Published online Dec 28, 2017. doi: 10.4254/wjh.v9.i36.1352
Peer-review started: October 6, 2017
First decision: November 7, 2017
Revised: November 17, 2017
Accepted: December 5, 2017
Article in press: December 6, 2017
Published online: December 28, 2017
To determine how sustained virological response at 12 wk (SVR12) with direct acting antivirals (DAAs) for the treatment of hepatitis C virus (HCV) infection affects chronic kidney disease (CKD) progression.
A retrospective analysis was performed in patients aged ≥ 18 years treated for HCV with DAAs at the VA Greater Los Angeles Healthcare System from 2014-2016. The treatment group was compared to patients with HCV from 2011-2013 who did not undergo HCV treatment, prior to the introduction of DAAs; the control group was matched to the study group in terms of age, gender, and ethnicity. Analysis of variance and co-variance was performed to compare means between SVR12 subgroups adjusting for co-variates.
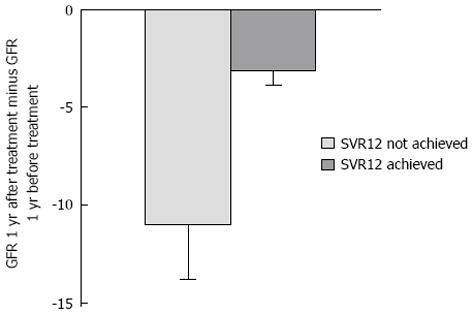
Five hundred and twenty-three patients were evaluated. When comparing the rate of change in estimated glomerular filtration rate (eGFR) one-year after HCV treatment to one-year before treatment, patients who achieved SVR12 had a decline in GFR of 3.1 mL/min ± 0.75 mL/min per 1.73 m2 compared to a decline in eGFR of 11.0 mL/min ± 2.81 mL/min per 1.73 m2 in patients who did not achieve SVR12 (P = 0.002). There were no significant clinical differences between patients who achieved SVR12 compared to those who did not in terms of cirrhosis, treatment course, treatment experience, CKD stage prior to treatment, diuretic use or other co-morbidities. The decline in eGFR in those with untreated HCV over 2 years was 2.8 mL/min ± 1.0 mL/min per 1.73 m2, which was not significantly different from the eGFR decline noted in HCV-treated patients who achieved SVR12 (P = 0.43).
Patients who achieve SVR12 have a lesser decline in renal function, but viral eradication in itself may not be associated improvement in renal disease progression.
Core tip: In hepatitis C patients treated with direct acting antivirals, there is a lesser decline in renal function in those who are treated and achieved sustained virological response at 12 wk (SVR12) compared to those who do not achieve SVR12. However, the decline in renal function is no different between those who achieve SVR12 and those who are never treated. This suggests that viral eradication may not be associated improvement in the progression of renal disease and other factors, such as cryoglobulinemia, may be implicated in renal disease progression.
- Citation: Aby ES, Dong TS, Kawamoto J, Pisegna JR, Benhammou JN. Impact of sustained virologic response on chronic kidney disease progression in hepatitis C. World J Hepatol 2017; 9(36): 1352-1360
- URL: https://www.wjgnet.com/1948-5182/full/v9/i36/1352.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i36.1352
Hepatitis C virus (HCV) is a significant public health issue that affects around 3 million individuals in the United States[1]. The prevalence of chronic HCV infection in veterans affairs (VA) healthcare users is more than 2-fold higher than the general United States population, thus being the nation’s largest provider for HCV care[2,3].
The consequences of HCV infection extend beyond the liver, including renal complications such as membranoproliferative glomerulonephritis (MPGN) in the setting of cryoglobulinemia[4]. Patients with HCV were found to have a five-fold increase in the odds of developing MPGN compared with individuals who were not infected[5]. Chronic HCV infection has also been associated with reductions in glomerular filtration rate (GFR) < 60 mL/min per 1.73 m2, development of end-stage renal disease (ESRD), and a rapid decline in renal function[4,6-9]. Interestingly, the duration of chronic HCV infection influences the risk of developing chronic kidney disease (CKD)[10]. Previous systematic reviews suggest a relationship between HCV infection and higher incidence of low estimated GFR (eGFR)[11]. In a meta-analysis of nearly 3 million individuals, chronic HCV infection predicted a 51% increase in the risk of proteinuria and a 43% increase in the incidence of CKD[11]. CKD is an important public health problem as it increases the likelihood of adverse outcomes and is associated with high healthcare costs[12].
Given that HCV infection is associated with CKD progression, the aim of our study was to determine if the achievement of sustained virological response at 12 wk (SVR12) with interferon-free, direct acting antivirals (DAAs) impacts the progression of CKD. We hypothesize that viral eradication would result in a reduction in CKD progression. No previous study has rigorously investigated whether eradication of HCV infection with newer DAA therapies is associated with improved renal function.
Data source and study population: The VA Greater Los Angeles Healthcare System (VAGLAHS) institutional review board approved this study. Data were abstracted using the Corporate Data Warehouse, a national repository of patient data, for all patients evaluated at the VAGLAHS. A retrospective medical records review was performed by reviewing those patients over 18 years of age who initiated hepatitis C treatment with interferon-free DAAs from January 1st, 2014 to June 1st, 2016. The control group consisted of patients over 18 years of age who did not undergo hepatitis C treatment from January 1st, 2011 to January 1st, 2013, prior to the introduction of DAAs; the control group was matched to the study group in terms of age, gender and ethnicity.
Demographic data, including age, gender, body mass index (BMI) and ethnicity, were obtained at the initial visit. Baseline laboratory data were collected at the time of initial visit. Serum creatinine and estimated GFR were collected yearly for two consecutive years before and one year after treatment. Patients were excluded if there was incomplete kidney function data one year after treatment. Patients were also excluded if they were lost to follow-up or died within 1 year of treatment. The diagnoses of comorbidities were based on International Classification of Disease, Ninth Revision and/or Tenth Revision, Clinical Modification (ICD-9 CM/ICD-10 CM) and use of anti-hypertensive or diabetes medications. The ICD-9/ICD-10 codes that were used were 250.00-250.93/E08-E13 for diabetes mellitus and 401.0, 401.1 and 401.9/I10 for essential hypertension. Cirrhosis and diuretic use were determined through chart review of hepatology provider notes. Patients receiving hemodialysis therapy were excluded. HCV patients were identified by ICD-9/ICD-10 coding, 070.0-0.70.1/B18.2 and B19.2. The primary outcome of our study was SVR12, which was defined as an undetectable HCV RNA (< 15 IU/mL) 8, 12 wk or beyond the conclusion of treatment[13].
Patient characteristics were measured by continuous and categorical variables. The baseline stage of kidney disease was measured with the GFR at the time of treatment as defined by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative[14]. The mean values of baseline characteristic were analyzed using student’s t-test. Proportions were compared using χ2 test. Medians were compared using the Wilcoxon rank-sum test. The mean GFR of the control group and the treatment group was tested for a normal distribution by using a kernel density estimation. Mean GFR between groups were compared using analysis of variance. Analyzed covariates included gender, age by tertile, ethnicity, treatment experience, HCV genotype, treatment regimen, baseline kidney disease, diuretic use, and the presence of such comorbidities as obesity, hypertension, diabetes, heart failure (CHF), coronary artery disease (CAD), and peripheral artery disease (PAD). In addition, the change in eGFR from 1-year prior to DAA initiation was calculated and compared to the change in eGFR between DAA initiation and 1-year post-DAAs; a paired t-test was performed. A P value of < 0.05 was considered as significant. Data analysis was done using STATA® v14.2.
A total of 523 patients met inclusion criteria for the study. Baseline characteristics of the cohort are presented in Table 1. The majority of patients were white males with a mean age of 62.7 (SE ± 0.3) years. A total of 48.6% had cirrhosis and 22.4% were treatment-experienced. Thirty-two percent had diabetes, 68.5% had hypertension, 10.1% had CAD, 4.2% had CHF and 2.9% had been diagnosed with PAD. The most common genotype was genotype 1a (53.2%) followed by genotype 1b (28.1%). The most common HCV treatment regimen was a combination of ledipasvir with sofosbuvir followed by sofosbuvir plus ribavirin. The majority of patients were CKD stages 1 or 2 prior to HCV treatment.
| All patients (n = 523) | SVR12 not achieved (n = 38) | SVR12 achieved (n = 485) | P value | |
| Age (mean, yr) (SE) | 62.7 (0.3) | 60.5 (1.1) | 63.0 (0.3) | 0.02a |
| Gender (%) | ||||
| Male (n = 512) | 97.9 | 94.7 | 97.9 | 0.81 |
| Female (n = 11) | 2.1 | 5.3 | 2.1 | |
| Ethnicity (%) | ||||
| White (n = 278) | 53.2 | 52.6 | 56.3 | 0.78 |
| Black or African American (n = 174) | 33.3 | 34.3 | 33.2 | |
| American Indian or Alaska Native (n = 11) | 2.1 | 2.6 | 2.1 | |
| Asian (n = 4) | 0.8 | 2.6 | 0.6 | |
| Native Hawaiian or other pacific islander (n = 5) | 1.0 | 0.0 | 1.0 | |
| Unknown/declined to answer (n = 51) | 9.8 | 7.9 | 9.9 | |
| Cirrhosis (%) | ||||
| Non-cirrhotic (n = 269) | 51.4 | 47.2 | 51.7 | 0.31 |
| Cirrhosis (n = 254) | 48.6 | 52.8 | 48.3 | |
| Treatment experience (%) | ||||
| Treatment naive (n = 406) | 77.6 | 73.7 | 77.9 | 0.54 |
| Treatment experienced (n = 117) | 22.4 | 26.3 | 22.1 | |
| HCV genotype (%) | ||||
| HCV genotype 1a (n = 278) | 53.2 | 50.7 | 53.3 | 0.44 |
| HCV genotype 1b (n = 147) | 28.1 | 22.2 | 28.6 | |
| HCV genotype 2 (n = 48) | 9.2 | 12.6 | 8.9 | |
| HCV genotype 3 (n = 40) | 7.6 | 17.1 | 6.9 | |
| HCV genotype 4 (n = 6) | 1.1 | 0.0 | 1.2 | |
| HCV genotype 6 (n = 1) | 0.2 | 0.0 | 0.2 | |
| Combination (n = 3) | 0.6 | 0.0 | 0.6 | |
| Treatment (%) | ||||
| Dasabuvir, ombitasvir, paritaprevir and ritonavir (n = 104) | 19.9 | 23.7 | 19.6 | 0.68 |
| Ledipasvir and sofosbuvir (n = 200) | 38.2 | 44.7 | 37.7 | |
| Simeprevir (n = 55) | 10.5 | 5.3 | 10.9 | |
| Sofosbuvir + Ribavirin (n = 164) | 31.4 | 26.3 | 31.8 | |
| Obesity (%) | ||||
| BMI < 30 (n = 284) | 54.3 | 63.5 | 53.6 | 0.25 |
| Obese (n = 239) | 45.7 | 36.5 | 46.4 | |
| Hypertension (%) | ||||
| No hypertension (n = 177) | 33.8 | 47.5 | 32.8 | 0.07 |
| Hypertension (n = 346) | 68.5 | 52.5 | 67.2 | |
| Diabetes (%) | ||||
| No diabetes (n = 358) | 31.5 | 73.5 | 68.1 | 0.47 |
| Diabetes (n = 165) | 30.9 | 26.5 | 31.9 | |
| Congestive heart failure (%) | ||||
| No congestive heart failure (n = 501) | 95.8 | 97.4 | 95.7 | 0.2 |
| Congestive heart failure (n = 22) | 4.2 | 2.6 | 4.3 | |
| Coronary artery disease (%) | ||||
| No coronary artery disease (n = 469) | 89.7 | 94.7 | 89.3 | 0.12 |
| Coronary artery disease (n = 53) | 10.1 | 2.6 | 10.7 | |
| Peripheral arterial disease (%) | ||||
| No peripheral arterial disease (n = 508) | 97.1 | 94.7 | 97.2 | 0.99 |
| Peripheral arterial disease (n = 15) | 2.9 | 5.3 | 2.7 | |
| Baseline CKD before treatment (%) | ||||
| Stage 1 CKD (n = 263) | 50.3 | 60.5 | 49.5 | 0.24 |
| Stage 2 CKD (n = 218) | 41.7 | 39.5 | 41.9 | |
| Stage 3 CKD (n = 41) | 7.8 | 0.0 | 8.5 | |
| Stage 4 CKD (n = 1) | 0.2 | 0.0 | 0.2 | |
| Diuretic use (%) | ||||
| No diuretic use (n = 367) | 70.2 | 71.1 | 70.1 | 0.9 |
| Diuretic use (n = 156) | 29.8 | 28.9 | 29.9 |
Within the treated groups, there was a significant difference in age between patients who achieved SVR12 compared to those who did not, with the group who achieved SVR12 being slightly older (P = 0.02). There were no other significant clinical differences between patients who achieved SVR12 compared to those who did not in terms of gender, ethnicity, cirrhosis, treatment course, treatment experience, CKD stage prior to treatment, diuretic use or other co-morbidities.
The control group consisted of 439 patients who were not treated for HCV and followed from January 1st, 2011 to January 1st, 2013. These patients were not treated for HCV given that DAAs were not available at VAGALHS during that time period. Baseline characteristics of the study population and control groups are shown in Table 2. The control group was matched to the treatment group by age, gender, and ethnicity. The control group was not statistically different from the cohort of HCV treated patients in terms of age, gender, ethnicity, HCV genotype, diabetes, CAD, PAD, CKD stage prior to treatment, and diuretic use. The median MELD score (interquartile range) for the cirrhotic patients at baseline was 8.4 (7.49-9.72) in the treatment group compared to 7.7 (6.43-9.16) in the control group that did not undergo treatment; there were no significant differences in MELD score between groups (P = 0.19). There were significantly more patients with cirrhosis and obesity (BMI > 30 kg/m2) in the cohort who underwent HCV treatment compared to the control group (P = 0.001, 0.005 respectively). The control group, however, had significantly more patients with hypertension and CHF compared to the cohort who underwent HCV treatment (P = 0.02, 0.001 respectively).
| All patients (n = 523) | Control patients (n = 439) | P value | |
| Age (mean, yr) (SE) | 62.8 (0.3) | 63.2 (0.3) | 0.13 |
| Gender (%) | |||
| Male | 97.9 | 98.1 | 0.75 |
| Female | 2.1 | 1.9 | |
| Ethnicity (%) | |||
| White | 53.2 | 55.4 | 0.3 |
| Black or African American | 33.3 | 35.1 | |
| American Indian or Alaska Native | 2.1 | 2.5 | |
| Asian | 0.8 | 0.5 | |
| Native Hawaiian or other pacific islander | 1.0 | 0.9 | |
| Unknown/declined to answer | 9.8 | 5.7 | |
| Cirrhosis (%) | |||
| Non-cirrhotic | 51.4 | 72.0 | 0.001a |
| Cirrhosis | 48.6 | 28.0 | |
| HCV genotype (%) | |||
| HCV genotype 1a | 53.2 | 55.9 | 0.31 |
| HCV genotype 1b | 28.1 | 22.8 | |
| HCV genotype 2 | 9.2 | 11.8 | |
| HCV genotype 3 | 7.6 | 6.7 | |
| HCV genotype 4 | 1.1 | 1.7 | |
| HCV genotype 6 | 0.2 | 0.0 | |
| Obesity (%) | |||
| BMI < 30 | 54.3 | 63.3 | 0.005a |
| Obese | 45.7 | 36.7 | |
| Hypertension (%) | |||
| No hypertension | 33.8 | 26.7 | 0.02a |
| Hypertension | 68.5 | 73.3 | |
| Diabetes (%) | |||
| No diabetes | 68.5 | 64.0 | 0.15 |
| Diabetes | 31.5 | 36.0 | |
| Congestive heart failure (%) | |||
| No congestive heart failure | 95.8 | 90.4 | 0.001a |
| Congestive heart failure | 4.2 | 9.6 | |
| Coronary artery disease (%) | |||
| No coronary artery disease | 89.7 | 88.4 | 0.46 |
| Coronary artery disease | 10.1 | 11.6 | |
| Peripheral arterial disease (%) | |||
| No peripheral arterial disease | 97.1 | 95.2 | 0.09 |
| Peripheral arterial disease | 2.9 | 4.8 | |
| Baseline CKD before treatment (%) | |||
| Stage 1 CKD | 50.3 | 47.1 | 0.56 |
| Stage 2 CKD | 41.7 | 37.8 | |
| Stage 3 CKD | 7.8 | 5.4 | |
| Stage 4 CKD | 0.2 | 0.4 | |
| Diuretic use (%) | |||
| No diuretic use | 70.2 | 67.2 | 0.3 |
| Diuretic use | 29.8 | 32.8 |
When comparing the rate of change in eGFR one-year after HCV treatment compared to one-year before treatment, patients who achieved SVR12 had a decline in GFR of 3.1 mL/min ± 0.75 mL/min per 1.73 m2 compared to a decline in eGFR of 11.0 mL/min ± 2.81 mL/min per 1.73 m2 in patients who did not achieve SVR12 (P = 0.002; Figure 1). In those who achieved SVR12, the change in eGFR 1-year prior to treatment was -6.2 mL/min ± 1.06 mL/min per 1.73 m2 compared to -1.8 mL/min ± 0.75 mL/min per 1.73 m2 in the year following DAA therapy; those who achieved SVR12 had a lesser decline in renal function following DAA treatment (P = 0.002). In those who were treated with DAAs but did not achieve SVR12, the change in eGFR 1-year prior to treatment was -5.4 mL/min ± 2.79 mL/min per 1.73 m2 compared to -7.42 mL/min ± 2.2 mL/min per 1.73 m2 in the year following DAA therapy (P = 0.62). In the control group, the decline in eGFR over two years was 2.8 mL/min ± 1.0 mL/min per 1.73 m2. This decline in eGFR in untreated patients over two years was not significantly different from the eGFR decline noted in patients who achieved SVR12 after HCV treatment (P = 0.43).
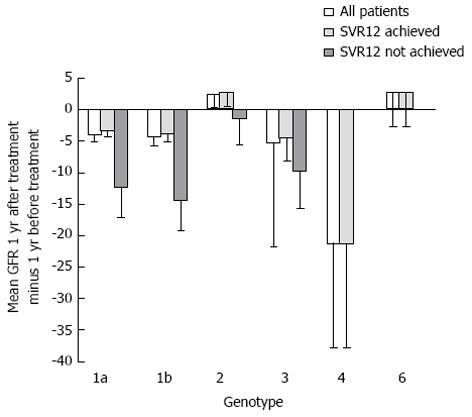
Figure 2 demonstrates the rate of change in eGFR one-year after HCV treatment compared to one-year before treatment stratified by genotype. In patients with genotype 1a and 1b, there was less of a decline in eGFR between one-year before HCV treatment compared to one-year after treatment in patients who achieved SVR12 compared to those who did not (P = 0.02). There was no significant difference in eGFR decline between patients who achieved SVR12 and those who did not in genotypes 2 (n = 48) and 3 (n = 40).
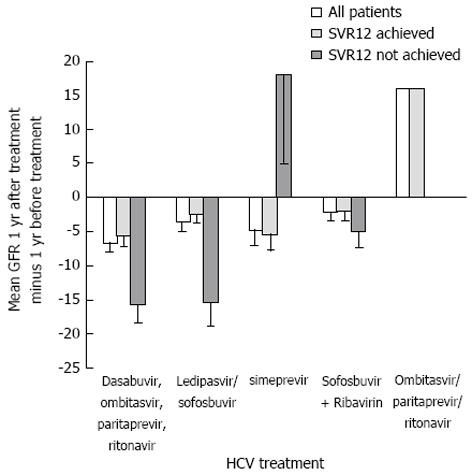
Figure 3 shows the rate of change in eGFR one-year after HCV treatment compared to one-year before treatment separated out by treatment type. In patients treated with dasabuvir, ombitasvir, paritaprevir, and ritonavir and ledipasvir/sofosbuvir, there was less of a decline in eGFR between one-year before HCV treatment compared to one-year after treatment in patients who achieved SVR12 compared to those who did not (P = 0.005). In patients treated with sofosbuvir, there was not statistically significant difference in the rate of change in eGFR between those achieved SVR12 compared to those who did not (P = 0.68) although the decline in eGFR was less in those who achieved SVR12.
In this single-center cohort of Veterans, we demonstrate that patients who achieved SVR12 with interferon-free DAAs had a reduced progression of renal disease that was statistically significant compared to patients who did not achieve SVR12. However, there were no significant differences in renal function decline between patients who were not treated with DAAs compared to those who were treated and achieved SVR12.
While there appears to be an association between HCV infection and progression CKD, the mechanism of HCV-induced kidney injury continues to be debated. One hypothesis is that HCV triggers immune and inflammatory responses locally, within vascular tissues, or potentially systemically through inflammatory mediators, causing atherothrombosis and thus progression of CKD[11]. Immune complex deposition with HCV proteins and anti-HCV antibodies may provoke kidney injury[15]. HCV RNA and related proteins have been found in mesangial cells and the existence of these HCV-related proteins in the mesangium is associated higher proteinuria, which may suggest HCV infection causes direct mesangial injury[16]. Another thought is that HCV seropositive status induces accelerated atheromatous disease at the kidney level[11]. There is also clinical and laboratory evidence that suggests that HCV infection may be associated with insulin resistance and susceptibility to diabetes, which may lead to endothelial dysfunction and oxidative stress[11,17,18].
There was no significant difference in renal function decline between those who were treated for HCV and achieved SVR12 and those who were not treated for HCV. These results are similar to previous studies. A meta-analysis looking at the effect of antiviral therapy on HCV-associated CKD showed that HCV RNA clearance with interferon based therapy was not associated with a decrease in serum creatinine in the group that achieved SVR12 compared to the group that did not[19]. However, those who achieved SVR12 did have a decrease in protein excretion[19]. There was inadequate data on proteinuria, given the retrospective design and given proteinuria is infrequently ordered by physicians at our center, thus we were unable to determine the impact of SVR12 on proteinuria.
The fact that there were no significant differences in renal function decline between patients who were not treated with DAAs compared to those who were treated and achieved SVR12, suggests that viral eradication may not be associated improvement in the progression of renal disease. In patients with MPGN and type II cryoglobulinemia, there may be virological clearance with DAA therapy, but there may be persistence of cryoglobulinemia, which may lead to persistent renal decline. Circulating cryoglobulins are detected in a large number of patients with HCV, however, only a minority of patients will experience clinical manifestations, thus some cases of cryoglobulinemia may remain undetected[20]. A recent study by Emery et al[21], showed that despite high SVR rates after DAA treatment in patients with HCV associated mixed cryoglobulinemia only 29.4% of symptomatic patients had complete cryoprecipitate clearance despite achievement of SVR12. Work by Gragnani et al[22] showed a 100% SVR12 rate, however reported that only 34% of patients had full complete response, defined as disappearance of all the baseline symptoms, with follow-up to 24 wk. However, a recent case series suggests that in patients with HCV and mixed cryoglobulinemia syndrome treated with DAAs that there is an improvement in renal function, even in patients not concomitantly treated with immunosuppression[23].
Another explanation as to why achievement of SVR12 may not improve renal disease progression is that patients may have intrinsic renal disease prior to treatment, such as MPGN, and these patients will have CKD progression despite achieving SVR12; this has been previously described in the literature in case reports[24]. However, other reports have suggested that DAA therapy can result in successful treatment of HCV-associated MPGN with improvement in creatinine and proteinuria[25]. Furthermore, the patient population studied was unique - it is comprised of Veterans who are predominantly male, older in age, have a higher prevalence of CKD compared to the general population, and often have significant co-morbidities associated with CKD, such as diabetes mellitus, hypertension, vascular disease, and cancer[26]. Given the high prevalence of CKD and associated co-morbidities in this veteran population, CKD progression may have occurred despite SVR12 given the other presence of co-morbidities that drive CKD progression.
An alternative explanation could also be that although HCV clearance may have renal sparing effects, there many be a component of direct nephrotoxicity due to DAA therapy. In patients treated with Viekira or ledipasvir and sofosbuvir, there was a greater decline in eGFR in those who did not achieve SVR12 compared to those who achieved SVR12. However, the sample sizes for each treatment group are too small to make any definitive conclusions. Previous treatment with interferon-based therapy was associated with acute kidney injury, however kidney injury has not been attributed to any DAA therapy[27]. Sofosbuvir’s circulating metabolite GS-331007 is renally cleared, thus there is a concern of Sofosbuvir use in patients with eGFR < 30 mL/min, but further work is needed to investigate the cases of kidney injury in patients following Sofosbuvir treatment[28].
There is a greater decline in renal function in those who were treated with DAAs and did not achieve SVR12 compared to those who were never treated. However, the group that did not achieve SVR12 following treatment had a greater proportion of cirrhotic patients when compared to the control group who did not undergo treatment. Given that the group who did not achieve SVR12 had a greater proportion of patients with cirrhosis, this group may have been more ill and thus had a higher propensity to undergo complications, such as hepatorenal syndrome, which may contribute to worsening renal function.
Our study has a number of limitations. First, this is a single center study restricted to Veteran health care users; therefore, the results may not be generalizable to non-Veteran populations, given the higher prevalence of baseline CKD and only a few women. Its retrospective nature may result in bias due to confounding variables, including unmeasured patient characteristics. The Modification of Diet in Renal Disease (MDRD) equation used to estimate GFR might be less accurate among patients in hepatitis C and cirrhosis because of abnormalities in protein metabolism as well as muscle wasting. In patients with cirrhosis, serum creatinine is a poor measure of GFR, however it is often used as a surrogate marker[29,30]. Finally, our follow-up time was short due to the recent introduction of DAAs. For our treatment cohort, there was not enough eGFR data two years following treatment, thus we were only able to evaluate eGFR changes one year following treatment. It is possible that the strength and degree of the associations described in the study might differ if the follow up period was extended.
Our study may have implications for clinical practice. Clinicians may be prompted to discuss the need for ESRD surveillance in their patients with HCV prior to treatment with DAAs. The current KDIGO guidelines suggest the patients with HCV be tested annually for proteinuria and eGFR, however the guideline is rated weak given it is based on expert judgment[31]. Given the lack of strong guidelines, it is likely that patients with HCV are not being screened for ESRD.
In summary, we found that there was a lesser decline in renal function in patients who achieved SVR12 compared to those who did not, however there were no significant differences in renal function decline between patients who were not treated compared to those who were treated and achieved SVR12. Additional research is needed to confirm these results in multi-institutional studies with longer duration of follow-up. Further work is required to develop screening guidelines for kidney disease in patients with HCV.
Hepatitis C virus (HCV) is a significant public health issue in the United States and worldwide. The consequences of HCV infection extend beyond the liver, including renal complications. Patients with HCV are at risk for renal function decline and developing end-stage renal disease (ESRD). Chronic kidney disease (CKD) is an important public health problem as it increases the likelihood of adverse outcomes and is associated with high healthcare costs.
Given HCV infection places patients at risk for renal function decline and developing ESRD, it is valuable to understand how the clearance of HCV infection with interferon free, direct acting antiviral (DAA) therapy affects chronic kidney progression. Given the recent introduction of DAA therapy, the impact of HCV clearance on kidney disease has not been fully established.
The authors’ principal aim was to determine if the achievement of sustained virological response at 12 wk (SVR12) with interferon-free, DAAs impacts the progression of CKD.
The authors retrospectively analyzed medical records of adult patients who initiated hepatitis C treatment with interferon-free DAAs from 2014 to 2016 at the VA Greater Los Angeles Healthcare System. The control group consisted of adult patients who did not undergo hepatitis C treatment, prior to the introduction of DAAs, from 2011 to 2013. Baseline demographic and clinical data were collected. The rate of change in estimated glomerular filtration rate (eGFR) one-year after HCV treatment compared to one-year before treatment was compared between patients who achieved SVR12 to those who did not. The change in eGFR was recorded over two years in patients who did not undergo treatment and compared to those who underwent DAA treatment.
The findings of the analysis suggest that patients who achieved SVR12 with interferon-free DAAs had a reduced progression of renal disease that was statistically significant compared to patients who did not achieve SVR12. However, there were no significant differences in renal function decline between patients who were not treated with DAAs compared to those who were treated and achieved SVR12. The control group was not statistically different from the cohort of HCV treated patients, except that the there were significantly more patients with cirrhosis and obesity in the cohort who underwent HCV treatment compared to the control group. The control group, however, had significantly more patients with hypertension and congestive heart failure compared to the cohort who underwent HCV treatment.
There is a lesser decline in renal function in patients who achieved SVR12 compared to those who did not, however there were no significant differences in renal function decline between patients who were not treated compared to those who achieved SVR12. There are several possible explanations for the lack of improvement of CKD progression with viral eradication, such as immune factors related to cyroglobulins, intrinsic renal disease prior to therapy, and that the control group had significantly more patients with cirrhosis compared to the treatment group.
Additional research is needed to confirm these results in multi-institutional studies with longer duration of follow-up.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Grasso A, Hoare M S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 556] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 2. | Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, Stenhouse A, Kling MA, Hrushesky W, Zeilman C. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev. 2015;37:131-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. 2013;61:623-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O’Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Su FH, Su CT, Chang SN, Chen PC, Sung FC, Lin CC, Yeh CC. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kidney Dis. 2012;60:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2012;6:369-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rogal SS, Yan P, Rimland D, Lo Re V 3rd, Al-Rowais H, Fried L, Butt AA; Electronically Retrieved Cohort of HCV Infected Veterans Study Group. Incidence and Progression of Chronic Kidney Disease After Hepatitis C Seroconversion: Results from ERCHIVES. Dig Dis Sci. 2016;61:930-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C Virus Infection Increases the Risk of Developing Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2015;60:3801-3813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034-2047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1113] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 13. | Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, Wang J, Chuang SM, Ma J, McNally J, Stamm LM, Brainard DM, Symonds WT, McHutchison JG, Beavers KL, Jacobson IM, Reddy KR, Lawitz E. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-S266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Blé M, Aguilera V, Rubín A, García-Eliz M, Vinaixa C, Prieto M, Berenguer M. Improved renal function in liver transplant recipients treated for hepatitis C virus with a sustained virological response and mild chronic kidney disease. Liver Transpl. 2014;20:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology. 1997;25:1237-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [PubMed] [Cited in This Article: ] |
| 18. | Vanni E, Abate ML, Gentilcore E, Hickman I, Gambino R, Cassader M, Smedile A, Ferrannini E, Rizzetto M, Marchesini G. Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C. Hepatology. 2009;50:697-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Feng B, Eknoyan G, Guo ZS, Jadoul M, Rao HY, Zhang W, Wei L. Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant. 2012;27:640-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol. 2010;8:1017-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, Wong D, Janssen HLA, Feld JJ. Efficacy and Safety of Direct Acting Antivirals for the Treatment of Mixed Cryoglobulinemia. Am J Gastroenterol. 2017;112:1298-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, Perez M, Ceccotti G, Colantuono S, Mitrevski M. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology. 2016;64:1473-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 23. | Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 24. | Chowdhury R, Tsen A. Recurrent Mixed Cryoglobulinemia Despite Sustained Virologic Response to Treatment: A Case Report. Am J Kidney Dis. 2017;70:301-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Obata F, Murakami T, Miyagi J, Ueda S, Inagaki T, Minato M, Ono H, Nishimura K, Shibata E, Tamaki M. A case of rapid amelioration of hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis treated by interferon-free directly acting antivirals for HCV in the absence of immunosuppressant. CEN Case Rep. 2017;6:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Patel N, Golzy M, Nainani N, Nader ND, Carter RL, Lohr JW, Arora P. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Barsoum RS, William EA, Khalil SS. Hepatitis C and kidney disease: A narrative review. J Adv Res. 2017;8:113-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Noell BC, Besur SV, deLemos AS. Changing the face of hepatitis C management - the design and development of sofosbuvir. Drug Des Devel Ther. 2015;9:2367-2374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201-205. [PubMed] [Cited in This Article: ] |
| 30. | Schück O, Gottfriedova H, Maly J, Jabor A, Stollova M, Bruzkova I, Skibova J, Ryska M, Spicak J, Trunecka P. Glomerular filtration rate assessment in individuals after orthotopic liver transplantation based on serum cystatin C levels. Liver Transpl. 2002;8:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;S1-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |