Published online Feb 28, 2016. doi: 10.4254/wjh.v8.i6.331
Peer-review started: July 3, 2015
First decision: August 26, 2015
Revised: October 7, 2015
Accepted: December 19, 2015
Article in press: December 23, 2015
Published online: February 28, 2016
AIM: To evaluate addition of boceprevir to peginterferon/ribavirin (PR) in Russian patients with chronic hepatitis C virus (HCV).
METHODS: Treatment-naive (TN) and treatment-experienced (TE) patients (who had failed prior treatment with PR for ≥ 12 wk) with chronic HCV genotype 1 infection were enrolled in this placebo-controlled, double-blind study. All patients initially received PR for 4 wk. Patients randomized to control treatment then received PR for an additional 44 wk. TN patients randomized to triple therapy received boceprevir (800 mg three times daily) plus PR for 24 wk and then further therapy according to treatment week 8 (TW8) HCV RNA levels. TE patients received boceprevir plus PR for 32 wk and then further therapy according to TW8 HCV RNA levels. Treatment was discontinued for TN patients with detectable HCV RNA at TW24 and TE patients with detectable HCV RNA at TW12 because of futility. The primary efficacy end point was sustained virologic response (SVR) defined as undetectable HCV RNA 24 wk after completing all study therapy.
RESULTS: SVR was 74.8% in the boceprevir plus PR arm compared with 46.2% in the control arm, with a stratification-adjusted treatment difference of 29.2% (95%CI: 16.4-41.5; P < 0.0001). Rates of SVR were higher in the boceprevir arm in both TN and TE patient groups (TN 78.4% vs 56.3%; TE 69.4% vs 30.0%). Within TE patients, the rates of SVR were higher with boceprevir plus PR compared with PR, regardless of treatment failure type (null responder, partial responder, and relapser). Most patients receiving boceprevir plus PR in both TN (86%) and TE (71%) populations were eligible for reduced treatment duration. Anemia was increased in patients receiving boceprevir plus PR vs PR alone (47.2% vs 24.4%); there was a corresponding increase in ribavirin dose reduction and erythropoietin use. Among patients receiving boceprevir plus PR, SVR rates were similar in patients with anemia (< 10 g/dL) and those without anemia (71.2% vs 77.4%).
CONCLUSION: Regulatory approval has been obtained for boceprevir plus PR in Russian patients with HCV genotype 1 infection based on the results of this study.
Core tip: Compared to the standard-of care treatment with peginterferon and ribavirin (PR), addition of boceprevir to PR results in a significant increase in rates of sustained virologic response achieved with substantially shorter treatment durations across a broad cross-section of patients with chronic hepatitis C virus infection in Russia.
- Citation: Isakov V, Nikitin I, Chulanov V, Ogurtsov P, Lukyanova E, Long J, Wahl J, Helmond FA, The P08160 Trial Investigators. Boceprevir plus peginterferon/ribavirin for treatment of chronic hepatitis C in Russia. World J Hepatol 2016; 8(6): 331-339
- URL: https://www.wjgnet.com/1948-5182/full/v8/i6/331.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i6.331
Boceprevir is an orally administered, serine protease inhibitor of the hepatitis C virus (HCV) nonstructural protein 3 protease[1]. The addition of boceprevir to peginterferon and ribavirin (PR) improves rates of sustained virologic response (SVR) in adult patients with HCV genotype 1 (GT1) infection[2,3]. In the phase 3 SPRINT-2 study in previously untreated patients and the RESPOND-2 study in patients who had failed previous treatment, the addition of boceprevir to PR increased SVR rates compared with PR alone. In both studies, the implementation of response-guided therapy (RGT) permitted a shortened treatment duration for patients with an early response to therapy. In SPRINT-2, 44% of patients receiving boceprevir RGT required only 28 wk of treatment with triple therapy, and the SVR rate in this group was 96%[3]. Similarly, in RESPOND-2, 46% of patients had undetectable HCV RNA at treatment week 8 (TW8) and were eligible for a shortened 36-wk treatment regimen: SVR in this population was 86%[2]. In these studies, the safety profile of boceprevir plus PR largely resembled the safety profile of PR alone, with the notable exceptions of increased rates of dysgeusia and anemia in patients receiving boceprevir.
According to the World Health Organization (WHO), there were an estimated 5.8 million patients with HCV infection in Russia in 2010, accounting for 4.1% of the total Russian population[4]. In Western countries, treatment of HCV infection has advanced dramatically over the last 5 years with the introduction of new targeted therapies that substantially shorten treatment duration and improve SVR rates[5,6]. However, in resource-constrained countries, standard treatment protocols are lacking, and PR dual therapy frequently remains the cornerstone of treatment[7,8]. Recent guidelines from the WHO note the low rates of treatment uptake for patients in low- and middle-income countries. The aim of this study was to evaluate the safety and efficacy of boceprevir plus PR therapy in treatment-naive (TN) and treatment-experienced (TE) Russian patients with chronic HCV GT1 infection.
This was a randomized, placebo-controlled, double-blind clinical trial (ClinicalTrials.gov identifier, NCT01425203; protocol P08160), carried out in accordance with the Declaration of Helsinki, current guidelines on Good Clinical Practice, and local ethical and legal requirements. All patients provided voluntary written informed consent before trial entry.
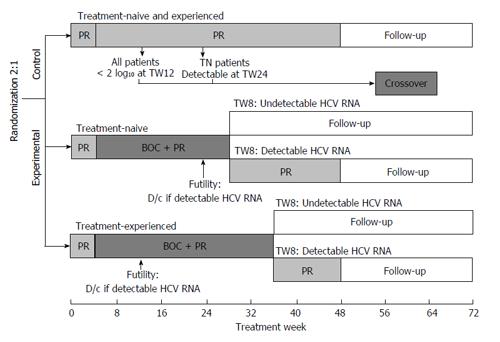
Patients were randomized in a 2:1 ratio to receive experimental or control therapy, stratified by previous treatment (naive vs experienced) and interleukin-28B (IL28B) status (CC allele vs non-CC allele) (Figure 1). All patients initially received PR [peginterferon alfa-2b (1.5 μg/kg per week) plus ribavirin (800-1400 mg/d)] for 4 wk. Patients in the control arm then received PR for an additional 44 wk. In the experimental arm, TN patients received boceprevir [800 mg three times daily (TID)] plus PR for 24 wk and then further therapy according to TW8 HCV RNA levels. Patients with undetectable HCV RNA at TW8 concluded treatment at week 28 while those with detectable HCV RNA at TW8 continued therapy with PR from weeks 28-48. TE patients received boceprevir (800 mg TID) plus PR for 32 wk and then further therapy according to TW8 HCV RNA levels. Patients with undetectable HCV RNA at TW8 concluded treatment at week 36, while those with detectable HCV RNA at TW8 continued PR therapy from weeks 36-48. Treatment was discontinued for TN patients with detectable HCV RNA at TW24 and TE patients with detectable HCV RNA at TW12 because of futility. Patients in the control arm (PR only) who failed treatment because of the futility rule could cross over to receive triple therapy. TN patients with < 2 log10 decline in HCV RNA at TW12, or with detectable HCV RNA at TW24 could cross over to receive boceprevir plus PR for 32 wk. TE patients with detectable HCV RNA at TW12 could also cross over to receive boceprevir plus PR for 32 wk. Duration of further therapy depended on HCV RNA detectability at crossover week 4 (COW4). Crossover treatment duration was 32 (COW4 HCV RNA undetectable) or 44 wk (COW4 HCV RNA detectable).
The study population included TN and TE adult patients with chronic HCV infection (enrollment ratio 60:40). TN patients had received no previous therapy for HCV infection, whereas TE patients were required to have received prior treatment with PR for ≥ 12 wk without interruption or dose reduction. Inclusion criteria for the study included a baseline viral load of ≥ 10000 IU/mL, and a liver biopsy consistent with chronic HCV infection. Cirrhotic patients were required to have an ultrasound within 6 mo of screening with no evidence of hepatocellular carcinoma. Exclusion criteria included a platelet count of < 100000/mm3; hemoglobin levels < 12 g/dL for females or < 13 g/dL for males; human immuno-deficiency virus or hepatitis B virus infection; previous discontinuation of PR due to a treatment-related adverse event (AE); or decompensated liver disease, including a history or presence of ascites, bleeding varices, or hepatic encephalopathy.
The primary efficacy end point was SVR, defined as undetectable HCV RNA 24 wk after completing treatment in randomized patients who received at least 1 dose of any trial medication. HCV RNA was detected using COBAS® AmpliPrep/COBAS® TaqMan® HCV Test, version 1.0 (Roche Diagnostics, Basel Switzerland); lower limit of quantification = 43 IU/mL; limit of detectability = 18.0 IU/mL. The key secondary end point was the achievement of SVR in randomized patients who received at least 1 dose of boceprevir or boceprevir placebo therapy. Other end points included the relationship between early virologic response and SVR (summarized using the proportion of patients who achieved SVR among those with undetectable HCV RNA at TW4, TW8 or TW12), the proportion of patients with virologic breakthrough (undetectable HCV RNA and subsequent HCV RNA above the limit of quantification while on study therapy), the proportion with incomplete virologic response (> 1 log10 increase in HCV RNA from nadir value while on study therapy), and safety.
The statistical methods of this study were reviewed by Jianmin Long from Merck and Co., Inc. Analyses were based on the full analysis set population, which included all randomized and treated patients. Target enrollment was 70 patients in the PR control group and 140 in the boceprevir plus PR arm, providing 98% power to demonstrate the superiority of boceprevir plus PR vs PR at an overall 1-sided, 2.5% alpha level, if the underlying difference in SVR was 30%. The power and sample size calculations were based on the assumption of an underlying response rate of 30% for the PR control arm. The minimum criterion for success was that the P value for the comparison of SVR between the boceprevir plus PR arm and the control PR arm was < 0.05. An interim analysis was performed when all patients had completed at least 8 wk of treatment or had discontinued therapy. The results of this interim analysis were used as the basis for regulatory submission in Russia.
Achievement of SVR was summarized using descriptive statistics. The primary statistical comparison was conducted on the full analysis set using the stratified Miettinen and Nurminen method at alpha level of 0.05 adjusted for stratification factors (IL28B genotype CC vs non-CC and TN vs TE) as specified at the time of randomization. Multiplicity adjustment for controlling the type 1 error for the primary and key secondary comparisons was based on the step-down approach. The key secondary comparison was tested only if the statistical significance of the primary comparison reached an alpha level of 0.05. Any patient with missing data at, or after follow-up week 24, and undetectable HCV RNA at follow-up week 12, was considered a sustained virologic responder. For efficacy analyses, patients in the PR control arm who rolled over to the crossover arm were considered as failures at and after the time of the crossover.
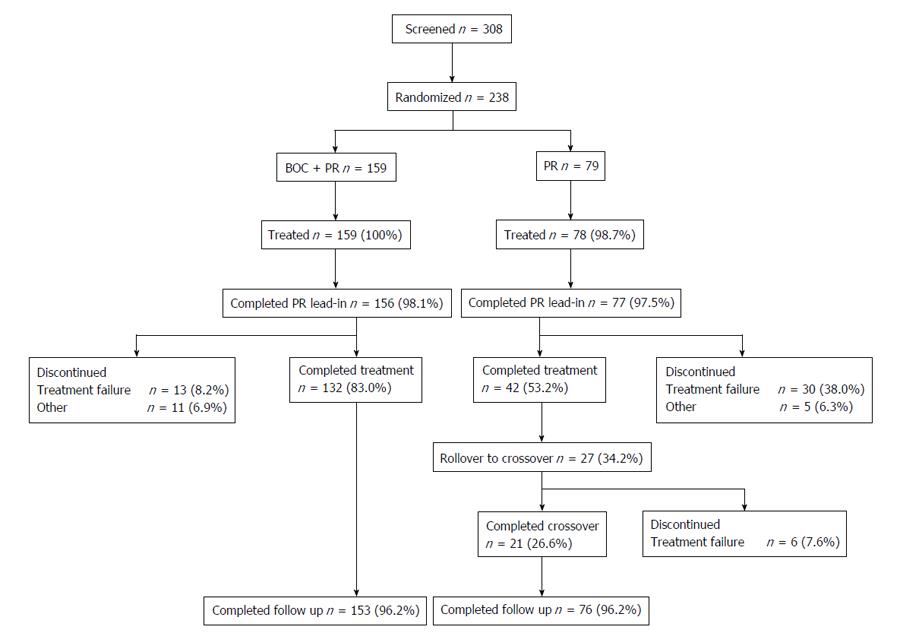
A total of 238 patients were randomly assigned: 159 were assigned to receive boceprevir plus PR and 79 were assigned to PR (Figure 2). One patient assigned to PR did not receive any study medication and was therefore excluded from the full analysis set population. Four patients discontinued during lead-in (boceprevir plus PR, n = 3; PR, n = 1), yielding 233 patients in the modified intent-to-treat data set. Fifty-nine patients (boceprevir plus PR, n = 24; PR, n = 35) discontinued after adding boceprevir/placebo, with the most common reason for discontinuation being treatment failure (5% of patients receiving boceprevir plus PR and 34% of those receiving PR alone were discontinued based on futility criteria, Figure 2). Twenty-seven patients in the PR control arm entered crossover because of treatment failure at the futility time points. In total, 229 patients entered the follow-up phase (Figure 2). The majority of patients were white, with GT1b infection, and the IL28B non-CC genotype (Table 1). Few patients were cirrhotic. Compliance rates with boceprevir therapy were high (97.5% of patients had ≥ 80% compliance).
| Boceprevir plus PR (n = 159) | PR (n = 78) | |
| Sex | ||
| Male | 94 (59.1) | 45 (57.7) |
| Female | 65 (40.9) | 33 (42.3) |
| Age (yr), mean (SD) | 38.6 (9.8) | 38.1 (10.0) |
| Race | ||
| White | 158 (99.4) | 77 (98.7) |
| Asian | 1 (0.6) | 1 (1.3) |
| Ethnicity | ||
| Not Hispanic or Latino | 159 (100) | 78 (100) |
| Weight (kg), mean (SD) | 78.1 (16.6) | 78.5 (16.8) |
| BMI (kg/m2), mean (SD) | 25.9 (4.2) | 26.0 (4.4) |
| Previous treatment | ||
| Naive | 97 (61.0) | 48 (61.5) |
| Experienced | 62 (39.0) | 30 (38.5) |
| IL28B genotype | ||
| CC allele | 22 (13.8) | 11 (14.1) |
| Non-CC allele | 137 (86.2) | 67 (85.9) |
| HCV genotype | ||
| GT1a | 4 (2.5) | 0 (0) |
| GT1b | 155 (97.5) | 78 (100) |
| Baseline HCV RNA | ||
| ≤ 800000 IU/mL | 89 (56.0) | 53 (67.9) |
| > 800000 IU/mL | 70 (44.0) | 25 (32.1) |
| Hemoglobin (g/dL), mean (SD) | 15.0 (1.5) | 14.9 (1.5) |
| Liver histology | ||
| Cirrhosis | 7 (4.4) | 2 (2.6) |
| No cirrhosis | 152 (95.6) | 76 (97.4) |
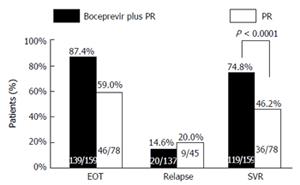
SVR at follow-up week 24 was higher in the boceprevir plus PR arm compared with the control arm [74.8% (119/159) vs 46.2% (36/78)], with a stratification-adjusted treatment difference of 29.2% (95%CI: 16.4-41.5; P < 0.0001) (Figure 3). The end of treatment response rate was 87.4% (139/159) for the boceprevir plus PR arm, and 59.0% (46/78) for the PR control arm. The relapse rate was 14.6% (20/137) for the boceprevir plus PR arm, and 20.0% (9/45) for the PR control arm.
Rates of virologic breakthrough were 3.8% (6/159) in the boceprevir plus PR arm, and 5.1% (4/78) in the PR control arm. No patients in the PR control arm exhibited virologic rebound. Incomplete virologic response/rebound rate in the boceprevir plus PR arm was 3.1% (5/159). Five patients with incomplete virologic response had samples sequenced, of which 3 samples had variants detected (V36M, n = 1; T54A, n = 2; T54S, n = 1; T54T, n = 2). Similarly, 5 patients with virologic breakthrough had samples sequenced, of which 3 had detectable HCV variants (T54A, n = 1; T54S, n = 1; T54T, n = 2; V55A, n = 1).
All patients with undetectable HCV RNA at TW4 in both treatment arms attained SVR (Table 2). In both treatment arms, all patients received PR alone for the first 4 wk of therapy. The proportions of patients with < 1 log drop [boceprevir 43/159 (27%) and PR 22/78 (28%)] and ≥ 1 log drop [boceprevir 90/159 (57%) and PR 45/78 (58%)] in HCV RNA at TW4 were similar in both treatment arms. However, SVR was higher in patients receiving boceprevir + PR compared with PR within the subgroups of patients with < 1 log drop in HCV RNA at TW4 (46.5% vs 0%) and those with ≥ 1 log drop in HCV RNA at TW4 (83.3% vs 57.8%).
| Boceprevir plus PR (n = 159) | PR (n = 78) | |
| Treatment naive | 76/97 (78.4) | 27/48 (56.3) |
| Treatment experienced | 43/62 (69.4) | 9/30 (30.0) |
| Null responder | 8/17 (47.1) | 1/6 (16.7) |
| Partial responder | 5/8 (62.5) | 1/4 (25.0) |
| Relapser | 30/37 (81.1) | 7/20 (35.0) |
| Treatment naive | ||
| IL28B CC genotype | 19/20 (95.0) | 11/11 (100.0) |
| IL28B non-CC genotype | 57/77 (74.0) | 16/37 (43.2) |
| Treatment experienced | ||
| IL28B CC genotype | 2/2 (100.0) | 0/0 |
| IL28B non-CC genotype | 41/60 (68.3) | 9/30 (30.0) |
| SVR according to baseline HCV RNA | ||
| All patients | ||
| ≤ 800000 IU/mL | 71/89 (79.8) | 25/53 (47.2) |
| > 800000 IU/mL | 48/70 (68.8) | 11/25 (44.0) |
| Treatment naive | ||
| ≤ 800000 IU/mL | 45/52 (86.5) | 16/27 (59.3) |
| > 800000 IU/mL | 31/45 (68.9) | 11/21 (52.4) |
| Treatment experienced | ||
| ≤ 800000 IU/mL | 26/37 (70.3) | 9/26 (34.6) |
| > 800000 IU/mL | 17/25 (68.0) | 0/4 (0) |
| SVR according to TW4 response | ||
| TW4 < 1 log drop | 20/43 (46.5) | 0/22 (0) |
| TW4 ≥ 1 log drop | 75/90 (83.3) | 26/45 (57.8) |
| TW4 undetectable | 23/23 (100) | 10/10 (100) |
| Missing | 1/3 | 0/1 |
| SVR according to TW8 response | ||
| TW8 undetectable | 115/139 (82.7) | 29/33 (87.9) |
| TW8 detectable | 4/16 (25) | 7/44 (15.9) |
| Missing | 0/4 | 0/1 |
| SVR according to presence of anemia | ||
| Yes | 47/66 (71.2) | 6/11 (54.5) |
| No | 72/93 (77.4) | 30/67 (44.8) |
| SVR according to EPO use | ||
| Yes | 10/15 (66.7) | 3/3 (100) |
| No | 109/144 (75.7) | 33/75 (44) |
| SVR according to ribavirin dose reduction | ||
| Yes | 46/67 (68.7) | 12/17 (70.6) |
| No | 73/92 (79.4) | 24/61 (39.3) |
A TW8 interim analysis was submitted for regulatory approval in Russia. In this analysis, rates of undetectable HCV RNA at TW8 in the boceprevir RGT and PR arms were 91% (88/97) vs 48% (23/48) in TN patients and 82% (51/62) vs 33% (22/67) in TE patients. Overall, the rates of undetectable HCV RNA at TW8 in all patients were higher in patients receiving boceprevir plus PR compared with control therapy (87.4% vs 42.3%, P < 0.0001). SVR rates in patients with undetectable HCV RNA at TW8 were similar between treatment arms [boceprevir + PR 82.7% (115/139) vs PR 87.9% (29/33)].
SVR rates are presented by previous treatment and response, and IL28B genotype (Table 2). SVR rates were higher in patients receiving boceprevir plus PR compared with PR in both TN (78.4% vs 56.3%) and TE (69.4% vs 30.0%) subgroups. Within TE patients, the rates of SVR were higher with boceprevir plus PR compared with PR, regardless of treatment failure type (null responder, partial responder, and relapser). SVR rates were high among all patients with IL28B CC genotype, regardless of treatment arm or previous treatment history. Conversely, the rates of SVR in patients with IL28B CT or TT genotypes were higher with boceprevir plus PR compared with PR alone (Table 2). SVR rates were also higher with boceprevir compared with PR, regardless of baseline viral load. SVR was 87% in TN patients with baseline viral load ≤ 800000 IU/mL. Among patients receiving boceprevir, rates of SVR were generally higher in TN patients with low viral load compared with those with high baseline viral load (86.5% vs 68.9%); however, SVR was similar in TE patients with high vs low baseline viral load receiving boceprevir (70.3% vs 68.0%) (Table 2).
Among patients receiving boceprevir plus PR, SVR rates were similar in patients with anemia (< 10 g/dL) and those without anemia (71.2% vs 77.4%). SVR rates were also relatively similar in boceprevir recipients requiring erythropoietin (EPO) for anemia management and those not using EPO (66.7% vs 75.7%, Table 2), and in those who received ribavirin dose reduction and those who did not (68.7% vs 79.4%).
The SVR rates for the crossover group are presented in Table 3. Overall, 70.4% of patients who crossed over from PR alone to boceprevir plus PR had SVR at follow-up week 24.
| SVR | |
| Total | 19/27 (70.4) |
| TN TW12 failure (< 2 log decline HCV RNA) | 8/11 (72.7) |
| TE TW12 failure (detectable HCV RNA) | 11/16 (68.8) |
| TN TW24 failure (detectable HCV RNA) | 0/0 |
The reported AEs were consistent with the known safety profile of boceprevir (Table 4), with treatment-emergent AEs noted frequently in both treatment arms (97.5% in the boceprevir plus PR arm and 91.0% in the PR control arm). The number of patients discontinuing treatment because of AEs was 4.4% in the boceprevir plus PR arm (n = 7, of which 5 were considered treatment related) and 2.6% in the PR control arm (n = 2, of which 1 was considered treatment related). Serious AEs were reported in 10.7% (n = 17, of which 12 were considered drug related) and 11.5% (n = 9, of which 5 were considered drug related) of patients in the boceprevir plus PR and PR arms, respectively. Dose modifications due to an AE were reported in 56% (89/159) in the boceprevir plus PR arm, and 33.3% (26/78) for PR alone. There were no deaths reported during the study.
| Boceprevir plus PR (n = 159) | PR (n = 78) | |
| Any AE | 155 (97.5) | 71 (91.0) |
| Neutropenia | 84 (52.8) | 31 (41.0) |
| Pyrexia | 77 (48.4) | 36 (46.2) |
| Anemia | 75 (47.2) | 19 (24.4) |
| Leukopenia | 62 (39.0) | 25 (32.1) |
| Dysgeusia | 59 (37.1) | 3 (3.8) |
| Asthenia | 44 (27.7) | 23 (29.5) |
| Headache | 43 (27.0) | 25 (32.1) |
| Influenza-like illness | 39 (24.5) | 14 (17.9) |
| Nausea | 39 (24.5) | 9 (11.5) |
| Anemia | ||
| 8.5-10 g/dL | 56 (35.2) | 9 (11.5) |
| < 8.5 g/dL | 10 (6.3) | 2 (2.6) |
| Ribavirin dose reduction | 65 (40.9) | 14 (17.9) |
| EPO use | 15 (9.4) | 3 (3.8) |
| Serious AE | 17 (10.7) | 9 (11.5) |
| Discontinued because of an AE | 7 (4.4) | 2 (2.6) |
| Dose modification due to an AE | 89 (56.0) | 26 (33.3) |
Anemia was reported at a higher rate in patients receiving boceprevir plus PR compared with those receiving PR alone (47.2% vs 24.4%). However, few patients in either treatment group had on-treatment hemoglobin levels < 8.5 g/dL (boceprevir + PR 6.3% vs PR 2.6%). EPO use was reported for 9.4% of patients receiving boceprevir plus PR and 3.8% of those receiving PR alone. Ribavirin dose reduction was required for 65 patients (40.9%) receiving boceprevir plus PR and 14 patients (17.9%) receiving PR alone.
Data from the present study indicate that, similar to activity seen in Western populations, boceprevir added to PR results in a marked improvement in SVR rates compared with PR alone in TN and TE Russian patients with HCV GT1 infection. The high rate of undetectable HCV RNA at TW8 in TN and TE patients receiving boceprevir plus PR resulted in a high proportion of patients being deemed eligible for RGT with consequent reductions in their treatment durations. The treatment effect (i.e., difference in response between boceprevir plus PR and PR alone) was comparable between this study in Russian patients, and the phase 3 trials (Table 5). However, whereas 42%-46% of patients receiving boceprevir RGT in the phase 3 studies had undetectable HCV RNA at TW8, in the present study 87.4% of boceprevir recipients had undetectable HCV RNA at TW8. This suggests that the proportion of Russian patients eligible for shortened treatment duration may be higher than reported in the phase 3 studies, and is suggestive of a favorable cost/efficacy ratio in Russian patients. Response rates were particularly high among patients with favorable disease characteristics such as the IL28B CC genotype. In patients with this genotype, SVR rates were high regardless of treatment regimen; however, patients with the IL28B non-CC genotype derived a substantial benefit from boceprevir therapy.
| Russian patients | SPRINT-2 | RESPOND-2 | ||||
| RGT of BOC | PR | RGT of BOC | PR | RGT of BOC | PR | |
| TN | ||||||
| EOT | 89/97 (91.8) | 33/48 (68.8) | 277/366 (76) | 191/363 (53) | - | - |
| SVR | 76/97 (78.4) | 27/48 (56.3) | 242/366 (66) | 137/363 (38) | - | - |
| Relapse | 13/89 (14.6) | 6/33 (18.2) | 24/265 (9) | 39/176 (22) | - | - |
| TE | ||||||
| EOT | 50/62 (80.6) | 13/30 (43.3) | - | - | 114/162 (70.4) | 25/80 (31) |
| SVR | 43/62 (69.4) | 9/30 (30) | - | - | 107/161 (66) | 17/80 (21) |
| Relapse | 7/48 (14.6) | 3/12 (25.0) | - | - | 14/121 (12) | 8/25 (32) |
The tolerability profile seen with boceprevir in Russian patients was consistent with the established tolerability profile documented in Western patients. The majority of AEs were associated with PR therapy. As seen in Western patients, anemia was increased with boceprevir, and there was also a corresponding increase in the use of anemia management strategies (ribavirin dose reduction and EPO use) among patients receiving boceprevir. In SPRINT-2 and RESPOND-2, approximately 3%-8% of patients receiving boceprevir plus PR had hemoglobin levels < 8.0 g/dL: EPO use was required in 41%-46% of patients, and 21% required dose reduction due to anemia[2,3]. In the present study, 6.3% of patients receiving boceprevir plus PR had nadir hemoglobin < 8.5 g/dL. There were also differences in the rates of anemia management strategies with lower rates of EPO use (9.4%) but higher rates of dose reduction (41%) in the present study compared with the phase 3 studies in Western patients[2,3]. These differences between studies are a reflection of the different anemia management strategies. In the phase 3 protocols, investigators were free to choose between ribavirin dose reduction and EPO use as a first-line strategy while in the present study ribavirin dose reduction was the first-line strategy and EPO use was the second-line strategy.
Response rates in this study are higher for both boceprevir plus PR and PR alone, compared with rates seen in previous phase 3 studies (Table 5). This increase in response may be explained by differences in the patient populations enrolled in the current study and the phase 3 studies[2,3]. Compared with patients enrolled in the boceprevir phase 3 studies, more Russian patients were aged ≤ 40 years (62% vs 13%), had baseline viral load ≤ 800000 IU/mL (60% vs 14%), and had HCV GT1b infection (98% vs 35%).
Data from the present study support the use of boceprevir in Russian patients with HCV GT1 infection. However, boceprevir-based triple therapy may not be appropriate for all patients with GT1 infection. Patients with low viral load at baseline who achieve undetectable HCV RNA at TW4 may achieve high SVR rates with 24-wk of therapy with PR alone and would not require the addition of boceprevir[9]. Despite the world-wide acceptance of interferon-free regimens as a standard of care due to the near 100% efficacy and low adverse events rate, some patients will continue to receive interferon-based treatment. This is due largely to the fact that the approval of interferon-free regimens is not immediately followed by total reimbursement in many countries, or that access to these regimens is dependent on the stage of the liver disease, prioritizing treatment of cirrhotic patients[10-12]. Easy-to-treat patients can be successfully treated with interferon-based regimens which may be easier to access through reimbursement.
In conclusion, data from the present study support the use of boceprevir plus PR for the treatment of Russian patients with HCV GT1 infection. The safety and efficacy profile of boceprevir in Russian patients was generally similar to that previously reported in phase 3 studies in Western patients; however, this treatment may be more cost-effective in Russia as approximately 88% of patients had undetectable HCV RNA at TW8, suggesting that a higher proportion of Russian patients receiving boceprevir plus PR would be eligible for reduced treatment duration with RGT compared with Western patients. Regulatory approval has been obtained for boceprevir in Russia based on the results of this study.
Medical writing and editorial assistance were provided by Tim Ibbotson, PhD, of ApotheCom, Yardley, PA, United States.
In the treatment of hepatitis C virus (HCV), genotype 1 infection, peginterferon plus ribavirin is associated with low efficacy and poor tolerability. Phase 3 studies have shown that addition of a direct-acting antiviral agent such as boceprevir can improve efficacy and shorten treatment durations.
The safety and efficacy of boceprevir plus peginterferon and ribavirin in Russian patients with HCV infection is currently unknown.
In the present study, patients receiving boceprevir plus peginterferon and ribavirin achieved significantly higher rates of sustained virologic response compared with patients treated with peginterferon and ribavirin alone. Patients receiving boceprevir-based therapy frequently required substantially shorter treatment durations compared to patients receiving PR alone alone. Rates of anemia were higher among patients receiving boceprevir.
Regulatory approval has been obtained for boceprevir in Russia based on the results of this study.
This manuscript evaluates the efficacy and safety of boceprevir plus peginterferon and ribavirin in treatment-naïve and treatment-experienced Russian patients with HCV genotype 1 infection.
P- Reviewer: Chuang WL, Messori A, Sirin G S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
| 1. | Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob Agents Chemother. 2006;50:1013-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 3. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1949] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 4. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 929] [Article Influence: 71.5] [Reference Citation Analysis (2)] |
| 5. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 6. | American Association for the Study of Liver Diseases, Infectious Diseases Society of America, International Antiviral Society-USA. Recommendations for testing, managing, and treating hepatitis C. AASLD/IDSA/IAS-USA Web site. [Accessed 2014-05-26]. Available from: http://hcvguidelines.org/news/hcv-guidance. [Cited in This Article: ] |
| 7. | Umar M, Khan AG, Abbas Z, Arora S, Asifabbas N, Elewaut A, Esmat G, Foster G, Fried M, Goh KL, Hamama TB, Imawari M, Isakov V, Krabshuis J, LaBrecque D, Lemair A, Malfertheiner P, Ryder S, Schiedermaier P, Stimac D, Tandon R, Villamil F, Zapata R, Ferenci P; World Gastroenterology Organisation. World Gastroenterology Organisation global guidelines: diagnosis, management and prevention of hepatitis C April 2013. J Clin Gastroenterol. 2014;48:204-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. WHO Web site. [Accessed 2014-06-26]. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en. [Cited in This Article: ] |
| 9. | Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, Ibranyi E, Weiland O, Noviello S, Brass C. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Sadler MD, Lee SS. Revolution in hepatitis C antiviral therapy. Br Med Bull. 2015;113:31-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 12. | Stepanova M, Younossi ZM. Interferon-Free Regimens for Chronic Hepatitis C: Barriers Due to Treatment Candidacy and Insurance Coverage. Dig Dis Sci. 2015;60:3248-3251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |