Published online May 8, 2016. doi: 10.4254/wjh.v8.i13.597
Peer-review started: January 8, 2016
First decision: March 1, 2016
Revised: March 13, 2016
Accepted: March 24, 2016
Article in press: March 25, 2016
Published online: May 8, 2016
AIM: To identify factors that influence long-term liver function following radiofrequency ablation (RFA) in patients with viral hepatitis-related hepatocellular carcinoma.
METHODS: A total of 123 patients with hepatitis B virus- or hepatitis C virus-related hepatocellular car-cinoma (HCC) (n = 12 and n = 111, respectively) were enrolled. Cumulative rates of worsening Child-Pugh (CP) scores (defined as a 2-point increase) were examined.
RESULTS: CP score worsening was confirmed in 22 patients over a mean follow-up period of 43.8 ± 26.3 mo. Multivariate analysis identified CP class, platelet count, and aspartate aminotransferase levels as signi-ficant predictors of a worsening CP score (P = 0.000, P = 0.011 and P = 0.024, respectively). In contrast, repeated RFA was not identified as a risk factor for liver function deterioration.
CONCLUSION: Long-term liver function following RFA was dependent on liver functional reserve, the degree of fibrosis present, and the activity of the hepatitis condition for this cohort. Therefore, in order to maintain liver function for an extended period following RFA, suppression of viral hepatitis activity is important even after the treatment of HCC.
Core tip: This study was conducted to identify risk factors for liver function deterioration following radio-frequency ablation (RFA) in patients with hepatocellular carcinoma (HCC) and viral hepatitis. A total of 123 patients with hepatitis B virus- or hepatitis C virus-related HCC were enrolled. Cumulative rates of wor-sening Child-Pugh (CP) scores (defined as a 2-point increase) following RFA were examined. CP class, platelet count, and aspartate aminotransferase levels were identified as significant predictors of a worsening CP score. Suppression of viral hepatitis activity with anti-viral therapy is important even after the treatment of HCC in order to maintain liver function following RFA.
- Citation: Honda K, Seike M, Oribe J, Endo M, Arakawa M, Syo H, Iwao M, Tokoro M, Nishimura J, Mori T, Yamashita T, Fukuchi S, Muro T, Murakami K. Risk factors for deterioration of long-term liver function after radiofrequency ablation therapy. World J Hepatol 2016; 8(13): 597-604
- URL: https://www.wjgnet.com/1948-5182/full/v8/i13/597.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i13.597
Hepatocellular carcinoma (HCC) is one of the most common malignant neoplasms worldwide[1] and most cases of HCC involve patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV)[2-4]. Radio-frequency ablation (RFA) is currently recognized as an effective local treatment for HCC[5,6] and has been shown to be a relatively low risk procedure[7-9]. However, deterioration of liver function has been observed during the long-term follow-up of these patients[10-12]. Therefore, the risk factors that contribute to deterioration of liver function need to be identified. Although a few reports have investigated changes in long-term liver function following RFA[10-12], long-term liver function in patients with viral hepatitis-related HCC is still uncertain. The goal of this study was to identify risk factors for liver function deterioration in patients with HCC and viral hepatitis.
This retrospective cohort study was based on data obtained from a prospective database maintained by the Oita University and Oita Medical Center. Between January 2002 and December 2010, 479 patients underwent percutaneous RFA for HCC at these two institutions. This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Ethics Committee of Oita University and Oita Medical Center.
A diagnosis of HCC was based on vascular findings obtained by dynamic computed tomography (CT) using early arterial uptake followed by washout in the porto-venous and equilibrium phase. For patients with an uncertain diagnosis, a fine-needle biopsy was perfor-med. Prior to RFA, patients with hyper vascular tumors underwent transarterial chemoembolization. All abla-tions were performed with a single needle electrode (COVIDIEN, Cool-tip RF Ablation System, Ireland). Furthermore, all RFA procedures were performed percu-taneously with ultrasound guidance, and diazepam and pentazocine were routinely administered prior to insertion of the electrode. If necessary, physiological saline was infused into the chest or abdominal cavity to induce artificial pleural effusion or ascites to avoid injury to adjacent organs, or to facilitate visualization of the tumor. Effects of RFA were confirmed by dynamic CT three days after treatment. If the ablated margin was insufficient, additional ablation was performed until a sufficient ablated margin was obtained.
Inclusion criteria for patient selection in the present study included: (1) HCC occurring due to HBV- or HCV-related chronic liver disease; (2) first occurrence of HCC; (3) the presence of up to four nodules per patient, with each nodule having a diameter less than 5 cm; and (4) the presence of tumors only in the liver, with complete necrosis achieved by treatment with RFA. Of the 479 patients treated for HCC, 356 patients were excluded from this study due to: Non-B or non-C HCC (n = 77), recurrent HCC (n = 80), complete necrosis was not obtained (n = 4), advanced HCC (n = 33), simultaneous other malignancies (n = 8), nephrotic syndrome or advanced chronic kidney disease (n = 7), portal thrombus (n = 3), chronic debilitating disease (n = 1), poor food intake (n = 2), breakthrough hepatitis by resistant HBV (n = 2), treatment with warfarin (n = 3), received albumin around the same time as RFA treatment (n = 1), started interferon (IFN) therapy up to 1 year after RFA treatment (n = 17), uncontrollable progression of HCC up to 1 year after RFA treatment (n = 4), death due to other disease within 1 year (n = 2), documents not stored by the electronic system (n = 75), a follow-up period less than one year (n = 27), and treatment with a nucleoside analog within six months of RFA treatment (n = 10). The latter was included based on reports that significant improvement in liver function had been observed within six months of lamivudine treatment for decompensated cirrhotic HBV patients[13,14]. Although it was also reported that albumin levels increased during the first two years of IFN treatment for chronic hepatitis C patients with sustained virological response (SVR)[15], none of the patients in the current cohort met this criterion.
For the resulting 123 patients enrolled in this study, two groups were established in order to examine the influence of viral hepatitis activity. The first group included nine HBV patients who achieved complete remi-ssion of hepatitis (defined as a normal range of trans-aminase levels) by treatment with an oral nucleoside such as lamivudine, adefovir, or entecavir, two patients with non-active HBV, and four HCV patients who re-ceived IFN therapy and achieved a SVR. This group was referred to as the remission of viral hepatitis (RVH) group. The second group consisted of one HBV patient and 107 HCV patients with active hepatitis, and this group was referred to as the chronic active hepatitis (CAH) group.
The starting point for observation was the first day that patients underwent RFA. Follow-up periods concluded when recurrent HCC(s) were no longer able to be con-trolled with RFA. In addition, follow-up periods were ended when liver function was found to be deteriorating due to another disease, when treatment with IFN was initiated, when treatment with a nucleoside analog was initiated, when recurrent tumors were treated by surgery, or when a thrombus formed in the portal vein. During the follow-up period, abdominal CT or ultrasonography was performed every four months and blood assays were performed monthly.
All quantitative variables are presented as the mean ± SD. The endpoint used was a 2-point increase in Child-Pugh (CP) scoring. The cumulative rate of worsening CP scores (defined as a 2-point increase) was also cal-culated, and cumulative proportion curves were gene-rated using the Kaplan-Meier method. Independent factors that influenced a worsening CP score were identified by univariate and multivariate analysis using Cox’s proportional hazards model. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics version 20.0 for Windows.
A total of 123 patients (71 males, 52 females) with HBV infection (n = 12) or HCV infection (n = 111) were enrolled in this study. Additional characteristics of this cohort are provided in Table 1. Of the HBV patients, 9/12 were treated with nucleoside analogs [lamivudine (n = 1), lamivudine plus adefovir dipivoxil (n = 4), and entecavir (n = 4)] at least six months prior to RFA therapy. There were also two patients with non-active HBV carriers, and one HBV patient had an active case of hepatitis at the time of RFA. Of the HCV patients, 4/111 achieved a post-SVR state with IFN therapy. The CP class A group consisted of 102 patients which included: An active HBV carrier (n = 1), inactive HBV carriers that did not receive nucleoside analog treatment (n = 2), inactive HBV carriers that received nucleoside analog treatment (n = 8), patients with active hepatitis C (n = 87), and SVR patients with hepatitis C (n = 4). The CP class B group included an inactive HBV carrier who received nucleoside analog treatment (n = 1), and active hepatitis C patients (n = 20). During the follow-up period, the frequency of RFA treatment for recurrent tumors included a single treatment (n = 32), two treatments (n = 23), three treatments (n = 9), four treatments (n = 5), five treatments (n = 3), and six treatments (n = 2). There were 49 patients that did not receive any RFA treatment.
| Gender (male/female) | 71/52 |
| Age (yr) | 69.7 ± 8.0 |
| Hepatitis (HBV/HCV) | 12/111 |
| CP score (5/6/7/8) | 79/22/15/7 |
| CP class (A/B/C) | 102/21/0 |
| Size of tumor (mm) | 20.6 ± 7.7 |
| No. of tumor(s) (1/2/3/4) | 78/30/13/2 |
| Total bilirubin (mg/dL) | 0.97 ± 0.4 |
| Albumin (g/dL) | 3.7 ± 0.6 |
| Prothrombin time (%) | 90.5 ± 15 |
| Platelet count (104/μL) | 11.1 ± 5.0 |
| AST (IU/L) | 58.2 ± 32.1 |
| ALT (IU/L) | 53.0 ± 39.7 |
| Hepatitis condition RVH group/CAH group | 13/110 |
| Prior TACE with TACE/without TACE | 110/13 |
The follow-up period was ended for patients of this cohort due to: Loss of local control of tumor progression with RFA (n = 21), death or worsening of liver function due to another disease or accident (n = 7), induction of IFN therapy for HCV infection (n = 5), surgical treatment for recurrent tumors (n = 1), emergence of a portal thrombus (n = 1), and administration of a nucleoside analog for HBV infection (n = 1). In the latter case, a patient with HBV was enrolled in the CAH group since he initially refused treatment with entecavir. However, 12 mo later he consented to receive entecavir as a treatment, and consequently, the follow-up period for this case ended after 12 mo.
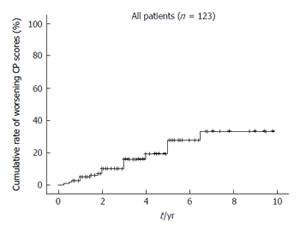
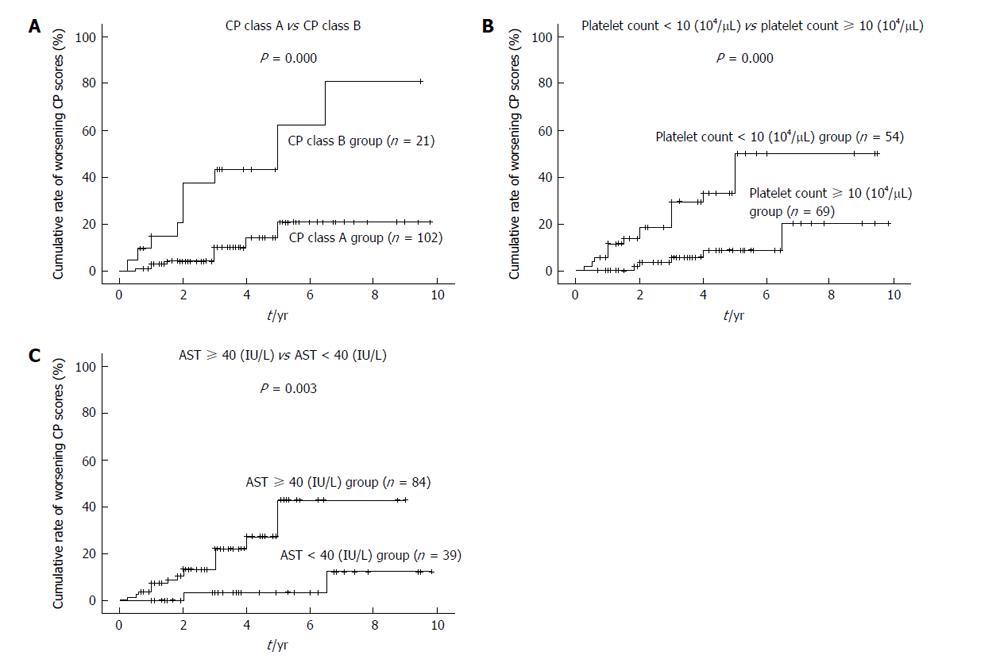
A worsening CP score was confirmed for 22 patients during a mean follow-up period of 43.8 ± 26.3 mo. Moreover, the 1-, 2-, 3-, 5- and 7-year cumulative rates for worsening CP scores calculated according to the Kaplan-Meier method were 2.4%, 6.9%, 10.0%, 19.3% and 33.2%, respectively (Figure 1). The variables listed in Table 1, as well as the frequency of RFA for recurrent HCC, were selected as factors for analysis using Cox’s proportional hazards model. In contrast, the type of infection (HBV or HCV), and the presence of an active hepatitis condition (RVH or CAH), were excluded from this analysis, since none of the patients in HBV or RVH group exhibited at least a two point increase in CP scores during the follow-up period. Risk factors that were found to contribute to worsening CP scores following RFA are listed in Tables 2 and 3. In a univariate analysis performed, CP class, total bilirubin, albumin, prothrombin time, platelet count, levels of as-partate aminotransferase (AST), and levels of alanine aminotransferase were found to be associated with a worsening CP score (Table 2). Accordingly, these factors were selected for multivariate analysis. Frequency of RFA treatments for recurrent HCC was not found to be associated with deterioration of long-term liver function. Since total CP class, bilirubin, albumin, and prothrombin time are factors that indicate liver function, CP class was selected as a factor representative of these variables. In the multivariate analysis performed, CP class, platelet count, and AST were identified as significant predictors of a worsening CP score (Table 3) (P = 0.000, P = 0.011 and P = 0.024, respectively). Cumulative rates of worsening CP scores were generated using the Kaplan-Meier method and are shown in Figure 2.
| Variable | HR | 95%CI | P-value |
| Gender (female vs male) | 1.93 | 0.83-4.47 | 0.128 |
| Age (yr) (< 70 vs≥ 70) | 1.05 | 0.45-2.44 | 0.906 |
| CP class (B vs A) | 5.03 | 2.17-11.7 | 0.000 |
| Size of tumor (mm) (≥ 20 vs < 20) | 2.01 | 0.84-4.81 | 0.116 |
| Number of tumors (≥ 2 vs 1) | 1.47 | 0.62-3.47 | 0.379 |
| Total bilirubin (mg/dL) (≥ 1.0 vs < 1.0) | 3.48 | 1.35-8.99 | 0.010 |
| Albumin (g/dL) (< 3.5 vs≥ 3.5) | 8.52 | 3.12-23.2 | 0.000 |
| Prothrombin time (< 80% vs≥ 80%) | 2.66 | 1.14-6.23 | 0.024 |
| Platelet count (104/μL) (< 10 vs≥ 10) | 5.04 | 1.86-13.7 | 0.001 |
| AST (IU/L) (≥ 40 vs < 40) | 7.06 | 1.57-31.8 | 0.011 |
| ALT (IU/L) (≥ 35 vs < 35) | 4.01 | 1.32-12.2 | 0.015 |
| Prior TACE vs no TACE | 1.05 | 0.24-4.48 | 0.952 |
| Frequency of RFA treatments for recurrent HCC (≥ 2 vs < 2) | 1.51 | 0.64-3.53 | 0.344 |
| Variable | HR | 95%CI | P-value |
| CP class (B vs A) | 5.07 | 2.13-12.1 | 0.000 |
| Platelet count (104/μL) (< 10 vs≥ 10) | 3.83 | 1.36-10.8 | 0.011 |
| AST (IU/L) (≥ 40 vs < 40) | 7.01 | 1.30-37.9 | 0.024 |
| ALT (IU/L) (≥ 35 vs < 35) | 1.21 | 0.35-4.19 | 0.761 |
Subpopulational analyses were also performed with respect to HBV, HCV, RVH and CAH. For the HBV group (n = 12, mean follow-up period: 64.0 ± 28.7 mo, CP class A (n = 11), CP class B (n = 1), CAH (n = 1), RVH (n = 11), platelet count: (10.4 ± 4.3) × 104/μL, AST: 26.3 ± 5.4 IU/L, frequency of RFA treatment after initial treatment (0/1/2/3 times): 4/6/1/1 patients, res-pectively), none of the patients exhibited deterioration of long-term liver function.
For the HCV group [n = 111, mean follow-up period: 41.6 ± 25.2 mo, CP class A (n = 91), CP class B (n = 20), CAH (n = 107), RVH (n = 4), platelet count: (11.1 ± 5.1) × 104/μL, AST: 61.6 ± 31.9 IU/L, frequency of RFA treatment after initial treatment (0/1/2/3/4/5/6 times): 45/26/22/8/5/3/2 patients, respectively], CP class and platelet count were both identified as signi-ficant predictors of worsening CP scores in the multi-variate analysis performed (P = 0.000 and P = 0.009, respectively) (Table 4). None of the patients in the SVR group (n = 4) exhibited worsening of CP scores.
| Variable | HR | 95%CI | P-value |
| CP class (B vs A) | 4.90 | 2.05-11.7 | 0.000 |
| Platelet count (104/μL) (< 10 vs≥ 10) | 3.96 | 1.40-11.2 | 0.009 |
| AST (IU/L) (≥ 40 vs < 40) | 5.25 | 0.98-28.0 | 0.052 |
| ALT (IU/L) (≥ 35 vs < 35) | 1.11 | 0.33-3.73 | 0.865 |
For the RVH group [n = 15, mean follow-up period: 65.4 ± 28.0 mo, HBV (n = 11), HCV (n = 4), CP class A (n = 14), CP class B (n = 1), platelet count: (11.7 ± 5.1) × 104/μL, AST: 25.7 ± 5.1 IU/L, frequency of RFA treatment after initial treatment (0/1/2/3 times): 8/6/0/1 patients, respectively], none of the patients exhibited worsening CP scores.
For the CAH group [n = 108, mean follow-up period: 40.8 ± 24.7 mo, HBV (n = 1), HCV (n = 107), CP class A (n = 88), CP class B (n = 20), platelet count: (11.0 ± 5.0) × 104/μL, AST: 62.7 ± 31.6, frequency of RFA treatment after initial treatment (0/1/2/3/4/5/6 times): 41/26/23/8/5/3/2 patients, respectively], CP class B and patients with a platelet count < 10 × 104/μL were associated with CP worsening (P = 0.000 and P = 0.010, respectively).
Treatment of HCC generally involves a surgical approach and/or a non-surgical approach. In the latter case, transarterial embolization, radiation therapy, chemo-therapy, and local puncture therapy are the main options available. While percutaneous ethanol injection therapy[16] is a type of local puncture therapy that has been performed since the 1980s, local ablative therapy such as microwave coagulation therapy[17] and RFA therapy were subsequently developed. Currently, RFA is the main form of local puncture therapy administered due to its ability to provide local control of HCC. The less invasive approach of RFA also represents a key advantage of RFA over surgical resection. However, since the recurrence rate of HCC following radical treatment is generally high, repeated RFA treatments are often needed. There have been reports that the application of repeated RFA for the treatment of recurrent tumors can increase the chances of long-term survival[8,9,18].
A few reports have referred to the influence of RFA on liver function. For example, Koda et al[10] reported that liver function in patients with low pre-treatment CP scores transiently deteriorated within the first month of observation, while patients with high pre-treatment CP scores exhibited a greater extent of deterioration over a longer term of observation, approximately 6 mo. In a study by Kuroda et al[11], changes in liver function were monitored one year after RFA, and it was observed that a CP score of 9 or higher represented a major risk factor for aggravation of liver function following RFA. Furthermore, in another report by Yokoyama et al[12], the influence of RFA treatments on long-term liver function was investigated. Approximately 15% of CP class A or CP class B patients were observed to progress to CP class C five years after RFA treatment. However, the factors that influence on long-term liver function in patients with viral hepatitis-related HCC following RFA is still uncertain. There are various factors that may contribute to changes in liver function. Since tumor progression is an obvious factor that aggravates the liver function of HCC patients, the current analyses were performed with patients where tumor progression could be excluded. Based on the analyses performed, CP class B patients, patients with a platelet count < 10 × 104/μL, and patients with AST levels ≥ 40 IU/L, were found to be significantly associated with a worsening of liver function after RFA. These results suggest that worsening of long-term liver function after RFA is dependent on liver function, the degree of fibrosis present, and the activity of a patient’s hepatitis condition. However, repeated RFA was not found to be a factor that aggravates long-term liver function.
None of the RVH patients exhibited CP worsening, thereby suggesting that liver function can be maintained in RVH patients if HCC is controlled. This result also suggests that short-term functional damage of the liver that is caused by RFA does not influence long-term liver function. However, since almost all of the RVH patients in the present study belonged to the CP class A group, additional studies are needed to clarify whether long-term liver function is affected following RFA for RVH patients with poor liver function.
Nucleoside analogs such as lamivudine or entecavir are used to treat active cases of hepatitis B by inhibiting DNA synthesis with termination of the nascent proviral DNA chain. As a result, levels of both serum HBV-DNA and transaminase concentrations are rapidly reduced. When viral suppression is prolonged, this can result in histological improvement, including regression of fibrosis[19-22], and in patients with HBV-related HCC, liver function has improved[23-26]. For hepatitis C patients, IFN therapy has previously been the only treatment found to reduce levels of virus. For example, peginterferon plus ribavirin treatment has been a standard therapy for HCV infection until recently when telaprevir or sime-previr combined therapy was shown to improve the efficacy of IFN therapy[27,28]. However, since many cases of HCV-related HCC involved elderly patients, or a cirrhotic liver, there were many patients who could not receive radical treatment for HCV when HCC was detected. Other direct-acting antiviral agents have recently been investigated, and these have been found to increase SVR ratios[29,30]. Correspondingly, it is possible for HCC patients who are difficult to treat with IFN to be treated with IFN-free therapies.
While liver resection and RFA are still the standard treatments for many HCC patients, the long-term effects of surgical resection vs RFA remain controversial[31-33]. Thus, when many patients of HCV-related HCC become able to be treated with IFN-free therapies, this issue may be re-evaluated. In addition, further studies are needed to evaluate treatment modalities with respect to coexisting hepatitis conditions.
In conclusion, the results of the present study indicate that long-term liver function following RFA is dependent on functional reserve of the liver, the degree of fibrosis present, and hepatitis activity. Since viral eradication or suppression is currently the most effective method to improve these factors, anti-viral therapy is important even after the treatment of HCC.
There are only a few reports that have examined liver function following radiofrequency ablation (RFA). In particular, long-term liver function following RFA in patients with viral hepatitis-related hepatocellular carcinoma (HCC) has not been well studied.
In the present study, long-term liver function in patients with viral hepatitis-related HCC that underwent RFA was found to be dependent on the functional reserve of the liver, the degree of fibrosis, and hepatitis activity.
In previous studies, liver functional reserve at the time of RFA treatment was identified as a risk factor for liver function deterioration following RFA. Here, the authors demonstrate that the degree of liver fibrosis and hepatitis activity are also associated with deterioration of liver function following RFA.
The strong and safe treatment regimen for patients with hepatitis B or hepatitis C that the authors have developed has the potential to maintain liver function following RFA treatment of patients with viral hepatitis-related HCC.
This is a very well done study, it demonstrates that RFA seems to be a well tolerated therapy without relationship with deterioration of liver function.
P- Reviewer: Tijera MFH, Xu Z, Zhong JH S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2337] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 3. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1927] [Cited by in F6Publishing: 1898] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 4. | Taura N, Fukushima N, Yastuhashi H, Takami Y, Seike M, Watanabe H, Mizuta T, Sasaki Y, Nagata K, Tabara A. The incidence of hepatocellular carcinoma associated with hepatitis C infection decreased in Kyushu area. Med Sci Monit. 2011;17:PH7-P11. [PubMed] [Cited in This Article: ] |
| 5. | Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 508] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 861] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 7. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multi-center study. Radiology. 2003;226:441-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1003] [Cited by in F6Publishing: 912] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 8. | Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, Silini EM, Dionigi P, Calliada F, Quaretti P. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 10. | Koda M, Ueki M, Maeda Y, Mimura KI, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma. Hepatol Res. 2004;29:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kuroda H, Kasai K, Kakisaka K, Yasumi Y, Kataoka K, Ushio A, Miyamoto Y, Sawara K, Oikawa K, Kondo K. Changes in liver function parameters after percutaneous radiofrequency ablation therapy in patients with hepatocellular carcinoma. Hepatol Res. 2010;40:550-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Yokoyama K, Anan A, Iwata K, Nishizawa S, Morihara D, Ueda S, Sakurai K, Iwashita H, Hirano G, Sakamoto M. Limitation of repeated radiofrequency ablation in hepatocellular carcinoma: proposal of a three (times) × 3 (years) index. J Gastroenterol Hepatol. 2012;27:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Tseng PL, Lu SN, Tung HD, Wang JH, Changchien CS, Lee CM. Determinants of early mortality and benefits of lamivudine therapy in patients with hepatitis B virus-related decompensated liver cirrhosis. J Viral Hepat. 2005;12:386-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol. 2000;33:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Longitudinal changes of the laboratory data of chronic hepatitis C patients with sustained virological response on long-term follow-up. J Viral Hepat. 2012;19:e97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Seki T, Nonaka T, Kubota Y, Mizuno T, Sameshima Y. Ultra-sonically guided percutaneous ethanol injection therapy for hepatocellular carcinoma. Am J Gastroenterol. 1989;84:1400-1407. [PubMed] [Cited in This Article: ] |
| 17. | Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817-825. [PubMed] [Cited in This Article: ] |
| 18. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 19. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1288] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 20. | Suzuki Y, Kumada H, Ikeda K, Chayama K, Arase Y, Saitoh S, Tsubota A, Kobayashi M, Koike M, Ogawa N. Histological changes in liver biopsies after one year of lamivudine treatment in patients with chronic hepatitis B infection. J Hepatol. 1999;30:743-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histolo-gical outcome during long-term lamivudine therapy. Gastroen-terology. 2003;124:105-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 22. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 717] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 23. | Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Park JW, Koh DW, Lee WJ, Kim CM. Efficacy of lami-vudine on hepatitis B viral status and liver function in patients with hepatitis B virus-related hepatocellular carcinoma. Liver Int. 2009;29:203-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Yoshida H, Yoshida H, Goto E, Sato T, Ohki T, Masuzaki R, Tateishi R, Goto T, Shiina S, Kawabe T. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008;2:89-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Kobashi H, Miyake Y, Ikeda F, Yasunaka T, Nishino K, Moriya A, Kubota J, Nakamura S, Takaki A, Nouso K. Long-term outcome and hepatocellular carcinoma development in chronic hepatitis B or cirrhosis patients after nucleoside analog treatment with entecavir or lamivudine. Hepatol Res. 2011;41:405-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 916] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 28. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 29. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1329] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 30. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 31. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepato-cellular carcinoma. Ann Surg. 2006;243:321-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 1039] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 32. | Huang J, Hernandez-Alejandro R, Croome KP, Yan L, Wu H, Chen Z, Prasoon P, Zeng Y. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in Childs A cirrhotics-a retrospective study of 1,061 cases. J Gastrointest Surg. 2011;15:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, Bagante F, Turcato G, D’Onofrio M, Iacono C. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301-311; discussion 311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |