Published online Aug 27, 2013. doi: 10.4254/wjh.v5.i8.439
Revised: June 10, 2013
Accepted: July 18, 2013
Published online: August 27, 2013
AIM: To evaluate the efficacy of the aspartate aminotransferase/platelet ratio index (APRI) and neutrophil-lymphocyte (N/L) ratio to predict liver damage in chronic hepatitis B (CHB).
METHODS: We analyzed 89 patients diagnosed with CHB by percutaneous liver biopsy and 43 healthy subjects. Liver biopsy materials were stained with hematoxylin-eosin and Masson’s trichrome. Patients’ fibrosis scores and histological activity index (HAI) were calculated according to the Ishak scoring system. Fibrosis score was recognized as follows: F0-1 No /early-stage fibrosis, F2-6 significant fibrosis, F0-4 non-cirrhotic and F5-6 cirrhotic. Significant liver fibrosis was defined as an Ishak score of ≥ 2. APRI and N/L ratio calculation was made by blood test results.
RESULTS: The hepatitis B and control group showed no difference in N/L ratios while there was a significant difference in terms of APRI scores (P < 0.001). Multiple logistic regression analysis revealed that the only independent predictive factor for liver fibrosis in CHB was platelet count. APRI score was significantly higher in cirrhotic patients than in non-cirrhotic patients. However, this significance was not confirmed by multiple logistic regression analysis. The optimum APRI score cut-off point to identify patients with cirrhosis was 1.01 with sensitivity, specificity, positive predictive value and negative predictive value of 62% (36%-86%), 74% (62%-83%), 29% (13%-49%) and 92% (82%-97%), respectively. In addition, correlation analyses revealed that N/L ratio has a negative and significant relationship with HAI (r = -0.218, P = 0.041).
CONCLUSION: N/L ratio was negatively correlated with HAI. APRI score may be useful to exclude cirrhosis in CHB patients.
Core tip: Due to the limitations of liver biopsy, the use of non-invasive markers has emerged in recent years. The aspartate aminotransferase/platelet ratio index (APRI) is used to determine chronic hepatitis C patients with advanced fibrosis. Neutrophil-lymphocyte (N/L) ratio is higher in patients with advanced fibrosis and considered as a novel non-invasive marker to predict advanced disease in non-alcoholic steatohepatitis. This study showed that N/L ratio is negatively correlated with HAI in chronic hepatitis B (CHB). APRI score may be useful to exclude cirrhosis in CHB patients.
- Citation: Celikbilek M, Dogan S, Gursoy S, Zararsız G, Yurci A, Ozbakır O, Guven K, Yucesoy M. Noninvasive assessment of liver damage in chronic hepatitis B. World J Hepatol 2013; 5(8): 439-444
- URL: https://www.wjgnet.com/1948-5182/full/v5/i8/439.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i8.439
Hepatitis B virus (HBV) infection is a major health problem all over the world, and is thought to affect 350-400 million people. Disease can be found in a wide range from inactive carrier state to cirrhosis and hepatocellular carcinoma (HCC)[1]. Disease morbidity and mortality in chronic hepatitis B (CHB) depends on the continuation of viral replication and progression of the disease to cirrhosis and HCC[2]. The goal of treatment is to prevent progression of the disease to advanced stages like cirrhosis and HCC. Establishing the status of hepatic fibrosis is important to decide the treatment[2]. Liver biopsy gives more accurate results about liver damage and fibrosis stage. Low patient compliance because of the invasive nature of liver biopsy, the occurrence of bleeding and pain, as a result of faulty sampling and missing pathological evaluation, differences between pathologists in the evaluation of biopsies and the limited use of biopsy in the monitoring of treatment are the limitations of liver biopsy[3,4]. For these reasons, non-invasive tests are needed to determine liver damage and fibrosis in CHB.
The aspartate aminotransferase/platelet ratio index (APRI) has been used to determine chronic hepatitis C (CHC) patients with advanced fibrosis[5]. APRI also predicts significant fibrosis in CHB[6]. The neutrophil-lymphocyte (N/L) ratio can be calculated easily from complete blood counts and is an easily accessible marker which indicates the state of inflammation in the body. It is considered to evaluate disease prognosis in HCC[7,8]. Alkhouri et al[9] found that the N/L ratio is higher in patients with non-alcoholic steatohepatitis (NASH) and advanced fibrosis. They also suggested that the N/L ratio can be used as a novel non-invasive marker to predict advanced disease in NASH. In our study, we evaluated APRI and the N/L ratio, which are cheap and easily accessible markers, to determine hepatic damage and fibrosis in patients with CHB. This study aimed to evaluate the efficacy of the N/L ratio to predict significant fibrosis in CHB for the first time in the literature.
This study was conducted between January 2007 and November 2008 at Erciyes University Medical Faculty in the Department of Gastroenterology. We retrospectively analyzed 89 patients diagnosed with CHB by percutaneous liver biopsy. Inclusion criteria were accepted as follows: positive surface antigen of HBV for at least 6 months, HBV DNA ≥ 2.000 IU/mL, patients with pre-treatment liver biopsies, the lack of HIV, HCV and hepatitis D virus infections, the lack of other liver diseases, lack of HCC and lack of alcohol use. The control group consisted of 43 individuals with normal liver tests without systemic disease. All cases were evaluated for clinical and medical background. Our study was conducted in accordance with the principles of the Helsinki Declaration. Erciyes University’s Medical Faculty Ethics Committee approved the study.
APRI and N/L ratio calculation is made by blood test results at least 1 mo prior to liver biopsy. The APRI score was calculated with the formula (AST/40)/platelet (109/L) × 100[5]. The N/L ratio was calculated using the values of neutrophils and lymphocytes obtained from the patients complete blood counts.
Liver biopsy materials were stained with hematoxylin-eosin and Masson’s trichrome. All of the liver biopsies were examined by experienced pathologists. Patients’ fibrosis scores and histological activity index (HAI) were calculated according to the Ishak scoring system[10]. Fibrosis score was recognized as follows: F0-1 No/early-stage fibrosis, F2-6 significant fibrosis, F0-4 non-cirrhotic and F5-6 cirrhotic. Significant liver fibrosis was defined as an Ishak score of ≥ 2. This score is also defined as a histologic indication of treatment[11].
MedCalc (Version 9.2.0.1) and IBM SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, United States) softwares were used for all analyses. The Shapiro-Wilk’s test was used and histogram and q-q plots were examined to assess the data normality. Accordingly, either an independent samples t test or Mann-Whitney U tests were used to compare the differences of continuous variables between groups. χ2 analyses were used to compare the differences of categorical variables. Results are expressed as frequencies and percentages, mean ± SD or median (25th and 75th percentiles). Moreover, univariate and multivariate logistic regression analyses were performed and ORs with 95%CI were calculated in order to indentify the risk factors of significant fibrosis and cirrhosis. Significant variables at a P < 0.10 level in univariate analysis were taken to multivariate analysis and backward stepwise elimination was used at a P < 0.10 stringency level to identify the independent risk factors of significant fibrosis and cirrhosis. Receiver operating characteristic (ROC) curves were plotted for the N-L ratio and APRI score to detect significant fibrosis and cirrhosis. The areas also, cut-offs were determined for each variable. Sensitivity, specifity, positive predictive rate, negative predictive rate and accuracy rate diagnostic measures were calculated and Kappa tests were performed for the N/L ratio and APRI score for the given cut-off value. Speaman’s rank test was used for correlation analysis. A P < 0.05 probability level was considered statistically significant.
In this study 89 patients with hepatitis B and 43 healthy subjects with no systemic disease were included as a control group. There were 31 (72%) females and 12 (28%) males in the control group and also 39 (44%) females and 50 (56%) males in the patient group. The demographic and laboratory data of the hepatitis B and control group are summarized in Table 1.
| Variable | Control (n = 43) | Hepatitis B patients (n = 89) | P value |
| Gender (female/male) | 31 (72.1)/12 (27.9) | 39 (43.8)/50 (56.2) | 0.004 |
| Age (yr) | 35.4 ± 12.64 | 41.5 ±1 3.02 | 0.012 |
| Platelet count (103μL) | 272.0 (242-342) | 179.0 (147-231) | < 0.001 |
| Total bilirubin (mg/dL) | 0.6 (0.4-0.8) | 0.8 (0.6-1) | 0.009 |
| AST (IU/L) | 17.0 (14-20) | 45.0 (30-68) | < 0.001 |
| ALT (IU/L) | 16.0 (11-19) | 56.0 (36-111) | < 0.001 |
| AP (IU/L) | 69.0 (53-79) | 72.0 (61-90) | 0.086 |
| GGT (IU/L) | 17.0 (14-27) | 29.0 (21-50) | < 0.001 |
| Neutrophil count | 4.3 (3.4-4.9) | 3.4 (2.9-4.7) | 0.004 |
| Lymphocyte count | 2.1 (1.6-2.4) | 2.0 (1.6-2.4) | 0.355 |
| N/L | 2.1 (1.5-2.8) | 1.9 (1.4-2.3) | 0.123 |
| APRI score | 0.1 (0.1-0.1) | 0.6 (0.3-1.1) | < 0.001 |
The hepatitis B and control group showed no difference in N/L ratios while there was a significant difference in terms of APRI scores (P < 0.001). In addition, as expected, platelet count, AST, ALT and GGT values were significantly different from those of the control group (P < 0.001). While platelet count was lower, AST, ALT and GGT levels were higher in the patient group (Table 1).
In CHB patients, when significant fibrosis was compared with early-stage fibrosis, a significant difference in platelet count and INR values was found (P < 0.05). Multiple logistic regression analysis revealed that the only independent predictive factor for liver fibrosis in CHB was platelet count. The APRI score was found to be higher in CHB with significant fibrosis but this increase was not found to be statistically significant (Table 2).
| Variable | Between group comparisons | Logistic regression analysis | |||
| No/mild fibrosis (n = 34) | Significant fibrosis (n = 55) | P value | Univariate OR (95%CI) | Multivariate OR (95%CI) | |
| Gender (female/male) | 16 (47.1)/18 (52.9) | 23 (41.8) /32 (58.2) | 0.792 | 1.2 (0.5-2.9) | - |
| Age (yr) | 40.2 ± 11.7 | 42.2 ± 13.7 | 0.473 | 1.01 (0.9-1.05) | - |
| HGB | 14.4 ± 2.08 | 14.6 ± 1.8 | 0.735 | 1.04 (0.8-1.3) | - |
| Platelet Count (103μL) | 203 (176-232) | 171 (115-227) | 0.010 | 0.9 (0.9-1) | 0.9 (0.9-1) |
| INR | 1.07 ± 0.1 | 1.13 ± 0.1 | 0.045 | 34.5 (1.01-1183.1) | - |
| Albumine | 4.06 ± 0.3 | 4.01 ± 0.3 | 0.466 | 0.6 (0.1-2.3) | - |
| Total bilirubin (mg/dL) | 0.7 (0.5-0.9) | 0.8 (0.6-1.1) | 0.110 | 3.05 (0.8-10.8) | - |
| AST (IU/L) | 41.5 (27-73) | 47 (32-68) | 0.447 | 1 (0.9-1.01) | - |
| ALT (IU/L) | 57 (33-132) | 54 (36-106) | 0.866 | 1 (0.9-1.01) | - |
| AP (IU/L) | 71.5 (62-89) | 73 (61-100) | 0.630 | 1.01 (0.9-1.02) | - |
| GGT (IU/L) | 27 (19-48) | 36 (23-52) | 0.119 | 1.01 (0.9-1.03) | - |
| HBV DNA | 346 (1.9-1000) | 75.7 (1.8-1000) | 0.838 | 1 (0.9-1.01) | - |
| HBeAg (negative/positive) | 30 (88.2)/4 (11.8) | 45 (81.8)/10 (18.2) | 0.611 | 1.6 (0.4-5.8) | - |
| Neutrophil count | 3.2 (2.7-4.8) | 3.5 (3.04-4.7) | 0.569 | 0.9 (0.6-1.2) | - |
| Lymphocyte count | 1.9 (1.4-2.3) | 2 (1.6-2.4) | 0.630 | 0.9 (0.5-1.7) | - |
| N/L | 1.9 (1.3-2.5) | 1.8 (1.5-2.2) | 0.859 | 0.8 (0.5-1.3) | - |
| APRI score | 0.5 (0.3-0.9) | 0.7 (0.3-1.4) | 0.060 | 1.2 (0.7-2.1) | - |
Cirrhotic patients were found to be more elderly compared to the non-cirrhotic patients (P < 0.05). The APRI score was significantly higher in cirrhotic patients than in non-cirrhotic patients (P < 0.05). However, this significance was not confirmed by multiple logistic regression analysis (Table 3).
| Variable | Between group comparisons | Logistic regression analysis | |||
| Non-cirrhotic (n = 76) | Cirrhotic (n = 13) | P value | Univariate OR (95%CI) | Multivariate OR (95%CI) | |
| Gender (female/male) | 33 (43.4)/43 (56.6) | 6 (46.2)/7 (53.8) | 0.999 | 1.1 (0.3-3.6) | - |
| Age (yr) | 40.2 ± 12.3 | 49.08 ± 15.04 | 0.022 | 1.06 (1.01-1.1) | 1.06 (1-1.11) |
| HGB | 14.5 ± 2.03 | 14.6 ± 1.37 | 0.891 | 1.0 (0.7-1.39) | - |
| Platelet count (103μL) | 190.5 (152-233.5) | 152 (117-175) | 0.051 | 0.9 (0.9-1) | - |
| INR | 1.1 ± 0.1 | 1.1 ± 0.09 | 0.250 | 6.3 (0.09-478.1) | - |
| Albumine | 4.04 ± 0.3 | 3.9 ± 0.4 | 0.210 | 0.2 (0.04-2.01) | - |
| Total bilirubin (mg/dL) | 0.8 (0.6-1) | 1 (0.6-1.3) | 0.389 | 2.09 (0.5-8.7) | - |
| AST (IU/L) | 41.5 (28-65) | 66 (40-79) | 0.078 | 1 (0.9-1.01) | - |
| ALT (IU/L) | 54 (33-124) | 69 (51-97) | 0.419 | 1 (0.9-1.01) | - |
| AP (IU/L) | 72 (61-89.5) | 82 (62-116) | 0.225 | 1.02 (1-1.03) | 1.02 (1-1.04) |
| GGT (IU/L) | 28 (20-50.5) | 47 (26-50) | 0.189 | 1.01 (1-1.02) | - |
| HBV DNA | 269.5 (2-1000) | 10.1 (0.4-1000) | 0.339 | 1 (0.9-1.01) | - |
| HbeAg (negative/positive) | 65 (85.5)/11 (14.5) | 10 (76.9)/3 (23.1) | 0.423 | 1.7 (0.4-7.4) | - |
| Neutrophil count | 3.4 (2.8-4.7) | 3.8 (3.04-4.1) | 0.468 | 1.05 (0.7-1.5) | - |
| Lymphocyte count | 2 (1.6-2.4) | 2 (1.5-2.2) | 0.493 | 0.6 (0.2-1.5) | - |
| N/L | 1.8 (1.3-2.2) | 2.04 (1.6-2.8) | 0.160 | 1.3 (0.8-2.07) | - |
| APRI score | 0.5 (0.3-1.08) | 1.1 (0.7-1.7) | 0.047 | 1.2 (0.8-2.05) | - |
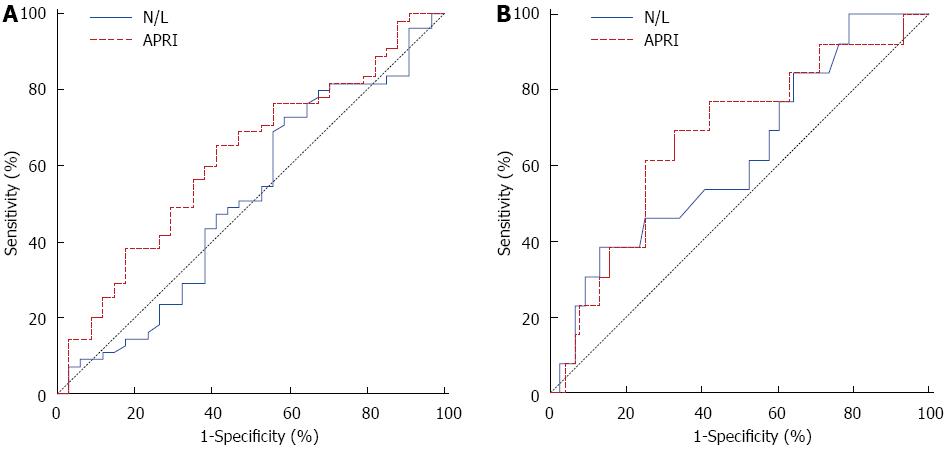
ROC curve analysis suggested that the optimum APRI score cut-off point to identify patients with cirrhosis was 1.01 with sensitivity, specificity, positive predictive value and negative predictive value of 62% (36%-86%), 74% (62%-83%), 29% (13-49) and 92% (82-97) respectively (Figure 1, Table 4). In general, the accuracy of the APRI score to determine patients with cirrhosis is 72%. In addition, correlation analyses revealed that the N/L ratio has a negative and significant relationship with HAI (r = -0.218, P = 0.041).
| Variable | Diagnostic measures | Kappa test | |||||
| SEN (95%CI) | SPE (95%CI) | PPR (95%CI) | NPR (95%CI) | AR (95%CI) | κ | P value | |
| Significant fibrosis | |||||||
| N/L ( ≤ 2.18) | 0.73 (0.59-0.84) | 0.41 (0.25-0.59) | 0.67 (0.53-0.78) | 0.48 (0.29-0.67) | 0.61 (0.50-0.71) | 0.143 | 0.174 |
| APRI (> 0.56) | 0.65 (0.51-0.78) | 0.56 (0.38-0.73) | 0.71 (0.56-0.83) | 0.50 (0.33-0.67) | 0.62 (0.51-0.72) | 0.209 | 0.048 |
| Cirrhosis | |||||||
| N/L (> 2.58) | 0.38 (0.14-0.68) | 0.87 (0.77-0.64) | 0.33 (0.12-0.62) | 0.89 (0.80-0.95) | 0.80 (0.70-0.88) | 0.238 | 0.024 |
| APRI (> 1.01) | 0.62 (0.36-0.86) | 0.74 (0.62-0.83) | 0.29 (0.13-0.49) | 0.92 (0.82-0.97) | 0.72 (0.61-0.81) | 0.238 | 0.011 |
In CHB patients with cirrhosis the APRI score was significantly higher but this significance was not confirmed by multiple logistic regression analysis. The APRI score was higher in significant fibrosis but it was not statistically significant. While the N/L ratio was not related with significant fibrosis and cirrhosis, it was found to be negatively correlated with HAI in patients with CHB.
Due to the limitations of liver biopsy, the use of non-invasive markers has emerged in recent years[12,13]. In these studies, positive results were obtained with Fibrotest and Fibroscan to determine advanced fibrosis and cirrhosis in patients with CHB and CHC. Studies have conflicting results with regard to the use of APRI score to predict significant fibrosis in CHB patients. In their study Wai et al[14] suggest that APRI score, which is used to predict significant fibrosis and cirrhosis in CHC, was not suitable for patients with CHB. They explain this by the presence of a fluctuating course with acute attacks in CHB patients while the progression of fibrosis in CHC is more quiet. Yilmaz et al[15] also confirmed this and concluded that in patients with CHC the APRI score showed good accuracy for the assessment of liver fibrosis, but not in those with CHB. In contrast to these findings, Shin et al[6] studied a large number of CHB patients and suggested a strong positive linear correlation between fibrosis and APRI. Kim et al[16] also concluded that APRI score correlated significantly to fibrosis stage. Güzelbulut et al[17] found that the areas under the ROC curves of the APRI score to predict significant fibrosis and cirrhosis were 0.77 and 0.78, respectively. They also mentioned that APRI score is more accurate in the prediction of the absence of both significant fibrosis and cirrhosis with negative predictive values of over 90%. In a recent meta-analysis, Jin et al[18] suggested that APRI score showed limited value in predicting CHB related significant fibrosis and cirrhosis and the areas under the ROC curves of APRI score were 0.79 and 0.75, respectively. In our study, we did not find statistically significant relation with APRI score and significant fibrosis. APRI score was significantly higher in cirrhotic patients and the accuracy of APRI score to determine patients with cirrhosis was 72%. In our study, as in that of Güzelbulut et al[17], the accuracy of the APRI score in the prediction of the absence of cirrhosis was high with negative predictive values of over 90%. Our study results also showed a statistical association between age and cirrhosis (P = 0.022). This is an expecting result because the liver damage increases gradually in proportion to the exposure to HBV infection. Patients with CHB above 40 years can be at increased risk of mortality because of liver disease. This can be explained by the increased cirrhosis rates with older age as a host risk factor[19].
With recent evidence, the APRI score, which is used to predict significant fibrosis and cirrhosis in CHC, did not seem as effective in determining fibrosis and cirrhosis in patients with CHB. This can be attributed to differences in the histopathological findings and course of disease. Regenerative nodules are wider in CHB than in CHC[16]. Piecemeal necrosis is more localized and less severe in CHC than in CHB[16]. Hepatic steatosis is an important factor in CHC histology[20]. Disease progression shows a fluctuating course with acute attacks in CHB patients while the progression of fibrosis in CHC is more quiet[16]. For all these reasons, non-invasive markers shown to be effective in CHC should be validated in CHB before use.
The prognosis of patients, who are infected with HBV, depends on the patient’s immune response[21]. The hepatitis B virus can be eliminated with a moderate immune response, whereas an excessive response may result in liver damage. HBV persists in the body due to the low-grade immune response. N/L ratio, which is a cheap and easily accessible marker, shows the body’s immune response[9]. This ratio provides information about two important immune pathways like neutrophils responsible for ongoing inflammation and lymphocytes which have a regulatory role in immune response. Lymphocytes have an impact on liver fibrosis in CHB[22,23]. Alkhouri et al[9] showed a relation between N/L ratio and advanced fibrosis in patients with non-alcoholic steatohepatitis. Consequently, N/L ratio may be considered as an important non-invasive marker of liver damage in response to HBV infection.
To our knowledge, our study is the first to evaluate the N/L ratio in CHB disease. In our study, we found a negative and significant relationship between HAI with N/L ratio. This negative relationship demonstrates the important role of lymphocytes in liver damage in CHB. According to our findings fibrosis stage and cirrhosis were not associated with N/L ratio.
All the spectra of biopsies of patients with CHB give rise to the study of the relationship between histological findings with the APRI score and the N/L ratio. The case-control nature of the present study and the number of cases were the limitations of this study.
As with other non-invasive markers APRI and N/L ratio are readily available and inexpensive tests. However, APRI and N/L ratio were not adequate tests to determine either significant fibrosis or cirrhosis in CHB according to our study. For the first time in the literature, this study showed that N/L ratio was negatively correlated with HAI. APRI score may be useful to exclude cirrhosis in CHB patients. Comprehensive and prospective studies are needed to determine the diagnostic value of non-invasive tests for liver damage in CHB.
Liver biopsy is the standard method to assess liver histology in chronic hepatitis B (CHB) disease. Due to the limitations of liver biopsy, the use of non-invasive markers has emerged in recent years. The aspartate aminotransferase/platelet ratio index (APRI) is used to determine chronic hepatitis C (CHC) patients with advanced fibrosis. Neutrophil-lymphocyte (N/L) ratio is higher in patients with advanced fibrosis and considered as a novel non-invasive marker to predict advanced disease in non-alcoholic steatohepatitis. But up to now, no study evaluated the efficacy of N/L ratio to predict liver damage in CHB.
The APRI has been used to determine CHC patients with advanced fibrosis. APRI also predicts significant fibrosis in CHB. The N/L ratio can be calculated easily from complete blood counts and is an easily accessible marker which indicates the state of inflammation in the body. The N/L ratio is higher in patients with non-alcoholic steatohepatitis (NASH) and advanced fibrosis. Also the N/L ratio may be used as a novel non-invasive marker to predict advanced disease in NASH. The research hotspot is to evaluate the N/L ratio to determine hepatic damage and fibrosis in patients with CHB and compare its effectiveness with APRI.
In the present study, the APRI score was significantly higher in CHB patients with cirrhosis. The APRI score was higher in significant fibrosis but it was not statistically significant. While the N/L ratio was not related with significant fibrosis and cirrhosis, it was found to be negatively correlated with HAI in patients with CHB.
The study results suggest that the N/L ratio is negatively correlated with HAI. APRI score may be useful to exclude cirrhosis in CHB patients.
The APRI score was calculated with the formula (AST/40)/platelet (109/L) × 100. The N/L ratio was calculated using the values of neutrophils and lymphocytes obtained from the patients complete blood counts.
The authors provide an interesting and potentially important manuscript describing noninvasive assessment of liver Fibrosis in CHB. The authors showed that the platelet count is a unique independent predictive factor for liver fibrosis in CHB.
P- Reviewers Cheng P, Cheng XW, Estrabaud E S- Editor Zhai HH L- Editor A E- Editor Ma S
| 1. | Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 1122] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 3. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [PubMed] [Cited in This Article: ] |
| 4. | Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55:569-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2976] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 6. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Chen L, Zhang Q, Chang W, Du Y, Zhang H, Cao G. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer. 2012;48:1977-1987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TA, Yerian L, Zein NN, Feldstein AE. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3521] [Cited by in F6Publishing: 3596] [Article Influence: 124.0] [Reference Citation Analysis (1)] |
| 11. | Papatheodoridis GV, Manesis EK, Manolakopoulos S, Elefsiniotis IS, Goulis J, Giannousis J, Bilalis A, Kafiri G, Tzourmakliotis D, Archimandritis AJ. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection. Hepatology. 2008;48:1451-1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready. Lancet. 2010;375:1419-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Poynard T, Ngo Y, Munteanu M, Thabut D, Ratziu V. Noninvasive Markers of Hepatic Fibrosis in Chronic Hepatitis B. Curr Hepat Rep. 2011;10:87-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, Mak B, Oo AM, Lim SG. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666-672. [PubMed] [Cited in This Article: ] |
| 15. | Yilmaz Y, Yonal O, Kurt R, Bayrak M, Aktas B, Ozdogan O. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease: APRI in chronic liver disease. Hepat Mon. 2011;11:103-106. [PubMed] [Cited in This Article: ] |
| 16. | Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, Cho SW, Han KH, Chon CY, Moon YM. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int. 2007;27:969-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Güzelbulut F, Sezıklı M, Akkan-Çetınkaya Z, Yaşar B, Özkara S, Kurdaş-Övünç AO. AST-platelet ratio index in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis B. Turk J Gastroenterol. 2012;23:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012;12:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in F6Publishing: 2114] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 20. | Sebastiani G, Vario A, Guido M, Alberti A. Sequential algorithms combining non-invasive markers and biopsy for the assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2007;13:525-531. [PubMed] [Cited in This Article: ] |
| 21. | Lohse AW, Weiler-Normann C, Tiegs G. Immune-mediated liver injury. J Hepatol. 2010;52:136-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology. 2011;53:219-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Mohamadkhani A, Bastani F, Sotoudeh M, Sayehmiri K, Shahnazari P, Montazeri G, Poustchi H. Influence of B cells in liver fibrosis associated with hepatitis B virus harboring basal core promoter mutations. J Med Virol. 2012;84:1889-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |