Published online Jun 8, 2015. doi: 10.4254/wjh.v7.i10.1325
Peer-review started: August 15, 2014
First decision: November 27, 2014
Revised: December 1, 2014
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: June 8, 2015
Non-alcoholic fatty liver disease (NAFLD) represents the most common and emerging chronic liver disease worldwide. It includes a wide spectrum of liver diseases ranging from simple fatty liver to non-alcoholic steatohepatitis (NASH), which may progress to fibrosis and more severe liver complications such as cirrhosis, hepatocellular carcinoma and liver mortality. NAFLD is strongly associated with obesity, insulin resistance, hypertension, and dyslipidaemia, and is now regarded as the liver manifestation of the metabolic syndrome. The increased mortality of patients with NAFLD is primarily a result of cardiovascular disease and, to a lesser extent, to liver related diseases. Increased oxidative stress has been reported in both patients with NAFLD and patient with cardiovascular risk factors. Thus, oxidative stress represents a shared pathophysiological disorder between the two conditions. Several therapeutic strategies targeting oxidative stress reduction in patients with NAFLD have been proposed, with conflicting results. In particular, vitamin E supplementation has been suggested for the treatment of non-diabetic, non-cirrhotic adults with active NASH, although this recommendation is based only on the results of a single randomized controlled trial. Other antioxidant treatments suggested are resveratrol, silybin, L-carnitine and pentoxiphylline. No trial so far, has evaluated the cardiovascular effects of antioxidant treatment in patients with NAFLD. New, large-scale studies including as end-point also the assessment of the atherosclerosis markers are needed.
Core tip: Non-alcoholic fatty liver disease (NAFLD) represents the most common chronic liver disease, including a wide spectrum of conditions ranging from simple fatty liver to non-alcoholic steatohepatitis, cirrhosis, hepatocellular carcinoma and liver mortality. NAFLD is considered the liver manifestation of the metabolic syndrome. The increased mortality of patients with NAFLD is primarily a result of cardiovascular disease (CVD). Oxidative stress represents a shared pathophysiological disorder between NAFLD and CVD. Several antioxidant treatments have been proposed in patients with NAFLD, with conflicting results, but no trial has evaluated their cardiovascular effects in this setting. Further studies are needed.
- Citation: Polimeni L, Del Ben M, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol 2015; 7(10): 1325-1336
- URL: https://www.wjgnet.com/1948-5182/full/v7/i10/1325.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i10.1325
Non-alcoholic fatty liver disease (NAFLD)[1] is a very frequent condition rising striking proportions in recent years, with prevalence of 20%-30%[1] in the general population, and 70%-90% in obese or diabetic patients. Non-alcoholic steatohepatitis (NASH) represents one of the most frequent conditions leading to liver transplantation and is projected to eventually become the first one in the next years[2].
NAFLD comprises different conditions, including simple steatosis and NASH. It is noteworthy that in some cases NAFLD patients may develop cirrhosis and hepatocellular carcinoma[3]. Moreover, patients with NAFLD show a higher risk for cardiovascular disease (CVD)[4], and cardiovascular mortality. Actually, patients with NAFLD have a greater probability to experience CVD rather than liver related complications.
NAFLD shows a strong association with many metabolic disorders such as insulin resistance, obesity, dyslipidaemia and hypertension and for this reason is considered the hepatic expression of the metabolic syndrome (MetS)[5]. MetS is a cluster of metabolic and CVD risk factors[6], characterized by low grade chronic inflammation and systemic oxidative stress. However, NAFLD and NASH have a complex pathogenesis and the fatty liver infiltration may be caused by several different mechanisms[3,7].
According to the “two-hit” theory[8], insulin resistance (IR) is believed to play an essential role in the early stages of steatosis. However, it is under discussion whether IR and hyperinsulinemia cause liver steatosis or NAFLD itself promotes hyperinsulinemia because of an inadequate degradation of insulin[5,9]. By contrast, oxidative stress seems to be one of the most important mechanisms leading to hepatic injury in NAFLD, playing a fundamental role in the progression from simple steatosis to NASH. It has been demonstrated that the augmented generation of reactive oxygen species (ROS) can induce lipid peroxidation leading to inflammation and fibrogenesis through the activation of stellate cells[10]. Moreover, ROS inhibit hepatocytes secretion of very low density lipoprotein (VLDL), inducing liver fat accumulation. ROS can also promote hepatic insulin resistance and necro-inflammation and activate several intracellular pathways that can lead to hepatocyte apoptosis[11]. At the same time, sound evidence has been generated that oxidative stress centrally contributes to atherothrombosis and is involved at all stages of atherosclerotic plaque evolution. Therefore, we speculate that increased oxidative stress may represent the missing link between NAFLD and CVD (Table 1).
| NAFLD is the most common and emerging chronic liver disease worldwide |
| NAFLD is considered the hepatic manifestation of the metabolic syndrome |
| Patients with NAFLD are at increased risk of cardiovascular morbidity and mortality and cardiovascular disease is the major cause of death |
| Chronic oxidative stress is considered one of the key mechanisms responsible for both liver damage progression in NAFLD and atherosclerotic disease |
| Therapeutic strategies targeting oxidative stress reduction in patients with NAFLD have been proposed, although based on only one RCT |
| No trial so far, has evaluated the cardiovascular effects of antioxidant treatment in patients with NAFLD |
Recent published data showed an increased risk for CVD associated to NAFLD. Söderberg et al[12] reported in a 28-year follow-up study of subjects with an elevation of liver enzymes an higher risk of mortality in patients with NAFLD than in the general population. Moreover, in this study, the first cause of mortality was represented by CVD while liver disease was only the third one[13].
Two major studies, based on ultrasonography detection of steatosis, investigated the association between NAFLD and CVD. The first, carried out in a large North American database, reported an higher prevalence of CVD risk factors and events in patient with NAFLD (n = 2492) in comparison with those without. Nonetheless, the rate of CVD mortality during a follow up period of 14 years was not increased in subjects with NAFLD[14]. In the second, a Japanese 5-year prospective study of 1221 healthy subjects, patients with NAFLD (n = 231) showed an increased incidence of CVD events (1.0% vs 5.2%; P < 0.001) and NAFLD emerged as an independent predictor of CVD[15].
Few prospective studies used liver biopsy based diagnosis, and investigated the correlation between hepatic inflammation and atherosclerosis[16,17]. In a Swedish study, subjects with NAFLD at liver biopsy have been followed-up for about 14 years. Patients with steatohepatitis showed an higher mortality rate than those with simple steatosis[18]. In a further study performed in Japan, ultrasound screening for steatosis was performed in 625 subjects who underwent coronary angiography. Significant stenosis (≥ 50%) was more prevalent in subjects with steatosis (84.6%), than in those without (64.1%). However, after 87 wk of follow-up there were no differences in the incidence of fatal CVD, non-fatal myocardial infarction, and coronary revascularization[19].
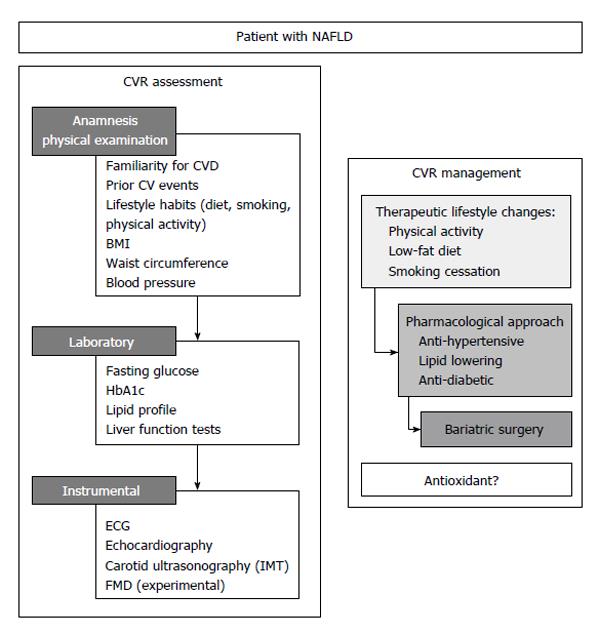
The above reported survival data suggest that a correct CV risk stratification is a fundamental step in the management of patients with NAFLD. This will include an accurate anamnesis, physical examination, laboratory and instrumental analyses (Figure 1).
Indeed, the recognition of the presence of early signs of atherosclerosis is crucial for an effective prevention strategy. Two surrogate markers of atherosclerosis have been studied so far in patients with NAFLD: the carotid intima-media thickness (IMT) and the brachial artery flow-mediated dilation (FMD).
IMT: The use of ultrasound to assess the presence of carotid plaques, or to measure the common carotid IMT is a common screening tool to evaluate the presence of early systemic atherosclerosis. Several studies investigated IMT and carotid atherosclerosis in subjects with NAFLD[20]. In a meta-analysis, Sookoian et al[20] observed in 3497 subjects a significantly higher IMT (+13%) in subjects with steatosis (n = 1427), as compared with patients without fatty liver (n = 2070). Moreover, also in pediatric population, available data showed an association between an increased IMT and NAFLD[21]. However, there are conflicting data supporting an independent role of NAFLD for increased IMT[22]. In a small study, Mohammadi et al[23] found an independent correlation between NAFLD and IMT. Instead, in a German study, after adjustment for CVD risk factors, NAFLD did not independently predict increased IMT[24]. Similarly, in Kim’s study, authors observed an increased IMT only in metabolic patients and speculated that NAFLD could be a marker of more severe MetS[25]. The correlation between NAFLD severity and IMT is unclear and the three major liver biopsy-based studies showed conflicting data. In a Greek study, NAFLD subjects had significantly higher cIMT (0.79 ± 0.18 mm vs 0.67 ± 0.13 mm, P = 0.01), compared to controls and there were no differences observed between NAFLD and NASH[26]. Conversely, Brea et al[27] and Targher et al[28] studies reported a close association between histology of NAFLD and IMT. Several studies investigated also carotid plaques prevalence in patients with NAFLD reporting conflicting data. In the Sookoian et al[20] systematic meta-analysis, the relative risk for carotid plaques in patients with NAFLD is about twice as compared to control subjects.
FMD: Brachial artery FMD is a non-invasive test to evaluate endothelial dysfunction, a clinical marker of early CVD abnormalities[29]. So far, few studies evaluated the relationship between FMD and NAFLD[23,26,30]. Vlachopoulos et al[26] found a reduced FMD in patients with NAFLD (1.9% ± 2.1% vs 4.8% ± 2.4% in controls, P < 0.001); in Mohammadi’s study FMD was 6.4% in patients and 15.7% in controls (P < 0.001); Thakur et al[30] reported a significantly greater degree of FMD impairment in NAFLD patients than controls (OR = 11.7; 95%CI: 1.4-96.5). Villanova’s[31] study described a correlation between NAFLD severity and impaired FMD; in fact, lower FMD value were observed in patients with NAFLD/NASH. Finally, among 250 obese children, those with NAFLD and transaminase elevation had significantly impaired FMD[32]. Despite FMD has been shown to be able to predict CV events in some settings[33], it is not commonly used in clinical practice mostly due to its variability. Thus, its utility is now limited to experimental clinical trials.
As mentioned above, in subjects with NAFLD the main cause of death is represented by CVD complications[4]. However, It is still under debate if NAFLD is associated with CVD as a result of the coexistence of multiple CVD risk factors, or if NAFLD independently confers a higher CVD risk, acting as a pro-atherogenic stimulus[34-36]. In fact, most patients with NAFLD are obese or overweight, and many of them have arterial hypertension, diabetes and atherogenic dyslipidemia, thus outlining the clinical features of the MetS. Therefore, NAFLD is usually considered an hepatic expression of MetS[37,38].
NAFLD prevalence is significantly higher in obese patients than in individuals with normal magnetic resonance imaging (BMI) and without metabolic risk factors (80% and 16%, respectively)[39,40] and a significant correlation between NAFLD and BMI has been reported[41]. Many evidences suggest that the distribution of body fat plays a more important role in obesity-associated comorbidities than the total body fat mass. Visceral adipose tissue (VAT) compared with subcutaneous fat (SCF) is a better predictor of hepatic steatosis and is associated with histological severity in NAFLD independent of IR and hepatic steatosis[42]. VAT is more lipolitically active on a per unit weight basis and exhibits greater IR than SCF[43-45], causing enhanced peripheral lipolysis resulting in surplus-free fatty acid turn over into the liver[46]. Furthermore, increased production of pro-inflammatory adipokines and a converse decrease in protective adipokines is more evident in VAT compared with SCF[47]. Waist circumference and waist-to-hip ratio are anthropometric measurements of central adiposity, which correlate well with VAT[48]. On the basis of recent findings, central adiposity could be an independent predictor of hepatic steatosis, as it predicts increased levels of hepatic enzymes independently from BMI[49,50].
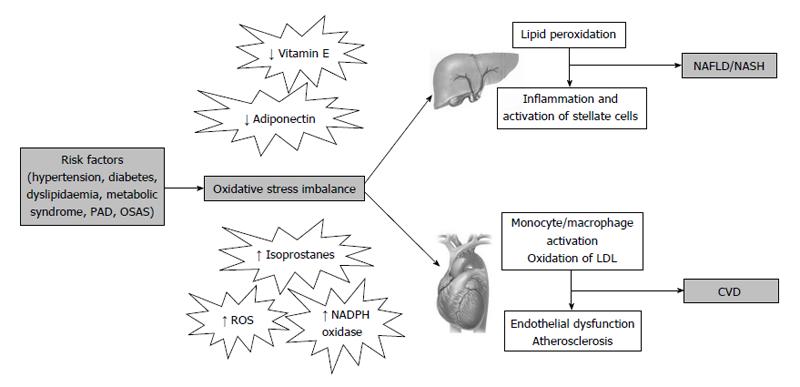
Among mechanisms linking CVD risk with hepatic steatosis, the most prominent factors seem to be insulin resistance, low-grade chronic inflammation and atherogenic dyslipidemia[34,51]. In addition, increased oxidative stress may also represent a shared pathophysiological condition between the two conditions (Figure 2). In fact, we have previously described increased oxidative stress in a number of chronic diseases, such as MetS, hypercholesterolemia, obesity, peripheral artery disease and obstructive sleep apnoea syndrome, all associated to increased CVD risk.
Oxidative stress plays a key role in the initiation and progression of both NAFLD and atherosclerosis. An excessive ROS production is responsible for the oxidation of LDL[52], which may promote the transformation of macrophages into foam cells, which represent the first step in the formation of the atherosclerotic lesion. On the other hand, in patients with NAFLD, ROS may also cause lipid peroxidation which may be followed by inflammation, and activation of stellate cells leading to fibrogenesis[8].
In fact, in many clinical studies, elevated systemic markers of oxidative stress and lipid peroxidation have been found in patients with NAFLD[53-59].
Recently, in two studies carried out in patients with NAFLD[60,61], we found increased oxidative stress in vivo, by measuring urinary 8-iso-prostaglandin F2α (8-iso-PGF2α), which derives from the non-enzymatic oxidation of arachidonic acid[62], and serum levels of soluble NOX2-derived peptide (sNOX2-dp), which is an indicator of NOX2 activation, a NADPH oxidase isoform involved in ROS generation[63,64]. In vivo measurement of 8-iso-PGF2α in urine is a validated and widely accepted reliable biomarker of oxidative stress in health and diseases[65]; a recent study from our group demonstrated that 8-iso-PGF2α production is partly a result of activation of NOX2[65]. Accordingly, we demonstrated that patients with genetically determined low oxidative stress, disclosed impaired formation of urinary 8-iso-PGF2α[63].
Increased values of urinary 8-iso-PGF2α and serum sNOX2-dp levels have been detected also in subjects with cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, obesity, sleep apnoea syndrome, atrial fibrillation and MetS[66-71], which are all commonly present in patients with NAFLD.
Moreover, we also have demonstrated changes of systemic markers of oxidative stress after modulation of risk factors. For example, in patients with familial or polygenic hypercholesterolemia, statin treatment was associated with a parallel decrease of serum cholesterol and urinary 8-iso-PGF2α values[72,73]. Accordingly, in patients with MetS who had lost at least 5% of their initial weight, a decrease in serum NOX2-dp and urinary 8-iso-PGF2α levels was found, with concomitant increase in the levels of antioxidant molecules such as vitamin E (α-tocopherol) and adiponectin[74]. Finally, in subjects with sleep breathing disorders, treatment with nasal continuous positive air pressure significantly decreased oxidative stress[75].
Oxidative stress may initiate the atherosclerotic process as it has a negative influence on endothelial cells[76-78]. Endothelial dysfunction predisposes patients to experience a CV event[79,80].
FMD is the most often non-invasive test used for assessing endothelial function, regulated by nitric oxide release[29]. Impaired FMD has been found in several CV and metabolic diseases related to chronic low-grade inflammation and oxidative stress[67,69,75,81-84]. Improvement of FMD with a coexistent decrease of NOX2 activation has been observed in patients with MetS after moderate weight loss[69]. Impaired endothelial function was suggested also in NAFLD subjects[31,85].
In conclusion, oxidative stress may increase CVD risk in patients with NAFLD, both by contributing to the pathogenesis of single CVD risk factors, or by inducing endothelial dysfunction. Therefore, oxidative stress may represent an attractive target for improving CVD prevention in these patients, as it may represent a common mechanism underlying multiple CVD risk factors[86]. In particular, targeting platelet NOX may represent a useful complementary anti-thrombotic approach for these patients[87]. Moreover, antioxidant therapy may also be useful to decrease lipid peroxidation and liver disease progression in NAFLD.
Based on the above evidences, several therapeutic strategies targeting oxidative stress reduction in patients with NAFLD have been proposed. Notably, none of these studies took into consideration cardiovascular implications. Moreover, a meta-analysis evaluating the benefits and harms of antioxidant supplements in this clinical subset has reported no enough data to express a conclusive and widely accepted opinion on antioxidant therapy use in NAFLD patients[88]. Note that, only a minority of the randomized controlled trials (RCTs) report pre-treatment and post-treatment histological data to support therapeutic efficacy of antioxidant therapy in biopsy-proven NAFLD or NASH.
Vitamin E is a lipophilic molecule with antioxidant activity that prevents membrane damage by ROS. Low levels of antioxidants including vitamin E have been observed in subjects with NASH compared to healthy individuals[89]. We confirmed this finding, demonstrating reduced blood values of α-tocopherol/cholesterol in 254 patients with NAFLD compared with 56 patients without NAFLD. Notably, similarly reduced vitamin E/chol values were obtained in a subgroup of 20 patients with biopsy-proven NASH (unpublished data).
The effects of vitamin E have been investigated in several experimental murine models of NAFLD showing an improvement of NASH and a reduction in oxidative stress markers, hepatic stellate cell activation, and histologic fibrosis in mice supplemented with vitamin E[90-92].
The effects of vitamin E or vitamin E plus other drugs on the liver damage, in patients with biopsy-proven NASH, have been investigated in few small studies showing conflicting results[93-95].
Recently, two large multicenter RCTs investigated the efficacy of vitamin E in subjects with NAFLD. In the PIVENS trial, adult patients with aggressive NASH and without diabetes or cirrhosis, high-dose vitamin E supplementation (800 UI q.d.) significantly improved NASH histology compared to pioglitazone or placebo treatment groups; however, increased insulin resistance and plasma triglyceride levels were reported[93]. By contrast, the TONIC trial found no differences between vitamin E (400 IU bid), metformin (500 mg bid), or placebo in inducing sustained decrease of ALT level in children with NAFLD, even if α-tocopherol showed a significant improvement of NASH[96].
Based only on the results of the above single positive RCT[93], recent guidelines suggest supplementation with high-dose vitamin E (800 UI q.d.) to treat NASH in patients without diabetes[97]. However, over the last few years, concerns about the safety of vitamin E supplementation have been raised. Indeed, a systematic review indicated that α-tocopherol might increase hemorrhagic stroke risk[98] and in another study it has been reported an increased prostate cancer incidence in healthy men taking vitamin E (400 IU q.d.) over 7 years[99]. Moreover, no benefits in cardiovascular mortality or cerebrovascular events have been demonstrated for vitamin E supplementation[100]. Finally, recent publications reported no benefits by α-tocopherol supplementation for cardiovascular prevention and no evidence for an association with increased all-cause mortality[101,102].
Therefore, even though there are some evidences about the efficacy of vitamin E supplementation in NAFLD/NASH, there are concerns about its safety. Further studies taking in to consideration cardiovascular implications and long-term safety of vitamin E supplementations are needed.
A possible alternative could be the simultaneous supplementation using smaller doses of several small molecule antioxidants in association, such as vitamin A, vitamin E, vitamin C, glutathione, etc. A further possibility could be the simultaneous supplementation using a small molecule antioxidant with a trace element (zinc, copper, or selenium) that may increase expression of an enzymatic antioxidant. However, further studies are needed to support the potential roles of these therapeutic approaches.
Several studies have been conducted investigating the effect of other antioxidant drugs in NASH, but with inconclusive results.
In recent years, many studies have found promising properties of Resveratrol (trans-3,4’,5-trihydroxystilbene) for NAFLD treatment. Resveratrol is a stilbene naturally occurring in several plants and extracted from red grapes. Resveratrol has a well-documented strong capacity against oxidation and inflammation, improves insulin sensitivity and glucose tolerance and reduces plasma lipids[103,104]. Some in vitro studies performed in different cell models of steatosis demonstrated that resveratrol shows anti-lipidogenic effects at doses between 10 and 50 μmol/L. The mechanism of action underlying this effect may be mediated by a decrease of de novo lipogenesis[105-108].
Many in vivo studies demonstrated positive effects on liver steatosis in animal models, showing efficacy both for fatty liver prevention and treatment[108,109]. The doses used in these studies have been generally very high, ranging from 0.5 to 450 mg/kg per day. Data deriving from these studies suggest that the reduction of oxidative stress also contributes to this positive effect.
So far, only one human study investigating the effects of resveratrol supplementation on the liver has been conducted. In this small-randomized double-blind crossover design study, 11 healthy obese male volunteers received 150 mg resveratrol per day or placebo for 1 mo. Plasma alanine aminotransferases (ALT) concentration and intrahepatic lipid content were significantly lower after 30 d of resveratrol supplementation in comparison to placebo[110].
Based on its potent effects on oxidative stress and inflammation, as well as its wide availability, resveratrol has become one of the most interesting candidate for the prevention of fatty liver diseases. Interestingly, a role of resveratrol in CVD protection has been demonstrated in primary and secondary prevention with an improvement of CVD risk markers, such as endothelial function, echocardiographic parameters and cytokines expression in different settings[111]. Nevertheless, there is a lack of long-term randomized clinical trials using resveratrol and improvement of markers of cardiovascular risk does not necessarily coincide with clinical benefits in patients management.
Recently, there has been a renewed interest for silybin, a natural product deriving from - Silybum marianum. Even if its therapeutic efficacy has been questioned for years, silybin is commonly used as hepato-protective agent. Silybin therapeutic efficacy has been demonstrated in several studies performed in different types of experimental liver injury[112-114]. Notably, in a recent study, silybin administration decreased oxidative stress and improved both liver and myocardial injury in an experimental murine model of NAFLD[115]. Moreover, some studies showed a positive effect of silybin on fibrosis and oxidation[116,117]. In a recent large multicenter RCT, a combination of silybin, vitamin E and phosphatidylcholine reduced hepatic damage and IR[118].
L-carnitine, an endogenous substance precursor of carnitine-palmitoyltransferase 1, is involved in the mitochondrial β-oxidation that affects mitochondrial function. L-carnitine has potent antioxidant properties, being a free radical scavenger, and thus may protect tissues from oxidative damage[119,120].
In a recent study, l-carnitine supplementation reduced tumor necrosis factor (TNF)-α, liver function parameters, plasma glucose levels and histological scores in NASH[121]. However, even if a majority of studies have shown that l-carnitine supplementation can improve factors associated with MetS and CVD[122-125], recent evidences suggested that dietary l-carnitine may accelerate atherosclerosis via gut microbiota metabolites[126]. Thus, further research is necessary to investigate the effect of chronic l-carnitine supplementation on both atherosclerosis and chronic fatty liver disease.
Pentoxiphylline (PTX) is a methylxanthine derivative with anti-oxidant and anti-inflammatory properties. In fact, pentoxiphylline suppresses TNF-α gene transcription and acts as a hydroxyl and peroxyl radical scavenger[127]. Moreover, it has been proven that it increases red blood cell flexibility, reduces blood viscosity and decreases platelet aggregation. PTX it is commonly used for the treatment of intermittent claudication in Western countries and several small clinical trials have reported beneficial effects of PTX supplementation on multiple surrogate clinical markers in subjects with chronic heart failure[128-131].
Indeed, recent evidences showed that PTX can decrease free-radical-mediated lipid oxidation and can improve histological features of NASH[132,133], such as steatosis, lobular inflammation and fibrosis. Thus, PTX may represent a new strategy for treating NAFLD. However, more studies are suggested to confirm its efficacy and the cardiovascular effects in this setting.
Today, a large body of clinical and epidemiological data support an association between NAFLD and increased CVD risk, which seems independent of traditional risk factors and the features of the MetS[134]. Indeed, NAFLD mortality is mainly due to CVD rather than liver related diseases. Chronic oxidative stress is considered a major pathogenic factor for hepatic damage in NAFLD/NASH and for atherosclerosis evolution. The increase of ROS production and the decrease of antioxidant factors produce oxidative stress.
In our recent studies, we found increased markers of oxidative stress in subjects with liver steatosis. In fact, patients with NAFLD had an higher concentration 8-iso-PGF2α in urines, widely considered as a valid test to evaluate oxidative stress in vivo and of serum sNOX2-dp which plays a major role in the production of ROS. Together, we found that both simple steatosis and NASH are related with decreased serum levels of vitamin E/chol when compared to controls, suggesting that oxidative stress imbalance could also occur in initial stages of fatty liver disease. Taken together, these results suggest that patients with NAFLD may have a chronic systemic oxidative stress, which may lead to a reduction in natural antioxidant pool including vitamin E.
Moreover, oxidative stress is implicated in cardiovascular diseases and in all stages of atherosclerotic plaque evolution. Increased ROS play a role in endothelial dysfunction and in high-CVD risk diseases, such as MetS, hypercholesterolemia, overweight/obesity, peripheral artery disease, sleep apnoea syndrome. Therefore, increased oxidative stress may well represent a possible link between NAFLD and CVD, and constitute an attractive target for antioxidant therapy. In fact, antioxidant therapy may have a favourable effect both on cardiovascular risk factors and liver histology.
Currently, vitamin E supplementation is recommended to treat non-diabetic, non-cirrhotic adults with active NASH, although this indication is supported only by the findings of a single RCT[93] and a meta-analysis has reported insufficient evidence supporting or refusing a favourable role of antioxidants for the treatment of NAFLD[88]. Moreover, no trial so far, has assessed the cardiovascular benefits of antioxidant treatment in individuals with fatty liver. New, large-scale RCTs including as end-point also the assessment of the atherosclerosis markers are needed.
P- Reviewer: Koch TR, Moralioglu S, Sicari R S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 2. | Kemmer N, Neff GW, Franco E, Osman-Mohammed H, Leone J, Parkinson E, Cece E, Alsina A. Nonalcoholic fatty liver disease epidemic and its implications for liver transplantation. Transplantation. 2013;96:860-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013;48:434-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 600] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 4. | Del Ben M, Baratta F, Polimeni L, Angelico F. Non-alcoholic fatty liver disease and cardiovascular disease: epidemiological, clinical and pathophysiological evidences. Intern Emerg Med. 2012;7 Suppl 3:S291-S296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Fiorello S, Cavallo MG, Zalunardo B, Lirussi F, Alessandri C. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7515] [Cited by in F6Publishing: 7808] [Article Influence: 410.9] [Reference Citation Analysis (0)] |
| 7. | Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Maccioni D, Antonini TM, Alessandri C. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3655] [Cited by in F6Publishing: 3609] [Article Influence: 164.0] [Reference Citation Analysis (2)] |
| 9. | Del Ben M, Polimeni L, Brancorsini M, Di Costanzo A, D’Erasmo L, Baratta F, Loffredo L, Pastori D, Pignatelli P, Violi F. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur J Intern Med. 2014;25:566-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2011;15:1325-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 13. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 184] [Cited by in F6Publishing: 177] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 14. | Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 15. | Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 373] [Cited by in F6Publishing: 384] [Article Influence: 22.6] [Reference Citation Analysis (4)] |
| 16. | Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52:1156-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1647] [Cited by in F6Publishing: 1624] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 19. | Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721-1727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Hyperuricaemia and non-alcoholic fatty liver disease (NAFLD): a relationship with implications for vascular risk? Curr Vasc Pharmacol. 2011;9:698-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Mohammadi A, Sedani HH, Ghasemi-Rad M. Evaluation of carotid intima-media thickness and flow-mediated dilatation in middle-aged patients with nonalcoholic fatty liver disease. Vasc Health Risk Manag. 2011;7:661-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 174] [Cited by in F6Publishing: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima-media thickness according to the presence of metabolic syndrome. Atherosclerosis. 2009;204:521-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: a pilot study. Am J Hypertens. 2010;23:1183-1189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Al-Qaisi M, Kharbanda RK, Mittal TK, Donald AE. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vasc Health Risk Manag. 2008;4:647-652. [PubMed] [Cited in This Article: ] |
| 30. | Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, Pandey RM, Vikram NK. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223:507-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 452] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 32. | Pacifico L, Anania C, Martino F, Cantisani V, Pascone R, Marcantonio A, Chiesa C. Functional and morphological vascular changes in pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1643-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Perri L, Pastori D, Pignatelli P, Violi F, Loffredo L. Flow-mediated dilation is associated with cardiovascular events in non-valvular atrial fibrillation patients. Int J Cardiol. 2015;179:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 316] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 35. | Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Sookoian S, Castaño GO, Burgueño AL, Rosselli MS, Gianotti TF, Mallardi P, Martino JS, Pirola CJ. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010;209:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 38. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1907] [Cited by in F6Publishing: 1849] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 39. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1515] [Article Influence: 116.5] [Reference Citation Analysis (1)] |
| 40. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 840] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 41. | Pagadala M, Zein CO, McCullough AJ. Predictors of steatohepatitis and advanced fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13:591-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, Peduto T, Chisholm DJ, George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 43. | Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Thamer C, Machann J, Haap M, Stefan N, Heller E, Schnödt B, Stumvoll M, Claussen C, Fritsche A, Schick F. Intrahepatic lipids are predicted by visceral adipose tissue mass in healthy subjects. Diabetes Care. 2004;27:2726-2729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Mårin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjöström L, Björntorp P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41:1242-1248. [PubMed] [Cited in This Article: ] |
| 46. | Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288:E454-E461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 47. | Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 48. | Rankinen T, Kim SY, Pérusse L, Després JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801-809. [PubMed] [Cited in This Article: ] |
| 49. | Kral JG, Schaffner F, Pierson RN, Wang J. Body fat topography as an independent predictor of fatty liver. Metabolism. 1993;42:548-551. [PubMed] [Cited in This Article: ] |
| 50. | Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Nseir W, Shalata A, Marmor A, Assy N. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig Dis Sci. 2011;56:3439-3449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 207] [Article Influence: 20.7] [Reference Citation Analysis (2)] |
| 53. | Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 54. | Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2010;43:815-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C, Sutti S, Povero D, Maina V, Novo E. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med. 2010;26:471-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Irie M, Sohda T, Iwata K, Kunimoto H, Fukunaga A, Kuno S, Yotsumoto K, Sakurai K, Iwashita H, Hirano G. Levels of the oxidative stress marker γ-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J Int Med Res. 2012;40:924-933. [PubMed] [Cited in This Article: ] |
| 58. | Pirgon Ö, Bilgin H, Çekmez F, Kurku H, Dündar BN. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2013;5:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Gaens KH, Niessen PM, Rensen SS, Buurman WA, Greve JW, Driessen A, Wolfs MG, Hofker MH, Bloemen JG, Dejong CH. Endogenous formation of NΕ-(carboxymethyl)lysine is increased in fatty livers and induces inflammatory markers in an in vitro model of hepatic steatosis. J Hepatol. 2012;56:647-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Del Ben M, Polimeni L, Baratta F, Bartimoccia S, Carnevale R, Loffredo L, Pignatelli P, Violi F, Angelico F. Serum Cytokeratin-18 Is Associated with NOX2-Generated Oxidative Stress in Patients with Nonalcoholic Fatty Liver. Int J Hepatol. 2014;2014:784985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Del Ben M, Polimeni L, Carnevale R, Bartimoccia S, Nocella C, Baratta F, Loffredo L, Pignatelli P, Violi F, Angelico F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014;14:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem. 2003;10:1723-1740. [PubMed] [Cited in This Article: ] |
| 63. | Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol. 2013;33:406-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Basili S, Raparelli V, Napoleone L, Del Ben M, Merli M, Riggio O, Nocella C, Carnevale R, Pignatelli P, Violi F. Polyunsaturated fatty acids balance affects platelet NOX2 activity in patients with liver cirrhosis. Dig Liver Dis. 2014;46:632-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Carnevale R, Iuliano L, Nocella C, Bartimoccia S, Trapè S, Russo R, Gentile MC, Cangemi R, Loffredo L, Pignatelli P. Relationship between platelet and urinary 8-Iso-PGF2α levels in subjects with different degrees of NOX2 regulation. J Am Heart Assoc. 2013;2:e000198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 67. | Loffredo L, Martino F, Carnevale R, Pignatelli P, Catasca E, Perri L, Calabrese CM, Palumbo MM, Baratta F, Del Ben M. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J Pediatr. 2012;161:1004-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Davì G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, Basili S, Ciabattoni G, Patrono C. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 2003;107:3199-3203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Angelico F, Loffredo L, Pignatelli P, Augelletti T, Carnevale R, Pacella A, Albanese F, Mancini I, Di Santo S, Del Ben M. Weight loss is associated with improved endothelial dysfunction via NOX2-generated oxidative stress down-regulation in patients with the metabolic syndrome. Intern Emerg Med. 2012;7:219-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Loffredo L, Carnevale R, Perri L, Catasca E, Augelletti T, Cangemi R, Albanese F, Piccheri C, Nocella C, Pignatelli P. NOX2-mediated arterial dysfunction in smokers: acute effect of dark chocolate. Heart. 2011;97:1776-1781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Pignatelli P, Pastori D, Carnevale R, Farcomeni A, Cangemi R, Nocella C, Bartimoccia S, Vicario T, Saliola M, Lip GY. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb Haemost. 2015;113:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Davies SS, Roberts LJ. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, Ferrante E, Ciabattoni G, Scarpini F, Ghezzi A. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis. 2011;214:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Del Ben M, Angelico F, Cangemi R, Loffredo L, Carnevale R, Augelletti T, Baratta F, Polimeni L, Pignatelli P, Violi F. Moderate weight loss decreases oxidative stress and increases antioxidant status in patients with metabolic syndrome. ISRN Obes. 2012;2012:960427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Del Ben M, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med. 2012;12:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation. 2009;120:1616-1622. [PubMed] [Cited in This Article: ] |
| 77. | Violi F, Sanguigni V, Loffredo L, Carnevale R, Buchetti B, Finocchi A, Tesauro M, Rossi P, Pignatelli P. Nox2 is determinant for ischemia-induced oxidative stress and arterial vasodilatation: a pilot study in patients with hereditary Nox2 deficiency. Arterioscler Thromb Vasc Biol. 2006;26:e131-e132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012;302:H499-H505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 79. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [PubMed] [Cited in This Article: ] |
| 80. | Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640-642. [PubMed] [Cited in This Article: ] |
| 81. | Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Poisa P, Porteri E, Agabiti-Rosei C, Paderno V, Belotti E. Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612-1618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 347] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 83. | Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 84. | Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769-1775. [PubMed] [Cited in This Article: ] |
| 85. | Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, Coskunpinar E, Kahraman OT, Mesci B, Ulasoglu C. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741-2748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 87. | Violi F, Pignatelli P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb Haemost. 2014;111:817-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;CD004996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with Nonalcoholic Steatohepatitis (NASH). Eur J Med Res. 2011;16:76-78. [PubMed] [Cited in This Article: ] |
| 90. | Nan YM, Wu WJ, Fu N, Liang BL, Wang RQ, Li LX, Zhao SX, Zhao JM, Yu J. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand J Gastroenterol. 2009;44:1121-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Phung N, Pera N, Farrell G, Leclercq I, Hou JY, George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int J Mol Med. 2009;24:171-180. [PubMed] [Cited in This Article: ] |
| 92. | Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:641-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2215] [Cited by in F6Publishing: 2194] [Article Influence: 156.7] [Reference Citation Analysis (1)] |
| 94. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 457] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 95. | Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 96. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 765] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 97. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2413] [Cited by in F6Publishing: 2449] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 98. | Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 99. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1218] [Cited by in F6Publishing: 1124] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 100. | Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1614] [Cited by in F6Publishing: 1355] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 101. | Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials. 2009;6:28-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Gerss J, Köpcke W. The questionable association of vitamin E supplementation and mortality--inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy-le-grand). 2009;55 Suppl:OL1111-OL1120. [PubMed] [Cited in This Article: ] |
| 103. | Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2705] [Cited by in F6Publishing: 2659] [Article Influence: 147.7] [Reference Citation Analysis (0)] |
| 104. | Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663-673. [PubMed] [Cited in This Article: ] |
| 105. | Gnoni GV, Paglialonga G. Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur J Clin Invest. 2009;39:211-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 106. | Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009;380:644-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 107. | Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 486] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 108. | Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 109. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 110. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 901] [Cited by in F6Publishing: 917] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 111. | Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective. Ann N Y Acad Sci. 2013;1290:37-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 112. | Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941-1949. [PubMed] [Cited in This Article: ] |
| 113. | Crocenzi FA, Sánchez Pozzi EJ, Pellegrino JM, Favre CO, Rodríguez Garay EA, Mottino AD, Coleman R, Roma MG. Beneficial effects of silymarin on estrogen-induced cholestasis in the rat: a study in vivo and in isolated hepatocyte couplets. Hepatology. 2001;34:329-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003;39:333-340. [PubMed] [Cited in This Article: ] |
| 115. | Salamone F, Galvano F, Marino Gammazza A, Paternostro C, Tibullo D, Bucchieri F, Mangiameli A, Parola M, Bugianesi E, Li Volti G. Silibinin improves hepatic and myocardial injury in mice with nonalcoholic steatohepatitis. Dig Liver Dis. 2012;44:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 116. | Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 117. | Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol. 2011;17:2288-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 233] [Cited by in F6Publishing: 223] [Article Influence: 17.2] [Reference Citation Analysis (2)] |
| 118. | Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52:1658-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 119. | Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78:803-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 616] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 120. | Cuturic M, Abramson RK, Moran RR, Hardin JW, Frank EM, Sellers AA. Serum carnitine levels and levocarnitine supplementation in institutionalized Huntington’s disease patients. Neurol Sci. 2013;34:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G, Galvano F. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis--a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338-1345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 122. | Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G, Orisio S, Remuzzi G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-L-carnitine therapy. Hypertension. 2009;54:567-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 123. | Capaldo B, Napoli R, Di Bonito P, Albano G, Saccà L. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14:191-195. [PubMed] [Cited in This Article: ] |
| 124. | Malaguarnera M, Vacante M, Motta M, Malaguarnera M, Li Volti G, Galvano F. Effect of L-carnitine on the size of low-density lipoprotein particles in type 2 diabetes mellitus patients treated with simvastatin. Metabolism. 2009;58:1618-1623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Johri AM, Heyland DK, Hétu MF, Crawford B, Spence JD. Carnitine therapy for the treatment of metabolic syndrome and cardiovascular disease: evidence and controversies. Nutr Metab Cardiovasc Dis. 2014;24:808-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2783] [Cited by in F6Publishing: 2830] [Article Influence: 257.3] [Reference Citation Analysis (0)] |
| 127. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 287] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 128. | Sliwa K, Woodiwiss A, Kone VN, Candy G, Badenhorst D, Norton G, Zambakides C, Peters F, Essop R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation. 2004;109:750-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 129. | Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P. Randomised investigation of effects of pentoxifylline on left-ventricular performance in idiopathic dilated cardiomyopathy. Lancet. 1998;351:1091-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 130. | Skudicky D, Sliwa K, Bergemann A, Candy G, Sareli P. Reduction in Fas/APO-1 plasma concentrations correlates with improvement in left ventricular function in patients with idiopathic dilated cardiomyopathy treated with pentoxifylline. Heart. 2000;84:438-439. [PubMed] [Cited in This Article: ] |
| 131. | Skudicky D, Bergemann A, Sliwa K, Candy G, Sareli P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation. 2001;103:1083-1088. [PubMed] [Cited in This Article: ] |
| 132. | Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 133. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 134. | Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |