. Propofol target-controlled infusion for sedated gastrointestinal endoscopy: A comparison of propofol alone versus propofol-fentanyl-midazolam. Kaohsiung J Med Sci. 2015;31:580-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Schroeder C, Kaoutzanis C, Tocco-Bradley R, Obear J, Welch KB, Winter S, Cleary RK. Patients Prefer Propofol to Midazolam Plus Fentanyl for Sedation for Colonoscopy: Results of a Single-Center Randomized Equivalence Trial. Dis Colon Rectum. 2016;59:62-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Li S, Yu F, Zhu H, Yang Y, Yang L, Lian J. The median effective concentration (EC50) of propofol with different doses of fentanyl during colonoscopy in elderly patients. BMC Anesthesiol. 2016;16:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc. 2002;55:815-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK. Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003;1:425-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Riphaus A, Stergiou N, Wehrmann T. Sedation with propofol for routine ERCP in high-risk octogenarians: a randomized, controlled study. Am J Gastroenterol. 2005;100:1957-1963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Fanti L, Agostoni M, Arcidiacono PG, Albertin A, Strini G, Carrara S, Guslandi M, Torri G, Testoni PA. Target-controlled infusion during monitored anesthesia care in patients undergoing EUS: propofol alone versus midazolam plus propofol. A prospective double-blind randomised controlled trial. Dig Liver Dis. 2007;39:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Kongkam P, Rerknimitr R, Punyathavorn S, Sitthi-Amorn C, Ponauthai Y, Prempracha N, Kullavanijaya P. Propofol infusion versus intermittent meperidine and midazolam injection for conscious sedation in ERCP. J Gastrointestin Liver Dis. 2008;17:291-297. [PubMed] [Cited in This Article: ] |
| 44. | Dewitt J, McGreevy K, Sherman S, Imperiale TF. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: a prospective, randomized trial. Gastrointest Endosc. 2008;68:499-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Schilling D, Rosenbaum A, Schweizer S, Richter H, Rumstadt B. Sedation with propofol for interventional endoscopy by trained nurses in high-risk octogenarians: a prospective, randomized, controlled study. Endoscopy. 2009;41:295-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Daza JF, Tan CM, Fielding RJ, Brown A, Farrokhyar F, Yang I. Propofol administration by endoscopists versus anesthesiologists in gastrointestinal endoscopy: a systematic review and meta-analysis of patient safety outcomes. Can J Surg. 2018;61:226-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Narula N, Masood S, Shojaee S, McGuinness B, Sabeti S, Buchan A. Safety of Propofol versus Nonpropofol-Based Sedation in Children Undergoing Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:6501215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J Gastrointest Endosc. 2019;11:193-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 49. | Lin OS. Sedation for routine gastrointestinal endoscopic procedures: a review on efficacy, safety, efficiency, cost and satisfaction. Intest Res. 2017;15:456-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | de Moura EGH, Ribeiro IB, Frazão MSV, Mestieri LHM, de Moura DTH, Dal Bó CMR, Brunaldi VO, de Moura ETH, Nunes GC, Bustamante FAC, Dos Passos Galvão Neto M, Matuguma SE, Bernardo WM, Santo MA. EUS-Guided Intragastric Injection of Botulinum Toxin A in the Preoperative Treatment of Super-Obese Patients: a Randomized Clinical Trial. Obes Surg. 2019;29:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Josino IR, Madruga-Neto AC, Ribeiro IB, Guedes HG, Brunaldi VO, de Moura DTH, Bernardo WM, de Moura EGH. Endoscopic Dilation with Bougies versus Balloon Dilation in Esophageal Benign Strictures: Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:5874870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | De Moura DTH, Coronel M, Ribeiro IB, Farias GFA, Choez MA, Rocha R, Toscano MP, De Moura EGH. The importance of endoscopic ultrasound fine-needle aspiration in the diagnosis of solid pseudopapillary tumor of the pancreas: two case reports. J Med Case Rep. 2018;12:107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Ribeiro IB, Rezende DT, Madruga Neto AC, Ide E, Furuya CK, De Moura DTH, De Moura EGH. Endoscopic dual therapy for giant peptic ulcer hemorrhage. Endoscopy. 2018;50:E316-E317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Nishizawa T, Suzuki H. Propofol for gastrointestinal endoscopy. United European Gastroenterol J. 2018;6:801-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Sethi S, Wadhwa V, Thaker A, Chuttani R, Pleskow DK, Barnett SR, Leffler DA, Berzin TM, Sethi N, Sawhney MS. Propofol versus traditional sedative agents for advanced endoscopic procedures: a meta-analysis. Dig Endosc. 2014;26:515-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Passos ML, Ribeiro IB, de Moura DTH, Korkischko N, Silva GLR, Franzini TP, Bernando WM, de Moura EGH. Efficacy and safety of carbon dioxide insufflation versus air insufflation during endoscopic retrograde cholangiopancreatography in randomized controlled trials: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E487-E497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Lôbo MRA, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019;S0004-28032019005002102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Serrano JPR, de Moura DTH, Bernardo WM, Ribeiro IB, Franzini TP, de Moura ETH, Brunaldi VO, Salesse MT, Sakai P, De Moura EGH. Nonsteroidal anti-inflammatory drugs versus placebo for post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E477-E486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Kayaaltı S, Kayaaltı Ö. Safety of applying midazolam-ketamine-propofol sedation combination under the supervision of endoscopy nurse with patient-controlled analgesia pump in colonoscopy. World J Clin Cases. 2018;6:1146-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Wu Z, Li J, Wang C, Yang J, Chen X, Yang W, Xiong Z, Peng X. Characterization of cardiovascular depression effect for propofol during anesthesia induction period on morbidly obese patients. Biomed Pharmacother. 2018;106:618-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Wang D, Chen C, Chen J, Xu Y, Wang L, Zhu Z, Deng D, Chen J, Long A, Tang D, Liu J. The use of propofol as a sedative agent in gastrointestinal endoscopy: a meta-analysis. PLoS One. 2013;8:e53311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |