Published online Nov 27, 2014. doi: 10.4254/wjh.v6.i11.793
Revised: September 9, 2014
Accepted: October 1, 2014
Published online: November 27, 2014
Ascites and hyponatremia are the most common complications in patients with liver cirrhosis and develop as a consequence of a severe impairment of liver function and portal hypertension. Increasing evidences support the central role of renal function alterations in the pathogenesis of hydroelectrolytic imbalances in cirrhotic patients, thus implying a dense cross-talk between liver and kidney in the systemic and splanchnic vascular homeostasis in such subjects. Since Arginin Vasopressin (AVP) hyperincretion occurs at late stage of cirrhosis and plays an important role in the development of refractory ascites, dilutional hyponatremia and finally hepato-renal syndrome, selective antagonists of AVP receptors V2 (vaptans) have been recently introduced in the therapeutic algorithm of advanced cirrhotic patients. Despite the promising results of earlier phase-two studies, randomized controlled trials failed to find significant results in terms of efficacy of such drugs both in refractory ascites and hyponatremia. Moreover, concerns on their safety profile arise, due to the number of potentially severe side effects of vaptans in the clinical setting, such as hypernatremia, dehydration, renal impairment, and osmotic demyelination syndrome. More robust data from randomized controlled trials are needed in order to confirm the potential role of vaptans in the management of advanced cirrhotic patients.
Core tip: Increasing evidences support the central role of renal function alterations in the pathogenesis of hydroelectrolytic imbalances in cirrhotic patients. Since Arginin Vasopressin (AVP) plays an important role in the development of refractory ascites, dilutional hyponatremia and hepato-renal syndrome, selective antagonists of AVP receptors V2 (vaptans) have been recently introduced in the therapeutic algorithm of advanced cirrhotic patients. Despite the promising results of earlier phase-two studies, randomized controlled trials failed to find significant results in terms of efficacy. Moreover, concerns on their safety profile arise. More robust data from randomized controlled trials are needed.
- Citation: Facciorusso A, Amoruso A, Neve V, Antonino M, Prete VD, Barone M. Role of vaptans in the management of hydroelectrolytic imbalance in liver cirrhosis. World J Hepatol 2014; 6(11): 793-799
- URL: https://www.wjgnet.com/1948-5182/full/v6/i11/793.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i11.793
Ascites is the most common complication in patients with cirrhosis and develops as a consequence of a severe impairment of liver function and portal hypertension[1]. In fact, there is substantial evidence that severe portal hypertension is the main disorder in the occurrence of ascites in cirrhosis as ascitic patients have significantly higher portal pressure than those without ascites. In particular, an hepatic venous pressure gradient (estimation of the intrahepatic vascular resistance) of more than 12 mmHg has been found to cause the occurrence of ascites in cirrhotics[2,3].
Increasing evidences support the central role of renal function alterations in the pathogenesis of hydroelectrolytic imbalances in cirrhotic patients, thus implying a dense cross-talk between liver and kidney in the systemic and splanchnic vascular homeostasis in such subjects.
Each step of this cross-talk could represent a potential target for the pharmacological management of cirrhotic patients.
It is now evident that ascites is related more to alterations in the arterial vascular compartment and in kidneys than in the portal venous system[4].
Ascites has been traditionally considered as a consequence of backward transmission of the increased intrahepatic hydrostatic pressure into the splanchnic microcirculation and of the decrease in intravascular oncotic pressure because of the impaired hepatic synthesis of albumin.
More recently, the splanchnic arterial vasodilatation secondary to portal hypertension has been found as the central event of ascites formation in cirrhosis[5]. Such mechanism simultaneously induces two different types of events: a “forward” increase in capillary pressure because of a greater inflow of blood at high pressure into the splanchnic microcirculation with consequent passage of fluid into the peritoneal cavity, and the impairment of systemic hemodynamics and renal function, which leads to sodium and water retention[6].
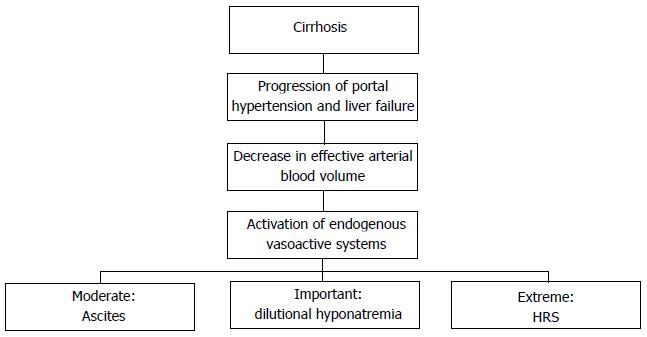
Splanchnic and systemic vasodilatation is due to the excretion by sinusoids of vasoactive mediators, for instance nitric oxid (NO), together with glucagon and other vasodilator molecules[7]. As a consequence of this process, the efforts made by the organism in order to obviate to the imbalance in favor of vasoactive molecules generate a well described chain of processes leading to a “vicious” circle and hence an impairment of the above cited pathological events. Particularly, the followings steps are reported: (1) “underfilling” of effective arterial blood volume; (2) impairment of renal perfusion and deterioration of glomerular filtration rate; (3) release of catecholamines by sympathetic nervous system (SNS) as response to decreased volemia leading to increase in cardiac output and renal vasoconstriction; (4) hyperactivation of Renin-Angiotensin-Aldosterone system (RAAS) and secondary hyperaldosteronism; and (5) release of Adiuretin (ADH), also called arginin vasopressin (AVP), and decrease in levels of Atrial Natriuretic Peptide.
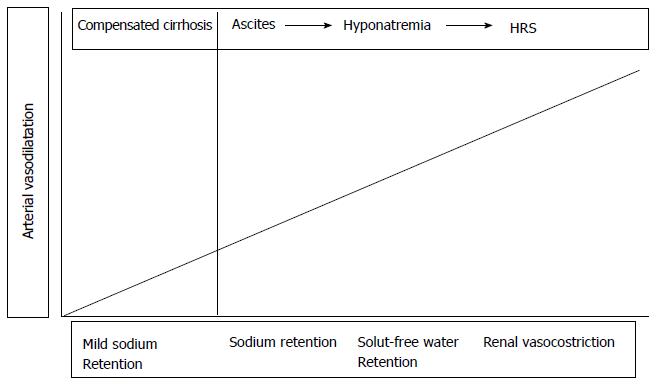
Initially, as long as cirrhosis is compensated and patients don’t develop any hydro-electrolytic imbalance, the retained fluid volume suppresses renal reuptake of sodium and water and resets fluid balance, thus leading, together with the augmented cardiac output and cathecolamine incretion, to a general increase in arterial volemia. However, as the disease progresses the effective arterial blood volume isn’t maintained any longer by the aforementioned mechanisms and ascites occurs as a consequence of the “vicious circle” due to the continuous retention of water and sodium by the kidneys[7,8].
Due to the above cited mechanisms, the decompensated cirrhotic patient, although the marked hydrosaline reuptake, presents hypovolemia, periferic arterial vasodilatation and tachycardia.
In the earlier phases of ascites onset, the RAAS and the SNS are not activated, hence the cause of sodium retention in this period is still unclear. Afterward, the two systems increase the release of mediators thus leading to further reduction in urine sodium excretion[7].
Finally, hyperincretion of ADH occurs and this explains the late onset of hyponatremia in decompensated cirrhotic patients. In fact, ADH is less sensitive than the SNS and RAAS to changes in the effective volemia[9].
Finally, in this setting, hepato-renal syndrome (HRS) occurs as extreme consequence of such imbalances (see below). The mechanisms leading to ascites occurrence are described in Figure 1.
As above specified, activation of RAAS has a key role in the occurrence of hydroelectrolytic imbalances in cirrhotics. Such activation, as well that of SNS and the hyperincretion of ADH, is a homeostatic response aimed at maintaining blood pressure at normal levels in cirrhotic patients with ascites. In fact, the infusion of selective antagonists of angiotensin II or antidiuretic hormone (V1 antagonists) to experimental animals or ascitic patients leads to a profound hypotensive response secondary to a decrease in peripheral vascular resistance[10]. Among the main stimuli leading to the activation of these systems, arterial vasodilatation and secondary arterial hypotension play a pivotal role.
Other system implied in the pathogenesis of renal sodium retention is the SNS, which increases sodium reabsorption in the proximal tubule, loop of Henle, and distal tubule[11]. As decompensated liver diseases progresses, patients develop a decreased renal ability to excrete free water. This water dilutes the interior milieu and produces hyponatremia and hypo-osmolality. Water retention and dilutional hyponatremia develop months after the onset of sodium retention and ascites and are secondary to non-osmotic hypersecretion of AVP from the neurohypophysis in response to the reduced effective intravascular volume in cirrhosis[12,13]. Higher levels of AVP are responsible for water reabsorption in the distal collecting duct of the kidney. Water retention in patients with dilutional hyponatremia is a part of the positive fluid balance and contributes to the occurrence of ascites[12].
Interestingly, the renal synthesis of prostaglandin E2 is increased in cirrhotics to counteract the water-retaining effect of AVP and hence Non Steroid Anti-Inflammatory Drugs may worsen the renal excretion of solute-free water in these patients[14,15].
As previously stated, the clinical consequence of solute-free water excretion impairment is the development of hyponatremia. This type of hyponatremia is referred to as dilutional hyponatremia because it occurs in the setting of increased total body water and dilution of extracellular fluid volume.
Hyponatremia in cirrhosis and ascites has gained attention as a strong prognostic marker, particularly in patients awaiting liver transplantation, given that several reports indicate that when serum sodium concentration if combined with the Model for End-Stage Liver Disease (MELD) score improves the prognostic accuracy of MELD in patients listed for orthotopic liver transplantation[16-18].
In most patients the degree of hyponatremia is mild and the condition is asyntomatic and needs no specific therapy.
Renal vasoconstriction is the renal functional abnormality that develops later in patients with cirrhosis and ascites[19,20].
The occurrence of renal vasoconstriction in patients with cirrhosis and ascites is clinically relevant for several reasons. First, a significant proportion of these patients have refractory ascites, as sodium and water excretion are markedly impaired. Second, it predisposes to the development of HRS[21].
An increased activity of vasoconstrictor factors (mainly plasma renin activity and norepinephrine) and reduced activity of renal vasodilator factors acting on the renal circulation play the most important role in the pathogenesis of HRS because renal vasoconstriction in cirrhosis occurs in the absence of morphologic changes in the kidney[22].
The pathogenesis of renal vasoconstriction in cirrhosis is also related to changes in systemic hemodynamics. The most accepted theory considers renal vasoconstriction as the consequence of the extreme underfilling of the systemic arterial circulation due to marked vasodilatation of the splanchnic circulation, which activates homeostatic vasoconstrictor systems, whose effect on the kidney vasculature cannot be counterbalanced by either renal or systemic vasodilators[23,24].
Progression of functional renal alterations paralleled with cirrhosis course is shown in Figure 2.
Due to the aforementioned circulatory dysfunctions and activation of neuro-humoral systems leading to sodium and water retention, there has been an increasing interest in research on drugs that may improve circulatory and renal function in cirrhotics with refractory ascites and/or hyponatremia. Among such drugs, many affords have been sustained in developing and testing selective antagonists of the V2-receptors of vasopressin, known as vaptans.
AVP is a neuropeptide hormone synthesized by two hypothalamic nuclei (supraoptic and paraventricular nuclei) and secreted by the posterior pituitary in response to an increase in plasma tonicity or decrease in plasma volume[25].
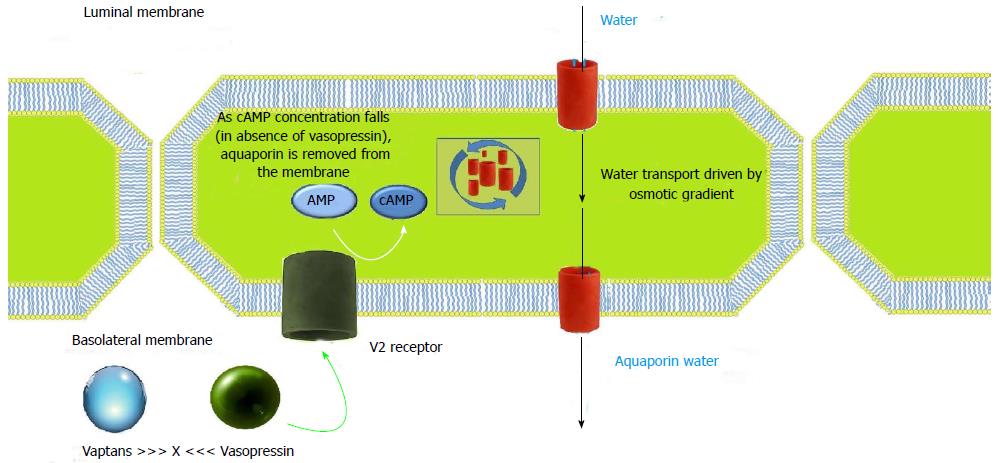
The actions of AVP are mediated by three receptor subtypes: V1a, V1b and V2, all of them being G protein-coupled receptors and classified by their location[26]. V1a receptors are present on vascular smooth muscle cells, myocardium, platelets, and hepatocytes, and mediate vasoconstriction, platelet aggregation, and glycogenolysis[25,27,28]. V1b receptors have little selective distribution in the central nervous system. V2 receptors are expressed in principal cells of the renal collecting duct system. As shown in Figure 3, they mobilize intracellular vesicles of aquaporin 2 to the apical plasma membrane of collecting duct cells causing an increase in the reabsorption of free water. AVP acts on V2 receptors on the basolateral surface of principal cells resulting in activation of adenylyl cyclase. This leads to protein kinase activation resulting in preformed cytoplasmic vesicles called aquaporins getting inserted into the luminal membrane. They span the luminal membrane and permit movement of water down an osmotic gradient. The water absorbed is returned to the systemic circulation across the basolateral membrane. When the effect of AVP has worn off, water channels are removed from the luminal membrane by endocytosis, aggregate within clathrin-coated pits, and are returned to the cytoplasm[29].
Orally and intravenously active non-peptide vasopressin receptor antagonists are called vaptans. They cause aquaresis, that is, excretion of solute-free urine (Figure 3). They differ from the diuretics as they promote excretion of water without the loss of electrolytes and hence are categorized as aquaretics.
Two phase-2 studies have recently tested satavaptan associated to diuretics finding a therapeutic benefit[30,31]. Such result was confirmed in the setting of ascites recurrence after large-volume paracentesis (LVP) in another phase-2 study[32]. Unfortunately phase-3 randomized, placebo-controlled studies found a non-superiority and a worse safety profile of satavaptan in combination with diuretics in ascitic patients with even an increased morbidity and mortality for unknown reasons[33].
Therefore, both European and American guidelines suggest as first-line therapy of refractory ascites repeated LVPs + albumin (8 g/L of ascites removed) and Transjugular Intrahepatic Porto-Systemic Shunt as rescue therapy for patients with very frequent requirement of LVPs[34,35]. Main characteristics of vaptans recently tested in clinical trials are reported in Table 1.
| Name | Receptor selectivity | Administration | Dose (mg) | Half-life (h) |
| Conivaptan | V1aR/V2R | Oral, IV | 40-80 | 31-78 |
| Tolvaptan | V2R | Oral | 15-60 | 6-8 |
| Lixivaptan | V2R | Oral | 50-400 | 7-10 |
| Satavaptan | V2R | Oral | 5-25 | 14-17 |
| Mozavaptan | V2R | Oral | 30-60 | - |
Due to the aforementioned prognostic impact of hyponatremia in cirrhotic patients and the well-known association between low serum sodium and comorbidities (neurological complications, above all), in the last years many efforts in order to define an effective and safe therapeutic algorithm for dilutional hyponatremia have been made.
The main therapy of hyponatremia, consisting of increasing solute-free water excretion, has recently been implemented with the introduction of vaptans[36,37]. A number of evidences show that a short-therapy with vaptans (1 wk to 1 mo) ameliorates solute-free water excretion and leads to the increase in serum sodium levels in 45%-82% of patients without particular side effects on renal function, urine sodium, circulatory function, and activity of RAAS[38-41].
Thirst is the main complication related to vaptans. Other possible consequences of vaptan use in cirrhotics are hypernatremia, dehydration, kidney failure, and osmotic demyelination syndrome due to an unregulated increase in serum sodium levels. On the other hand, these concerns found little confirm in the aforementioned studies and their low frequency makes such considerations more theoretical assumptions than real problems[38-41]. Nevertheless, in light of these reported complications, therapy with vaptans should always be started under medical control with an “in-hospital” regime and serum sodium shouldn’t increase of more than 8-10 mmol/L per day[34]. Furthermore, vaptan therapy should be avoided in individuals affected by encephalopathy or who cannot guarantee an appropriate uptake of water due to the risk of dehydration and hypernatremia. The metabolism of these drugs is on charge of hepatic CYP3A enzymes; therefore, an unexpected increase in hematic vaptan levels could be due to drugs or compounds known as strong inhibitors of CYP3A such as ketoconazole, grapefruit juice, and clarithromycin. On the other hand, inducers of the CYP3A system, such as rifampicin, barbiturates, and phenytoin, may lead to a severe impairment of vaptan efficacy.
Tolvaptan was approved in the United States and Europe for the treatment of severe hypervolemic hyponatremia (< 125 mmol/L) due to SIADH, while for other conditions such as cirrhosis, ascites, and heart failure, only the American Food and Drug Administration (FDA) had licensed the drug.
Tolvaptan should be started with 15 mg/d and titrated progressively to 30 and 60 mg/d, if needed, following the serum sodium concentration. In the above reported studies, concerns raised only for some reported cases of gastrointestinal bleeding. Except for the aforementioned event, the safety profile of the drug resulted acceptable but clinicians should be aware that robust long-term data are lacking. However, in a recent placebo-controlled and open-label extension study of chronically administered tolvaptan in patients with autosomal dominant polycystic kidney disease, three cases of serious liver injury attributed to tolvaptan were observed[42]. Therefore, in a recently published safety announcement, FDA has forbidden the use of tolvaptan in patients with underlying liver disease, including cirrhosis, because the ability to recover may be impaired[43].
Conivaptan has also been licensed by FDA for the short term (5 d) intravenous treatment of hypervolemic hyponatremia but in cirrhosis data form randomized trials are lacking.
Given the narrow therapeutic window of these drugs, current practical guidelines state that management of symptomatic hyponatremia relies on infusion of saline and removal of the underlying etiologic mechanism (usually due to diuretic therapy)[34,35]. Vaptan therapy should be reserved to cases of severe hypervolemic hyponatremia (< 125 mmol/L) and should be introduced under careful medical monitoring in hospital regime. Patients may be discharged when sodium levels’ stabilization is reached and no further adjustments of vaptan dose are needed. The proper length of therapy with vaptans is still unclear and concerns raise for long-term courses (> 1 mo)[34].
Vaptans represent a modern and promising therapeutic tool in the management of hydroelectrolytic imbalances in cirrhotic patients. Their safety profile and efficacy need further validation by randomized controlled trials.
P- Reviewer: Dang SS, He JY, Ikura Y, Penkova-Radicheva MP, Wong GLH S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Schiff ER, Sorrell MF, Maddrey WC. Schiff’s Diseases of the Liver. 10th ed. Amsterdam: Lippincott Williams and Wilkins 2007; 529. [Cited in This Article: ] |
| 2. | Rector WG. Portal hypertension: a permissive factor only in the development of ascites and variceal bleeding. Liver. 1986;6:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Morali GA, Sniderman KW, Deitel KM, Tobe S, Witt-Sullivan H, Simon M, Heathcote J, Blendis LM. Is sinusoidal portal hypertension a necessary factor for the development of hepatic ascites? J Hepatol. 1992;16:249-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1131] [Cited by in F6Publishing: 984] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 371] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 360] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Facciorusso A, Nacchiero MC, Rosania R, Laonigro G, Longo N, Panella C, Ierardi E. The use of human albumin for the treatment of ascites in patients with liver cirrhosis: item of safety, facts, controversies and perspectives. Curr Drug Saf. 2011;6:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterol Rep (Oxf). 2014;2:21-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Claria J, Jiménez W, Arroyo V, La Villa G, López C, Asbert M, Castro A, Gaya J, Rivera F, Rodés J. Effect of V1-vasopressin receptor blockade on arterial pressure in conscious rats with cirrhosis and ascites. Gastroenterology. 1991;100:494-501. [PubMed] [Cited in This Article: ] |
| 11. | Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Cárdenas A, Ginès P. Portal hypertension. Curr Opin Gastroenterol. 2009;25:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ginés P, Berl T, Bernardi M, Bichet DG, Hamon G, Jiménez W, Liard JF, Martin PY, Schrier RW. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 206] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Pérez-Ayuso RM, Arroyo V, Camps J, Rimola A, Gaya J, Costa J, Rivera F, Rodés J. Evidence that renal prostaglandins are involved in renal water metabolism in cirrhosis. Kidney Int. 1984;26:72-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014;29:1356-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 19. | Ginés P, Rodés J. Clinical disorders of renal function in cirrhosis with ascites. eds. Ascites and renal dysfunction in liver disease. Malden: Blackwell Science 1999; 36–62. [Cited in This Article: ] |
| 20. | Moore CM, Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229-236. [PubMed] [Cited in This Article: ] |
| 22. | Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 23. | Leithead JA, Hayes PC, Ferguson JW. Review article: advances in the management of patients with cirrhosis and portal hypertension-related renal dysfunction. Aliment Pharmacol Ther. 2014;39:699-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013;38:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Reilly RF, Jackson EK. Regulation of renal function and vascular volume. Goodman and Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw Hill 2011; 701–719. [Cited in This Article: ] |
| 26. | Aditya S, Rattan A. Vaptans: A new option in the management of hyponatremia. Int J Appl Basic Med Res. 2012;2:77-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Barbano B, Sardo L, Gigante A, Gasperini ML, Liberatori M, Giraldi GD, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kumar P, Clark M, editors . Kumar and Clark’s Clinical Medicine. 7th ed. Edinburgh: Saunders Elsevier 2009; 649–663. [Cited in This Article: ] |
| 30. | Ginès P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48:204-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Ginès P, Wong F, Watson H, Terg R, Bruha R, Zarski JP, Dudley F. Clinical trial: short-term effects of combination of satavaptan, a selective vasopressin V2 receptor antagonist, and diuretics on ascites in patients with cirrhosis without hyponatraemia--a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010;31:834-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Wong F, Gines P, Watson H, Horsmans Y, Angeli P, Gow P, Minini P, Bernardi M. Effects of a selective vasopressin V2 receptor antagonist, satavaptan, on ascites recurrence after paracentesis in patients with cirrhosis. J Hepatol. 2010;53:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Wong F, Bernardi M, Horsmans Y, Cabrijan Z, Watson H, Ginès P. Effects of satavaptan, an oral vasopressin V2 receptor antagonist, on management of ascites and morbidity in liver cirrhosis in a long-term, placebo-controlled study. J Hepatol. 2009;50:S42–S43. [DOI] [Cited in This Article: ] |
| 34. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1070] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 35. | Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 488] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 36. | Quittnat F, Gross P. Vaptans and the treatment of water-retaining disorders. Semin Nephrol. 2006;26:234-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Ginès P. Vaptans: a promising therapy in the management of advanced cirrhosis. J Hepatol. 2007;46:1150-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099-2112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 902] [Cited by in F6Publishing: 795] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 39. | O’Leary JG, Davis GL. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transpl. 2009;15:1325-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Gerbes AL, Gülberg V, Ginès P, Decaux G, Gross P, Gandjini H, Djian J. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology. 2003;37:182-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407-2418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1037] [Cited by in F6Publishing: 1000] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 43. | Available from: http://www.fda.gov/Drugs/DrugSafety/ucm350062.htm. [Cited in This Article: ] |