Published online Sep 8, 2017. doi: 10.4254/wjh.v9.i25.1073
Peer-review started: Janaury 7, 2017
First decision: April 6, 2017
Revised: April 29, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: September 8, 2017
To characterize peripheral blood natural killer (NK) cells phenotypes by flow cytometry as potential biomarker of liver fibrosis in human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfected patients.
Peripheral mononuclear cells from 24 HIV/HCV (HBV negative) coinfected and 5 HIV/HCV/HBV seronegative individuals were evaluated. HIV/HCV coinfected patients were divided in to groups: G1, patients with METAVIR F0-F2 and G2, patients with METAVIR F3-F4. NK surface cell staining was performed with: Anti-CD3(APC/Cy7), anti-CD56(PE/Cy5), anti-CD57(APC), anti-CD25(PE), anti-CD69(FITC), anti-NKp30(PE), anti-NKp46(PE/Cy7), anti-NKG2D(APC), anti-DNAM(FITC); anti-CD62L (PE/Cy7), anti-CCR7(PE), anti-TRAIL(PE), anti-FasL(PE), anti CD94(FITC). Flow cytometry data acquisition was performed on BD FACSCanto, analyzed using FlowJo software. Frequency of fluorescence was analyzed for all single markers. Clinical records were reviewed, and epidemiological and clinical data were obtained.
Samples from 11 patients were included in G1 and from 13 in G2. All patients were on ARV, with undetectable HIV viral load. Liver fibrosis was evaluated by transient elastography in 90% of the patients and with biopsy in 10% of the patients. Mean HCV viral load was (6.18 ± 0.7 log10). Even though, no major significant differences were observed between G1 and G2 regarding NK surface markers, it was found that patients with higher liver fibrosis presented statistically lower percentage of NK cells than individual with low to mild fibrosis and healthy controls (G2: 5.4% ± 2.3%, G1: 12.6% ± 8.2%, P = 0.002 and healthy controls 12.2% ± 2.7%, P = 0.008). It was also found that individuals with higher liver fibrosis presented lower CD4 LT count than those from G1 (G2: 521 ± 312 cells/μL, G1: 770 ± 205 cells/μL; P = 0.035).
Higher levels of liver fibrosis were associated with lower percentage of NK cells and LTCD4+ count; and they may serve as noninvasive biomarkers of liver damage.
Core tip: Approximately 2.3 million individuals with human immunodeficiency virus are coinfected with hepatitis C virus (HCV). The high cost of HCV treatment restricts its use. It is crucial to identify patients with advanced liver fibrosis with an urgent need of treatment. The aim of this study was to identify natural killer (NK) phenotypes as a biomarker for liver fibrosis. We observed that those subjects with higher fibrosis are those with lower percentage of NK cells and also with lower LTCD4+ count. These constitute two simple parameters that might be performed in a routine laboratory test and used in clinical practice as biomarkers for liver fibrosis.
- Citation: Laufer N, Ojeda D, Polo ML, Martinez A, Pérez H, Turk G, Cahn P, Zwirner NW, Quarleri J. CD4+ T cells and natural killer cells: Biomarkers for hepatic fibrosis in human immunodeficiency virus/hepatitis C virus-coinfected patients. World J Hepatol 2017; 9(25): 1073-1080
- URL: https://www.wjgnet.com/1948-5182/full/v9/i25/1073.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i25.1073
Chronic hepatitis C virus (HCV) infection affects 115 million individuals worldwide and is a common cause of chronic hepatitis, which may eventually progress to cirrhosis and hepatocellular carcinoma[1]; whereas currently 36.9 million people are living with human immunodeficiency virus (HIV)/aids [2]. Because of overlapping pathways of transmission, approximately 2.3 million individuals worldwide are estimated to be coinfected with both viruses[3]. Direct antiviral agents (DAA) are a major development in the treatment of HCV infection, with cure rates higher than 90%[4]. However, the high cost of DAA regimens and competing public health priorities have prompted a worldwide discussion whether all patients should have access to the new therapies without restriction. In many countries, new DAA regimens are therefore reserved for patients with advanced fibrosis or cirrhosis[5,6].
Liver fibrosis is a response to a wound-healing process triggered by various types of chronic liver injuries, among them HCV infection[7]. Liver fibrosis is well characterized by abnormal accumulation of extracellular matrix, and hepatic stellate cells (HSCs) are considered to be the major type of cells responsible for liver fibrosis. Such profibrotic role might be down-regulated by natural killer (NK) cells either directly through induction of HSC apoptosis or indirectly via production of IFN-γ. Increased peripheral NK cell-mediated cytotoxicity has been associated with less liver fibrosis during HCV infection and likely reflects this mechanism[7]. HIV infection per se has a strong suppressive effect on anti-HCV activity of NK cells[8].
NK cells are lymphoid cells that are primary responders to microbial infections and tumor cells[9]. Phenotypically, NK cells are defined as CD3-CD56+cells with variable expression of CD16, depending on cell subpopulation of NK cells. They comprise approximately 5%-20% of peripheral lymphoid cells, but up to 30%-50% of intrahepatic lymphoid cells. NK cell activation is regulated by cell surface receptors that become engaged by cognate ligands expressed on target cells by cytokines, and by Toll-like receptors (TLRs)[9-11].
Different techniques to asses liver fibrosis have been developed, from liver biopsy (gold standard) to non-invasive studies (transient liver elastography; patented and nonpatented biomarkers - FIB4, FibroTest, APRI, etc). Liver biopsy is invasive and has risk of complications[12]. In addition, liver biopsy may be limited by the size of the specimen obtained as well as sampling, intraobserver, and interobserver variability[13]. On the other hand, there are many unresolved issues regarding the accuracy (especially in HIV/HCV-coinfected patients) of noninvasive studies[14], and in low-resource countries there is an important barrier to access to these methods (in particular liver elastography and patented biomarkers).
The identification of noninvasive liver fibrosis biomarkers is still an open research area. In this context, we reasoned that the study of the phenotype of peripheral blood cells may unravel interesting clues towards the identification of such biomarkers. Some evidence indicates that the characteristics of the immune cells, including NK cells, observed in peripheral blood are similar to those seen in liver with relatively lower levels of magnitude. Accordingly, the aim of this study was to characterize peripheral blood NK cell phenotypes by flow cytometry as potential biomarker for liver fibrosis in patients chronically coinfected with hepatitis C and HIV.
Informed consent was obtained from each subject. The study protocol is in line with the ethical guidelines of the Declaration of Helsinki and was approved by the ethics review committee of Fundación Huésped (Buenos Aires, Argentina).
Cryopreserved peripheral blood mononuclear cells (PBMC) from 24 HIV/HCV-coinfected individuals and 5 HIV/HCV-seronegative individuals (healthy controls, HC) were used in this study. HIV/HCV-coinfected patients and healthy control individuals enrolled in this study were not acutely or chronically infected with HBV; they denied current use of recreational drugs or alcohol intake. HIV/HCV-coinfected patients were divided into two groups based on their level of liver fibrosis (group 1: Patients with METAVIR score F0 to F2 on liver biopsy or transient elastography - FibroScan®-; and group 2: Patients with METAVIR score F3-F4). Hepatic fibrosis was evaluated by liver biopsy in 10% of patients and by transient hepatic elastography in 90% of patients. All healthy control individuals presented F0-F1 fibrosis according to transient liver elastography (less than 5 kPa). Clinical records were reviewed, and epidemiological and clinical data were obtained.
Cryopreserved PBMC were thawed and stained with fluorochrome-conjugated antibodies distributed in five different panels (depending on PBMC availability) to evaluate expression of different markers on NK cells detailed in Table 1. Staining was performed for 30 min at 4 °C. Samples were washed, fixed in 1% paraformaldehyde and acquired in a FACS Canto flow cytometer (BD Biosciences). Data were analyzed using the FlowJo software (TreeStar, Ashland, Oregon, United States). NK cell populations were defined according to the corresponding isotype control.
| Antibody | Fluorochrome | Clone | Provider |
| All panels | |||
| Anti-CD3 | APC/Cy7 | SK7 | BioLegend |
| Anti-CD56 | PE/Cy5 | 679.1Mc7 | Beckman Coulter |
| Panel 1 | |||
| Anti-CD57 | APC | HNK-1 | BioLegend |
| Anti-CD25 | PE | BC96 | BioLegend |
| Anti-CD69 | FITC | FN50 | BioLegend |
| Panel 2 | |||
| Anti-NKp30 | PE | P30-15 | Biolegend |
| Anti-NKp46 | PE/Cy7 | 9E2 | Biolegend |
| Anti-NKG2D | APC | 1D11 | Biolegend |
| Anti-DNAM | FITC | TX25 | Biolegend |
| Panel 3 | |||
| Anti-CD62L | PE/Cy7 | DREG-56 | Biolegend |
| Anti-CCR7 | PE | G043H7 | Biolegend |
| Panel 4 | |||
| Anti-TRAIL | PE | S35-934 | BD Bioscience |
| Panel 5 | |||
| Anti-FasL | PE | NOK-1 | Biolegend |
| Anti CD94 | FITC | DX22 | Biolegend |
Plasma viral load levels (Abbott RealTime HIV-1 RNA version 3; Abbott Molecular, Inc., Des Plaines, IL, United States) were assessed in HIV-infected subjects and CD4+ T-cell counts (flow cytometry double platform, BD FACSCanto; BD Biosciences, San Diego/California, United States) were assessed in HIV and HIV-negative individuals.
For categorical variables, both χ2 and Fisher’s exact test were applied. For continuous variables, the nonparametric Kruskal-Wallis and Mann-Whitney test were used. Area under the receiving operating curve (ROC) was used to calculate the cut-off point in NK cell percentage with the best sensitivity of high liver fibrosis. Statistical analyses were performed using the Statistical Package for the Social Sciences software version 19.0 (SPSS Inc., Chicago, IL, United States).
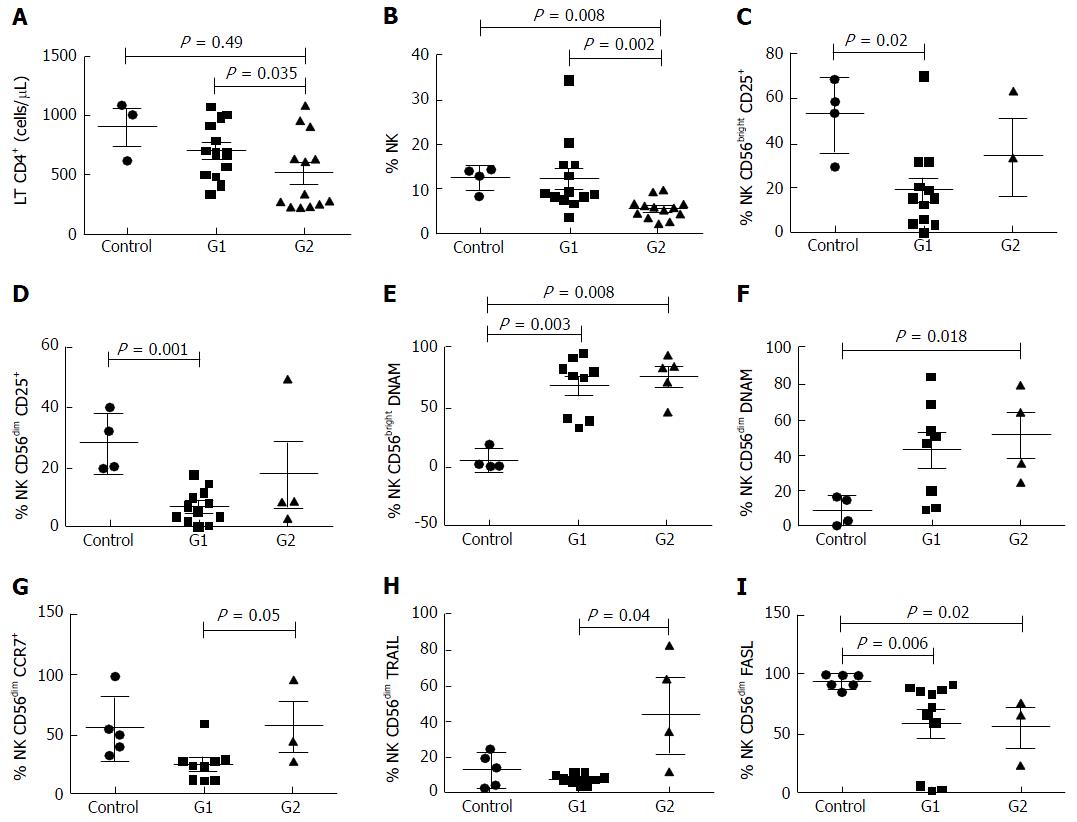
Patient characteristics at the time of liver fibrosis assessment are shown in Table 2. Individuals from Group 1 (n = 11, 46%) presented low to mild liver fibrosis (METAVIR F0-F2) whereas patients included in Group 2 (n = 13, 54%) had severe fibrosis (METAVIR F3-F4). Forty percent of patients had previously received HCV treatment with pegylated interferon and ribavirin (with no differences between groups); a median of 6.25 ± 1.48 years before sample collection; none of them achieved sustained virological response. The mean age was 46.9 years (± 8.4); 83% were male. Patients from group 2 were older than those with lower METAVIR score (P = 0.028). No differences were found between groups regarding gender or mean time of known HIV and HCV infection. The mean HCV viral load was 6.18 ± 0.70 log, with no differences between the two groups (G1: 6.54 ± 0.24; G2: 6.18 ± 0.7). HCV genotype 1 was identified in 90% of the patients, the rest presented infection by genotype 3. All patients were on antiretroviral treatment with undetectable HIV viral load, with no differences in the time on ARV therapy between groups. Patients with higher fibrosis presented lower CD4+ T cell count (521 ± 312 cells/μL) than those from group 1 (770 ± 205 cells/μL, P = 0.035) There was no difference in the CD4+ T cell count between group 1 and healthy controls (P = 0.49).
| Characteristics | Group 1 | Group 2 | Control | P value |
| n = 11 | n = 13 | n = 5 | ||
| Age (yr)1 | 46.3 (3.9) | 52.2 (4.5) | 31 (4.8) | 0.02 |
| Male/female | 9/2 | 11/2 | 3/2 | 0.85 |
| CD4 cell count1 | 770 (205) | 521 (312) | 910 (251) | 0.03 |
| CD8 cell count1 | 1079 (475) | 657 (339) | NA | 0.24 |
| METAVIR F0-F2 | 100% | 0 | 100% | NC |
| METAVIR F3-F4 | 0 | 100% | 0 | NC |
| TGP1 | 79.7 (74.2) | 99 (71.4) | NA | 0.41 |
| Time of known HIV infection1 | 18 (6.52) | 17.3 (3.8) | NC | 0.60 |
| Time of known HCV infection1 | 14.3 (7.0) | 13.8 (4.6) | NC | 0.65 |
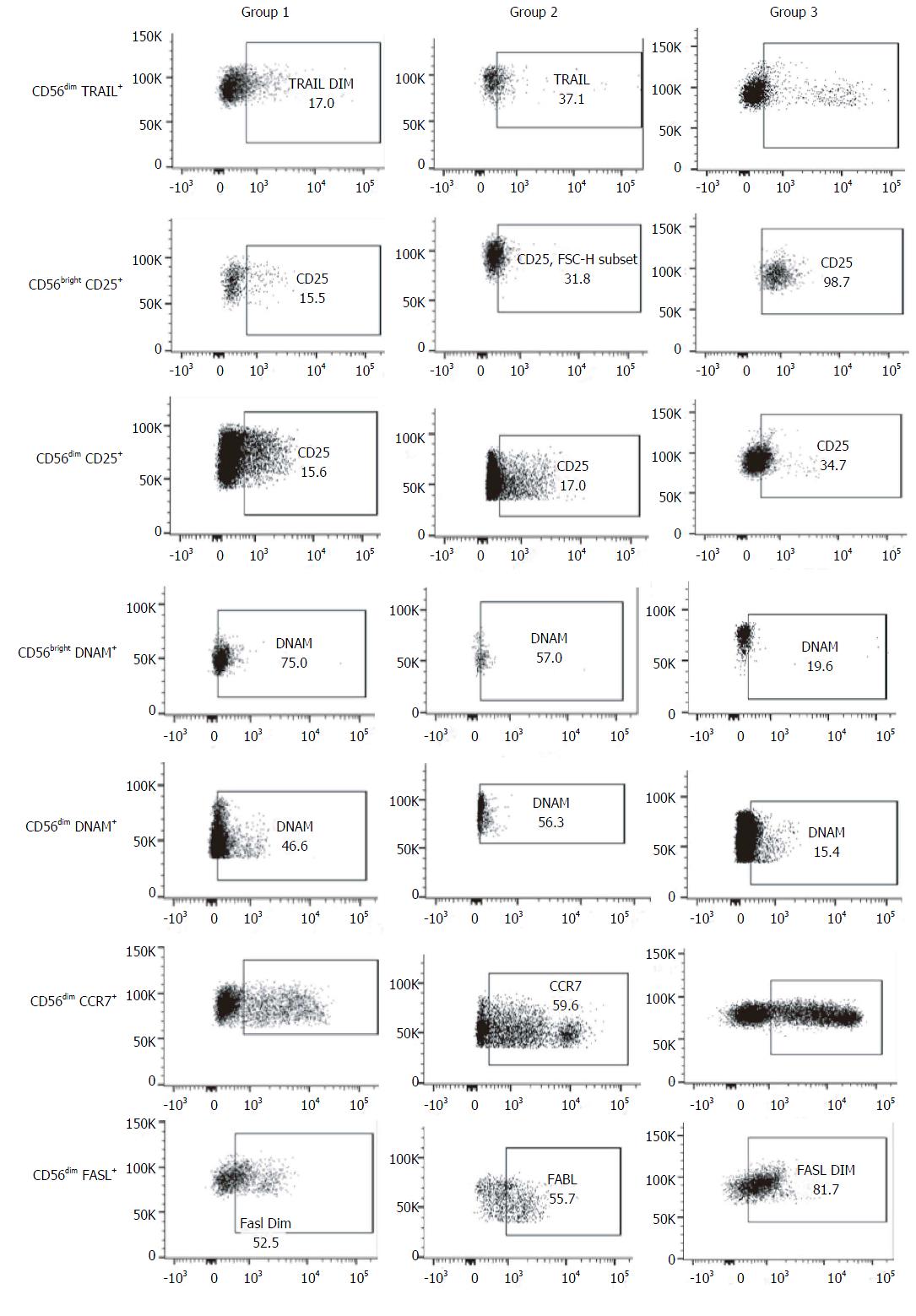
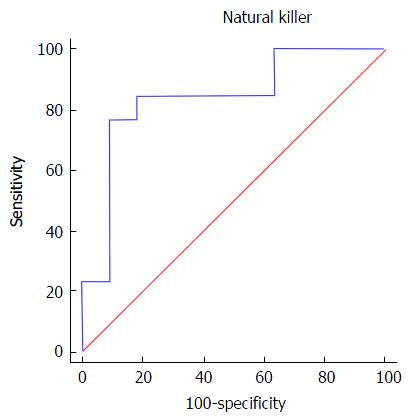
Regarding NK cells, a lower percentage was found in samples from patients of group 2 (5.4% ± 2.3%) compared both with patients from group 1 (12.6% ± 8.2%, P = 0.002) and healthy controls (12.2% ± 2.7%, 0.008) (Figures 1 and 2). With ROC curve analysis a cut-off of a NK cell percentage lower than 6.6% was determined to have 90% sensitivity and 77% specificity to predict the presence of METAVIR F3-F4 (Figure 3).
The percentage of CD56bright NK cells (G1: 11.7% ± 8.0%, G2: 7.1% ± 4.0%, HC: 6.8% ± 3.6%) and CD56dim NK cells (G1: 88.2% ± 7.6%, G2: 73.7% ± 40.1%, HC: 92.9% ± 3.6%) did not present differences among the three groups studied.
As the function of NK is regulated by an array of activating and inhibitory receptors, we also evaluated the NK cell activating receptors[15] NKp46 (CD335), NKp30 (CD337), NKG2D (CD314) and DNAM (CD226), the activation markers CD69 and CD25, and other molecules involved in NK cell effector functions, terminal differentiation and cytotoxicity such as CD94, TRAIL, CD57[16], Fas-L (CD178), CCR7 (CD197) and CD62L.
When compared with healthy controls, samples from patients included in group 2 presented a higher frequency of CD56bright DNAM-1+ NK cells (76.2% ± 18.5% vs 4.6% ± 8.5%, P = 0.008) and CD56dim DNAM-1+ NK cells (42% ± 29% vs 6.0% ± 8.1%, P = 0.018). The same differences were observed between group 1 and healthy controls, both in the percentage of CD56bright DNAM-1+ NK cells (71.2% ± 23% vs 4.6% ± 8.5%, P = 0.003) and in the percentage of CD56dim DNAM-1+ NK cells (41% ± 28% vs 6.0% ± 8.1%, P = 0.013).
Additionally, samples from group 1 exhibited higher percentage of CD56bright CD25+ NK cells (53.1% ± 16.6% vs 19.4% ± 18.9%, P = 0.029) and CD56dim CD25+ NK cells (28.3% ± 10.2% vs 7.1% ± 5.6%, P = 0.001) than healthy controls. These results show the possible consequence of a higher activation degree in NK cells from subjects with chronic infection. Of note, there were no differences in the frequency of these NK cells subsets between group 1 and 2. Moreover, no differences were observed in the other activator molecules evaluated (NKp46, NKp30, NKG2D, CD69) neither between group 1 and 2 nor between controls and HCV-infected subjects.
No differences in surface expression of CD94 were observed between the 3 groups. The frequency of these molecules was very high in CD56bright NK cells in all the samples evaluated (G1: 77.1% ± 28.2%, G2: 91.7% ± 1.2%, controls: 77.4% ± 36.7%), whereas in CD56dim NK cells this molecule was stained in less than 50% (G1: 48.4% ± 21.4%, G2: 36.9% ± 19.7%, controls: 31.6% ± 16.9%).
The frequency of CD56dim TRAIL+ NK cells was higher in samples from group 2 than those from group 1 (29.4% ± 31.7% vs 7.5% ± 3.1%, P = 0.04), while no differences were observed between coinfected patients and healthy controls.
Nevertheless, the percentage of CD56dim FasL+ NK cells was lower in samples from HCV/HIV-coinfected patients (G1: 27.2% ± 19.8%, P = 0.001; G2: 36.9% ± 19.7%, P = 0.01) than those from healthy controls (69.3% ± 18.2%), without detecting differences between groups 1 and 2.
In addition, there was a trend towards a higher percentage of CD56dim CCR7+ NK cells in samples from patients with advanced fibrosis than in samples from patients with lower fibrosis (G2: 56.4% ± 36.2% vs G1: 24.4% ± 14.6%; P = 0.05). Regarding the CD62L expression, there were no differences in CD56bright NK cells between groups (G1: 61.8% ± 24.9%; G2: 87% ± 15.1%; P = 0.09).
In this study we found that patients with advanced fibrosis presented lower LT CD4+ cell counts than subjects with low to mild fibrosis. All the patients were on successful antiretroviral treatment. Even though there are controversial data whether the presence of HCV is a factor that alters LT CD4 recovery with ARV, it can be hypothesized that patients with higher chances to develop liver fibrosis are those with lower LT CD4+ cell recovery after HIV treatment. Such a poor HAART-mediated LT CD4+ cell recovery may contribute to an impaired stimulation of NK cells, and consequently a diminished anti-fibrotic activity by their action on hepatic stellate cells, favoring an accelerated liver fibrosis progression in HIV/HCV patients[17]. Yi et al[18] and other groups have observed that NK cells negatively regulated liver fibrosis. NK cells isolated from HCV-infected patients efficiently induced apoptosis of activated HSCs in TRAIL-, FasL-, and NKG2D-dependent manners[19]. NKp46high NK cell subset potentially suppresses HCV replication and HCV-associated liver damage, leading to amelioration of liver fibrosis.
It has been described that HIV/HCV coinfection can modulate the peripheral NK phenotype[20]. In our study, we also observed differences in the NK phenotype particularly between control and HIV/HCV-coinfected patients which resemble those reported previously[21,22]. We found a lower percentage of CD56dim FasL+ NK cells in HCV/HIV-coinfected patients compared to healthy controls. This finding could reflect a lower NK cell capacity to exert cytotoxic activity in patients with chronic HIV and HCV infection compared to non-infected individuals that could ultimately lead to a decreased capacity to regulate HSC.
Regarding HIV/HCV-coinfected individuals, no differences were observed in NK cell phenotypes according to the different degrees of liver fibrosis. Nevertheless, we could observe a statistically significant difference in the percentage of peripheral blood NK cells in patients with high scores compared to patients with low liver fibrosis. Patients with advanced fibrosis have lower percentage of NK cells than those with low fibrosis scores. Moreover, we observed that a percentage of NK cells lower than 6.6% had 90% sensitivity and 77% specificity to predict the presence of advance fibrosis (METAVIR F3-F4). This observation could indicate, for the first time, that the evaluation of the NK cells compartment is a potential biomarker for fibrosis staging in HIV/HCV-coinfected patients.
In the era of direct antiviral agents with high efficacy for the treatment of chronic HCV, one of the main treatment access barriers for many patients is the high cost of these drugs, and where these barriers exist the assessment of liver fibrosis is mandatory to ensure treatment access. In this study, we have observed that those subjects with higher fibrosis are those with lower absolute count both of LT CD4+ and lower percentage of NK cells. Although additional research is needed to confirm our findings, the evaluation of these two parameters that can be performed in a routine laboratory test may be helpful in improving the available noninvasive methods for liver fibrosis staging.
Different techniques to assess liver fibrosis have been developed, from liver biopsy (gold standard) to non-invasive studies (transient liver elastography; patented and non-patented biomarkers - FIB4, FibroTest, APRI, etc). Liver biopsy is invasive and has risk of complications. In addition, liver biopsy may be limited by the size of the specimen obtained as well as sampling, intra and inter-observer variability. On the other hand, there are many unresolved issues regarding the accuracy [especially in human immunodeficiency virus (HIV)/ hepatitis C virus (HCV)-coinfected patients] of noninvasive studies, and in low-resource countries there is an important barrier to access to these methods (in particular liver elastography and patented biomarkers).
The identification of non-invasive liver fibrosis biomarkers is still an open research area. In this context, the authors reasoned that the study of the phenotype of peripheral blood cells may unravel interesting clues towards the identification of such biomarkers. Some evidence indicates that the characteristics of the immune cells, including natural killer (NK) cells, observed in peripheral blood are similar to those seen in liver with relatively lower levels of magnitude. Accordingly, the aim of this study was to characterize peripheral blood NK cell phenotypes by flow cytometry as potential biomarker for liver fibrosis in patients chronically coinfected with hepatitis C and HIV.
In the era of direct antiviral agents with high efficacy for the treatment of chronic HCV, one of the main treatment access barriers for many patients is the high cost of these drugs, and where these barriers exist, the assessment of liver fibrosis is mandatory to ensure treatment access. In this study, the authors have observed that those subjects with higher fibrosis are those with lower absolute count both of LT CD4+ and lower percentage of NK cells. Although additional research is needed to confirm their findings, the evaluation of these two parameters that can be performed in a routine laboratory test may be helpful in improving the available noninvasive methods for liver fibrosis staging.
The data in this study suggested that LTCD4 and NK cells could be used as potential non-invasive biomarkers of the level of liver fibrosis in HIV-HCV coinfected patients. These parameters could improve the accuracy of the available non-invasive methods to measure liver fibrosis.
NK cells, and CD4 T lymphocytes (LTCD4) are involved in the immunological control of hepatic stellate cells that are the responsible of liver fibrosis development.
The identification of noninvasive liver fibrosis biomarkers is still an open research area. NK cells and LTCD4+ count; are two simple parameters, that might be perform in a routine laboratory test and may serve as noninvasive biomarkers of liver fibrosis, identifying patients in need for HCV therapy in the short term.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tsoulfas G S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1325] [Cited by in F6Publishing: 1311] [Article Influence: 131.1] [Reference Citation Analysis (0)] |
| 2. | UNAIDS. How AIDS changes everything-MDG 6: 15 lessons of hope from the AIDS response. UNAIDS, 2015. . [Cited in This Article: ] |
| 3. | Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 4. | Zoulim F, Liang TJ, Gerbes AL, Aghemo A, Deuffic-Burban S, Dusheiko G, Fried MW, Pol S, Rockstroh JK, Terrault NA. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut. 2015;64:1824-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | AAEEH. Actualización de las Recomendaciones para el Tratamiento de la Hepatitis Crónica por Virus C 2014. Asociación Argentina para el Estudio de las Enfermedades del Hígado. Available from: http://www.aaeeh.org.ar/neweb/docs/AAEEH-Guia-tto-Hepatitis-C-Cronica.pdf. [Cited in This Article: ] |
| 6. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 877] [Cited by in F6Publishing: 890] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 7. | Yi HS, Eun HS, Lee YS, Jung JY, Park SH, Park KG, Choi HS, Suh JM, Jeong WI. Treatment with 4-methylpyrazole modulated stellate cells and natural killer cells and ameliorated liver fibrosis in mice. PLoS One. 2015;10:e0127946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Goeser F, Glässner A, Kokordelis P, Wolter F, Lutz P, Kaczmarek DJ, Schwarze-Zander C, Boesecke C, Strassburg CP, Rockstroh JK. HIV mono-infection is associated with an impaired anti-hepatitis C virus activity of natural killer cells. AIDS. 2016;30:355-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Howell J, Visvanathan K. The role of natural killer cells in hepatitis C infection. Antivir Ther. 2013;18:853-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol. 2007;179:3472-3479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors. 2010;36:274-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Eisenberg E, Konopniki M, Veitsman E, Kramskay R, Gaitini D, Baruch Y. Prevalence and characteristics of pain induced by percutaneous liver biopsy. Anesth Analg. 2003;96:1392-1396, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1504] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 14. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1177] [Cited by in F6Publishing: 1178] [Article Influence: 130.9] [Reference Citation Analysis (0)] |
| 15. | Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, Locatelli F, Mingari MC. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol. 2014;164:253-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol. 2013;4:422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Glässner A, Eisenhardt M, Kokordelis P, Krämer B, Wolter F, Nischalke HD, Boesecke C, Sauerbruch T, Rockstroh JK, Spengler U. Impaired CD4+ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol. 2013;59:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Yi HS, Jeong WI. Interaction of hepatic stellate cells with diverse types of immune cells: foe or friend? J Gastroenterol Hepatol. 2013;28 Suppl 1:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Glässner A, Eisenhardt M, Krämer B, Körner C, Coenen M, Sauerbruch T, Spengler U, Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest. 2012;92:967-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Bhardwaj S, Ahmad F, Wedemeyer H, Cornberg M, Schulze Zur Wiesch J, van Lunzen J, Sarin SK, Schmidt RE, Meyer-Olson D. Increased CD56(bright) NK cells in HIV-HCV co-infection and HCV mono-infection are associated with distinctive alterations of their phenotype. Virol J. 2016;13:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325-35.e1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149:190-200.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |