Published online May 18, 2016. doi: 10.4254/wjh.v8.i14.632
Peer-review started: January 1, 2016
First decision: February 2, 2016
Revised: February 19, 2016
Accepted: March 9, 2016
Article in press: March 14, 2016
Published online: May 18, 2016
Autoimmune phenomena are common in patients with chronic hepatitis C. Management of chronic hepatitis C/autoimmune hepatitis syndrome has until recently been problematic due to the adverse effects of interferon on autoimmune processes and immunosuppression on viral replication. In this report we describe 3 patients with chronic hepatitis C/autoimmune hepatitis overlap syndrome who responded rapidly to direct acting anti-viral therapy. The resolution of the autoimmune process supports a direct viral role in its pathophysiology.
Core tip: Autoimmune phenomena are common in patients with chronic hepatitis C, and occasionally patients with chronic hepatitis C have concomitant features of autoimmune hepatitis (AIH). Management of these patients has until recently been problematic due to the adverse effects of interferon on autoimmune processes and immunosuppression on viral replication. In this report we describe 3 patients with chronic hepatitis C/AIH overlap syndrome who responded rapidly to direct acting anti-viral therapy with prompt normalization of liver tests and progressive decrease in the serologic markers of AIH. The resolution of the autoimmune process supports a direct viral role in its pathophysiology.
- Citation: Sahebjam F, Hajdu CH, Nortey E, Sigal SH. Direct acting antiviral therapy is curative for chronic hepatitis C/autoimmune hepatitis overlap syndrome. World J Hepatol 2016; 8(14): 632-636
- URL: https://www.wjgnet.com/1948-5182/full/v8/i14/632.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i14.632
Chronic hepatitis C virus (HCV) infection has a worldwide prevalence of 2%-3% and is a leading cause of cirrhosis and hepatocellular carcinoma in Western countries[1]. In 40%-74% of patients, HCV is associated with autoimmune phenomena ranging from positive serologic markers to wide spread autoimmune diseases, including rheumatoid arthritis, mixed cryoglobulinemia, B-cell lymphoma, systemic lupus erythematosus, sicca syndrome, autoimmune thyroiditis, and autoimmune hepatitis (AIH).
AIH is a condition of unknown etiology characterized by a progressive inflammatory process with histopathologic changes that include interface hepatitis with a predominant lymphoplasmacytic infiltrate, elevated transaminases and immunoglobulin levels, and the presence of autoantibodies. To standardize diagnostic criteria, the International Autoimmune Hepatitis Group (IAHG) devised a scoring system to categorize patients as definite AIH, probable AIH and not AIH[2] in which points are distributed based on the presence of anti-nuclear antibody (ANA), anti smooth antibody (ASMA or F-Actin Antibody), anti-soluble liver/liver pancreas antigen, immunoglobulin G (IgG) level, liver histology and the absence of viral hepatitis.
In patients with chronic hepatitis C, markers of AIH are frequently present. Up to 40% of HCV patients may have positive ANA, SMA, and LKM-1 autoantibodies[3]. In most cases, titers are usually low, and cases with positive serologies are in general histologically indistinguishable from those without detectable antibodies. However, patients with an autoimmune overlap syndrome in whom liver biopsies reveal features of both chronic hepatitis C and inflammatory features characteristic of AIH are occasionally encountered[4].
The treatment of patients with HCV/AIH overlap syndrome has until recently been challenging. Because interferon (IFN) therapy for chronic HCV can trigger latent AIH and lead to severe hepatic failure, there are significant concerns about its use in patients with preexisting autoimmune processes[5]. Immunosuppression, on the hand, has an adverse effect on viral replication[6]. In this report, we present three patients with AIH/hepatitis C overlap syndrome in whom both processes rapidly responded to interferon-free antiviral therapy.
A 22-year-old Caucasian man with chronic hepatitis C presented with mild generalized fatigue and anhedonia. Risk factors for infection included intravenous drug use and possible vertical transmission. Physical examination did not reveal stigmata of advanced liver disease. Initial laboratory evaluation was remarkable for markedly elevated aspartate aminotransferase (AST) 314 U/L (normal, 15-46) and alanine aminotransferase (ALT) 608 U/L (normal, 15-65) levels, total bilirubin 0.6 mg/dL (normal, 0-1), albumin 4.5 g/dL (normal, 3.5-5), international normalized ratio 1.1, platelet count of 156 K/uL (normal 150-400), HCV RNA viral load of 1410000 IU/mL (6.15 logs), HCV genotype 1. Serological markers of AIH were remarkable for elevated IgG at 2100 mg/dL (normal, 700-1600), positive ANA (1:80), and positive F-actin antibody of 37 units (normal, 0-19).
Liver biopsy showed established cirrhosis with mild to moderate activity. Inflammatory infiltrates composed of lymphocytes with lymphoid aggregate formation, polymorphonuclear cells and scattered plasma cells were present in the portal tracts, interface and fibrous septae. A brisk lobular lymphoplasmacytic infiltrate with rare acidophil bodies was also present. Due to the severity of the inflammatory activity, the overall histologic appearance was suggestive of an autoimmune process with a simplified IAHG, diagnostic score of 6 (probable AIH).
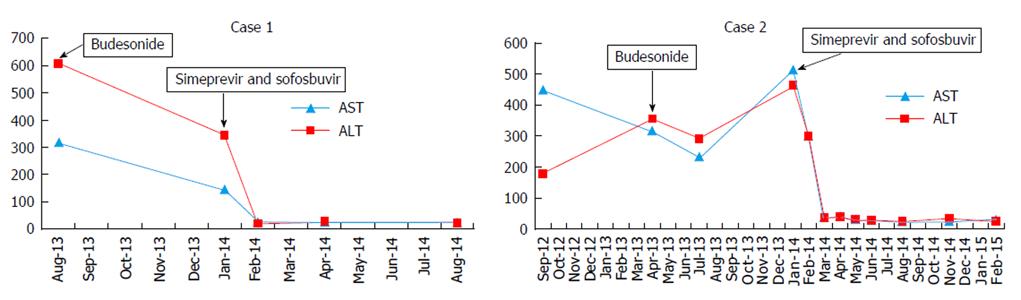
The patient was treated with budesonide 3 mg three times daily for two months with limited biochemical response (ALT, 343 U/L) and response in either the total protein level (8.2 g/dL, pre-; 7.6 g/dL, post-) or HCV viral load [1523252 IU/ML (6.18 logs)]. Budesonide was then discontinued, and a 12 wk interferon-free regimen of simeprevir and sofosbuvir started with prompt normalization of aminotransferase levels, normalization of the IgG level (1350 mg/dL), and achievement of a sustained response. F-Actin antibody titer did not change following treatment (37 U before, 36 U after) (Figure 1).
A 62-year-old man with chronic hepatitis C presented with generalized fatigue. Risk factors for hepatitis C infection included a blood transfusion at birth. Past medical history was significant for epilepsy and depression. Physical examination did not reveal stigmata of chronic liver disease. Laboratory evaluation was remarkable for AST 447 U/L, ALT 480 U/L, total bilirubin of 0.8 mg/dL, albumin 3.9 g/dL, international normalized ratio 1.4, HCV genotype 1, platelet count of 115 K/uL, HCV RNA viral load 1660000 IU/mL. Serologic markers of AIH were remarkable for elevated IgG at 3030 mg/dL, ANA titer 1:80, and F-actin antibody titer of 24 U.
Liver biopsy revealed mild to moderate portal infiltration consisting of lymphocytes with lymphoid aggregate formation, plasma cells and eosinophils. Interface activity was moderate with plasma cells easily identified and lobular inflammation was mild to moderate with acidophil bodies readily found. Macrovesicular steatosis in 30% to 40% of the specimen with focal ballooning degeneration and focal bridging fibrosis (stage 3) was also present. The moderate interface activity with numerous plasma cells was consistent with an autoimmune process with a simplified IAHG diagnostic score of 6 (probable AIH)[7].
The patient declined interferon therapy and was started on budesonide 3 mg twice daily without significant effect on ALT or IgG levels (Figure 1) and HCV viral load remain unchanged (1177912 IU/mL). After 6 mo, budesonide was tapered to 3 mg daily, and a repeat liver biopsy was performed which revealed persistent portal and lobular inflammation, worsening ballooning degeneration and progression to cirrhosis. Budesonide was discontinued, and interferon-free therapy with 12 wk of simeprevir and sofosbuvir initiated. Aminotransferase levels promptly normalized. HCV RNA was undetectable by treatment week 8, and a sustained virologic response was achieved. During and after completion of therapy, IgG and F-actin levels progressively decreased (2070 mg/dL, 15 units respectively), and ANA titer was negative one year after completion of antiviral therapy.
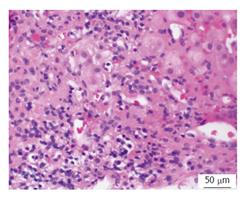
A 62-year-old African American woman with a history of alcoholism was referred for treatment of chronic hepatitis C. Risk factors included a history of intravenous drug abuse. Liver biopsy 3 years previously revealed stage IV fibrosis and moderate necroinflammatory activity with plasma cell component (Figure 2). Physical examination was significant for an enlarged left lobe of liver. Initial laboratory evaluation was remarkable for mildly elevated AST 68 U/L, ALT of 84 U/L levels, total bilirubin 0.8 mg/dL, albumin 3.6 g/dL, total protein 8.89 g/dL, international normalized ratio 1.2, platelet count of 85 K/uL, HCV RNA viral load of 285000 IU/mL (5.46 logs), HCV genotype 1a. Serological markers of AIH were remarkable for elevated IgG 3250 mg/dL, positive ANA, F-actin antibody titer of 37.
The patient was started on ledipasvir/sofosbuvir. Viral load became undetectable within 4 wk, and she achieved SVR with 12 wk of treatment. At the end of therapy, aminotransferase levels were normal (AST, 36; ALT, 27). ANA became negative, serum total protein decreased to normal level of 7.6 g/dL (normal, 6.3-8.2), and serum IgG decreased to 2300 mg/dL (normal, 700-1600). F-actin antibody titer also decreased to the normal range (17 U).
In this report, we present the response of HCV/AIH overlap syndrome to direct acting antiviral (DAA) therapy. The diagnosis of overlap syndrome was established by the presence of active viremia and characteristic biochemical, serologic, and histopathologic features of AIH. Although only a simplified AIH score of 6 was present, it is important to note that only a maximum score of 6 is possible if viral hepatitis component is not included. There were no biochemical or immunologic responses, but rather worsening pathologic changes in the one case in which a repeat liver biopsy was performed. In contrast, there was a prompt normalization of liver biochemistries and resolution of serologic features of AIH in response to DAA therapy in all three cases.
Although the pathogenesis of AIH is incompletely understood, a frequently cited mechanism is a reaction to viral infections in genetically susceptible persons. Cross-reaction between viral particles and liver auto-antigens has been proposed as a trigger mechanism of virus induced AIH. Activation of resting T cells by inducing the release of a variety of cytokines, and polyclonal activation of lymphocytes has also been proposed to play a role. An association with measles virus was first proposed in 1987 after identification of persistent measles virus genome in lymphocytes and high antibody titers in 12 of 18 patients with AIH[8]. Vento et al[9] reported the development of AIH in healthy relatives of patients with AIH that was associated with cases of infectious mononucleosis due to Epstein-Barr virus (EBV) infection. In these cases, the development and persistence of autoantibodies to the asialoglycoprotein receptor were documented, and it was proposed that cross reactivity between asialoglycoprotein and EBV antibodies caused an autoimmune reaction. Recently, high prevalence of hepatitis E antibody positivity was found in patients with AIH, compared to healthy, and individuals with HCV or HBV infection[10]. Other viral infections that have been associated with AIH include hepatitis B, varicella-zoster and rubella[11].
There are several proposed mechanisms in the pathogenesis of autoimmunity in HCV. HCV facilitates lymphotropism in which clonal B-lymphocyte expansion leads to widespread autoantibody production. The HCV envelope protein E2 is able to bind to the CD81 molecule expressed on hepatocytes and B-lymphocytes, resulting in a dysregulation of cytokines with an enhanced Th1 immune response. This may cause self-reactive lymphocytes to induce autoimmunity in the chronic HCV patient. Recently, cross-reactivity between CYP2E1 and specific sequences in HCV-NS5b protein has been shown responsible for the production of auto-antibodies targeting self-proteins[12].
There are no established treatment strategies for HCV/AIH overlap syndrome. IFN alone previously was avoided due to the concern about its potential to induce an autoimmune flare. Early reports of steroid therapy prior to the era in which HCV RNA testing was available frequently included RNA negative patients, making determination of efficacy difficult to assess. Several small series and case reports have advocated pre-treatment with corticosteroids with or without azathioprine followed by IFN-based therapy to prevent an IFN-induced flare[13-15].
The development of interferon-free direct acting antiviral regimens has revolutionized the treatment of HCV. These new treatments are potent, safe, and achieve rapid normalization of aminotransferase levels and viral suppression within the first few weeks of therapy. This is the first description of DAA therapy for HCV/AIH overlap syndrome. The rapid normalization of aminotransferase level and suppression of viral RNA followed by a gradual disappearance of autoimmune markers without immunosuppression supports the hypothesis that the viral infection triggers the autoimmune response. Based on our cases, we propose DAA agents as an initial treatment for patients with HCV/AIH overlap syndrome and early reassessment of response. Corticosteroids and immunosuppression should be reserved for those who are refractory to this approach.
Three patients presented for treatment of chronic hepatitis C.
Severe hepatitis with markedly elevated aminotransferase levels.
Chronic hepatitis C with severe activity or superimposed second process such as autoimmune hepatitis (AIH).
Positive anti-nuclear antibody and anti-smooth muscle antibody, elevated immunoglobulin G (IgG) level.
Liver biopsy reveal in all three cases prominent numbers of plasma cells compatible with AIH.
Treatment with steroids in the form of budesonide was not effective. However, there was prompt resolution of both the chronic hepatitis C and AIH with direct acting anti-viral therapy.
Reports have suggested an infectious precipitant for AIH. Previous therapeutic approach for the treatment of chronic hepatitis C/AIH have usually involved steroid therapy followed by interferon-based therapy with variable success.
In chronic hepatitis C/AIH overlap syndrome, hepatitis C viremia is present in patients with AIH as defined by the presence of anti-nuclear and anti-smooth muscle antibodies, elevated IgG levels, and lymphoplasmacytic infiltrates on liver biopsy.
Resolution of both the viral and AIH in response to direct acting antiviral therapy supports the hypothesis that the autoimmune process is caused by the viral infection.
In the present manuscript, the authors described 3 case patients with chronic HCV/AIH syndrome who were treated with direct acting antiviral (DAA). DAA treatment promptly induced the normalization of liver biochemistries and the resolution of serological features of AIH. The present report is potentially easily understandable and very interesting.
P- Reviewer: Ito H, Lau WY, Morales-Gonzalez J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2042] [Cited by in F6Publishing: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 2. | Yeoman AD, Westbrook RH, Al-Chalabi T, Carey I, Heaton ND, Portmann BC, Heneghan MA. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50:538-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Chrétien P, Chousterman M, Abd Alsamad I, Ozenne V, Rosa I, Barrault C, Lons T, Hagège H. Non-organ-specific autoantibodies in chronic hepatitis C patients: association with histological activity and fibrosis. J Autoimmun. 2009;32:201-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Czaja AJ, Carpenter HA. Histological findings in chronic hepatitis C with autoimmune features. Hepatology. 1997;26:459-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kogure T, Ueno Y, Fukushima K, Nagasaki F, Inoue J, Kakazu E, Matsuda Y, Kido O, Nakagome Y, Kimura O. Fulminant hepatic failure in a case of autoimmune hepatitis in hepatitis C during peg-interferon-alpha 2b plus ribavirin treatment. World J Gastroenterol. 2007;13:4394-4397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Calleja JL, Albillos A, Cacho G, Iborra J, Abreu L, Escartín P. Interferon and prednisone therapy in chronic hepatitis C with non-organ-specific antibodies. J Hepatol. 1996;24:308-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1132] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 8. | Robertson DA, Zhang SL, Guy EC, Wright R. Persistent measles virus genome in autoimmune chronic active hepatitis. Lancet. 1987;2:9-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Vento S, Guella L, Mirandola F, Cainelli F, Di Perri G, Solbiati M, Ferraro T, Concia E. Epstein-Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346:608-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Pischke S, Gisa A, Suneetha PV, Wiegand SB, Taubert R, Schlue J, Wursthorn K, Bantel H, Raupach R, Bremer B. Increased HEV seroprevalence in patients with autoimmune hepatitis. PLoS One. 2014;9:e85330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Kalvenes MB, Haukenes G, Nysaeter G, Kalland KH, Myrmel H. Raised levels of antibodies to human viruses at the clinical onset of autoimmune chronic active hepatitis. J Viral Hepat. 1995;2:159-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Sutti S, Vidali M, Mombello C, Sartori M, Ingelman-Sundberg M, Albano E. Breaking self-tolerance toward cytochrome P4502E1 (CYP2E1) in chronic hepatitis C: possible role for molecular mimicry. J Hepatol. 2010;53:431-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Azhar A, Niazi MA, Tufail K, Malek AH, Balasubramanian M, Araya V. A new approach for treatment of hepatitis C in hepatitis C-autoimmune hepatitis overlap syndrome. Gastroenterol Hepatol (N Y). 2010;6:233-236. [PubMed] [Cited in This Article: ] |
| 14. | Oeda S, Mizuta T, Isoda H, Kuwashiro T, Oza N, Iwane S, Takahashi H, Kawaguchi Y, Eguchi Y, Toda S. Efficacy of pegylated interferon plus ribavirin in combination with corticosteroid for two cases of combined hepatitis C and autoimmune hepatitis. Clin J Gastroenterol. 2012;5:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Schiano TD, Te HS, Thomas RM, Hussain H, Bond K, Black M. Results of steroid-based therapy for the hepatitis C-autoimmune hepatitis overlap syndrome. Am J Gastroenterol. 2001;96:2984-2991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |