Published online Apr 18, 2015. doi: 10.4254/wjh.v7.i5.806
Peer-review started: December 2, 2014
First decision: January 8, 2015
Revised: February 3, 2015
Accepted: March 5, 2015
Article in press: March 9, 2015
Published online: April 18, 2015
AIM: To investigate the efficacy, safety, and cost of treatment of direct acting antivirals (DAAs) with and without peg interferon alfa2a (P), and/or ribavirin (R) in treating hepatitis C virus (HCV) genotype 1 patients.
METHODS: MEDLINE was searched for randomized controlled trials (RCT) using DAAs for HCV treatment. Phase 1 trials and studies with investigational drugs on genotype 2 or 3, and on human immunodeficiency virus patients were excluded. Data were pooled for sustained virologic response (SVR), serious adverse effects, and drug discontinuation rate on various treatment arms in trials: P + R; 1st generation DAA (telaprevir or boceprevir) + P + R; 2nd generation DAA (sofosbuvir or simeprevir) + P + R; 2nd generation DAA + R; two 2nd generation DAA + R; and two 2nd gen DAA. Data were analyzed separately for each arm for treatment naive and non-responders (NR) to previous treatment. The cost of treatment with each regimen for achieving one SVR was also compared.
RESULTS: Twenty three RCTs (n = 9354, 62% male, 11% cirrhosis) were analyzed. All oral (P free) regimens with combination of 2 DAA achieved SVR above 95%. The cost of treatment to achieve an SVR with DAA based regimens was lower for NR compared to P+R regimen. However, the cost per SVR remained higher for treatment naive patients.
CONCLUSION: Second generation and emerging DAAs are promising agents in HCV treatment, with a very high level of safety and efficacy. An important drawback is their high cost. However, the present meta-analysis shows that the cost per SVR for non responders (but not for naive patients) was lower compared to P + R. This finding together with the superior safety profile and better compliance makes these drugs highly attractive. It is possible that further reduction in treatment duration may make them even more cost effective.
Core tip: Data are rapidly evolving on the efficacy and safety of newer oral direct acting antivirals (DAAs) for treating hepatitis C virus (HCV) infection. Second generation and emerging DAAs are promising agents in HCV treatment, with a very high level of safety and efficacy. An important drawback is their high cost. However, the present meta-analysis shows that the cost per sustained virologic response for non responders (but not for naive patients) was lower compared to peg interferon alfa2a + ribavirin. This finding together with the superior safety profile and better compliance makes these drugs highly attractive.
- Citation: Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol 2015; 7(5): 806-813
- URL: https://www.wjgnet.com/1948-5182/full/v7/i5/806.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i5.806
World Health Organization estimates that about 3% of the world’s population is infected with hepatitis C virus (HCV) and that there are more than 170 million chronic carriers who are at risk of developing liver cirrhosis and/or liver cancer[1]. Natural history suggests that amongst acute hepatitis C infected patients, 70%-90% go on to develop chronic hepatitis C infection. Of those with chronic HCV, 10%-20% progress to cirrhosis. HCV-associated cirrhosis leads to liver failure and death in about 20%-25% patients, and 1%-5% of persons with chronic hepatitis C will develop hepatocellular carcinoma[2,3]. Treatment for HCV infection is undergoing a rapid evolution, offering new hope to both treatment naïve HCV patients and patient who have not responded well to previous treatment. Numerous highly effective, but expensive, direct acting antiviral (DAA) drugs active against different targets are now available.
HCV is an enveloped, small, single-stranded RNA virus of the family Flaviviridae. Its genome was cloned in 1989. The virus undergoes co- and post translational cleavage by proteases of the host and virus to yield individual viral proteins[4]. The N-terminal consists of the nucleocapsid proteins and a small ion channel protein[5]. These are followed by the non-structural (NS) proteins NS2-NS5, which mediate intracellular aspects of viral functions. NS3 facilitates unwinding of the viral genome for replication. NS5b is the RNA-dependent RNA polymerase needed for viral replication. NS2, NS3, and NS4a proteins interact to mediate polyprotein processing. Based on genetic differences between isolates, the HCV species is classified into seven genotypes (1-7) with several subtypes within each genotype, which differ by 30%-35% of the nucleotide sites over the complete genome[6]. HCV subtypes 1a and 1b are most common and cause 60% of all HCV infection cases[7].
Before the introduction of DAAs, HCV was treated with peg interferon alfa2a (P), which is an immunomodulatory agent administered as subcutaneous injection. Subsequently, ribavirin(R), an oral antiviral nucleoside analog, was added to the regimen. These regimens have variable success rates. The newer agents, DAAs, target various stages of the HCV life cycle. They target HCV proteins, particularly the NS proteins, e.g., NS3/4A by telaprevir, boceprevir, simeprevir, faldaprevir, asunaprevir, and danoprevir [not Food and Drug Administration (FDA) approved]; NS5A by daclatasvir, and ledipasvir; and NS5B by sofosbuvir. DAAs can also be categorized as: 1st generation which includes telaprevir and boceprevir, and 2nd generation which include-sofosbuvir (SOF), simeprevir, ledipasvir, and daclatasvir.
There are several recent good quality clinical trials on DAAs for the treatment of HCV. But there is a paucity of good quality articles on comparison of efficacy and safety of these agents or meta-analysis on the data outcome of these newer agents. Moreover data on cost effectiveness is very limited[8-10]. We performed this study to examine the efficacy, safety, and cost of treatment of DAAs with and without P, and/or R in treating HCV genotype 1 patients.
The MEDLINE, National Library of Medicine through PubMed was searched for hepatitis C treatment, DAAs, and randomized control trials. The search was later expanded using MeSH terms telaprevir, boceprevir, sofosbuvir, simeprevir, and ledipasvir. The search was conducted for studies published in the English language between January 1, 1975, and April 15, 2014. In addition, we searched Scopus, and Google Scholar databases for the terms hepatitis C, and DAA and randomized control trial. References of identified articles were searched for additional relevant articles. Studies were included if they were randomized control trials in phase II, III or IV, on HCV genotype 1, published in English, used FDA-approved therapies that included SVR as a primary or secondary end point, and defined treatment-experienced patients using American Association for the Study of Liver Diseases definitions. Phase 1 trial, studies with investigational drugs or drugs not approved by FDA, patients with genotype 2 or 3, and human immunodeficiency virus were excluded.
The success rate for HCV treatment is measured as the sustained viral response (SVR), which is defined as the absence of detectable RNA of the HCV in blood or serum for at least 24 wk after discontinuing the treatment. Serious adverse events (SAE) were defined as side effects that lead to serious outcomes, and drug discontinuation rate (DDR) as the rate of drug discontinuation due to any cause.
Treatment naïve were defined as patient who have never received treatment for HCV and non-responders (NR) were defined as patient who have received prior treatment but have not responded to treatment in terms of not achieving SVR (include failed, partial responder and relapse).
Data including study design, participant demographics, stage of liver disease, treatment regimen and duration, SVR, SAE, and DDR were extracted and recorded on electronic data collection sheet. Data were pooled for various arms in trials: (1) Traditional only P + R; (2) 1st generation DAA + P + R; (3) 2nd generation DAA + P + R; (4) 2nd generation DAA + R (without P); two different 2nd generation DAAs + R (without P); and (5) Two different 2nd generation DAAs (without P or R).
Individual data for each outcome were entered into the Comprehensive meta-analysis software (Biostat, Englewood, NJ, United States). Pooled effects with 95%CI are reported. Data were analyzed separately for each arm for treatment naive and NR to previous treatment. The best SVR rate was used for analysis.
Cost effectiveness analysis was performed using the prevalent cost of DAAs as per our institutional pharmacy drug accrual cost. Cost of treatment for each regimen was calculated per week of treatment and was also compared for achieving one SVR. Data was reported in dollar amount.
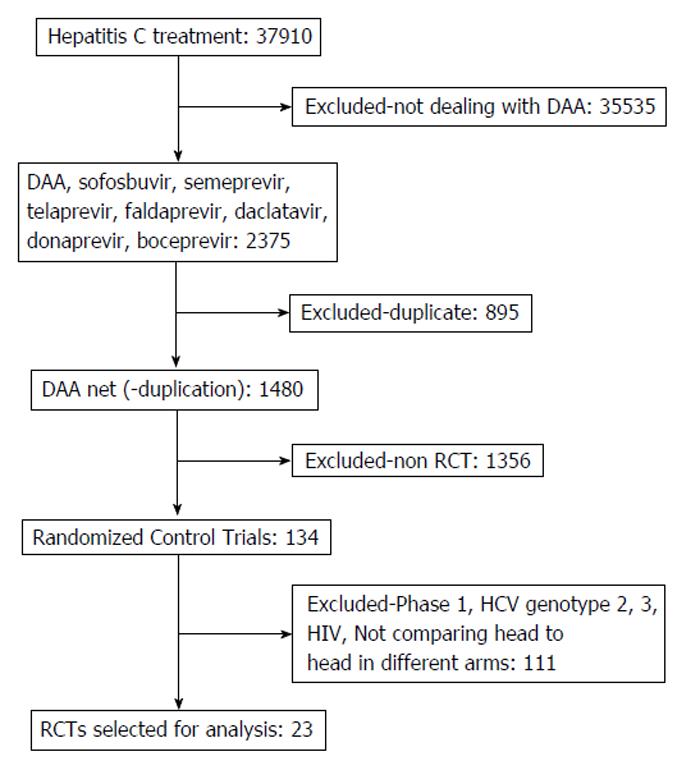
A flow diagram illustrating the study selection process is shown in Figure 1. One hundred thirty four relevant studies were screened and assessed for eligibility. After applying the inclusion and exclusion criteria, 23 studies were selected for analysis[11-33].
Table 1 summarizes the description of treatment regimen, number of participants, demographics, previous treatment status, and number of study arms, SVR, SAE and DDR. Baseline characteristics of the patients enrolled in the study demonstrated highly variable sample size, ranging from 40 to 1097 patients. Including all the studies, there were a total of 9354 patients, with 62% males and 11% cirrhotics. The average age of the study population was 50 years and the average body mass index was 27.
| Ref. | Previous treatment | No. of arms | Study arms/types | No. of patients (n) | Males (n) | Age median (yr) | Median BMI | Cirrhosis (n) |
| Afdhal et al[12] | Naïve | 4 | LED + SOF vs LED + SOF + R | 865 | 513 | 53 | 27 | 136 |
| Afdhal et al[11] | NR | 4 | LED + SOF vs LED + SOF + R | 440 | 287 | 56 | 28 | 88 |
| Bacon et al[13] | NR | 3 | P + R vs P + R + BOC | 403 | 268 | 53 | 28 | 49 |
| Flamm et al[14] | NR | 2 | P + R vs P + R + BOC | 201 | 140 | 53 | 28 | 33 |
| Fried et al[15] | Naïve | 5 | P + R vs P + R + SIM | 386 | 213 | 46 | 25 | 0 |
| Hézode et al[16] | Naïve | 4 | P + R vs TEL + P vs TEL + P + R | 323 | 192 | 45 | 24 | 1 |
| Jacobson et al[17] | Naïve | 3 | P + R vs P + R + TEL | 1088 | 636 | 49 | 26 | 68 |
| Kowdley et al[19] | Naïve | 3 | SOF vs SOF + R | 332 | 214 | 50 | 28 | 0 |
| Kowdley et al[18] | Naïve | 3 | LED + SOF vs LED + SOF + R | 647 | 375 | 52 | 28 | 0 |
| Kumada et al[20] | Naïve | 2 | P + R vs P + R + TEL | 189 | 99 | 54 | 23 | 0 |
| Kwo et al[21] | Naïve | 5 | P + R vs P + R + BOC | 520 | 305 | 45 | 37 | |
| Lawitz et al[22] | Naïve | 3 | P + R vs P + R + SOF | 121 | 73 | 49 | 27 | 0 |
| Lawitz et al[23] | NR | 2 | SOF + LED vs SOF + LED + R | 40 | 29 | 53 | 31 | 22 |
| Lawitz et al[23] | Naïve | 3 | SOF + LED vs SOF + LED + R | 60 | 37 | 48 | 29 | 0 |
| Marcellin et al[24] | Naïve | 4 | TEL + Palfa + R | 161 | 80 | 45 | 24 | 4 |
| McHutchison et al[25] | Naïve | 4 | P + R vs TEL + P + R | 250 | 157 | 49 | 27 | 51 |
| McHutchison et al[26] | NR | 4 | P + R vs TEL + P vs TEL + P + R | 453 | 306 | 52 | 28 | 74 |
| Osinusi et al[27] | Naïve | 2 | SOF + R vs SOF + low dose R | 50 | 33 | 55 | 29 | 13 |
| Pearlman et al[28] | Naïve | 2 | P + R vs P + R + BOC | 101 | 62 | 53 | 29 | 20 |
| Poordad et al[29] | Naïve | 3 | P + R vs P + R + BOC | 1097 | 656 | 49 | 100 | |
| Sherman et al[31] | Naïve | 3 | TEL + P + R (diff dur) | 440 | 271 | 51 | 42 | |
| Rodríguez-Torres et al[30] | Naïve | 4 | P + R vs P + R + SOF | 63 | 43 | 45 | 28 | 0 |
| Zeuzem et al[32] | NR | 3 | P + R vs P + R + TEL | 662 | 460 | 51 | 27 | 169 |
| Zeuzem et al[33] | NR | 7 | P + R vs P + R + SIM | 462 | 311 | 50 | 27 | 83 |
Table 2 summarizes the pooled outcome data. Data is expressed separately for treatment naïve and NR. Regimens were divided into P based regimens vs all oral, i.e., P free regimen, as regimen based on P requires weekly subcutaneous injections, while R and DAA are orally administered. Each subgroup was divided into regimens based without DAA, with 1st generation DAA and 2nd generation DAA.
| Regimen | Type | n | SVR (%) | SAE (%) | DDR (%) | Cost/wk ($) | Cost/SVR ($) |
| P + R | Naïve | 14 | 49.4 (42.7-56.2) | 10.1 (7.2-14.0) | 9 (5.3-14.9) | 900 | 87449 |
| P + R | NR | 5 | 18.5 (15.2-22.4) | 7.9 (5.5-11.3) | 3.5 (2.1-5.7) | 900 | 233514 |
| TEL or BOC based with P/R | Naïve | 8 | 74.5 (67.8-80.2) | 9.4 (6.7-13.0) | 11.9 (6.5-20.7) | 2300 | 148188 |
| TEL or BOC based with P/R | NR | 4 | 62.6 (55.9-68.7) | 13.7 (11.3-16.5) | 12.5 (9.8-15.8) | 2300 | 176358 |
| SOF or SIM based with P/R | Naïve | 9 | 90.3 (83.6-94.4) | 5.4 (1.9-12.5) | 2.5 (1.1-5.4) | 6900 | 91694 |
| SOF or SIM based with P/R | NR | 4 | 95.9 (91.5-98.1) | 6.8 (1.1-12.8) | 1.9 (0.5-7.1) | 6900 | 86340 |
| DAA + R | Naïve | 5 | 92.3 (82.9-96.7) | 3.1 (1.3-6.8) | 0.9 (0.3-2.6) | 12200 | 158613 |
| DAA +R | NR | 4 | 95.9 (91.5-98.1) | 3.3 (1.1-9.9) | 1.9 (0.5-7.1) | 12200 | 152659 |
| 2 DAA, No P/R | Naïve | 4 | 96.4 (93.6-98.0) | 1.9 (0.6-5.7) | 0.9 (0.3-2.7) | 12000 | 149378 |
| 2 DAA, No P/R | NR | 3 | 94.1 (88.9-97.0) | 2.3 (0.6-8.8) | 1.4 (0.3-6.5) | 12000 | 153029 |
P based regimen: Analysis of the pooled data of the traditional P + R regimen showed only 49.4% of patients with a CI of (42.7%-56.2%) had absence of detectable HCV RNA for at least 24 wk after discontinuing the treatment. This was associated with a high SAE of 10.1 (7.2%-14.0%) and DDR of 9 (5.3%-14.9%). Analysis favored DAA based regimens by showing that the addition of 1st generation DAA, i.e., boceprevir or telaprevir, increases the SVR to 74.5 (67.8%-80.2%), although it still had a high SAE of 9.4 (6.7%-13.0%) and higher DDR 11.9 (6.5%-20.7%). Regimens with a 2nd generation DAA showed a further increase in SVR to 90.3 (813.6%-94.4%), was associated with fewer side effects and less discontinuation rate with SAE of 5.4 (1.9%-12.5%) and DDR of 2.5 (1.1%-5.4%).
All oral regimens: This group included regimens with DAA with or without ribavirin. All medications were taken as oral only without any subcutaneous injections. 2nd generation DAAs, i.e., sofosbuvir, simeprevir, and ledipasvir, with R (either as single DAA or in combination of two DAAs showed a SVR of 92.3 (82.9%-96.7%) with a low SAE 3.1 (1.3%-6.8%) and low DDR of 0.9 (0.3%-2.6%). Pooled analysis showed that combining two DAAs without R, leads to a further increase in cure rates with SVR reaching 96.4 (93.6%-98.0%) with low SAE 1.9 (0.6%-5.7%) and lower DDR 0.9 (0.3%-2.7%). Comparing regimens with or without the use of ribavirin showed that the addition of R to DAAs did not change the SVR much, but added to the side effect profile with an increase in SAE.
P based regimens: Pooled data analysis demonstrated that all the above noted effects were more profound in treatment experienced individuals who had previously not responded to traditional P + R regimen. Repetition of another course of traditional P + R regimen showed a very low cure rate, with SVR of 18.5 (15.2%-22.4%) with a high SAE of 7.9 (5.5%-11.3%) and DDR 3.5 (2.1%-5.7%). The addition of a 1st generation DAA increased the SVR dramatically to 62.6 (55.9%-68.7%) but was associated with higher side effects, SAE of 13.7 (11.3%-16.5%) and higher DDR 12.5 (9.8%-15.8%). Similarly, regimens with 2nd generation DAA showed superior efficacy with an increase in SVR to 95.9 (91.5%-98.1%) with high SAE of 6.8 (1.1%-12.8%) and DDR of 1.9 (0.5%-7.1%).
All oral regimens: Analysis revealed that regimens with 2nd generation DAA with R in NR resulted in a marked increase in SVR of 95.9 (91.5%-98.1%), with an improvement in side effect profile if P was eliminated, as evident by low SAE of 3.3 (1.1%-9.9%) and low DDR 1.9 (0.5%-7.1%). Similar to naïve patients, combining two DAAs without R in NR lead to greater increase in SVR of around 95% (considering that the SVR was only 18% with the traditional regimen) with a value of 94.1 (88.9%-97.0%) with SAE 2.3 (0.6%-8.8%) and low DDR of 1.4 (0.3%-6.5%).
The efficacy and safety benefit of DAA did come with an added cost. Analysis of cost revealed that the overall cost of treatment was substantially higher with the newer DAA based regimens, around $6000 with single DAA and around $12000 with two DAAs as compared to $900 for P + R only per week. The cost for the newer combination pill of sofosbuvir + ledipasvir was around $9500 per week (as compared to adding 2 DAA separately, with a price tag of $12000).
Further cost effectiveness analysis of pooled data demonstrated that the cost per SVR was similar and even better for DAA based regimens, especially in NR (around $153k with two DAAs vs $233k for P + R for NR), likely related to the low SVR with the traditional regimen and high cost of recurrent treatments.
The traditional approach to treat hepatitis C infection was to use weekly injections of P with oral Ribavirin. This treatment was associated with low efficacy and significant side effect profile, often leading to high drug discontinuation rates. Analysis of the pooled data of traditional P + R regimen showed only 50% of patients achieved cure. This was also associated with a high rate of serious adverse events, 10% and drug discontinuation rate of 9.0%.
DAAs are exciting new treatments that target NS3/NS4a serine proteases, NS5a or the NS5b polymerase. The first generation DAAs, telaprevir and boceprevir significantly improved the SVR rates to over 60%, although with a considerable side effect profile.
The newer, 2nd generation DAAs, sofosbuvir, simeprevir, ledipasvir, and daclatasvir, have even higher cure rates. Several other DAAs are in development, some of them are awaiting approval by FDA while others are in the investigational stage. The analysis of pooled data favored DAA based regimens, with better efficacy rate and lower side effect profiles. The addition of a DAA to the traditional regimen in treatment naïve patients showed an improvement in cure rate in terms of SVR, from 50% to 75%. This improvement in SVR was even higher with the second generation DAAs, of around 90%.
The impact on SVR was even more profound with the addition of two second generation DAAs raising the cure rate above 95%. The all oral regimens not only increased the SVR above 90%, they are easier to administer and hence are likely to have better compliance. This beneficial effect was associated with a reduction in the serious side effect profile with decreasing SAE, from 10% to 1.5% with two DAAs. This resulted in better treatment completion rate and decreased drug discontinuation rates of DDR from 9.0% to 0.9% with two DAAs.
These differences were more evident in patients who have not responded favorably to previous treatment as compared to naïve patients, given the low SVR with traditional P + R regimen. SVR improved from 18.5% to 62.6%-95.9% with a single DAA and to around 95% with two DAAs. This provides new hope especially for patients who are intolerant or are ineligible to P based regimen.
Amongst all the oral regimens, DAA only regimens appear to be superior since the addition of R does not increase the SVR much, (94.1%-95.9%) but increases the SAE in both naïve (1.9%-3.1%) and NR (2.3%-3.3%), without altering DDR much. This analysis supports the recent AASLD/IDSA guidelines for the treatment of HCV infection[34].
The benefits of the second generation DAA are believed to be associated with an increase in the cost of treatment. On initial analysis it seems that the cost of treatment may go up by multiple folds from $900/wk without DAA to around $6000/wk for a single DAA based regimen and around $12000/wk for double DAA regimens. However, further analysis of the pooled data for cost per SVR showed only a doubling in the cost for naive patients ($87449 for P + R to $149378 for double DAA). By contrast, this analysis favors DAAs for NR ($233514 for P + R as compared to only $153029 for double DAA), perhaps due to the high cost of recurrent treatments for NR. The cost of combining two DAAs has gone down further, with the newer combination pill (sofosbuvir + ledipasvir) costing $121148 per SVR (as compared to $153029) in non responders. Also, it is important to note that this cost analysis has only taken into account the direct cost burden (with upfront cost of therapy only) and does not taken into consideration the indirect cost of the disease, its complications, treatment side effects and disease burden on the patient and society in terms of quality-adjusted life year (QALY). A recent article on cost effective analysis suggested that after taking the total duration of therapy and QALY, the shorter (12 wk) course of SOF/SMV is a more cost effective treatment (despite higher individual cost of drugs) for genotype 1 HCV then 24 wk SOF/RBV in IFN-ineligible/intolerant individuals[35,36].
Development of DAA is a very rapidly emerging field, multiple agents are in pipeline, some are being developed and some are in approval phase, summarized in Table 3. Since the performance of this meta-analysis, FDA has approved ombitasvir/paritaprevir/ritonavir with dasabuvir (Viekira Pak) on December 19, 2014 and many others are in development[37]. All included studies do carry an inherent selection bias, which also gets reflected in our meta-analysis by the inherent nature of a meta-analysis. Studies dealing with cirrhotic population in sufficient details are also limited. More future trials would be needed to address the problem of treating cirrhotic patients. Also, cost-efficiency calculations in our review reflect $ amount and cost in United States. It might not reflect cost in other countries as the cost of medication is different amongst individual countries and there is no international standard available to regulate them and it is governed by drug companies. Our analysis provides relative cost-effectiveness in United States.
| Currently FDA approved DAA | Under development butcurrently non-FDA approved |
| TEL | Daclatasvir, |
| BOC | Asunaprevir, |
| LED | Beclabuvir |
| SOF | Faldaprevir |
| SIM | Mericitabine |
| SOF/LED (Harvoni) | Tegobuvir |
| Ombitasvir/Paritaprevir/Ritonavir with Dasabuvir (Viekira Pak)1 | Grazoprevir with Elbasvir |
The newer DAAs and oral only regimens provide better efficacy and a favorable side effect profile. P free regimens comprising of 2 DAAs achieves SVR above 95%. The addition of R to the 2 DAAs increases the SAE and DDR without an increase in the efficacy. Although, an important drawback of DAAs is the high initial cost, the cost of achieving an SVR with DAA based regimens was lower for NR compared to P + R regimen. However, the cost per SVR remains high for treatment naive patients. It is possible that further reduction in treatment duration may make DDAs even more cost effective.
The newer all oral direct acting antivirals (DAAs) are promising agents for treating hepatitis C virus (HCV) infection. Data comparing efficacy, safety and cost of different drug regimens are limited.
In the era of new therapeutic options for hepatitis C, the current research hotspot is evaluate the efficacy and safety of these newer all oral direct acting antivirals.
Second generation and emerging DAAs are promising agents in HCV treatment, with a very high level of safety and efficacy. An important drawback is their high cost. Superiority is higher for non-responders.
This study suggests that emerging DAAs are promising for treatment of hepatitis C.
Direct antiviral agents are newly developed drugs against hepatitis C. They target various stages of the HCV life cycle and are taken orally.
The review is well done and interesting.
P- Reviewer: Antonelli A, Ferenci P, Wirth S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | WHO Hepatitis C Guidelines. [Cited 2014 Dec 1]. Available from: http: //www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. [Cited in This Article: ] |
| 2. | WHO Hepatitis C Fact sheet. [Cited 2014 Dec 1]. Available from: http: //www.who.int/mediacentre/factsheets/fs164/en/. [Cited in This Article: ] |
| 3. | Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 463] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [PubMed] [Cited in This Article: ] |
| 5. | Kato N. Genome of human hepatitis C virus (HCV): gene organization, sequence diversity, and variation. Microb Comp Genomics. 2000;5:129-151. [PubMed] [Cited in This Article: ] |
| 6. | Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1070] [Cited by in F6Publishing: 1058] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 7. | Delwart E, Slikas E, Stramer SL, Kamel H, Kessler D, Krysztof D, Tobler LH, Carrick DM, Steele W, Todd D. Genetic diversity of recently acquired and prevalent HIV, hepatitis B virus, and hepatitis C virus infections in US blood donors. J Infect Dis. 2012;205:875-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Lee LY, Tong CY, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66:342-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 10. | Cooper C, Lester R, Thorlund K, Druyts E, El Khoury AC, Yaya S, Mills EJ. Direct-acting antiviral therapies for hepatitis C genotype 1 infection: a multiple treatment comparison meta-analysis. QJM. 2013;106:153-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1065] [Cited by in F6Publishing: 1042] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 12. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1329] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 13. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 14. | Flamm SL, Lawitz E, Jacobson I, Bourlière M, Hezode C, Vierling JM, Bacon BR, Niederau C, Sherman M, Goteti V. Boceprevir with peginterferon alfa-2a-ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. Clin Gastroenterol Hepatol. 2013;11:81-87.e4; quiz e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 16. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 888] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 17. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1835] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 18. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 911] [Cited by in F6Publishing: 906] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 19. | Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, Afdhal N, Vierling JM, Gordon SC. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100-2107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 567] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 22. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 23. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 24. | Marcellin P, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, Drenth JP, Serfaty L, De Backer K, Van Heeswijk R. Telaprevir is effective given every 8 or 12 hours with ribavirin and peginterferon alfa-2a or -2b to patients with chronic hepatitis C. Gastroenterology. 2011;140:459-468.e1; quiz e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 916] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 26. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 27. | Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 240] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 28. | Pearlman BL, Ehleben C. Hepatitis C genotype 1 virus with low viral load and rapid virologic response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology. 2014;59:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1951] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 30. | Rodríguez-Torres M. Sofosbuvir (GS-7977), a pan-genotype, direct-acting antiviral for hepatitis C virus infection. Expert Rev Anti Infect Ther. 2013;11:1269-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 628] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 32. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1194] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 33. | Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hezode C, Hirschfield GM, Jacobson I, Nikitin I. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430-41.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | AASLD/ADSA guidelines for treatment of hepatitic C infection. [Cited 2014 Dec 1]. Available from: http://www.hcvguidelines.org. [Cited in This Article: ] |
| 35. | Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20:847-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014;60:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | FDA approves Viekira Pak to treat hepatitis C. [Cited 2015 Jan 25]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427530.htm. [Cited in This Article: ] |