INTRODUCTION
The incidence of non alcoholic fatty liver disease (NAFLD) and non alcoholic steatohepatitis (NASH) has dramatically increased in Western countries during the last decades[1-3]. Still, the diagnosis of NAFLD displays a problem since there is a known heterogeneity in the histological staging of lipid accumulation in the liver[4,5]. This problem is equally relevant for research laboratories studying mechanistic and therapeutic aspects of NAFLD and NASH.
A commonly used model for the investigation of NASH is the methionine choline deficient mouse model, which is histologically similar to human NASH regarding steatohepatitis and fibrosis[6]. The methionine choline deficient diet (MCD) model is well characterized regarding its effect on the expression of lipid regulators, such as lipogenic transcription factors and lipogenic enzymes[7,8]. The development of steatosis in the MCD model is attributable in part to impaired very low density lipoprotein (VLDL) secretion due to the deficiency of methionine and choline, which are the precursors for phosphatidylcholine, the main phospholipid coating VLDL particles[9].
An interesting but as yet less characterized steatosis model is the insulin-like growth factor 2 (Igf2) mRNA binding protein p62/IMP2-2/IGF2BP2-2 transgenic mouse model[10]. p62 was originally identified as an autoantigen in an HCC patient[11] and its expression correlates with poor prognosis in hepatocellular carcinoma (HCC)[12]. Hepatic p62 overexpression induces a microvesicular fatty liver[10], which is characterized by an absence of inflammatory processes and liver damage[10]. Still p62 overexpression amplifies murine NASH and fibrosis[13].
NAFLD, even in the absence of cirrhosis, can progress to HCC[14]. Increasing knowledge suggests that not only the increase in lipid accumulation itself but rather the hepatic lipid composition plays a dominant role in the development of both simple steatosis and steatohepatitis[15]. Lipid composition has in fact been shown to have a pathophysiological relevance in different metabolic diseases[15-18] as well as in cancer[19]. Accordingly, the pharmacologically reduced production of cholesterol by inhibition of hydroxy-methyl-glutaryl-coenzyme A reductase is discussed as a strategy for the chemoprevention and a slower progression of HCC[20]. The comparison of the lipidome of a murine NASH and HCC model with the human NASH and HCC lipidome found significant changes within several fatty acid signatures between the normal, NASH, and HCC lipidome[21]. Therefore, a more comprehensive characterization and understanding of pathophysiological lipidomic changes in liver diseases and common disease models seems mandatory.
For the investigation of lipid composition liquid chromatography-mass spectrometry (LC-MS) is state-of-the-art. However, due to high costs for the equipment and maintenance, the method is not suitable for routine analyses in clinical and research laboratories. Furthermore, the results obtained by LC-MS contain information in a level of detail too complex for most of the studies, in which rather general alterations in lipid classes are of interest. Thin layer chromatography (TLC) offers some advantages over LC-MS. For example, the possibility to apply many different samples on a single TLC plate is in practice often faster than LC[22]. 3D TLC was developed in the 1960s as a reliable method for lipid separation. However, a major limitation of the technique is the fact that it is possible to test only one sample per plate[23]. Since 3D TLC has a very low inter-plate reproducibility it is only suitable for qualitative measurements.
We herein present a rapid and low-cost quantitative 1D TLC, which can detect major lipid classes and can be used to quantitatively compare up to 12 samples per plate. Furthermore, we provide insight into changes of lipid composition in the p62 transgenic mouse model for the first time[13].
MATERIALS AND METHODS
Materials
Standard substances 1,3-diolein (D3627), L-α-lysophosphatidylcholine from egg yolk (L4129), cholesterol (C8667), glyceryl trioleate (T7140), 3-sn-phosphatidylethanolamine from bovine brain (P7693), L-α-phosphatidylcholine (P3556), 1,2-diacyl-sn-glycero-3-phospho-L-serine (P7769), non-hydroxy fatty acid ceramide from bovine brain (C2137), and stearic acid (85679) were purchased from Sigma-Aldrich (Taufkirchen, Germany). The standard substances were dissolved in chloroform/methanol [1:1 (v/v)] at a concentration of 1 mg/ml, aliquoted, and stored at -80 °C. TLC silica gel 60 F254 glass plates were purchased from Merck (105715, Merck, Darmstadt, Germany). All solvents were distilled prior to utilization.
Animal models
All animal procedures were performed in accordance with the local animal welfare committee. Mice were kept under stable conditions regarding temperature, humidity, food delivery, and 12 h day/night rhythm.
Steatosis model
p62 transgenic mice were established as described previously[10]. Mice carrying a liver enriched activator protein promoter under tetracycline transactivator control[24] were crossed with p62 transgenic mice, in which the human p62 is under the control of the transrepressive responsive element cytomegaly virus (TRE-CMVmin). The double positive offspring expresses p62 liver-specifically. The mice were sacrificed at an age between 2.5 and 5 wk.
Steatohepatitis model
Wild-type mice were fed either a methionine choline deficient (MCD, 960439, MP Biomedicals, Illkirch Cedex, France) or a methionine choline supplemented control diet (co, 960441, MP Biomedicals, Illkirch Cedex, France) for 3 wk.
Histology
For hematoxylin eosin (HE) staining 5 μm paraffin slides were rehydrated in a xylol/alcohol series, incubated for 10 min in hematoxylin, washed for 5 min under running water, and incubated for 2 min in eosin.
Extraction of bovine and murine liver lipids
Bovine liver was bought from a local butchery and directly freeze dried and stored at -80 °C. Lipids from snap-frozen murine or bovine liver samples were extracted by a modified version of a published method[25]. Briefly, 60 mg liver samples were lyophilized, 15 mg of the freeze-dried tissue was dispersed with 18 volumes of a mixture of hexane/2-propanol [3:2 (v/v)] for 10 min, and centrifuged at 4 °C and 10000 g for 10 min. The supernatant was transferred to a new vial and dried under a nitrogen stream, redissolved in an appropriate volume of chloroform/methanol [1:1 (v/v)], and applied in equal amounts onto the TLC plates.
1D TLC with two solvent system
The TLC plates were prewashed with a mixture of chloroform/methanol [2:1 (v/v)] to remove any contaminants and afterwards activated at 110 °C for 1 h. The samples and standard substances were applied onto the TLC plates and chromatographically separated with the first solvent system containing chloroform/methanol/acetic acid/water [50:30:8:3 (v/v/v/v)][26] to half of the plate. The TLC was dried and subjected to chromatography in a second solvent system consisting of heptane/diethyl ether/acetic acid [70:30:2 (v/v/v)][27] to the top of the plate[28].
Detection and quantification
The TLC plates were dried for 30 min under a nitrogen stream and first sprayed with 0.1% 2’,7’-dichlorofluorescein (DCF, 109676, Merck, Darmstadt, Germany) in methanol and afterwards with sulfuric acid/ethanol [1:1 (v/v)] followed by heating at 160 °C[23]. After drying the plates one UV image at 312 nm for DCF and one white top light image for sulfuric acid/ethanol was captured using the Biostep (Jahnsdorf, Germany) dark hood dh-4050 with transilluminator Biostep bioview (excitation 312 nm, UST-20M-8E) and an stationary fixed olympus digital camera (Hamburg, Germany) in combination with the Biostep argus X1 software (version 4.1.10). The unprocessed images in tiff format were quantified using the ImageJ 1.47i software[29].
Statistical analysis
Results are expressed as means ± SE. The statistical significance was determined by independent two-sample t-test and was considered as statistically significant when P values were less than 0.05. The Microsoft® Office Excel 2003 software (Microsoft Coperation, Redmond, United States) was used for statistical analyses.
RESULTS
Quantification of lipids on the TLC plate
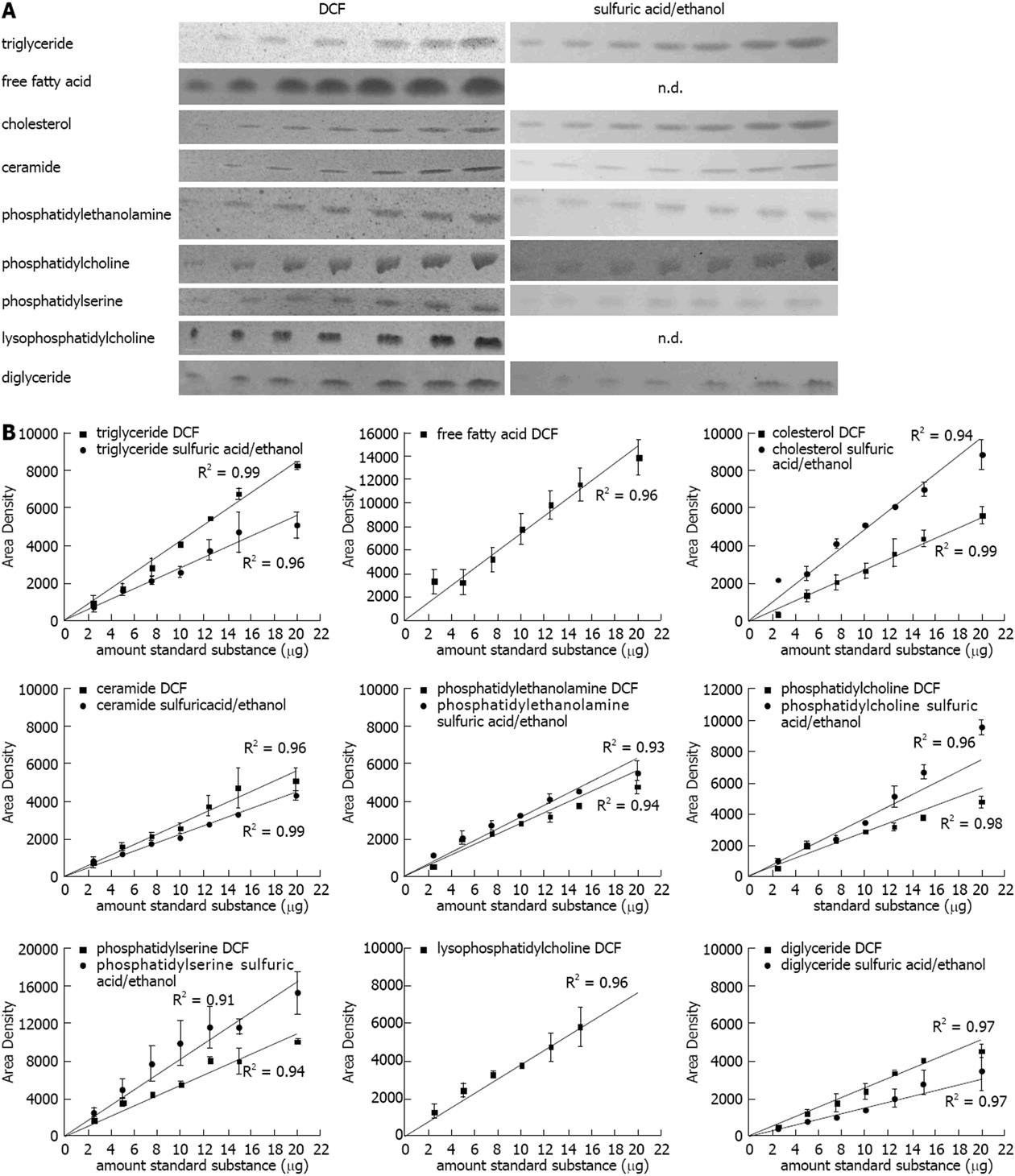
In order to check the linearity of the method used, lipid standards for triglyceride (TG), free fatty acid (FFA), cholesterol (CH), ceramide (CE), phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylserine (PS), lysophosphatidylcholine (LPC), and diglyceride (DG) (2.5, 5, 7.5, 10, 12.5, 15, 20 μg, each) were chromatographed, stained, and quantified according to our newly developed method described in the methods section. The DCF spray reagent was susceptible to all subjected lipids, the sulfuric acid/ethanol spray reagent was susceptible to almost all substances except for the FFA stearic acid and LPC (Figure 1A). As expected the band intensities increased with higher amount of the standard substances (Figure 1A). The quantification revealed a strong correlation with R2 values close to one for all substances (Figure 1B).
Figure 1 Quantification of lipids on the thin layer chromatography plate.
A: Representative lipid dilution series (range: 2.5, 5, 7.5, 10, 12.5, 15, 20 μg) detected with 2’,7’-dichlorofluorescein (DCF) or sulfuric acid/ethanol; B: Quantification of the standard dilution series detected with DCF or sulfuric acid/ethanol and quantified with ImageJ. Results represent the mean ± SE from at least two independent thin layer chromatography plates. FFA: Free fatty acids.
Validation of the lipid extraction procedure
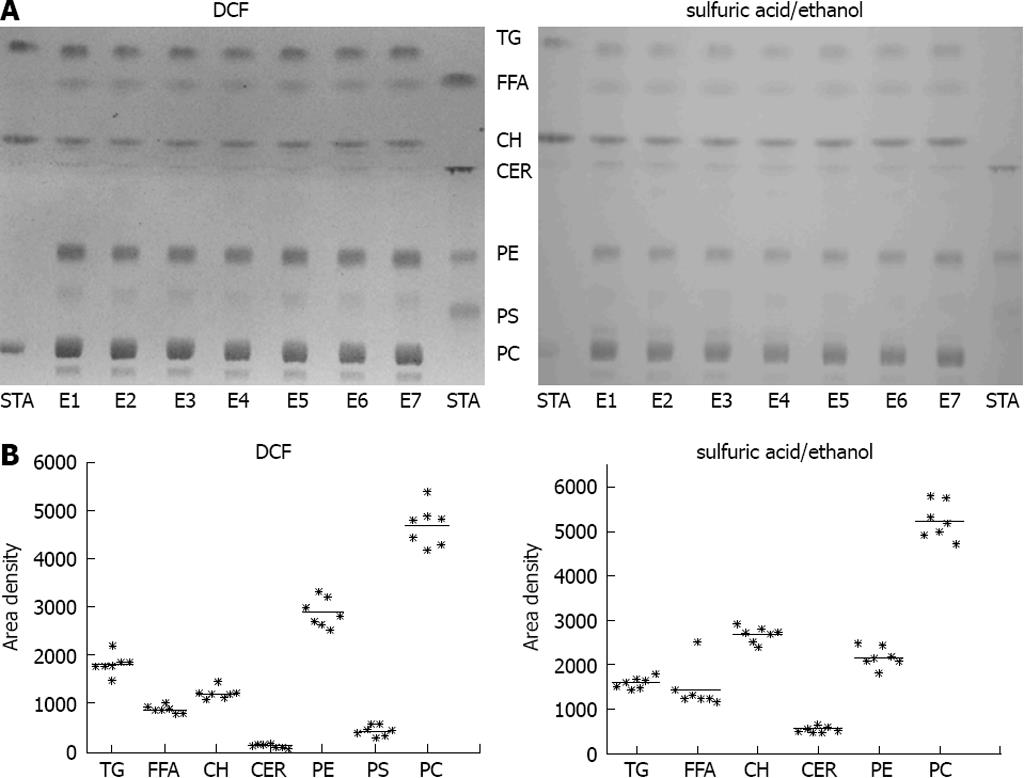
For validation of the reproducibility of the extraction procedure, freeze-dried tissue from bovine liver was extracted in seven independent extraction procedures and subjected to chromatography. The extraction procedure revealed a high reproducibility in all lipid classes investigated (Figure 2). PC, PE, TG and CH were most prominent in bovine liver (Figure 2).
Figure 2 Validation of the lipid extraction procedure.
A: Freeze-dried bovine liver was extracted in seven independent extraction procedures (E1-E7). STA: Standard substances co-chromatographed with the samples. left: detection with 2’,7’-dichlorofluorescein (DCF); right: detection with sulfuric acid/ethanol; B: Quantification of thin layer chromatography (TLC) with ImageJ detected with DCF (left) or sulfuric acid/ethanol (right).
Lipid quantification in different mouse models
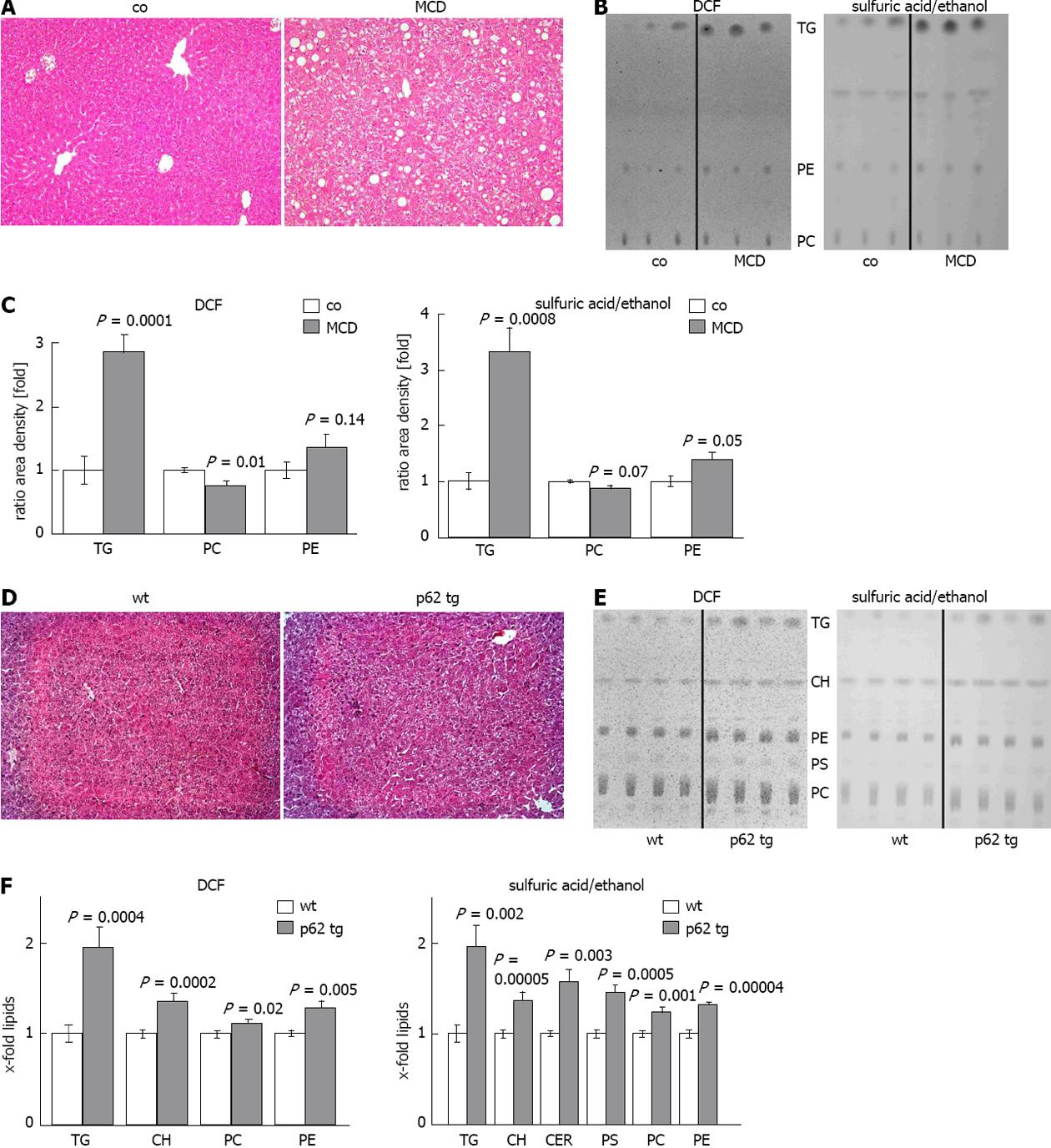
Steatohepatitis/MCD mouse model: In order to test whether altered lipid composition can be determined reliably we used a well established murine steatohepatitis model, for which altered lipid classes are known[30]. Livers from control and MCD fed mice were processed, extracted, and lipids were chromatographed and detected as mentioned above. Two independent TLC plates revealed a strong increase in TG with DCF and sulfuric acid/ethanol (Figure 3B, C). As the MCD model is characterized by choline deficiency, the levels of PC were significantly decreased, whereas the levels of PE were significantly increased (Figure 3B, C). We consequently observed a reduced PC/PE ratio by approximately one third (P = 0.003) with both detection methods. The other lipid classes investigated were not significantly changed (data not shown). Due to the high amount of TG in this model, the routinely subjected amount of lipid extract had to be reduced by five folds compared to normal tissues. Routinely used amounts led to overloading of the plates (data not shown).
Figure 3 Lipid quantification in different mouse models.
A: Representative hematoxylin eosin (HE) staining of control (co) or methionine choline deficient diet (MCD) fed mice (× 200); B: Representative thin layer chromatography (TLC) detected with 2’,7’-dichlorofluorescein (DCF) (left) or sulfuric acid/ethanol (right); C: Quantification of TLC with ImageJ, detected with DCF (left) or sulfuric acid/ethanol (right). Results represent the mean ± SE from at least two independent TLC plates with n = 7 in each group; D: representative HE staining of wildtype (wt) and p62 transgenic mice (p62 tg)(× 200); E: Representative TLC detected with DCF (left) or sulfuric acid/ethanol (right); F: Quantification of TLC with ImageJ detected with DCF (left) or sulfuric acid/ethanol (right). Results represent the mean ± SE from at least two independent TLC plates and n = 4 in each group.
Steatosis/p62transgenic mouse model: Since our method confirmed changes in lipid classes in the MCD steatohepatitis mouse model, we used it to characterize changes in lipid classes in the p62 steatosis model. The model shows distinct histologically proven microvesicular lipid incorporation in up to 58% of the animals[10] when specific lipid staining is performed. Accordingly, HE staining revealed a milder extent of steatosis compared to the MCD diet (Figure 3A, D). Two independent TLC plates revealed that all detected lipid classes were significantly increased in the livers of p62 transgenic animals (Figure 3E, F). FFA, DG and LPC were not detectable (Figure 3E). The strongest effect was observed for TG, which were increased approximately two folds in p62 trangenic animals compared to wildtype controls (Figure 3F). Interestingly, although the levels of both PC and PE were significantly increased, the PC/PE ratio was significantly decreased by about 10% (P = 0.05) with both detection methods. The same was true for the ratio of CH/PC, which was increased by approximately 23%, as validated by the DCF detection (P = 0.05).
DISCUSSION
Within this work we developed a rapid analytical method, which allows to quantify changes in hepatic lipid classes. The newly established method confirmed published findings for the lipid changes in a mouse NASH model and for the first time reports the lipid composition in the p62 transgenic steatosis model.
TLC method
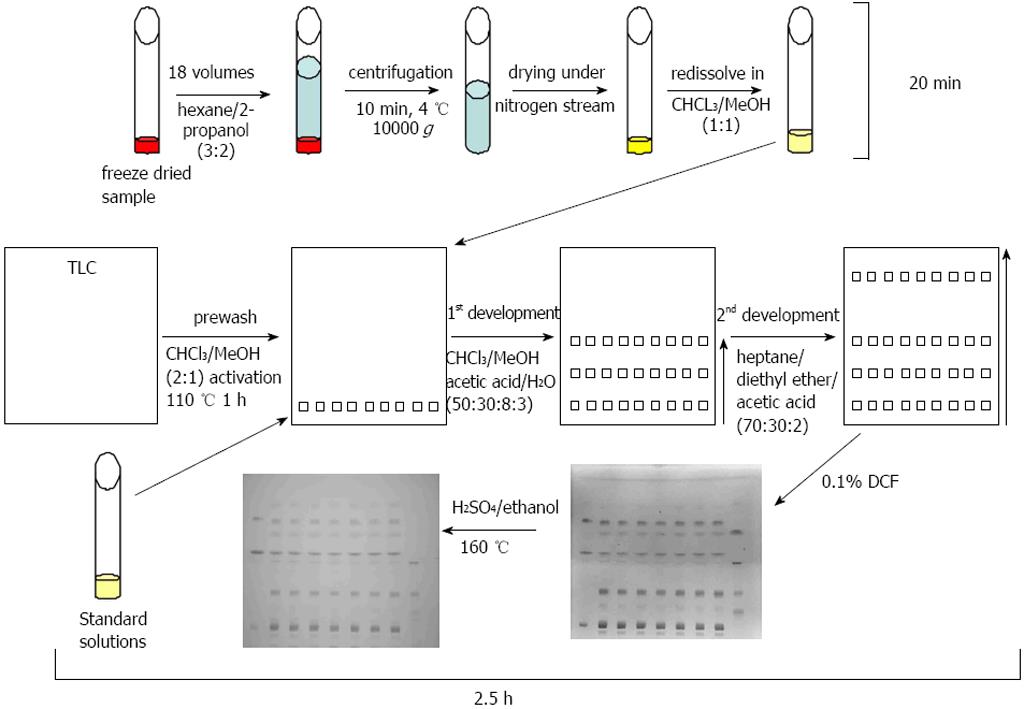
The one-dimensional TLC with a two-step solvent system and the detection with DCF or sulfuric acid/ethanol was able to separate and to detect the major lipid classes of TG, FFA, CH, CER, PE, PC, PS, LPC, and DG within a time period of 2.5 h (Figure 4). Standard curves revealed a high linearity of the standard substances from 2.5 to 20 μg. The chosen standard substances corresponded with the major lipid classes typically changed in NAFLD/NASH[31]. A lack of reactivity of saturated fatty acids towards a sulfuric acid/ethanol/hexane reagent was reported previously[32] and is in line with our finding that our FFA (the saturated fatty acid stearic acid) and LPC standard (which contains mostly palmitic acid and stearic acid) showed no staining with sulfuric acid/ethanol.
Figure 4 Thin layer chromatography method.
Samples were freeze-dried, extracted, centrifuged, and the supernatant was transferred to a new vessel and dried under gaseous nitrogen. In the meantime plates were prewashed and activated. Standard solutions and samples were subjected to the prewashed and activated thin layer chromatography (TLC) plates and developed with the first eluant system to half of the plate. After drying the TLC plate was subjected to a second eluant system in the same direction up to the top of the plate. After drying of the plates, lipids were visualised with 2’,7’-dichlorofluorescein (DCF) and afterwards with sulfuric acid/ethanol and heating to 160 °C.
Most publications only investigate an assortment of the most important lipids in liver diseases[18,26,27,31,33]. It is almost impossible to detect all abundant lipid species in tissues within one method, because the physicochemical properties of the broad spectrum of lipid classes are too variable[22]. The lipid class spectrum of the bovine liver extract was quite similar to published studies which showed that PC and PE are the main components of the bovine liver phospholipids[34,35].
The extraction procedure has the advantage to be quick and that it requires relatively low amounts of tissue (approximately 70 mg wet weight tissue) compared to other methods[33]. Additional advantages are a low contamination with non-lipids due to the high apolarity of the solvent mixture, a low toxicity, a low phospholipid degradation, and the possibility to use plastic materials[22,36]. Freeze-drying of the liver tissue samples reduces the enzymatic activity of potential lipid degrading enzymes[37]. Taken together, this method is an easy, cheap, and rapid screening method for up to 12 samples in parallel. In addition, it needs only little technical equipment. The TLC method allows detection from the applied crude lipid extracts without the need of additional purification steps.
Confirmation of known changes in lipid classes in the MCD NASH model
After establishing a reliable technique we sought to confirm known alterations in lipid classes in the MCD NASH model. The MCD-induced NASH has the advantage of a histological appearance highly similar to human NASH concerning steatosis, i.e., mixed inflammatory cell infiltrates, hepatocellular necrosis, and eventual pericellular fibrosis mimics[38]. We found strongly increased levels of TG, increased levels of PE, and decreased levels of PC, which led to a significantly decreased PC/PE ratio. Yao et al[39] reported decreased PC levels in choline deficient rat hepatocytes. Since PC biosynthesis is partly due to the methylation of PE by S-adenosyl methionine[40], it is not surprising that the lack of methionine in this model resulted in the accumulation of PE. An increase in TG[30] and a decreased mitochondrial PC/PE ratio[41] in the MCD diet was described previously. Therefore, our one-dimensional TLC method could well confirm known alterations in lipid classes in this dietary model of NASH.
Lipid composition in p62-induced steatosis
This is the first study, which clarifies the lipid composition in p62-induced steatosis. The increase in all detected lipid classes might be due to a p62-mediated activation of lipogenic genes induced by the lipogenic growth factor Igf2[42], which is highly overexpressed in p62 transgenic animals[10]. The p62-induced microvesicular steatosis is difficult to evaluate with simple histological H/E staining (Figure 3D). Still, our TLC method revealed strongly affected lipid accumulation also in histologically normal tissue and allows more reliable and quantitative statements.
Accumulation of TG in hepatocytes is a hallmark of NAFLD[43]. As expected, TG were the lipid class elevated to the highest degree in p62-induced fatty liver. Interestingly, Yetukuri et al[44] described a positive correlation between TG and CER in an ob/ob steatosis model. The precursor for TG[45], namely DG, were not detectable in our murine models, despite the fact that DG standard series revealed strong signals. A similar observation could be seen for FFA and LPC. Since the age of our investigated transgenic and control animals were 2.5 to 5 wk, the lack of abundance of some lipid species might be explained by the relatively young age. In this context Rappley et al[46] reported age-dependent changes in phospholipid levels in mouse brain.
An unaltered content of FFA in human NAFLD was described previously[18]. Since we saw weak signals for FFA, which were not elevated in the p62 transgenics, no elevation by p62 can be assumed.
One of the most complex investigations of the human NAFLD/NASH lipidome found in the literature reports elevated CH levels, and an increased ratio of CH to PC[18]. Accordingly, these results are in line with the findings in our p62 steatotic animals. On the other hand, the literature report also found decreased levels of PC and PE, whereas PS remained unaltered[18]. Since increased CH levels are often associated with enhanced PC synthesis[47], increased PC levels in our p62 transgenic animals might be explainable. Most notably, a decreased PC/PE ratio was observable in p62 transgenic animals although PC and PE levels were both increased. The distinct manipulation of the PC/PE ratio performed by Li et al[48] showed that an elevation of the ratio can in fact reverse steatohepatitis, but not steatosis. This observation strongly suggests that a decreased PC/PE ratio plays a role in the progression from steatosis to steatohepatitis. In fact, NASH patients were found to have decreased PC/PE ratios in the same study[48]. The responsible mechanisms are as yet only speculative and might involve the inhibition of the PE N-methyltransferase[48], which converts PE to PC. Among the lipids, which were elevated in the p62 transgenics, cholesterol[49] and ceramides[50] are highly cytotoxic. Although p62 overexpression induces a benign steatosis in the absence of inflammatory events, we speculate that the increased levels of CH and CER and the decreased PC/PE ratio might finally promote an inflammatory environment in the livers of p62 transgenic animals. In fact, p62 overexpression can promote the development of NASH and fibrosis[13].
In a conclusion, taken together, we have established a rapid technique to quantify altered lipid classes in experimental models of steatosis and steatohepatitis. The method confirmed known changes in the well-established MCD NASH model and for the first time revealed a distinctly altered lipid composition in the p62 steatosis model. The knowledge of changes in lipid composition might be helpful for the understanding of pathophysiological mechanisms in NAFLD and NASH.
COMMENTS
Background
Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis are mostly of benign appearance, but they are highly discussed as potential risk factors for the development of hepatocellular carcinoma (HCC). Hepatocellular carcinoma is a highly aggressive cancer type with high mortality, which is difficult to detect and to cure. Changes in lipid and fatty acid composition in disease progression to hepatocellular carcinoma are not well characterized, but are suggested to be of major importance. The high impact of these lipidomic changes needs appropriate in vivo models, and rapid and reliable methods to quantify the whole spectrum of lipid classes simultaneously.
Research frontiers
A state of the art method used for the identification of lipid classes is lipid chromatography coupled with mass spectrometric detection. This method is highly cost intensive, needs a lot of time for the establishment of the method and requires well-educated and experienced staff. Thin layer chromatography on the other hand represents a well-established technique, which allows a fast establishment in each laboratory within a really short time and allows a highly sensitive detection. As already mentioned, the lipidomic changes in the different states of liver disease progression in diverse in vivo mouse models and their potential correlation with human liver diseases are rare.
Innovations and breakthroughs
The thin layer chromatography of lipids is normally limited by the requirement to separately analyze either polar lipids or neutral lipids, each on one plate. Another method is the 3D thin layer chromatography, which allows only one sample per plate. Here the authors describe a fast screening method to chromatograph several samples on one plate, to separate the main polar and neutral lipid classes, to visualize them by two different staining methods, and to quantify them using the freely available ImageJ software within a short time. The authors proved the reliability of the method by comparing the obtained data from a methionine choline deficient non-alcoholic steatohepatitis mouse model to published data. The authors investigated the alterations in lipid classes in the p62/IMP2-2/IGF2BP2-2 transgenic mouse model for the first time and found interesting changes, which might indicate the progressive character of this steatosis model.
Applications
The described method can be used by all research laboratories for the lipidomic analyses of liver samples from the whole array of existing and newly developed experimental models for liver diseases. The method is not restricted to steatosis and steatohepatitis, but should also be useful for the analysis of HCC samples. While the gold standard for lipidomic analyses, i.e., lipid chromatography-mass spectrometry is a quite expensive method, the thin layer chromatographic method can also be used in laboratories, which have no access to respective high-end equipment. The p62 transgenic mouse model might be a potentially interesting model to investigate mechanisms of steatosis and disease progression. Further characterization and correlation to human data might help to understand the role of lipid changes in pathogenesis.
Terminology
Non alcoholic fatty liver disease: non alcoholic fatty liver disease is characterized by a strong accumulation of lipids, especially triglycerides, within hepatocytes; non alcoholic steatohepatitis: non alcoholic steatohepatitis is a steatotic liver, which is characterized by an inflammatory environment and might result in fibrosis; hepatocellular carcinoma: hepatocellular carcinoma is an aggressive form of liver cancer; thin layer chromatography: thin layer chromatography is a chromatographic method, glass or aluminium plates are coated mostly with silica gel and allows separation with different solvent systems.
Peer review
This study seems well done and well written about a subject of increasing relevance and judge that it deserves to be published.