Published online Oct 15, 2009. doi: 10.4253/wjge.v1.i1.45
Revised: March 20, 2009
Accepted: March 30, 2009
Published online: October 15, 2009
AIM: To evaluate the sensitivity (Sn), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) of 3 different techniques: high resolution white light endoscopy (WLE), Narrow Band Imaging (NBI) and Chromoendoscopy (CHR), all with magnification in differentiating adenocarcinomas, adenomatous and hyperplastic colorectal polyps.
METHODS: Each polyp was sequentially assessed first by WLE, followed by NBI and finally by CHR. Digital images of each polyp with each modality were taken and stored. Biopsies or polypectomies were then performed followed by blinded histopathological analysis. Each image was blindly graded based on the Kudo’s pit pattern (KPP). In the assessment with NBI, the mesh brown capillary network pattern (MBCN) of each polyp was also described. The Sn, Sp, PPV and NPV of differentiating hyperplastic (Type I & II-KPP, Type I-MBCN) adenomatous (Types III, IV-KPP, Type II-MBCN) and carcinomatous polyps (Type V-KPP, Type III-MCBN) was then compared with reference to the final histopathological diagnosis.
RESULTS: A total of 50 colorectal polyps (5 adenocarcinomas, 38 adenomas, 7 hyperplastic) were assessed. CHR and NBI [KPP, MBCN or the combined classification (KPP & MBCN)] were superior to WLE in the prediction of polyp histology (P < 0.001, P = 0.002, P = 0.001 and P < 0.001, respectively). NBI, using the MBCN pattern or the combined classification showed higher numerical accuracies compared to CHR, but this was not statistically significant (P = 0.625, 0.250).
CONCLUSION: This feasibility study demonstrated that this combined classification with NBI could potentially be useful in routine clinical practice, allowing the endoscopist to predict histology with higher accuracies using a less cumbersome and technically less challenging method.
- Citation: Singh R, Owen V, Shonde A, Kaye P, Hawkey C, Ragunath K. White light endoscopy, narrow band imaging and chromoendoscopy with magnification in diagnosing colorectal neoplasia. World J Gastrointest Endosc 2009; 1(1): 45-50
- URL: https://www.wjgnet.com/1948-5190/full/v1/i1/45.htm
- DOI: https://dx.doi.org/10.4253/wjge.v1.i1.45
Improvements in the resolution of imaging in video endoscopy over the years have resulted in a tremendous increase in the polyp detection rate in the colon. Although encouraging, this phenomenon has unfortunately provided additional burden to the pathologist, as most polyps, which are either biopsied or removed, are non-neoplastic in nature[1]. The distinction therefore between non-neoplastic and neoplastic colorectal polyps in vivo and a suitable technique, which allows this differentiation, could increase the efficiency of treatment by eliminating the cost associated with unnecessary biopsies and the risk with polypectomies. Chromoendoscopy (CHR) has long been propagated as a technique that improves prediction of polyp histology. However, due to failure of standardisation and the very nature of CHR being labour intensive, the technique has not had widespread acceptance in routine colonoscopy practice especially in the West. Narrow Band Imaging (NBI) is a novel endoscopic imaging technique that has recently come to the forefront[2]. It relies on altering the normal red, green and blue optical filters in the light source of the video endoscopy system that are used to make up sequential coloured frames of an endoscopic image. The relative contribution of the longer wavelength and deeper penetrating red light is negated and the superficial penetrating narrowed spectral blue and green wavelengths are used instead. This results in enhancement of the surface mucosal morphology whereby the microvascular and microstructural pit patterns are visualised in greater detail. Incorporated into the endoscope, NBI is relatively simple to use, involving the activation of a switch thus enabling the endoscopist to obtain images, which simulate chromoendoscopy almost instantaneously during the procedure. We embarked on this study to evaluate 3 different techniques which could potentially be used in colorectal cancer (CRC) screening: conventional high resolution white light endoscopy (WLE), NBI and CHR, all with magnification in predicting hyperplastic, adenomatous and carcinomatous colorectal polyps.
The study was approved by the Nottingham Research and Ethics Committee, UK. Patients undergoing colonoscopy for bowel symptoms, polyp surveillance and family history screening for bowel cancer were invited to participate in the study. All patients gave written informed consent.
All examinations were performed with the prototype NBI system (Olympus, Japan). This system is equipped with a red, green and blue (RGB) sequential illumination xenon light source (XCLV-260HP), a high resolution zoom colonoscope (CF-H260AZL/I), a video processor (XCV-260HP3P) and a high definition television monitor (Olympus OEV181H). The light source contains one rotating RGB filter and one NBI filter. The NBI filter can be placed between the RGB filter and the light source. It splits white light into two specific lights with narrowed bandwidths; blue (400-430 nm) and green (530-550 nm) while the contribution of the red light is taken out of the equation. This allows the blue and green lights, which have more superficial penetration to penetrate the superficial mucosal architecture leading to enhancement of both the pit patterns and vasculature. The insertion of the NBI filter between the RGB filter and the xenon lamp is achieved by activating a switch on the scope. The endoscopist can then alternate freely between WLE and NBI at any time. The magnification function is activated by depression of a lever on the colonoscope which activates a motorised zoom lens at the distal tip of the scope. The lever’s location on the endoscope simulates the “raiser bridge” on duodenoscopes and is relatively easy to use. By altering the focal distance of the lens, a maximal magnification of up to 115X can be achieved. Prior to endoscopy, a black rubber cap/hood (MB-046 Olympus, Japan) was fitted and adjusted to a distance of 2 mm from the tip of the endoscope. This was performed by visualising a thin rim of the cap on endoscopic views once it had been snugly fitted to the tip. This made it possible for the endoscopist to fix the mucosa to the endoscope before applying the zoom mode. Optimal focus of the area of interest was thus easily obtainable using this technique and the endoscopist was able to focus in or hone out effortlessly.
All patients were offered conscious sedation with intravenous Midazolam (2.5-5 mg) and/or Pethidine (25-50 mg). Bowel preparation was done with Senna tablets (80 mg) followed by Polyethylene Glycol (4 litres) the day prior to the procedure. All endoscopies were recorded using a Digital Video Cassette Recorder (Sony Mini DV GV-D1000E PAL). With such high magnification, it is imperative to visualise the mucosa clearly, hence liberal flushing was done with water mixed with a mucolytic agent, n-Acetylcysteine and a defoaming agent, Simethicone, during the procedure. If the colon exhibited excessive peristaltic activity, which interfered with the examination, an antispasmodic agent, Hyoscine butyl bromide (10-20 mg), was administered intravenously. Once a polyp was detected, it was examined in greater detail. Any overlying mucous or faeces was flushed with water until the mucosal surface of the polyp was clearly visualised. Each lesion was then evaluated sequentially first by WLE, then NBI and finally by CHR using 0.4% indigo carmine spray. All polyps were assessed in the magnification mode. This was done by gently applying the zoom function during the assessment. If the image was out of focus, the non-zoom mode could be applied to obtain an overview of the colon and re-identify the polyp before application of the zoom mode again. Digital images of each polyp with each modality were taken and stored as high quality JPEG files (200-300 kb, 1280 × 1024 pixel array and 32 bit colour). This was followed by either taking biopsies or performing polypectomy.
All images were subsequently transferred using a movie making software (U Lead Video Studio 7SE DVD, U Lead Systems Inc., USA) to another programme (Powerpoint; Microsoft; Redmond, Redmond, WA, USA). Each image was then blindly graded based on the standard Kudo’s pit pattern (KPP)[3] for assessment of colorectal polyps. In the assessment with NBI, the mesh brown capillary network pattern (MBCN) of each polyp was also described (Type I-absent pattern, Type II-regular capillary network, Type III-irregular capillary network)[4,5].
Biopsy or polypectomy specimens were processed with HE stains. These were reviewed by an expert gastrointestinal pathologist (PK) who was blinded to the endoscopic findings.
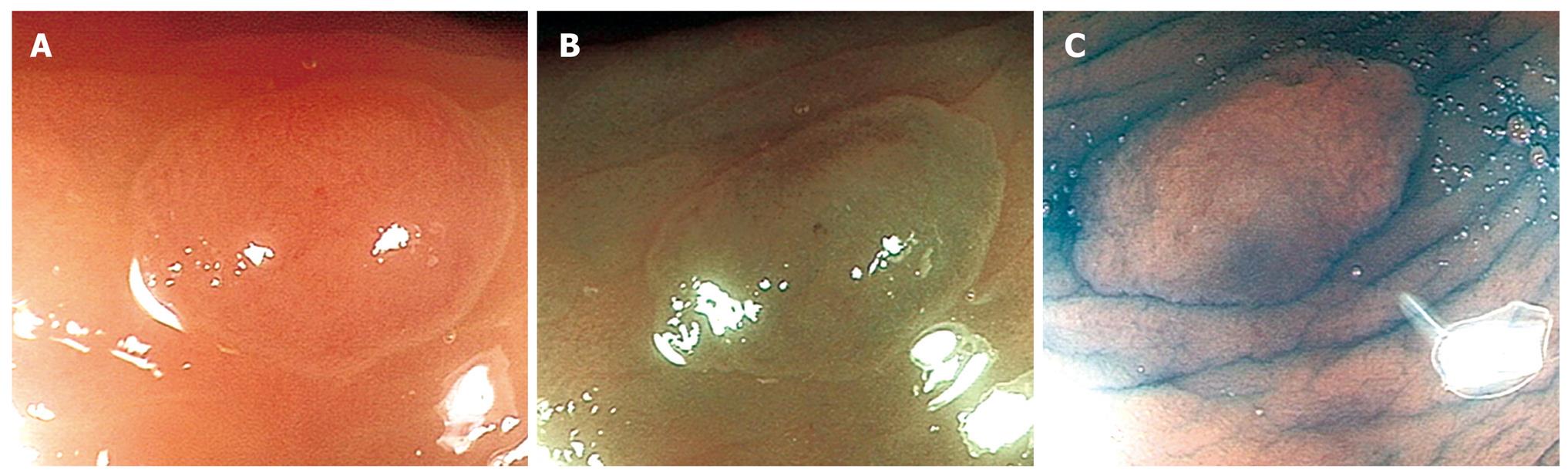
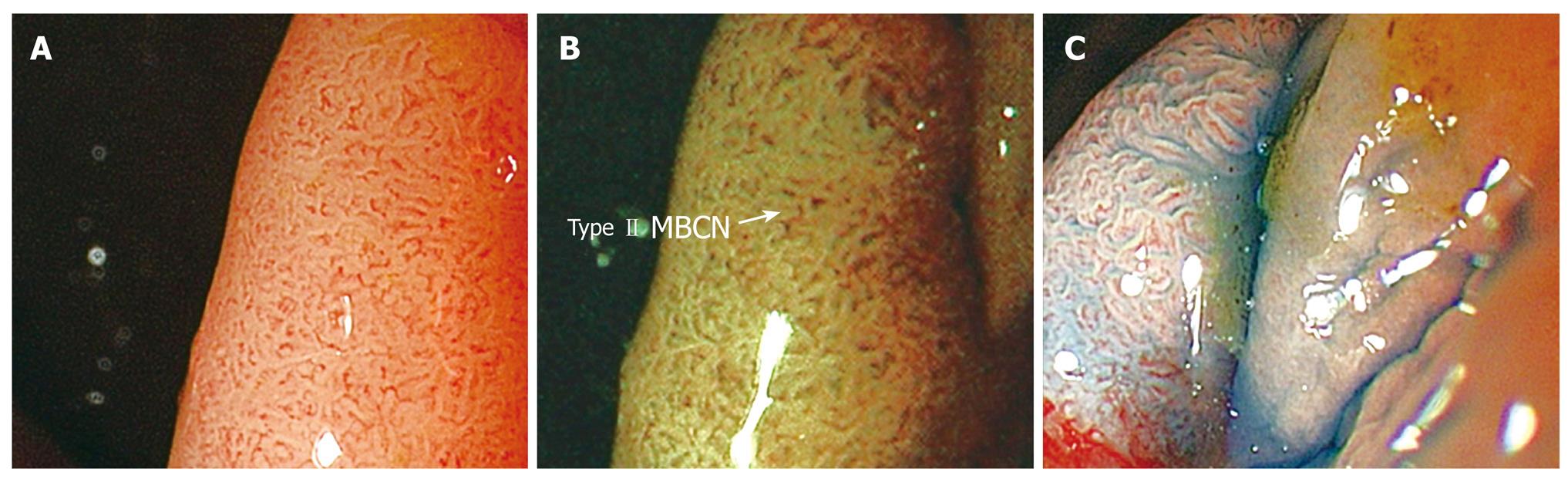
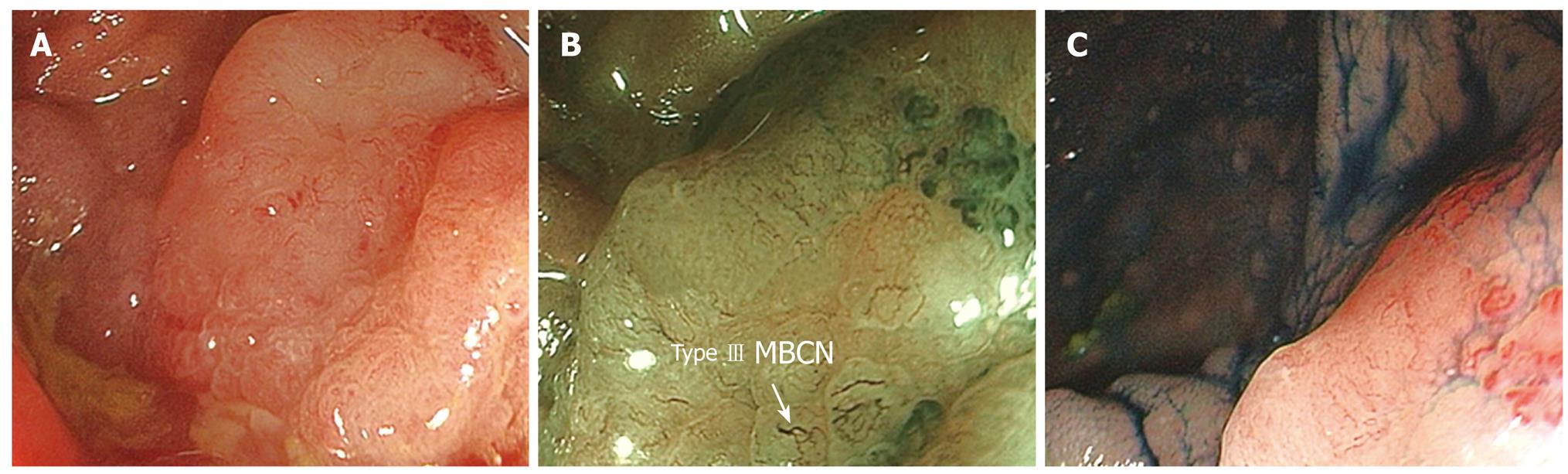
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS version 14, SPSS Inc, Chicago, Ill). The sensitivity (Sn), specificity (Sp), positive predictive value (PPV) and Negative Predictive Value (NPV) of differentiating hyperplastic (Type I& II-KPP, Type I-MBCN) (Figure 1A-C) from adenomatous (Types III, IV-KPP, Type II-MBCN) (Figure 2A-C) and carcinomatous (Type V-KPP, Type III-MCBN) (Figure 3A-C) polyps was then compared with reference to the final histopathological diagnosis.
A total of 50 colorectal polyps in 37 patients with a mean size of 15.2 mm (range 3-50) were assessed. Seventeen lesions were located in the proximal colon (Caecum 5, Ascending Colon 7, Transverse Colon 5), 15 in the distal (Descending Colon 2, Sigmoid Colon 13) and 18 in the rectum. Morphologically, according to the updated Paris classification of superficial neoplastic lesions[6], 19 were classified as Type 0-Is, 6 Type 0- Ip, 24-Type 0-IIa after that 1 Type 0-IIa-c. On final histopathological assessment, 7 polyps were hyperplastic, 38 adenomatous and 5 carcinomatous.
The performances of various modalities in the assessment of the polyps are depicted in Table 1. WLE, as expected was only modestly accurate in the prediction of polyp histology. NBI, using the KPP only correctly predicted 2 of 7 hyperplastic polyps, although it performed relatively well in the prediction of neoplastic polyps (adenomatous and carcinomatous polyps) (95%). In the assessment of the polyps using the combination of KPP and MBCN, the performance of NBI was numerically better than CHR. This combined approach correctly predicted all the hyperplastic and carcinomatous polyps although it failed to accurately predict 2 of the 38 adenomatous polyps. It was interesting to note that NBI using the MBCN pattern only performed better in the assessment of hyperplastic and adenomatous polyps compared to CHR although it did miss 1 out of the 5 carcinomatous polyps.
| WLE | NBI (KPP) | NBI (MBCN) | NBI (COMB) | CHR | |
| Hyperplastic (7) | 1 | 2 | 7 | 7 | 5 |
| Adenomatous (38) | 28 | 36 | 36 | 36 | 35 |
| Carcinoma (5) | 4 | 5 | 4 | 5 | 5 |
| Total (50) | 33 | 43 | 47 | 48 | 45 |
The Sn, Sp, PPV and NPV looking individually at the performances of each modality compared with the prediction of histology are depicted in Table 2. As expected, CHR was clearly superior to WLE in the prediction of polyp histology (P < 0.001). NBI, using either the KPP, MBCN or the combined classification, performed similarly to CHR in the prediction of polyp histology compared to WLE (P = 0.002, P = 0.001 and P < 0.001, respectively). NBI, using only the MBCN or the combined classification showed higher accuracies compared to CHR; although this proved to be statistically not significant (P = 0.625, P = 0.250).
| Sn | Sp | PPV | NPV | |
| WLE | ||||
| HP | 14.3 (2.6, 51.3) | 97.7 (87.9, 99.6) | 50 (9.5, 90.5) | 87.5 (75.3, 94.1) |
| A | 73.7 (58.0, 85.0) | 91.7 (64.6, 98.5) | 96.6 (82.8, 99.4) | 52.4 (32.4, 71.7) |
| C | 80.0 (37.6, 96.4) | 97.8 (88.4, 99.6) | 80 (37.6, 96.4) | 97.8 (88.4, 99.6) |
| Overall | 94.1 (80.9, 98.4) | 6.3 (1.1, 28.3) | 68.1 (53.8, 79.6) | 33.3 (6.1, 79.2) |
| NBI (KPP) | ||||
| HP | 28.6 (8.2, 64.1) | 100 (91.8, 100) | 100 (34.2, 100) | 89.6 (77.8, 95.5) |
| A | 94.7 (82.7, 98.6) | 100 (75.8, 100) | 100 (90.4, 100) | 85.7 (60.1, 96.0) |
| C | 100 (56.6, 100) | 97.8 (88.4, 99.6) | 83.3 (43.6, 97.0) | 100 (92.0, 100) |
| Overall | 100 (91.4, 100) | 22.2 (6.3, 54.7) | 85.4 (72.8, 92.8) | 100 (34.2, 100) |
| NBI (MCBN) | ||||
| HP | 100 (64.6, 100) | 97.7 (87.9, 99.6) | 87.5 (52.9, 97.8) | 100 (91.6, 100) |
| A | 94.7 (82.7, 98.6) | 91.7 (64.6, 98.5) | 97.3 (86.2, 99.5) | 84.6 (57.8, 95.7) |
| C | 80.0 (37.6, 96.4) | 97.8 (88.4, 99.6) | 80.0 (37.6, 96.4) | 97.8 (88.4, 99.6) |
| Overall | 95.2 (84.2, 98.7) | 87.5 (52.9, 97.8) | 97.6 (87.4, 99.6) | 77.8 (45.3, 93.7) |
| NBI (COMBINED) | ||||
| HP | 100 (64.6, 100) | 97.7 (87.9, 99.6) | 87.5 (52.9, 97.8) | 100 (91.6, 100) |
| A | 94.7 (82.7, 98.6) | 100 (75.8, 100) | 100 (90.4, 100) | 85.7 (60.1, 96.0) |
| C | 100 (56.6, 100) | 97.8 (88.4, 99.6) | 83.3 (43.6, 97.0) | 100 (92, 100) |
| Overall | 97.6 (87.7, 99.6) | 87.5 (52.9, 97.8) | 97.6 (87.7, 99.6) | 87.5 (52.9, 97.8) |
| CHR | ||||
| HP | 71.4 (35.9, 91.8) | 97.7 (87.9, 99.6) | 83.3 (43.6, 97.0) | 95.5 (84.9, 98.7) |
| A | 92.1 (79.2, 97.3) | 100 (75.8, 100) | 100 (90.1, 100) | 80.0 (54.8, 93.0) |
| C | 100 (56.6, 100) | 97.8 (88.4, 99.6) | 83.3 (43.6, 97.0) | 100 (92.0, 100) |
| Overall | 97.6 (87.4, 99.6) | 55.6 (26.7, 81.1) | 90.9 (78.8, 96.4) | 83.3 (43.6, 97.0) |
It is important to realise that in order for bowel cancer screening and surveillance to be optimised, a two pronged strategy which involves detection of polyps followed by an accurate assessment of its nature would be a logical if not ideal approach. There have been numerous studies looking at the ability of NBI in the detection of colonic polyps[7-9]. This study however was designed to answer the latter question i.e. predicting the histology of a polyp once it has been detected. The ability to discriminate neoplastic from non-neoplastic polyps could potentially result in a “one stop” approach[10] where hyperplastic polyps are ignored; adenomatous polyps are resected and carcinomatous polyps are biopsied for confirmation of histology and further management. NBI with magnification, a novel modality that is relatively easy to use could potentially be incorporated into standard practice given that the technology is now commercially available. A single push of a button enables the endoscopist to visualise the mucosa quickly and then decide which of the 3 approaches should be used.
The utility of this novel modality has been studied in two previous studies[11,12], which showed similar diagnostic accuracy compared to CHR in the prediction of polyp histology in the diagnosis of colorectal neoplasia. However, in a study performed by East et al[10] comparing the magnified pit pattern with NBI versus CHR for diminutive colonic polyps, the pit pattern classification on NBI was found to be not always identical with CHR. Vascular pattern intensity and a simple colour change on NBI were deemed to be as accurate as the KPP classification with CHR. The authors went on to suggest that assessing polyps with NBI using the KPP classification may need to be modified before it can be used.
Sano et al[4] elegantly proposed the MBCN pattern classification in their benchmark study describing the microvasculature surrounding the mucosal pits in colonic polyps. We hence attempted to gauge this classification in our cohort of patients. The results of our feasibility study suggest that NBI with the combination of the KPP and the MBCN network performed on par if not better than CHR in predicting the histology of colorectal polyps. It is interesting to note that NBI using the MBCN pattern alone proved to be more accurate than CHR in the assessment of hyperplastic and adenomatous polyps albeit reduced accuracies in prediction of carcinomatous polyps.
There were however some limitations in the study which needs to be addressed. The interpretation of the data could have been skewed by the small sample size especially in the hyperplasic and carcinomatous polyp arms. However, as this was a preliminary feasibility study, sample size calculations were not performed. Single photographs representing each polyp with each modality were evaluated. This methodology is certainly less accurate than real time endoscopy. To minimize this bias, we selectively chose the best image representing each modality. The polyps were also assessed by a single assessor and it would have been useful to perform inter/intraobserver assessments to demonstrate its reproducibility.
In conclusion, although numerically superior but not statistically, NBI with magnification performed similarly to CHR with magnification if both the KPP and MBCN criteria were combined and applied in the prediction of histology of non neoplastic and neoplastic colorectal polyps. This feasibility study demonstrated that, perhaps the combined classification could potentially be useful in routine clinical practice, allowing the endoscopist to predict histology with higher accuracies using a less cumbersome method.
The distinction between non neoplastic from neoplastic colorectal polyps during colonoscopy is paramount. This study evaluated the sensitivity, specificity, positive predictive value and negative predictive value of 3 different techniques: high resolution white light endoscopy, narrow band imaging (NBI) and chromoendoscopy (CHR), all with magnification in differentiating adenocarcinomas, adenomatous and hyperplastic colorectal polyps.
To date there have been a paucity of studies looking at the diagnostic capability of NBI with magnification in real time in assessing colorectal polyps. This study combined the novel concept of both the Kudo’s Pit Pattern (KPP) and the Mesh Brown Capillary Network (MBCN) classifications in predicting polyp histology.
NBI, using the MBCN pattern or the combined classification (KPP and MBCN together) showed higher numerical accuracies compared to CHR, but this was not statistically significant (P = 0.625, P = 0.250).
It may lead to significant implications to the practising gastroenterologist if narrow band imaging with magnification is readily available in future. Further larger scale, multi-center, randomized controlled trials would be of value to determine if this novel technology has a role in colorectal cancer screening and diagnosis.
Narrow band imaging: Altered and narrowed spectrum light used to accentuate the surface morphology accentuating the microvascular and microstructural appearance of the mucosa. Magnification: Optical technique used to magnify a given area by 125X.
Although primarily a feasibility study, this study is limited by its small sample size.
Peer reviewer: Yutaka Saito, MD, PhD, Head, Division of Endoscopy, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Li JL L- Editor Alpini GD E- Editor Ma WH
| 1. | Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343-348. [Cited in This Article: ] |
| 2. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [Cited in This Article: ] |
| 3. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [Cited in This Article: ] |
| 4. | Sano Y, Horimatsu T, Fu IK, Katagiri , A , Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow band imaging system. Dig Endosc. 2006;18:S44-S51. [Cited in This Article: ] |
| 5. | Singh R, Kaye PV, Ragunath K. Distinction between neoplastic and non-neoplastic colorectal polyps utilizing narrow band imaging with magnification: a novel technique to increase the efficacy of colorectal cancer screening? Scand J Gastroenterol. 2008;43:380-381. [Cited in This Article: ] |
| 6. | Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [Cited in This Article: ] |
| 7. | East JE, Suzuki N, Stavrinidis M, Guenther T, Thomas HJ, Saunders BP. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65-70. [Cited in This Article: ] |
| 8. | Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64. [Cited in This Article: ] |
| 9. | Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42-47. [Cited in This Article: ] |
| 10. | East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310-316. [Cited in This Article: ] |
| 11. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [Cited in This Article: ] |
| 12. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [Cited in This Article: ] |