Published online Nov 28, 2017. doi: 10.4254/wjh.v9.i33.1239
Peer-review started: July 19, 2017
First decision: August 15, 2017
Revised: September 1, 2017
Accepted: September 16, 2017
Article in press: September 16, 2017
Published online: November 28, 2017
Today, with the introduction of interferon-free direct-acting antivirals and outstanding progresses in the prevention, diagnosis and treatment of hepatitis C virus (HCV) infection, the elimination of HCV infection seems more achievable. A further challenge is continued transmission of HCV infection in high-risk population specially injecting drug users (IDUs) as the major reservoir of HCV infection. Considering the fact that most of these infections remain undiagnosed, unidentified HCV-infected IDUs are potential sources for the rapid spread of HCV in the community. The continuous increase in the number of IDUs along with the rising prevalence of HCV infection among young IDUs is harbinger of a forthcoming public health dilemma, presenting a serious challenge to control transmission of HCV infection. Even the changes in HCV genotype distribution attributed to injecting drug use confirm this issue. These circumstances create a strong demand for timely diagnosis and proper treatment of HCV-infected patients through risk-based screening to mitigate the risk of HCV transmission in the IDUs community and, consequently, in the society. Meanwhile, raising general awareness of HCV infection, diagnosis and treatment through public education should be the core activity of any harm reduction intervention, as the root cause of failure in control of HCV infection has been lack of awareness among young drug takers. In addition, effective prevention, comprehensive screening programs with a specific focus on high-risk population, accessibility to the new anti-HCV treatment regimens and public education should be considered as the top priorities of any health policy decision to eliminate HCV infection.
Core tip: Despite the outstanding progresses in the management of hepatitis C virus (HCV) infection, the elimination of HCV would be difficult due to the emergence of injection drug use as the main source of HCV transmission. Asymptomatic nature of HCV infection, restricted accessibility to diagnostic approaches and appropriate antiviral treatments in the injecting drug users (IDUs) community are the root cause of failure in control of HCV infection among IDUs. These circumstances create a strong demand for timely diagnosis and proper treatment of HCV-infected patients as well as raising general awareness of HCV infection through public education to mitigate the risk of HCV transmission.
- Citation: Taherkhani R, Farshadpour F. Global elimination of hepatitis C virus infection: Progresses and the remaining challenges. World J Hepatol 2017; 9(33): 1239-1252
- URL: https://www.wjgnet.com/1948-5182/full/v9/i33/1239.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i33.1239
With a global prevalence rate of 2.8%, equating to over 185 million infections, and more than 350000 deaths annually, hepatitis C virus (HCV) infection is undoubtedly considered a major public health problem[1]. Globally, an estimated 3 million to 4 million new cases of HCV infection emerge every year[1]. Furthermore, the HCV-related mortality is increasing and HCV infection is projected to be the most important leading cause of viral hepatitis-related mortality in the near future[1,2]. Apparently, the management of HCV infection faces several challenges. These challenges merit further attention if elimination of HCV infection is aimed to be achieved.
HCV is a member of the family Flaviviridae and the genus Hepacivirus. The HCV genome is a positive-stranded RNA, which encodes a core protein (C), two envelope glycoproteins (E1 and E2), and several non-structural proteins (NS1, NS2, NS3, NS4A, NS4B, NS5A and NS5B)[3,4]. This enveloped positive-stranded RNA virus is usually acquired through exposure to infected blood. This might happen through transfusion of blood and blood products, surgery, organ transplantation, intravenous drug use, tattooing, hemodialysis, unsafe injection practices, mother to fetus, and sexual intercourse[5-8]. However, sexual transmission of HCV is less common and most often observed among men who have sex with men and HIV-infected patients[9,10].
HCV is the causative agents of hepatitis C infection. This infection is characterized by an acute or chronic course in the host. The complications are preliminary asymptomatic, mild or severe, which spontaneously clear or slowly progress to chronic liver disease, cirrhosis and finally hepatocellular carcinoma (HCC) within about 20 years[11,12]. The clinical symptoms of acute HCV infection might include fever, fatigue, malaise, and gastrointestinal symptoms such as anorexia, nausea, vomiting, right upper quadrant pain, dark urine, grey-colored stool, and yellow skin and sclera of the eyes, the well-characterized symptoms of jaundice. These symptoms might appear from 3 to 12 wk after being infected. The clinical symptoms of chronic HCV infection might take decades to develop, and they are usually indicative of an advanced liver disease[13-15].
The long-term chronic HCV infection is capable of causing some extra hepatic manifestations with serious consequences, such as glomerulonephritis, diabetes mellitus, thyroid disorders, porphyria cutaneous tarda, mixed cryoglobulinemia, lichen planus, and B cell lymphoproliferative disorders[16-21]. These extrahepatic complications might outshine the hepatic manifestations of HCV infection, and the presence of HCV infection might be overlooked, paving the way for the silent development of advanced liver disease. Therefore, the possible role of HCV in the development of extrahepatic manifestations merits further attention.
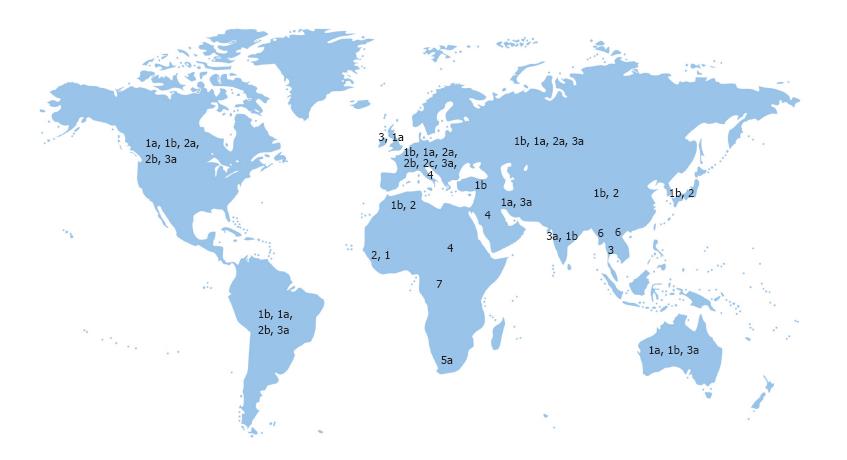
Due to genomic heterogeneity, there are 7 major genotypes and over 67 subtypes of HCV[1,22,23]. HCV genotype distribution varies by the route of transmission and geographical location[24,25]. In addition, pathogenicity, response to antiviral therapy and the duration of treatment can be influenced by different HCV genotypes[5,24,26]. The genotypes 1, 2 and 3 show a widespread distribution in almost all parts of the world. HCV genotype 4 has been traditionally restricted to a few countries in the Middle East and Africa and is more prevalent in Saudi Arabia, Bahrain, Jordan, Egypt and Ethiopia[1,27,28]. HCV genotype 5, 6 and 7 have been reported in South Africa, South East Asia and Central Africa, respectively[11,29,30] (Figure 1).
Genotype 1 is more prevalent among patients with history of blood and blood products transfusion, surgery, and dental procedure[24,25,27]. Infection with HCV genotype 2 is mainly associated with nosocomial transmission and prior dental treatment[1,22]. Genotype 3 is frequently found in the intravenous drug user communities and in those with history of tattooing and piercing[24,31,32]. Genotype 4 is mainly transmitted through high-risk sexual practices, especially among homosexual males, and intravenous drug use[1,22].
Infection with HCV genotype 3 is associated with a more rapid progression of fibrosis, a higher degree of steatosis, and a higher incidence of cirrhosis and hepatocellular carcinoma[1,22,31,33]. Spontaneous clearance is more often observed in infection with HCV genotype 1, while if patients remain HCV RNA positive, the disease progresses in a more aggressive manner than the other genotypes[11]. Genotypes 1 and 4 are associated with lower response rates and higher treatment duration in response to interferon (IFN) and ribavirin (RBV) combination therapy as compared to genotypes 2 and 3[6,24,34].
In addition to IFN-based therapies, the direct-acting antivirals (DAAs) have been developed, which specifically inhibit the function of viral proteins that are essential for viral replication[4,37,38]. These DAAs include NS3/4A protease inhibitors, NS5A replication complex inhibitors, nucleoside NS5B polymerase inhibitors, and non-nucleoside NS5B polymerase inhibitors (Table 1)[39-43]. These novel antiviral drugs, despite having considerable advantages over conventional IFN-based therapy, suffer from the resistance-associated mutations, which occur naturally during the replication of the virus and select under the pressure of DAAs. The emergence of HCV resistance-associated variants (RAVs) decreases the susceptibility to DAAs and finally results in treatment failure[38,44-46]. Assessment of resistance substitutions at pretreatment baseline in patients candidate for DAA therapy seems to be the best option to optimize first-line therapeutic strategies, to avoid the fitness of resistant variants as the predominant viral population and to prevent DAA failure due to baseline resistant variants. In addition, failing DAA-based therapy should be discontinued as soon as possible to avoid an increase in the frequency of RAVs, to preserve HCV re-treatment options. Finally, development of next-generation DAAs with higher resistance barrier is strongly recommended[45,47].
| Direct-acting antiviral agent | Generic name (abbreviation) | Code name | Trade name | Active against HCV genotype (based on clinical trial outcomes) | Combination therapy |
| NS3/4A protease inhibitors (-previr) | Telaprevir (TVR) | VX-950 | Incivek/Incivo | 1 | TVR + IFN ± RBV |
| Boceprevir (BOC) | SCH-503034 EBP-520 | Victrelis | 1 | BOC + IFN ± RBV | |
| Faldaprevir (FDV) | BI-201335 | - | 1 | FDV + Peg-IFN + RBV | |
| Simeprevir (SIM) | TMC-435 | Olysio | 1 and 4 | SIM + SOF ± RBV | |
| Vaniprevir (VNV) | MK-7009 | Vanihep | 1 | VNV + IFN ± RBV | |
| Asunaprevir (ASV) | BMS-650032 | Sunvepra | 1 and 4 | ASV + DCV | |
| Paritaprevir (PTV) | ABT-450 | Veruprevir | 1 and 4 | PTV+R+OBV+DAV ± RBV | |
| Voxilaprevir (VOX) | GS-9857 | - | Pan-genotypic antiviral activity | VOX + SOF + VPR | |
| Sovaprevir | ACH-1625 | - | 1 | Sovaprevir + ODV + RBV | |
| Grazoprevir (GZP) | MK-5172 | - | 1a, 1b, 4 and 6 | Zepatier (GZP + EBV) | |
| Danoprevir (DNV) | RG-7227 | - | 1 and 4 | DNV + PEG-IFN + RBV | |
| ITMN-191 | DNV + R + PEG-IFN + RBV | ||||
| ASC08 | |||||
| Deldeprevir (DDV) | ACH-2684 ACH-0142684 | - | 1 | DDV + ODV | |
| Neceprevir | |||||
| Narlaprevir (NVR) | SCH-900518 | Arlansa | 1 | NVR + R + PEG-IFN ± RBV | |
| Vedroprevir (VDV) | GS-9451 | - | 1 | VDV + LDV + SOF | |
| VDV + LDV + TGV + RBV | |||||
| Glecaprevir (GLE) | ABT-493 | - | Pan-genotypic antiviral activity | GLE + PIB ± RBV | |
| - | GS-9256 | - | 1 | GS-9256 + PEG-IFN + RBV | |
| GS-9256 + TGV + Peg-IFN ± RBV | |||||
| NS5A replication complex inhibitors (-Asvir) | Daclatasvir (DCV) | BMS-790052 | Daklinza | 1, 2 and 3 | Sovodak (DCV + SOF) ± RBV |
| DCV + VX-135 | |||||
| Ledipasvir (LDV) | GS-5885 | - | 1, 3, 4, 5 and 6 | Harvoni (LDV + SOF) ± RBV | |
| LDV + SOF ± (VDV or Radalbuvir) | |||||
| Ombitasvir (OBV) | ABT-267 | - | 1 and 4 | Viekira Pak (OBV + PTV + R + DSV) ± RBV | |
| Technivie (OBV + PTV + R) | |||||
| Elbasvir (EBV) | MK-8742 | - | 1a, 1b, 4 and 6 | Zepatier (EBV + GZP) ± RBV | |
| Velpatasvir (VPR) | GS-5816 | - | Pan-genotypic antiviral activity | Epclusa (VPR + SOF) ± RBV | |
| Odalasvir (ODV) | ACH-3102 | - | 1 | ODV + Sovaprevir + RBV | |
| Ravidasvir (RVD) | PPI-668 | - | 4 | RVD + SOF ± RBV | |
| ASC16 | |||||
| - | PPI-461 | - | 1 | - | |
| - | JNJ-56914845 | - | 1 | GSK2336805 + PEG-IFN + RBV | |
| GSK2336805 | GSK2336805 + VX-135 + SIM | ||||
| Samatasvir | IDX-18719 IDX-719 | - | 1, 2, 3 and 4 | Samatasvir + SIM + RBV | |
| MK-1894 | |||||
| - | BMS-824393 | - | 1 | BMS-824393 + PEG-IFN + RBV | |
| Pibrentasvir (PIB) | ABT-530 | - | Pan-genotypic antiviral activity | PIB + GLE ± RBV | |
| Ruzasvir (RZR) | MK-8408 | - | Pan-genotypic antiviral activity | RZR + UPR + GZP | |
| Nucleoside NS5B polymerase inhibitors | Sofosbuvir (SOF) | PSI-7977; | Sovaldi; Soforal | Pan-genotypic antiviral activity | SOF + IFN ± RBV |
| (-Buvir) | GS-7977 | Sovodak (DCV + SOF) ± RBV | |||
| Mericitabine (MCB) | RG-7128 | - | 1 and 4 | MCB + PEG-IFN + RBV | |
| RO5024048 | MCB + DNV | ||||
| MCB + R + DNV ± RBV | |||||
| - | VX-135 | - | 1 | VX-135 + GSK2336805 + SIM | |
| ALS-2200 | VX-135 + TVR + RBV | ||||
| VX-135 + DCV | |||||
| VX-135 + RBV | |||||
| VX-135 + SIM | |||||
| Valopicitabine | NM283 | - | 1 | Valopicitabine + Peg-IFN | |
| Non-nucleoside NS5B polymerase inhibitors (-Buvir) | Beclabuvir (BCV) | BMS-791325 | - | 1 | BCV+ ASV+ DCV |
| Dasabuvir (DAV) | ABT-333 | Exviera | 1 | DAV + OBV+ PTV + R ± RBV | |
| Lomibuvir | VX-222 | - | 1 | VX-222 + TVR + RBV | |
| VCH-222 | VX-222 + Filibuvir | ||||
| Filibuvir | PF-00868554, | - | 1 | Filibuvir + Peg-IFN + RBV | |
| PF-868554 | Filibuvir + VX-222 | ||||
| Setrobuvir (STV) | ANA-598 | - | 1 | STV + IFN + RBV | |
| RO-5466731 | STV + R + DNV + RBV ± MCB | ||||
| RG-7790 | |||||
| Nesbuvir (NBV) | HCV-796 | - | 1 | NBV +Peg-IFN + RBV | |
| VB-19796 | |||||
| Tegobuvir (TGV) | GS-9190 | - | 1 | TGV + GS-9256 +Peg-IFN ± RBV | |
| TGV + LDV + VDV + RBV | |||||
| Deleobuvir (DBV) | BI-207127 | - | 1 | DBV + PEG-IFN + RBV | |
| DBV + FDV | |||||
| DBV + FDV + RBV | |||||
| Uprifosbuvir (UPR) | MK-3682 | - | Pan-genotypic antiviral activity | UPR + RZR | |
| UPR + RZR + GZP | |||||
| Radalbuvir | GS-9669 | - | 1 | Radalbuvir + LDV + SOF | |
| AL-335 | ALS-335 | - | 1 | AL-335 + ODV + SIM |
Telaprevir and boceprevir are not recommended by WHO due to the frequent adverse effects and low cure rates[79].
Prior to the treatment, the infected individuals need to be identified. HCV infection is described by the presence of anti-HCV antibodies and HCV-RNA in plasma or serum with either elevated or normal levels of liver enzymes[29]. Anti-HCV antibodies are detected by using serological screening tests, including enzyme linked immunosorbent assay and recombinant immunoblot assay. Detection of anti-HCV antibodies indicates current or past HCV infection. An additional test called HCV RNA test or reverse transcriptase polymerase chain reaction assay (RT-PCR) is needed to determine if a person is currently infected with HCV[17,80-82].
However, those infected individuals with undetectable levels of HCV-RNA in serum or plasma might remain undiagnosed. In this condition, HCV-RNA can be detected in peripheral blood mononuclear cells (PBMCs) specimens, liver biopsies, and ultracentrifugated serum samples[81,83]. Serological screening tests might be negative or positive in these patients. This kind of infection is defined as occult HCV infection, which is a serious threat to blood safety[84,85]. Since, despite having undetectable level of HCV RNA, blood and blood products are potentially infectious[84,86]. In fact, the presence of blood donors with occult HCV infection can increase the risk of HCV transmission through blood transfusion and therefore is a potential source of HCV transmission in the society[87].
Despite having appropriate antiviral treatments and diagnostic approaches, diagnosis rate and access to treatment is considerably low especially in resource-limited settings. Perhaps the most promising strategy to control HCV infection is the development of a prophylactic vaccine[88,89]. Several vaccine candidates against HCV have been developed so far, including recombinant protein vaccine, peptide-based vaccine, virus-like particles, bacterial-vectored vaccine, viral-vectored vaccine, and DNA vaccine (Table 2)[29,88,90-96]. The currently developed vaccines against HCV, despite inducing strong humoral and cellular immune responses in preclinical animal models or clinical trials in humans, have not been approved for use in human beings[89,90,97]. The reason is high genomic diversity of HCV and viral escape from immune responses[88,90,93,98,99]. Targeting the conserved regions within HCV proteins might help to overcome this genetic variability[100].
| Type of vaccine | Vaccine structure/adjuvant | Stage of development | Outcome | Application | Developer | Year | Current status | Ref. |
| Recombinant protein vaccine | Recombinant E1 or E2/MF59 | 7 chimpanzees | Induce strong humoral immune response; complete protection in 5 chimpanzees | Prophylactic vaccine | Chiron/ Novartis | 1994 | Completed | [101] |
| Recombinant E1 or E2/Alum | 4 Chimpanzees | Induce antigen-specific T-helper cytokines in either E1 or | Therapeutic vaccine | BPRC | 2011 | Published | [102] | |
| Recombinant E1/Alum | Phase I 20 healthy volunteers | E2-vaccinated animals; clear HCV infection in only E1-vaccinated animals (neutralizing antibodies) Induce strong cellular and humoral anti-E1 responses | Therapeutic vaccine | Fujirebio Europe | 2004 | Published | [103] | |
| Recombinant E1 and E2/MF59 | Phase I 60 healthy volunteers | Induce humoral and cellular immune responses | Prophylactic vaccine | Novartis | 2010 | Completed | [104] | |
| Recombinant E1/Alum | Phase I/II 20 healthy volunteers and 35 patients with chronic HCV infection/122 HCV-infected patients | Induce HCV specific humoral and cellular immune responses (Th1 type); no change in HCV viral load | Therapeutic vaccine | Innogenetics/ GenImmune | 2003/2008 | Published | [103,105,106] | |
| HCV core protein/ISCOMATRIX | Phase I/IIa 30 healthy volunteers | Induce strong humoral immune responses in all except one patients; induce CD8+ T cell responses in 2 of 8 patients receiving the highest dose | Prophylactic vaccine | CSL Ltd | 2009 | Published | [107] | |
| GI5005: Inactivated recombinant Saccharomyces cerevisiae expressing NS3-core fusion protein/ GI-5005 plus SOC | Phase I/II 66 patients with chronic HCV infection/ | Improve SVR | Therapeutic vaccine | GlobeImmune | 2009/2010 | Completed | [108,109] | |
| Peptide-based vaccine | Peptide from core protein (C35-C44)/ISA51 | Phase I 26 patients with chronic HCV infection | Induce peptide-specific cellular and humoral immune responses in 15 of 25 patients; decline HCV viral load in 2 of 25 patients | Therapeutic vaccine | Karume University | 2009 | Published | [110] |
| Four peptides from E1, E2, NS3 and NS5A/Freund’s adjuvant | Phase I 12 nonresponder patients with chronic HCV infection | Induce peptide-specific cellular and humoral immune responses; decline HCV viral load in 3 patients | Therapeutic vaccine | Karume University | 2007 | Published | [111] | |
| Autologous dendritic cell delivered six CD8+ T cell epitope peptides from core, NS3 and NS4B | Phase I 6 nonresponder patients with chronic HCV infection | Induce transient T-cell response | Therapeutic vaccine | Burnet Institute + others | 2010 | Completed | [112] | |
| IC41: Five peptides from core, NS3, and NS4/Poly-L-arginine | Phase I/II 128 volunteers/60 non-responders with chronic HCV infection | Induce HCV-specific T-cell responses | Therapeutic vaccine | Intercell AG | 2006/2008 | Published | [113,114] | |
| IC41/Poly-L-arginine + imiquimod | Phase I 54 healthy volunteers | Induce significant T cell responses; low immunogenicity of topical imiquimod | Therapeutic vaccine | Intercell AG | 2010 | Published | [115] | |
| IC41 + imiquimod | Phase II 50 HCV-infected patients | Decline viral load; induce T cell responses | Therapeutic vaccine | Intercell AG | 2012 | Completed | [116] | |
| Virus-like particles | Recombinant HCV-like particles (HCV-LPs) containing core, E1, and E2/AS01B | 4 chimpanzees | Induce HCV-specific cellular immune responses; viral clearance | Prophylactic vaccine | NIH | 2007 | Published | [117] |
| Recombinant baculovirus containing core, E1 and E2 | Mice | Induce high titers of anti-E2 antibodies and strong HCV-specific cellular immune responses (CD8+ T and Th1 cells) | Prophylactic vaccine | NIH | 2001 | Published | [118] | |
| Bacterial-vectored vaccine | Attenuated Salmonella typhimurium containing NS3 gene | Mice | Induce long-lasting T-cell responses | Therapeutic vaccine | NIH | 2001 | Published | [119] |
| Viral-vectored vaccine | Recombinant adenoviral vectors and plasmid DNA expressing NS3-NS5B | 5 chimpanzees | Induce memory HCV-specific T cells; control of viremia | Prophylactic vaccine | NIH/Okairos | 2012 | Completed | [120] |
| Multiple adenoviral vectors (Ad5, Ad6, Ad24, ChAd32 and ChAd33) expressing NS3-NS5B proteins | Mice and rhesus macaque | Induce strong cellular immune responses; long-term maintenance of memory cells | Prophylactic vaccine | Okairos | 2006 | Published | [121] | |
| Recombinant vaccinia viruses (rVV) expressing core, E1, E2, P7, NS2 and NS3 | 4 chimpanzees | Induce cellular immune responses; reduce viral load; resolve HCV infection | Prophylactic vaccine | NYC Blood Center | 2008 | Published | [122] | |
| Recombinant adenoviral vectors (Ad6 and ChAd3) expressing NS3-NS5B proteins | Phase I 40 healthy volunteers | Induce sustained HCV-specific T cell responses | Prophylactic vaccine | Okairos | 2012 | Completed | [123] | |
| Adenovirus vector (Ad6 and ChAd3) expressing NS3-NS5B proteins | Phase I 36 healthy volunteers | Highly immunogenic; induce HCV specific T cell responses | Prophylactic vaccine | Okairos and Oxford University | 2009 | Published | [124] | |
| TG4040: MVA vector expressing NS3, NS4 and NS5B proteins | Phase I 15 patients with chronic HCV infection | Decline HCV viral load in 7 of 15 patients associated with T-cell response | Therapeutic vaccine | Transgene | 2009 | Withdrawn | [125] | |
| MVA and ChAd3 vectors expressing NS3, NS4, NS5A and NS5B proteins | Phase I/II Healthy at risk population (68/472 IDU) | July 28, 2018: Final data collection date | Prophylactic vaccine | NIAID | 2017 | Ongoing | [126] | |
| TG4040 + SOC | Phase II 153 patients with chronic HCV infection | Induce HCV- and MVA-specific T-cell responses; develop anti-MVA antibodies; increase rate of early virologic response | Therapeutic vaccine | - | 2014 | Published | [127] | |
| DNA vaccine | Recombinant DNA plasmid encoding E2 | 2 chimpanzees | Induce humoral and cellular immune responses; resolve the infection; prevent progression to chronicity | Prophylactic vaccine | NIAID/NIH | 2000 | Published | [128] |
| Recombinant DNA plasmid and adenovirus vector expressing core, E1, E2 and NS3-5 | 8 chimpanzees | Induce HCV-specific T-cell and long-lasting E2-specific antibody responses; reduce viral load | Prophylactic vaccine | NIH | 2005 | Published | [129] | |
| Recombinant DNA plasmids and MVA vector expressing core, E1, E2 and NS3 | 6 chimpanzees | Induce HCV-specific immune responses; reduce viral load; early control of acute HCV infection; fail to impact on chronicity | Prophylactic vaccine | Transgene | 2007 | Published | [130] | |
| CIGB-230: Plasmid expressing core/E1/E2 plus recombinant core protein | Phase I 15 non-responder patients with chronic HCV infection | Induce humoral and cellular immune responses; no viral clearance | Therapeutic vaccine | University of Montreal + others | 2009 | Published | [131] | |
| ChronVac-C: Plasmid expressing NS3 and NS4A delivered by in vivo electroporation | Phase I/IIa 12 HCV-infected patients | Decline HCV viral load in 4 of 6 patients receiving the highest dose with corresponding HCV-specific T-cell response in 3 patients | Therapeutic vaccine | Tripep AB | 2009 | Recruiting | [132] |
In the absence of an approved prophylactic vaccine for hepatitis C, reducing exposure to HCV through prevention seems to be the best option. This can be achieved through routine screening of donated blood for HCV markers, providing safe medical procedures, promoting risk-reduction counseling and services for at risk population, increasing public awareness and offering regular HCV testing to high-risk populations with the goal of breaking the cycle of HCV transmission in the society[7,9,82,133]. Despite the so-called improvements in the management of HCV infection, still a long way is ahead to achieve a world free of HCV infection. Here, the remaining challenges to eliminating HCV infection will be discussed.
For many years, IFN-based therapy, despite having frequent side effects, poor tolerability, suboptimal efficacy and prolonged treatment course, was recommended as the standard treatment for HCV infection[134,135]. Introduction of IFN-free DAAs has solved most of these problems in the treatment course of HCV infection. Switch the HCV treatment regimens from IFN-based therapy to DAA therapy is a desirable approach, yet encounter practical barriers such as high price and the restricted accessibility of DAAs[135-138]. Most of the time, the cost of antivirals rather than their effectiveness is the main driver in the treatment decisions. The use of these DAAs is far beyond the financial means of the most-in-need patients especially those who are IFN-intolerant or non-responder. While, equity in health demands that all patients with every socioeconomic status have equitable access to these treatment regimens. Currently, reducing treatment costs and providing DAAs with a relatively high health insurance coverage seem to be best options to improve access to DAA therapy[139].
Accessibility to DAAs, though, by itself is a superb health achievement, still alone might not be sufficient to mitigate the burden of HCV infection. A further challenge is continued transmission of HCV infection in high-risk population specially injecting drug users (IDUs) as the major reservoir of HCV infection[133,137,139]. Considering the fact that most of these infections remain undiagnosed, unidentified HCV-infected IDUs are potential sources for the spread of HCV infection in the society[133,139-141]. While, silent introduction of HCV infection into the community is a serious threat to the national effort to eliminate HCV infection, a threat that will increase with time. Therefore, timely diagnosis of HCV-infected patients through risk-based screening is of the greatest importance[126,133,137]. Screening of blood donations for hepatitis C initiated in the early 1990s has remarkably reduced the risk of HCV transmission through blood transfusion since then. Blood transfusion before the early 1990s was a major contributor to the HCV transmission, but today this risk has become minute[142]. However, it is far, far more difficult to screen IDUs, those who most need risk assessment. Despite the remarkable advantages, the cultural objections hinder screening progress, resulting in low diagnosis rate and, consequently, persistent silent spread of infection. On the other hand, the stigma of injecting drug use makes recognition of all HCV-infected IDUs impossible or logistically difficult at best[133]. In addition, establishment of HCV screening system with a specific focus on IDUs imposes high financial burden on the health system. Given the treatment expenses and dependence of these expenses on the stage of liver disease, screening of all at-risk populations seems much more affordable in a long run. Overall, in addition to interrupting unrecognized transmission of HCV, a part of costs expended in the treatment sector will also be saved with the prompt diagnosis and timely treatment of infected but asymptomatic patients[133,143]. While this process would demand allocation of adequate budgets and resources to integrate routine screening of high-risk population into national health programs.
As another solution, the coverage of needle and syringe exchange program should be expanded to increase the daily access to fresh needles and syringes among IDUs[144]. However, this program has not been very successful to control HCV transmission thus far, as the prevalence of HCV infection among IDUs is on the rise[139]. In fact, the overall focus on syringe sharing as the main vehicle for HCV spread has taken focus away from the other risk behaviors of IDUs such as the shared use of drug ampoules or the other injecting paraphernalia, engagement in high-risk sexual practices and the other drug-related harms[145]. These circumstances create a strong demand for precise surveillance of IDUs to obtain a reliable insight into risk behaviors of IDUs community, and subsequently harm reduction interventions should be tailored to the common risk behaviors among IDUs to mitigate the risk of HCV transmission. In addition, raising general awareness of HCV infection, diagnosis and treatment through public education should be the core activity of any harm reduction intervention, as the root cause of failure in control of HCV infection has been lack of awareness among young drug takers[133,141,146]. The growing number of IDUs and the relatively young age distribution of HCV-infected IDUs have evoke huge attention and provided a good opportunity to drive down the increasing trend of HCV-related mortality in near future through timely interventions and appropriate treatment[139,147].
The changes in HCV genotype distribution attributed to injecting drug use is another challenge in eliminating HCV infection. The changes in genotype distribution are so slight as to be unnoticeable but can have a deep impact on the epidemiology of HCV infection in a long run. These changes merit further attention if we want to properly manage the future burden of HCV infection. Globally, the most prevalent genotype is 1 (46%), followed by 3 (22%), 2 (13%) and 4 (13%)[35,137]. Over the last decade, however, a gradual decrease in the prevalence of genotype 1 and an increase in genotype 3 have been reported due to some changes in the route of transmission, risk factors, source of infection, human migration flow, and age distribution[148,149].
Blood transfusion before 1990 was the most important contributor to the spread of HCV, which has been reflected in the predominance of genotype 1 among older individuals[149,150]. In fact, screening for hepatitis C made blood transfusion remarkably safe since 1990s, paving the way for a gradual increase in the prevalence of genotype 3, which is mostly transmitted by IDU[148-150]. In recent years, IDU has become the main source of HCV transmission[35,137,144,145]. Globally, the estimated number of HCV-infected IDUs is up to 10.0 million (6.0-15.2 million), most of whom are young[35,139,144,147,151]. Meanwhile, the most common risk behavior of IDUs, syringe sharing, is more frequent among young drug injectors than in experienced and long-term injectors[152], amplifying the transmission of HCV among young IDUs population and favoring the continuous increase of HCV genotype 3. In addition to the change in the route of HCV transmission, the ongoing civil strife in the Middle East and the active migration flow from India, Afghanistan and Pakistan, where subtype 3a is endemic, have fuelled the increasing prevalence of genotype 3[148]. On the other hand, death of elderly HCV carriers is slowly driving down the prevalence of HCV genotype 1.
These changes in genotype distribution have profound effects on the prevalence of HCV infection, response to antiviral therapy, cost and duration of treatment, and future burden of HCV infection. Given the higher rates of sustained virological response (SVR) to IFN-based therapy, the first-line therapy in low- and middle-income countries, in patients with HCV genotype 3 as compared to genotype 1[149], an increase in the prevalence of genotype 3 beneficially affects the treatment course both in terms of duration and in terms of cost and brings high benefits on an individual level. However, this increase would impose a greater risk on a population level. In reality the rising prevalence of HCV infection along with the continuous increase in the number of IDUs outweigh this benefit. The disastrous interacting epidemics of HCV infection and IDU are harbinger of a forthcoming public health dilemma, presenting a serious challenge to control transmission of HCV infection. On the other hand, high prevalence of HCV infection among young IDUs is a cause for concern, paving the way for rapid spread of HCV in the community. The old story of hepatitis C has gotten a new scenario. The emergence of IDU as the main risk factor for transmission of HCV is a surrogate in this new scenario. If this scenario is to continue, the emergence of an uncontrollable epidemic of hepatitis C will be expected in the near future.
The global community has always been concerned about the future burden of HCV infection. Although action on this concern has started many years ago with great hopes to eliminate HCV infection, the success remains elusive and will become even more elusive if the current HCV management paradigm is to be continued. We believe that it is now time to reconsider the wisdom of the current management strategies, admit failure, and act with all the strength. If we want to succeed in eliminating HCV infection, a more integrated international effort will be required, involving health policy makers, healthcare practitioners, public health organizations, antiviral drug manufacturers, health insurance companies, and all major stakeholders. In addition, effective prevention, comprehensive screening programs with a specific focus on high-risk population, accessibility to the new anti-HCV treatment regimens and public education should be considered as the top priorities of any health policy decision to eliminate HCV infection. While waiting for a solution, prevalence of HCV infection continues to increase. If we do not want to encounter another uncontrollable public health dilemma, the time to act is now, tomorrow will be very late.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bock CT, Dang SS, El-Shabrawi M, Sanal MG, Takahashi T S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Petruzziello A, Marigliano S, Loquercio G, Cacciapuoti C. Hepatitis C virus (HCV) genotypes distribution: an epidemiological up-date in Europe. Infect Agent Cancer. 2016;11:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 610] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 3. | Falcón V, Acosta-Rivero N, González S, Dueñas-Carrera S, Martinez-Donato G, Menéndez I, Garateix R, Silva JA, Acosta E, Kourı J. Ultrastructural and biochemical basis for hepatitis C virus morphogenesis. Virus Genes. 2017;53:151-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Yang CHT, Yoo ER, Ahmed A. The Role of Direct-acting Antivirals in the Treatment of Children with Chronic Hepatitis C. J Clin Transl Hepatol. 2017;5:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Rehan HS, Manak S, Yadav M; Deepinder, Chopra D, Wardhan N. Diversity of genotype and mode of spread of Hepatitis C virus in Northern India. Saudi J Gastroenterol. 2011;17:241-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Farahat MA, Bahnasy KA, Abdo A, Kamal SM, Kassim SK, Eldin AS. Response Prediction for Chronic HCV Genotype 4 Patients to DAAs. IJACSA. 2016;1:173-178. [Cited in This Article: ] |
| 7. | Ferreira PM, Guimarães RA, Souza CM, Guimarães LC, Barros CV, Caetano KA, Rezza G, Spadoni L, Brunini SM. Exposure to hepatitis C virus in homeless men in Central Brazil: a cross-sectional study. BMC Public Health. 2017;17:90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Alter MJ. HCV routes of transmission: what goes around comes around. Semin Liver Dis. 2011;31:340-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Omata M, Kanda T, Wei L, Yu ML, Chuang WL, Ibrahim A, Lesmana CR, Sollano J, Kumar M, Jindal A. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Li Q, Yao Y, Shen Y, Cao D, Li Y, Zhang S, Cun W, Sun M, Yu J, Shi L. Assessment of HCV genotypes in Yunnan Province of Southwest China. Virus Genes. 2017;53:190-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Seto WK, Lai CL, Fung J, Hung I, Yuen J, Young J, Wong DK, Yuen MF. Natural history of chronic hepatitis C: genotype 1 versus genotype 6. J Hepatol. 2010;53:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Umumararungu E, Ntaganda F, Kagira J, Maina N. Prevalence of Hepatitis C Virus Infection and Its Risk Factors among Patients Attending Rwanda Military Hospital, Rwanda. Biomed Res Int. 2017;2017:5841272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Nepal A, Kunwar B. Evidence of Hepatitis C Virus Infection and Associated Treatment in Nepal. J Mol Biomark Diagn. 2016;7:1000270. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 15. | Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S-20S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 467] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Farshadpour F, Raherkhani R. Hepatitis C-related diabetes mellitus: A health dilemma too necessary to consider. Virol Research J. 2016;1:1-2. [Cited in This Article: ] |
| 17. | Taherkhani R, Farshadpour F. Epidemiology of hepatitis C virus in Iran. World J Gastroenterol. 2015;21:10790-10810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Hwang SJ, Chen LK. Chronic hepatitis C and diabetes mellitus. J Chin Med Assoc. 2006;69:143-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Rouabhia S, Malek R, Bounecer H, Dekaken A, Bendali Amor F, Sadelaoud M, Benouar A. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol. 2010;16:3427-3431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Jadoon NA, Shahzad MA, Yaqoob R, Hussain M, Ali N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol J. 2010;7:304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis. 2007;16:65-73. [PubMed] [Cited in This Article: ] |
| 22. | Kartashev V, Döring M, Nieto L, Coletta E, Kaiser R, Sierra S; HCV EuResist Study group. New findings in HCV genotype distribution in selected West European, Russian and Israeli regions. J Clin Virol. 2016;81:82-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 965] [Cited by in F6Publishing: 924] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 24. | Roman F, Hawotte K, Struck D, Ternes AM, Servais JY, Arendt V, Hoffman P, Hemmer R, Staub T, Seguin-Devaux C. Hepatitis C virus genotypes distribution and transmission risk factors in Luxembourg from 1991 to 2006. World J Gastroenterol. 2008;14:1237-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Keskın F, Çıftçı S, Türkoğlu S, Badur S. Transmission routes of chronic hepatitis C and their relation to HCV genotypes. Turk J Gastroenterol. 2010;21:396-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Farshadpour F, Makvandi M, Samarbafzadeh AR, Jalalifar MA. Determination of hepatitis C virus genotypes among blood donors in Ahvaz, Iran. Indian J Med Microbiol. 2010;28:54-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Al-Kubaisy W, Al-Naggar RA, Ibrahim NSN, Bobryshev YV, Al-Kubaisy MW. Is dental extraction a risk factor for contracting HCV infection: Abs, RNA and genotype detection. Oral Biol Dentist. 2014;2:7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Albenmousa A, Al Obary E, Bzeizi K. Treatment Options for HCV Genotype-4. J Infect Dis Ther. 2016;4:1000266. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Nouroz F, Shaheen S, Mujtaba G, Noreen S. An overview on hepatitis C virus genotypes and its control. EJMHG. 2015;16:291-298. [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Murphy DG, Sablon E, Chamberland J, Fournier E, Dandavino R, Tremblay CL. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J Clin Microbiol. 2015;53:967-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 - the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Salemovic D, Pesic-Pavlovic I, Jevtovic D, Bojovic K, Ranin J, Brmbolic B, Stanojevic M. Intravenous drug use - an independent predictor for HCV genotypes 3 and 4 infection among HIV/HCV co-infected patients. Arch Med Sci. 2017;13:652-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Chan A, Patel K, Naggie S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs. 2017;77:131-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Yee BE, Nguyen NH, Zhang B, Lin D, Vutien P, Wong CR, Lutchman GA, Nguyen MH. Sustained virological response and its treatment predictors in hepatitis C virus genotype 4 compared to genotypes 1, 2, and 3: a meta-analysis. BMJ Open Gastroenterol. 2015;2:e000049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1325] [Cited by in F6Publishing: 1311] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 36. | Ramia S, Eid-Fares J. Distribution of hepatitis C virus genotypes in the Middle East. Int J Infect Dis. 2006;10:272-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Kanda T, Nakamoto S, Nakamura M, Jiang X, Miyamura T, Wu S, Yokosuka O. Direct-acting Antiviral Agents for the Treatment of Chronic Hepatitis C Virus Infection. J Clin Transl Hepatol. 2014;2:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Bartels DJ, Sullivan JC, Zhang EZ, Tigges AM, Dorrian JL, De Meyer S, Takemoto D, Dondero E, Kwong AD, Picchio G. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013;87:1544-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Asselah T. A revolution in HCV treatment with direct-acting antivirals: from non-response to eradication. J Hepatol. 2012;57:455-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Tamori A, Enomoto M, Kawada N. Recent Advances in Antiviral Therapy for Chronic Hepatitis C. Mediators Inflamm. 2016;2016:6841628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Wyles D, Dvory-Sobol H, Svarovskaia ES, Doehle BP, Martin R, Afdhal NH, Kowdley KV, Lawitz E, Brainard DM, Miller MD. Post-treatment resistance analysis of hepatitis C virus from phase II and III clinical trials of ledipasvir/sofosbuvir. J Hepatol. 2017;66:703-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 487] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 43. | Wang S, Wang Y, Wang J, Sato T, Izawa K, Soloshonok VA, Liu H. The second-generation of highly potent hepatitis C virus (HCV) NS3/4A protease inhibitors: Evolutionary design based on tailor-made amino acids, synthesis and major features of bio-activity. Curr Pharm Des. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Alavian SM, Hajarizadeh B, Bagheri Lankarani K, Sharafi H, Ebrahimi Daryani N, Merat S, Mohraz M, Mardani M, Fattahi MR, Poustchi H. Recommendations for the Clinical Management of Hepatitis C in Iran: A Consensus-Based National Guideline. Hepat Mon. 2016;16:e40959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Esposito I, Trinks J, Soriano V. Hepatitis C virus resistance to the new direct-acting antivirals. Expert Opin Drug Metab Toxicol. 2016;12:1197-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Karbalaie Niya MH, Salman-Tabar S, Bokharaei-Salim F, Behmanesh M, Keyvani H. Prevalence of resistant associated variants (RAVs) in the naïve HCV patient candidate for direct acting antiviral (DAA) therapy. Microb Pathog. 2017;105:166-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB; American Association for Study of Liver Diseases. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 803] [Cited by in F6Publishing: 834] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 48. | Deutsch M, Papatheodoridis GV. Danoprevir, a small-molecule NS3/4A protease inhibitor for the potential oral treatment of HCV infection. Curr Opin Investig Drugs. 2010;11:951-963. [PubMed] [Cited in This Article: ] |
| 49. | Guedj J, Dahari H, Shudo E, Smith P, Perelson AS. Hepatitis C viral kinetics with the nucleoside polymerase inhibitor mericitabine (RG7128). Hepatology. 2012;55:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Greig SL. Sofosbuvir/Velpatasvir: A Review in Chronic Hepatitis C. Drugs. 2016;76:1567-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Tong L, Yu W, Chen L, Selyutin O, Dwyer MP, Nair AG, Mazzola R, Kim JH, Sha D, Yin J. Discovery of Ruzasvir (MK-8408): A Potent, Pan-Genotype HCV NS5A Inhibitor with Optimized Activity against Common Resistance-Associated Polymorphisms. J Med Chem. 2017;60:290-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Walker J, Crosby R, Wang A, Woldu E, Vamathevan J, Voitenleitner C, You S, Remlinger K, Duan M, Kazmierski W. Preclinical characterization of GSK2336805, a novel inhibitor of hepatitis C virus replication that selects for resistance in NS5A. Antimicrob Agents Chemother. 2014;58:38-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Adkison KK, Gan J, Elko-Simms L, Gardner S, Dumont E, Jones LS, Saunders J, Marbury T, Smith W, Berg J. Pharmacokinetics of hepatitis C virus NS5A inhibitor JNJ-56914845 (GSK2336805) in subjects with hepatic impairment. J Clin Pharmacol. 2015;55:1042-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Rodriguez-Torres M, Stoehr A, Gane EJ, Serfaty L, Lawitz E, Zhou A, Bourque M, Bhanja S, Strizki J, Barnard RJ. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment-experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1029-37.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Gane EJ, Rouzier R, Wiercinska-Drapalo A, Larrey DG, Morcos PN, Brennan BJ, Le Pogam S, Nájera I, Petric R, Tran JQ. Efficacy and safety of danoprevir-ritonavir plus peginterferon alfa-2a-ribavirin in hepatitis C virus genotype 1 prior null responders. Antimicrob Agents Chemother. 2014;58:1136-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Sarrazin C, Castelli F, Andreone P, Buti M, Colombo M, Pol S, Calinas F, Puoti M, Olveira A, Shiffman M. HCVerso1 and 2: faldaprevir with deleobuvir (BI 207127) and ribavirin for treatment-naïve patients with chronic hepatitis C virus genotype-1b infection. Clin Exp Gastroenterol. 2016;9:351-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Tao T, Jiang X, Chen Y, Song Y. Efficacy and Safety of Ledipasvir/Sofosbuvir with and without Ribavirin in Patients with Chronic Hepatitis C Virus Genotype 1 Infection: a meta-analysis. Int J Infect Dis. 2017;55:56-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Jensen CM, Holle LM. Ledipasvir-Sofosbuvir: A Once-Daily Oral Treatment Option for Chronic Hepatitis C Virus Genotype 1 Infection. Pharmacotherapy. 2016;36:562-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Sato K, Hosonuma K, Yamazaki Y, Kobayashi T, Takakusagi S, Horiguchi N, Kakizaki S, Kusano M, Ohnishi H, Okamoto H. Combination Therapy with Ombitasvir/Paritaprevir/Ritonavir for Dialysis Patients Infected with Hepatitis C Virus: A Prospective Multi-Institutional Study. Tohoku J Exp Med. 2017;241:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Flisiak R, Janczewska E, Wawrzynowicz-Syczewska M, Jaroszewicz J, Zarębska-Michaluk D, Nazzal K, Bolewska B, Bialkowska J, Berak H, Fleischer-Stępniewska K. Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther. 2016;44:946-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Calleja JL, Crespo J, Rincón D, Ruiz-Antorán B, Fernandez I, Perelló C, Gea F, Lens S, García-Samaniego J, Sacristán B. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: Results from a Spanish real-world cohort. J Hepatol. 2017;66:1138-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 62. | Crespo J, Calleja JL, Fernández I, Sacristan B, Ruiz-Antorán B, Ampuero J, Hernández-Conde M, García-Samaniego J, Gea F, Buti M. Real-World Effectiveness and Safety of Oral Combination Antiviral Therapy for Hepatitis C Virus Genotype 4 Infection. Clin Gastroenterol Hepatol. 2017;15:945-949.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Wilfret DA, Walker J, Adkison KK, Jones LA, Lou Y, Gan J, Castellino S, Moseley CL, Horton J, de Serres M. Safety, tolerability, pharmacokinetics, and antiviral activity of GSK2336805, an inhibitor of hepatitis C virus (HCV) NS5A, in healthy subjects and subjects chronically infected with HCV genotype 1. Antimicrob Agents Chemother. 2013;57:5037-5044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Cho BW, Kim SB, Song IH, Lee SH, Kim HS, Lee TH, Kang YW, Kim SH, Lee BS, Chae HB. Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection: a retrospective multi-institutional study. Clin Mol Hepatol. 2017;23:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, Gordon SC, Flamm SL, Fried MW, Bernstein DE. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 66. | Brieva T, Rivero A, Rivero-Juarez A. Pharmacokinetic drug evaluation of velpatasvir plus sofosbuvir for the treatment of hepatitis C virus infection. Expert Opin Drug Metab Toxicol. 2017;13:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Feld JJ, Ramji A, Shafran SD, Willems B, Marotta P, Huchet E, Vachon ML, Svarovskaia ES, Huang KC, Hyland RH. Ledipasvir-Sofosbuvir Plus Ribavirin in Treatment-Naive Patients With Hepatitis C Virus Genotype 3 Infection: An Open-Label Study. Clin Infect Dis. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Ramos H, Linares P, Badia E, Martín I, Gómez J, Almohalla C, Jorquera F, Calvo S, García I, Conde P. Interferon-free treatments in patients with hepatitis C genotype 1-4 infections in a real-world setting. World J Gastrointest Pharmacol Ther. 2017;8:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 69. | Wyles DL, Rodriguez-Torres M, Lawitz E, Shiffman ML, Pol S, Herring RW, Massetto B, Kanwar B, Trenkle JD, Pang PS. All-oral combination of ledipasvir, vedroprevir, tegobuvir, and ribavirin in treatment-naïve patients with genotype 1 HCV infection. Hepatology. 2014;60:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Kanda T, Yokosuka O, Omata M. Faldaprevir for the treatment of hepatitis C. Int J Mol Sci. 2015;16:4985-4996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 72. | Jensen DM, Brunda M, Elston R, Gane EJ, George J, Glavini K, Hammond JM, Le Pogam S, Nájera I, Passe S. Interferon-free regimens containing setrobuvir for patients with genotype 1 chronic hepatitis C: a randomized, multicenter study. Liver Int. 2016;36:505-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Jiang M, Zhang EZ, Ardzinski A, Tigges A, Davis A, Sullivan JC, Nelson M, Spanks J, Dorrian J, Nicolas O. Genotypic and phenotypic analyses of hepatitis C virus variants observed in clinical studies of VX-222, a nonnucleoside NS5B polymerase inhibitor. Antimicrob Agents Chemother. 2014;58:5456-5465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Yi G, Deval J, Fan B, Cai H, Soulard C, Ranjith-Kumar CT, Smith DB, Blatt L, Beigelman L, Kao CC. Biochemical study of the comparative inhibition of hepatitis C virus RNA polymerase by VX-222 and filibuvir. Antimicrob Agents Chemother. 2012;56:830-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Vince B, Hill JM, Lawitz EJ, O’Riordan W, Webster LR, Gruener DM, Mofsen RS, Murillo A, Donovan E, Chen J. A randomized, double-blind, multiple-dose study of the pan-genotypic NS5A inhibitor samatasvir in patients infected with hepatitis C virus genotype 1, 2, 3 or 4. J Hepatol. 2014;60:920-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | de Bruijne J, Bergmann JF, Reesink HW, Weegink CJ, Molenkamp R, Schinkel J, Tong X, Li J, Treitel MA, Hughes EA. Antiviral activity of narlaprevir combined with ritonavir and pegylated interferon in chronic hepatitis C patients. Hepatology. 2010;52:1590-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Lawitz E, Poordad F, Wells J, Hyland RH, Yang Y, Dvory-Sobol H, Stamm LM, Brainard DM, McHutchison JG, Landaverde C. Sofosbuvir-velpatasvir-voxilaprevir with or without ribavirin in direct-acting antiviral-experienced patients with genotype 1 hepatitis C virus. Hepatology. 2017;65:1803-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 378] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 79. | World Health Organization. Hepatitis C, WHO fact sheet; No. 164, updated April. 2017; Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. [Cited in This Article: ] |
| 80. | Shaheen MA, Idrees M. Evidence-based consensus on the diagnosis, prevention and management of hepatitis C virus disease. World J Hepatol. 2015;7:616-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 81. | Helaly GF, Elsheredy AG, El Basset Mousa AA, Ahmed HK, Oluyemi AE. Seronegative and occult hepatitis C virus infections in patients with hematological disorders. Arch Virol. 2017;162:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Easterbrook PJ, Roberts T, Sands A, Peeling R. Diagnosis of viral hepatitis. Curr Opin HIV AIDS. 2017;12:302-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 83. | Carreño V. Seronegative occult hepatitis C virus infection: clinical implications. J Clin Virol. 2014;61:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | De Marco L, Manzini P, Trevisan M, Gillio-Tos A, Danielle F, Balloco C, Pizzi A, De Filippo E, D’Antico S, Violante B. Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease. PLoS One. 2012;7:e43541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Jain P, Nijhawan S. Occult hepatitis C virus infection is more common than hepatitis B infection in maintenance hemodialysis patients. World J Gastroenterol. 2008;14:2288-2289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Carreño V, Bartolomé J, Castillo I, Quiroga JA. New perspectives in occult hepatitis C virus infection. World J Gastroenterol. 2012;18:2887-2894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, Taherkhani S, Nejadbolkheyr A. Prevalence and Trends of Transfusion-Transmissible Viral Infections among Blood Donors in South of Iran: An Eleven-Year Retrospective Study. PLoS One. 2016;11:e0157615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Baumert TF, Fauvelle C, Chen DY, Lauer GM. A prophylactic hepatitis C virus vaccine: a distant peak still worth climbing. J Hepatol. 2014;61:S34-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Abdelwahab KS, Ahmed Said ZN. Status of hepatitis C virus vaccination: Recent update. World J Gastroenterol. 2016;22:862-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Zingaretti C, De Francesco R, Abrignani S. Why is it so difficult to develop a hepatitis C virus preventive vaccine? Clin Microbiol Infect. 2014;20 Suppl 5:103-109. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Forns X, Bukh J, Purcell RH. The challenge of developing a vaccine against hepatitis C virus. J Hepatol. 2002;37:684-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Man John Law L, Landi A, Magee WC, Lorne Tyrrell D, Houghton M. Progress towards a hepatitis C virus vaccine. Emerg Microbes Infect. 2013;2:e79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol. 2015;21:11984-12002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Dunlop J, Owsianka A, Cowton V, Patel A. Current and future prophylactic vaccines for hepatitis C virus. Vaccine: Development and Therapy. 2015;2015:31-44. [Cited in This Article: ] |
| 95. | Yu CI, Chiang BL. A new insight into hepatitis C vaccine development. J Biomed Biotechnol. 2010;2010:548280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10:659-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 97. | Naderi M, Gholipour N, Zolfaghari MR, Moradi Binabaj M, Yegane Moghadam A, Motalleb G. Hepatitis C virus and vaccine development. Int J Mol Cell Med. 2014;3:207-215. [PubMed] [Cited in This Article: ] |
| 98. | Ogholikhan S, Schwarz KB. Hepatitis Vaccines. Vaccines (Basel). 2016;4:E6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Balasco N, Barone D, Sandomenico A, Ruggiero A, Doti N, Berisio R, Ruvo M, Vitagliano L. Structural versatility of hepatitis C virus proteins: implications for the design of novel anti-HCV intervention strategies. Curr Med Chem. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Li S, Plebanski M, Smooker P, Gowans EJ. Editorial: Why Vaccines to HIV, HCV, and Malaria Have So Far Failed-Challenges to Developing Vaccines Against Immunoregulating Pathogens. Front Microbiol. 2015;6:1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 421] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 102. | Verstrepen BE, Depla E, Rollier CS, Mares G, Drexhage JA, Priem S, Verschoor EJ, Koopman G, Granier C, Dreux M. Clearance of genotype 1b hepatitis C virus in chimpanzees in the presence of vaccine-induced E1-neutralizing antibodies. J Infect Dis. 2011;204:837-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | Leroux-Roels G, Depla E, Hulstaert F, Tobback L, Dincq S, Desmet J, Desombere I, Maertens G. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine. 2004;22:3080-3086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367-6373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 105. | Nevens F, Roskams T, Van Vlierberghe H, Horsmans Y, Sprengers D, Elewaut A, Desmet V, Leroux-Roels G, Quinaux E, Depla E. A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology. 2003;38:1289-1296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 106. | Wedemeyer H, Mazur W, Nevens F, Horsmans Y, Adler M, Blum H, Inglot M, Gerken G, Janczewska E, Roskams T. Factors influencing progression of liver fibrosis in patients with chronic hepatitis C: results of the 3-year T2S-918-HCV study with HCVE1 therapeutic vaccine. J Hepatol. 2008;48:S27-S28. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Drane D, Maraskovsky E, Gibson R, Mitchell S, Barnden M, Moskwa A, Shaw D, Gervase B, Coates S, Houghton M. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum Vaccin. 2009;5:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Habersetzer F, Baumert TF, Stoll-Keller F. GI-5005, a yeast vector vaccine expressing an NS3-core fusion protein for chronic HCV infection. Curr Opin Mol Ther. 2009;11:456-462. [PubMed] [Cited in This Article: ] |
| 109. | Pockros P, Jacobson I, Boyer TD, Schiff ER, Everson GT, Lee WM, Vierling JM, Lawitz E, Kugelmas M, Tsai N. GI-5005 Therapeutic vaccine plus Peg-IFN/Ribavirin improves sustained virologic response versus Peg-IFN/Ribavirin in prior non-responders with genotype 1 chronic HCV infection. Hepatology. 2010;52:404A-405A. [Cited in This Article: ] |
| 110. | Yutani S, Komatsu N, Shichijo S, Yoshida K, Takedatsu H, Itou M, Kuromatu R, Ide T, Tanaka M, Sata M. Phase I clinical study of a peptide vaccination for hepatitis C virus-infected patients with different human leukocyte antigen-class I-A alleles. Cancer Sci. 2009;100:1935-1942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Yutani S, Yamada A, Yoshida K, Takao Y, Tamura M, Komatsu N, Ide T, Tanaka M, Sata M, Itoh K. Phase I clinical study of a personalized peptide vaccination for patients infected with hepatitis C virus (HCV) 1b who failed to respond to interferon-based therapy. Vaccine. 2007;25:7429-7435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Gowans EJ, Roberts S, Jones K, Dinatale I, Latour PA, Chua B, Eriksson EM, Chin R, Li S, Wall DM. A phase I clinical trial of dendritic cell immunotherapy in HCV-infected individuals. J Hepatol. 2010;53:599-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Firbas C, Jilma B, Tauber E, Buerger V, Jelovcan S, Lingnau K, Buschle M, Frisch J, Klade CS. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine. 2006;24:4343-4353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 114. | Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, Blum H, Buschle M, Jelovcan S, Buerger V. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134:1385-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 115. | Firbas C, Boehm T, Buerger V, Schuller E, Sabarth N, Jilma B, Klade CS. Immunogenicity and safety of different injection routes and schedules of IC41, a Hepatitis C virus (HCV) peptide vaccine. Vaccine. 2010;28:2397-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Klade CS, Schuller E, Boehm T, von Gabain A, Manns MP. Sustained viral load reduction in treatment-naive HCV genotype 1 infected patients after therapeutic peptide vaccination. Vaccine. 2012;30:2943-2950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Elmowalid GA, Qiao M, Jeong SH, Borg BB, Baumert TF, Sapp RK, Hu Z, Murthy K, Liang TJ. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci USA. 2007;104:8427-8432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 118. | Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Wedemeyer H, Gagneten S, Davis A, Bartenschlager R, Feinstone S, Rehermann B. Oral immunization with HCV-NS3-transformed Salmonella: induction of HCV-specific CTL in a transgenic mouse model. Gastroenterology. 2001;121:1158-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Park SH, Shin EC, Capone S, Caggiari L, De Re V, Nicosia A, Folgori A, Rehermann B. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012;143:1048-60.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Fattori E, Zampaglione I, Arcuri M, Meola A, Ercole BB, Cirillo A, Folgori A, Bett A, Cappelletti M, Sporeno E. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming-boosting with novel adenoviral vectors based on different serotypes. Gene Ther. 2006;13:1088-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Youn JW, Hu YW, Tricoche N, Pfahler W, Shata MT, Dreux M, Cosset FL, Folgori A, Lee DH, Brotman B. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896-10905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 124. | Barnes E, Antonella F, Aston S, Smith K, Brown AC, Capone S, Ambrosio M, Ammendola V, Bartiromo M, Traboni C. Phase I trial of a highly immunogenic T-cell vaccine for hepatitis C virus based on novel adenoviral vectors from rare serotypes. Hepatology. 2009;50:397A-398A. [Cited in This Article: ] |
| 125. | Habersetzer F, Zarski J-P, Leroy V, Maynard-Muet M, Bronowicki J-P, Feray C, Hezode C, Fournillier A, Bain C, Inchauspe G, Honnet G, Trepo C. A novel vectorized HCV therapeutic vaccine (TG4040): results of a Phase I study in naive patients chronically infected by HCV. 44th Annual Meeting of the European Association for the Study of the Liver. 2009;April 23-26-Copenhagen, Denmark. [Cited in This Article: ] |
| 126. | Page K, Yu M, Cohen J, Evans J, Shumway M, Riley ED. HCV screening in a cohort of HIV infected and uninfected homeless and marginally housed women in San Francisco, California. BMC Public Health. 2017;17:171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Di Bisceglie AM, Janczweska-Kazek E, Habersetzer F, Mazur W, Stanciu C, Carreno V, Tanasescu C, Flisiak R, Romero-Gomez M, Fich A. Efficacy of immunotherapy with TG4040, peg-interferon, and ribavirin in a Phase 2 study of patients with chronic HCV infection. Gastroenterology. 2014;147:119-131.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Forns X, Payette PJ, Ma X, Satterfield W, Eder G, Mushahwar IK, Govindarajan S, Davis HL, Emerson SU, Purcell RH. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 129. | Youn JW, Park SH, Lavillette D, Cosset FL, Yang SH, Lee CG, Jin HT, Kim CM, Shata MT, Lee DH. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42:1429-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 130. | Rollier CS, Paranhos-Baccala G, Verschoor EJ, Verstrepen BE, Drexhage JA, Fagrouch Z, Berland JL, Komurian-Pradel F, Duverger B, Himoudi N. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 131. | Alvarez-Lajonchere L, Shoukry NH, Grá B, Amador-Cañizares Y, Helle F, Bédard N, Guerra I, Drouin C, Dubuisson J, González-Horta EE. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a Phase I clinical trial. J Viral Hepat. 2009;16:156-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Sallberg M, Frelin L, Diepolder H, Jung MC, Mathiesen I, Fons M, Hultcrantz R, Carlsson T, Weiland O. A first clinical trial of therapeutic vaccination using naked DNA delivered by in vivo electroporation shows antiviral effects in patients with chronic hepatitis C. 44th Annual Meeting of the European Association for the Study of the Liver;. 2009;April 23-26; Copenhagen, Denmark. [Cited in This Article: ] |
| 133. | Dillon JF, Lazarus JV, Razavi HA. Urgent action to fight hepatitis C in people who inject drugs in Europe. Hepatol Med Policy. 2016;1:2. [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 134. | Kottilil S, Wright M, Polis MA, Masur H. Treatment of hepatitis C virus infection: is it time for the internist to take the reins? Ann Intern Med. 2014;161:443-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Seo KI, Yun BC, Li WJ, Lee SU, Han BH, Park ET. Barriers to treatment of failed or interferon ineligible patients in the era of DAA: single center study. Clin Mol Hepatol. 2017;23:74-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 136. | McCance-Katz EF, Valdiserri RO. Hepatitis C Virus Treatment and Injection Drug Users: It Is Time to Separate Fact From Fiction. Ann Intern Med. 2015;163:224-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 137. | Robaeys G, Bielen R, Azar DG, Razavi H, Nevens F. Global genotype distribution of hepatitis C viral infection among people who inject drugs. J Hepatol. 2016;65:1094-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Lim SG, Aghemo A, Chen PJ, Dan YY, Gane E, Gani R, Gish RG, Guan R, Jia JD, Lim K. Management of hepatitis C virus infection in the Asia-Pacific region: an update. Lancet Gastroenterol Hepatol. 2017;2:52-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Moradpour D, Grakoui A, Manns MP. Future landscape of hepatitis C research - Basic, translational and clinical perspectives. J Hepatol. 2016;65:S143-S155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 140. | Grebely J, Bruggmann P, Treloar C, Byrne J, Rhodes T, Dore GJ; International Network for Hepatitis in Substance Users. Expanding access to prevention, care and treatment for hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26:893-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Taherkhani R, Farshadpour F. Lurking epidemic of hepatitis C virus infection in Iran: A call to action. World J Hepatol. 2017;9:1040-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Moyer VA; U. S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 143. | Aggarwal R, Chen Q, Goel A, Seguy N, Pendse R, Ayer T, Chhatwal J. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLoS One. 2017;12:e0176503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 144. | Davis SM, Daily S, Kristjansson AL, Kelley GA, Zullig K, Baus A, Davidov D, Fisher M. Needle exchange programs for the prevention of hepatitis C virus infection in people who inject drugs: a systematic review with meta-analysis. Harm Reduct J. 2017;14:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Midgard H, Weir A, Palmateer N, Lo Re V 3rd, Pineda JA, Macías J, Dalgard O. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65:S33-S45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 146. | U.S. Department of Health and Human Services. Combating the Silent Epidemic of Viral Hepatitis: Action Plan for the Prevention, Care, and Treatment of Viral Hepatitis. Technical consultation: hepatitis C virus infection in young persons who inject drugs. 2013. Available from: https://hepfree.nyc/wp-content/uploads/2014/04/hcv-and-young-pwid-consultation-report.pdf?x77964. [Cited in This Article: ] |
| 147. | Grebely J, Dore GJ. Prevention of hepatitis C virus in injecting drug users: a narrow window of opportunity. J Infect Dis. 2011;203:571-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 148. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 1066] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 149. | Dias PT, Hahn JA, Delwart E, Edlin B, Martin J, Lum P, Evans J, Kral A, Deeks S, Busch MP. Temporal changes in HCV genotype distribution in three different high risk populations in San Francisco, California. BMC Infect Dis. 2011;11:208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 150. | Yan Z, Fan K, Wang Y, Fan Y, Tan Z, Deng G. Changing pattern of clinical epidemiology on hepatitis C virus infection in southwest china. Hepat Mon. 2012;12:196-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 19] [Reference Citation Analysis (0)] |
| 151. | Morris MD, Shiboski S, Bruneau J, Hahn JA, Hellard M, Prins M, Cox AL, Dore G, Grebely J, Kim AY, Lauer GM, Lloyd A, Rice T, Shoukry N, Maher L, Page K; International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3). Geographic Differences in Temporal Incidence Trends of Hepatitis C Virus Infection Among People Who Inject Drugs: The InC3 Collaboration. Clin Infect Dis. 2017;64:860-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 152. | Oliveira ML, Yoshida CF, Telles PR, Hacker MA, Oliveira SA, Miguel JC, do O KM, Bastos FI. Trends in HCV prevalence, risk factors and distribution of viral genotypes in injecting drug users: findings from two cross-sectional studies. Epidemiol Infect. 2009;137:970-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |