Published online Dec 27, 2012. doi: 10.4254/wjh.v4.i12.365
Revised: October 15, 2012
Accepted: November 14, 2012
Published online: December 27, 2012
AIM: To estimate the prevalence of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections in women in Mali and to evaluate the performance of serological assays.
METHODS: Two prospective studies were conducted in 2009 and 2010 in Mali. They concerned first, 1000 pregnant women attending six reference health centers in Bamako (Malian capital) between May 26 and June 16, 2009; and secondly, 231 women over 50 years who consulted general practitioners of two hospitals in Bamako between October 25 and December 24, 2010. Blood samples were collected and kept frozen in good condition before analysis. All samples depicted as positive using HIV/HCV enzyme immuno-assay screening assays were submitted to confirmation analysis. Molecular markers of HCV were characterized.
RESULTS: The seroprevalence of HIV and HCV in the population of pregnant women was 4.1% and 0.2% respectively. Among older women the seroprevalence was higher and similar for HIV and HCV (6.1% vs 6.5%). The anti-HIV prevalence was not different in young and older women (4.1% vs 6.1%). In contrast, the anti-HCV prevalence was higher in older compared to younger women (6.5% vs 0.2%, P < 0.01). Of 2 pregnant women who were HCV seropositive, only one was polymerase chain reaction (PCR) reactive and infected by genotype 2, with a viral load of 1600 IU/mL. Regarding older women who were HCV seropositive, 13 out of 15 were PCR reactive, infected by genotype 1 or 2. Globally HCV genotype 2 was predominant. The positive predictive value (PPV) measured with VIKIA HIV test in young women was 100% therefore significantly higher than the 87.5% measured in older women (P < 0.05). Conversely, the PPV measured with Monolisa HCV assay in older women was 88.2% and higher than the 14.3% measured in younger women (P < 0.01).
CONCLUSION: Whereas HIV prevalence was similar in both subpopulations HCV was more frequent among older women (P < 0.01). The PPV of screening assays varied with the age of the subjects.
- Citation: Bouare N, Vaira D, Gothot A, Delwaide J, Bontems S, Seidel L, Gerard P, Gerard C. Prevalence of HIV and HCV infections in two populations of Malian women and serological assays performances. World J Hepatol 2012; 4(12): 365-373
- URL: https://www.wjgnet.com/1948-5182/full/v4/i12/365.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i12.365
Discovered in 1989 by Houghton and coworkers, the hepatitis C virus (HCV) is the leading cause of chronic hepatitis and cirrhosis in Europe and North America[1]. In these countries, before the introduction of preventive measures such as blood donor selection and screening of blood donations, blood transfusion was largely responsible for the transmission of HCV, and amounted for up to 1/3 of cases[1].
The estimation of HCV prevalence in the general population is imprecise because of the difficulty in collecting representative samples and the cost of such studies[1].
In 1999, WHO estimated that about 3% of the world population was infected with hepatitis C and that at least 170 million chronic carriers of the virus were at risk of complications of developing cirrhosis and hepatocellular carcinoma, including more than 5 million in Europe[1,2]. HCV transmission is mainly parenteral[3]. Vertical transmission (mother-to-child) of HCV is estimated to be less than 5%, but in case of human immunodeficiency virus (HIV) co-infection, the risk of mother-to-child transmission can reach 15% to 20%[4]. Similarly, HCV/HIV co-infection promotes the progression of hepatitis to cirrhosis[5]. In sub-Saharan Africa, the prevalence of HCV infection varies between 0.1% and 13.8 %[6].
As for Malian blood donors, HCV seroprevalence was reported at 3.30%[7]. In Mali, the seroprevalence of HCV is not well elucidated in the population of pregnant women, and even less in the general population. Molecular epidemiology of HCV is also unknown. In this country, while prevention and treatment programs are implemented for HIV, much remains to be done for viral hepatitis in general.
In the literature there are controversies about the performance of HCV enzyme immuno-assay (EIA) tests in sub-Saharan Africa. This has stimulated scientific interest to document HCV epidemiology as well as the performance of HCV EIA tests among women in Mali in order to guide public health decisions. According to UNAIDS epidemic update in 2007 an estimated 22.5 million (20.9-24.3 million) people living with HIV, or 68% of the world cases, are in sub-Saharan Africa[8]. West Africa, considered as the most populated region of this continent, has a postulated HIV prevalence between 1% and 5%[9]. This region is the epicenter of the HIV-2 epidemic[10].
HIV prevalence is 1.3% in the general population in Mali (EDSM-IV 2006, final report 2007)[11]. In Mali, to prevent HIV mother to child transmission few rapid tests exist for the screening of pregnant women who attend health centers for antenatal care. The VIKIA HIV 1/2 3rd generation test (BioMerieux) is both highly sensitive and specific, does not require complex equipment and therefore could facilitate HIV testing especially in poorer areas[12,13]. This prompted us to evaluate the effectiveness of this test in Malian women.
Our study was conducted in Bamako (Malian capital). Mali is a country located in West Africa which borders with Ivory Cost and Guinea Conakry to the South, Mauritania and Senegal to the West, Algeria to the North, Niger to the East and Burkina Faso to the South-East (Figure 1). Mali extends to 1 240 192 square kilometers and comprises a population estimated at 13 415 205 inhabitants, among which 8 649 035 live in rural areas and 4 766 170 in urban areas[14]. The choice of Bamako was warranted by the fact that this city achieved the maximum coverage rate of antenatal care (ANC) between 2006 and 2007, estimated at 85%-90%, with an ANC assiduity index at 2.12 to 2.21[15].
To estimate and compare the prevalence of both infections and evaluate HCV EIA performance, we undertook a study in two populations of women in Mali: (1) Pregnant women (or young women): this population, although exclusively female, is fairly representative of the general population because the patients are not selected on specific risk factors and are supposedly healthy[16]. Furthermore, these women can be easily contacted and represent the vector of the major modes of HIV transmission (sexual and vertical); (2) Women over 50 years attending general practice in two hospitals in Bamako: this second population is likely to be better informed on the epidemiology of HCV, as suggested by Ndong-Atome et al[17] who described an association between age and HCV seroprevalence.
Initially, this was a prospective study including pregnant women attending ANC in the six Health Centers of Reference in Bamako. Exclusion criteria were: refusal to participate in the study and treatment with heparin (an inhibitor of PCR). In a second step, the study was extended to women over 50 years attending general practice in two hospitals (CHU Gabriel Touré, and Hospital Mother and Child) in Bamako. The exclusion criteria were refusal, any physical or mental condition precluding investigation as well as treatment by heparin.
This study has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The study was approved ethically under the reference number 08-0006/INRSP-CE (Ethical Committee of National Institute of Public Health) of Mali. All participating subjects remained anonymous and gave voluntary informed consent.
Standard operating procedures were issued to control blood collection, preservation and transport. Physicians, researchers, midwives and laboratory technicians were trained in the application of procedures. Sample transport by drivers was supervised.
A sample of 15 mL of venous blood was collected in vacutainer tubes [(dry and ethylenediaminetetraacetic acid (EDTA)] from all pregnant women in the study. From these primary samples serum and plasma were separated by refrigerated centrifugation (5 °C) at 1500 rpm for 10 min. For women > 50 years, the samples were taken only in EDTA vacutainer tubes (10 mL/participant). The plasma was separated by centrifugation at 5000 rpm for maximum 5 min at room temperature. A delay of maximum 6 h between blood collection and freezing (-20 °C to -80 °C) was always respected by the strategy put in place to ensure the quality of the collection samples. A large number of aliquots (6 × 1 mL/pregnant woman; 4 × 1 mL/older woman) were harvested before analysis in order to avoid multiple freeze-thaw cycles.
All assays were performed according to the manufacturer’s instructions. The samples were analyzed after a single thaw. All tests were performed according to procedures accredited to EN15189 norm in the AIDS Reference Laboratory of the CHU-ULg.
Screening and confirmation of HIV infection: The rapid test VIKIA HIV1/2 (BioMérieux), a 3rd generation assay based on the principle of immunochromatography (ICT or lateral flow), known for its sensitivity and specificity[12] was used as first line test.
The INNO-LIA HIVI/II score based on the line immuno assay (LIA) principle was used to confirm positive results obtained with the first-line tests and to distinguish HIV-1 from HIV-2 infections (Innogenetics Gent, Belgium). The undetermined samples were tested with VIDAS DUO assay to search for HIV p24 Ag.
Screening and confirmation of HCV infection: (1) First-line test. For pregnant women, two EIA tests were used: Innotest HCV Ab IV (Innogenetics, Belgium), which detects HCV antibodies; Monolisa HCV Ag/Ab Ultra (Biorad, Belgium), which detects simultaneously HCV antibody and antigen. For women over 50 years, the combined test (Monolisa) was solely used as a screening test. Samples found positive in a first run were further analyzed in two additional replicates. Samples giving at least two positive replicates (test ratio ≥ 1) out of three with the screening test were considered “positive”. Samples which were positive in the first run but whose result was not reproduced at least once were considered “negative”; and (2) Second-line serological assay. INNO-LIA HCV (Innogenetics) was used to confirm samples found positive with the screening test. All series of tests were validated by a specific internal quality control material (Pelispy, Westburg). The test ratio value of internal quality control was encoded in MedLab QC software for run validation.
For pregnant women: HCV RNA was assayed using qualitative PCR (COBAS AMPLICOR hepatitis C, Roche Laboratory). Samples containing HCV RNA were further analyzed by quantitative PCR (COBAS AMPLICOR HCV MONITOR, Roche Laboratory).
For women over 50 years: HCV RNA was quantified using HCV m2000 Real Time PCR kit (Abbott) which became available for this part of the study. All samples tested positive by the combined test (Monolisa) but further found LIA negative, thus potentially indicative of very early infections, were submitted to PCR analysis. The infecting genotype was defined by the use of Versant HCV Genotype Assay test LiPA of Siemens Healthcare Diagnostics.
Results are presented as mean ± SD (range) for continuous variables and as frequencies (%) for categorical variables. Comparisons of categorical variables between groups were done using a χ2 test. Positive predictive values (PPV) were calculated in each group. Results were considered to be significant at the 5% level (P < 0.05). Calculations were done using SAS version 9.3 for Windows (SAS Institute, Cary, NC, United States).
In the pregnant women population (n = 1000), age ranged from 14 to 50 years; with a mean of 25.2 ± 6.3 years.
Prevalence of HIV: Out of 1000 pregnant women tested, 41 were confirmed HIV seropositive among 45 subjects with a positive screening assay; the rate of indeterminate results (VIKIA+, LIA Ind) was 8.9% (4/45). A unique profile was found for these 4 indeterminate results due to an isolated gp41 band with intensity between 0.5+ and 2+. These 4 undetermined were tested negative with VIDAS DUO. The rate of false positive results (VIKIA+, LIA-) was 0%. The HIV seroprevalence in this population was 4.1% (95%CI: 2.90%-5.30%). Among the confirmed positive samples, HIV-1 species represented 95.1% (39 HIV-1 and 2 HIV-2).
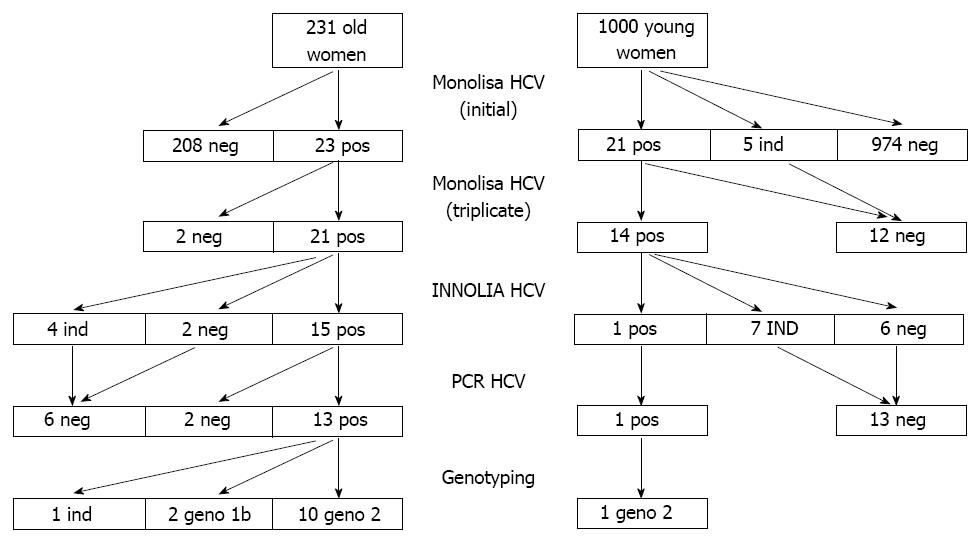
Prevalence of HCV: Two pregnant women out of 1000 (Table 1) were confirmed HCV positive, with one single proven case of active infection (HCV-RNA pos, 1600 IU/mL, genotype 2). The rates of samples found reproducibly positive with HCV EIA tests but confirmed negative by LIA (false positives) did not differ for INNOTEST (25/45; 55.6%) or MONOLISA (6/14; 42.9%) (P > 0.05).
| Subject | MONOLISA-HCV | INNOTEST-HCV | LIA-HCV | LIA-HCV | PCR HCV | Genotype HCV | |||||||||
| (test ratio value) | (test ratio value) | (reactivity score) | |||||||||||||
| X1 | X2 | X3 | X1 | X2 | X3 | C1 | C2 | E2 | NS3 | NS4 | NS5 | ||||
| AD3 | 5.84 | 6.12 | 6.08 | 5.50 | 5.71 | 5.58 | 4 | 1 | 0 | 3 | 2 | 0 | Pos | Pos | 2 |
| OM4 | 0.84 | 0.28 | 0.27 | 3.32 | 2.54 | 2.38 | 1 | 0 | 0 | 0 | 1 | 0 | Pos | Neg | - |
The OM4 sample giving an initial discrepant result between the two tests EIA HCV (Monolisa- Innotest+), was confirmed positive by LIA analysis (but with only two bands of weak intensity) but negative by PCR. The same sample analyzed in triplicates was found repeatedly negative with the Monolisa test. Based on the results of HCV LIA, the HCV seroprevalence measured in this population of young Malian women was very low: 0.2% (95%CI: 0.0%-0.4%).
In the 231 older women, ages ranged from 51 to 89 years; with a mean of 62.1 ± 8.6 years.
Prevalence of HIV: Out of 231 subjects, 14 were confirmed HIV seropositive among 19 found positive with the VIKIA test. The rate of indeterminate results (VIKIA+, LIA Ind) was 15.8%, i.e., 3 samples out of 19. Among these 3 samples, one was found with an isolated HIV1 gp41 band (at an intensity of 2+) and an isolated HIV2 gp36 band (at an intensity of 1+); in the 2 others, an isolated HIV1 gp41 band was detected at an intensity of 1+ and 2+, respectively. The rate of false positive results (VIKIA+, LIA-) was 10.5% (2/19). All indeterminate (3/3) and false positive (2/2) samples tested negative with VIDAS DUO HIV assay.
HIV seroprevalence in this population was 6.06% (95%CI: 2.96%-9.16%). Among the confirmed positive samples, HIV-1 species represented 64.3% (9 HIV-1 and 5 HIV-2). There was significantly more HIV-2 in women > 50 years (5/14) than in young females (2/41), P < 0.01.
Prevalence of HCV: Out of 231 subjects, 14 were confirmed seropositive out of 21 found reproducibly positive with the Monolisa assay. One sample showing an isolated HCV NS3 band (with an intensity of 4+) was found PCR positive. Taking this sample into account, the HCV seroprevalence measured in this population of older women was 6.49% (15/231) (95%CI: 3.31%-9.67%).
Viral HCV-RNA was detected in 13/15 seropositive women. Among them, 12 cases of active infection with genotypes 1 or 2 and viral loads ranging from 20 503 to 12 352 743 IU/mL were recorded. In one case, the viral load was < 12 IU/mL. Infection with HCV genotype 2 was much more represented, i.e., 10/12 (83.3%).
In this older population the rate of HCV false positives by ELISA was 2/21 (9.5%). This rate was significantly different from that of 6/14 (42.9%) measured in the younger population (P < 0.05). The HCV test algorithm and main results are depicted in Figure 2.
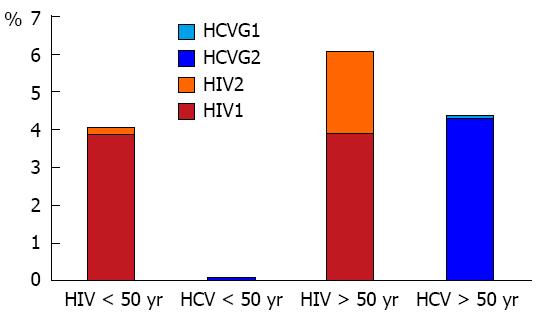
Considering the overall population of both young and older women, the estimated seroprevalences were 4.5% and 1.3% for HIV and HCV, respectively. The rate of active HCV infection was 1.1%.
Whereas the prevalence of HIV infection was not significantly different in the two series, the HCV seroprevalence was significantly higher in older women as was also the prevalence of HCV-RNA (P < 0.01). Among 13 HCV genotypes identified at all, there were 11 HCV genotype 2 (Table 2).
| Series | n | HIV Ab | HCV Ab | PCR-HCV | HCV genotype | ||
| % | % | % | 1 | 2 | other | ||
| Series 1 | 1000 | 4.10 | 0.20 | 0.10 | 0 | 1 | 0 |
| W ≤ 50 yr old | |||||||
| Series 2 | 231 | 6.06 | 6.49 | 5.60 | 2 | 10 | 0 |
| W > 50 yr old | |||||||
In young women, HIV infection was more common than HCV infection while HCV infection was more frequent in older women. The proportion of HIV1 was equal in both subpopulations, but HIV2 was more common in older women compared to pregnant ones. HCV genotype 2 was more frequent in older women (Figure 3).
Regardless of the two series, no HIV/HCV co-infection was detected but in each population, one case of “co-indeterminate” HCV/HIV results was found. The serum of a young woman initially depicted as doubtful and then negative using VIKIA test was analysed with LIA HIV for confirmation. It showed a co-indetermination with an isolated p17 HIV band (at an intensity of 2+) and an isolated band of low intensity (1+) for the HCV C1 protein. The sample of this young woman was found HIV negative with VIDAS DUO test. In the older women series, one sample showed an isolated NS3 HCV band with low intensity (0.5+) and an isolated gp41 HIV band (with an intensity of 2+).
Predictive value of VIKIA screening test for HIV: In the overall series of 1231 samples, 64 were found positive with the screening test: 55 were confirmed positive, 7 indeterminate and 2 negative giving a PPV of 96.5%.
Comparing the two series, the PPV measured with VIKIA HIV1/2 in young women (i.e., 100%) was significantly higher than that of 87.5% measured in older women (P < 0.05). However, when indeterminate results were included in the analysis, the PPV measured with VIKIA test in young women (i.e., 91%) was not different than the PPV of 73.7% found in the older cohort.
Predictive value of screening tests for HCV: Indeterminate results of LIA and/or PCR HCV were not considered for the calculation of predictive values. (1) Positive predictive value of Monolisa HCV test. In the whole series of 1231 samples, 35 were found reproducibly positive: 16 were confirmed seropositive by the LIA-HCV assay, 11 were indeterminate and 8 were confirmed negative, giving a PPV of 66.7% for the screening assay. However, the PPV (88.2%) measured with the Monolisa HCV test in women > 50 years old was significantly higher than that of 14.3% measured in young women (P < 0.01); (2) Predictive value of test ratio (TR) results measured with the HCV Monolisa test (Table 3). Using the Monolisa test, when the TR was found to be between 1 and 3 (i.e., TR ≤ 50% TRmax), the rate of positive confirmation was 1/9 with LIA-HCV, and 0/13 with PCR. By contrast, when the TR of the Monolisa test was greater than 3 (TR > 50% TRmax), the probability of positive confirmation was 15/16 with the LIA (P < 0.01) and 14/21 with PCR (P < 0.01, compared to lesser TR).
Predictive value of LIA-HCV bands: Out of 17 LIA positive profiles, NS3 and C1 bands were clearly predominant in 16/17 (94.1%); C2 and NS4 were represented in 15/17 (88.2%) and 8/17 (47.1%) respectively, whereas E2 and NS5 were poorly represented in 3/17 samples (17.6%).
The intensity of the most represented band, NS3, was analyzed with regard to its predictive value of a positive PCR result. There was an obvious association between the intensity of the NS3 HCV band and HCV viraemia. This association was highly significant when the NS3 intensity was ≥ 3 (P < 0.01). Similarly, there was a significant association between the viraemia of HCV and the coexistence of both C1 and NS3 bands, when the intensity was > 2 (P < 0.01). On the contrary, there was no significant association between the intensity of the C1 band and HCV viraemia (P > 0.05).
Sample No. 6 (Table 4) had a TR > 5 with two EIA tests (Monolisa HCV and Innotest HCV) and an isolated NS3 band (at 4+ intensity), and therefore was classified as indeterminate according to the manufacturers’ recommendations; it was later found to be positive for PCR-HCV-RNA. This result supports the hypothesis that any reactivity in an isolated NS3 band may be indicative of an HCV seroconversion[18].
| No. | HCV LIA profile (bands’ intensity) | PCR HCV | Viral load (IU/mL) | |||||
| C1 | C2 | E2 | NS3 | NS4 | NS5 | |||
| 1 | 4 | 3 | 0 | 4 | 1 | 0 | pos | 32 350 |
| 2 | 3 | 1 | 0 | 4 | 0 | 0 | pos | 1 442 298 |
| 3 | 1 | 1 | 0 | 0.5 | 0 | 0 | neg | Not detected |
| 4 | 4 | 2 | 0 | 4 | 0 | 0 | pos | 2 701 467 |
| 5 | 4 | 3 | 0 | 4 | 0.5 | 0.5 | pos | 3 247 624 |
| 6 | 0 | 0 | 0 | 4 | 0 | 0 | pos | 290 103 |
| 7 | 4 | 2 | 0 | 4 | 3 | 0 | pos | 98 440 |
| 8 | 4 | 4 | 0 | 4 | 0 | 0 | pos | 20 503 |
| 9 | 3 | 2 | 1 | 4 | 0 | 0 | pos | 683 959 |
| 10 | 3 | 1 | 0 | 2 | 0 | 0 | neg | Not detected |
| 11 | 3 | 1 | 0 | 4 | 0 | 2 | pos | 6 369 090 |
| 12 | 4 | 2 | 0 | 4 | 1 | 3 | pos | 831 422 |
| 13 | 4 | 3 | 2 | 4 | 0 | 0 | pos | 12 352 743 |
| 14 | 3 | 1 | 0 | 4 | 0.5 | 0 | pos | 4 019 864 |
| 15 | 2 | 3 | 4 | 4 | 4 | 0 | pos | < 12 |
| 16 | 4 | 1 | 0 | 3 | 2 | 0 | pos | 1600 |
| 17 | 1 | 0 | 0 | 0 | 1 | 0 | neg | Not detected |
| BP | 16 | 15 | 3 | 16 | 8 | 3 | ||
The HIV seroprevalence measured in these populations of young (4.1%) and older (6.1%) women was of the same magnitude. The overall HIV seroprevalence of 4.5% estimated by our study in women is higher than 1.3% that was notified for the general population of Mali[11]. Of note, the rate of HIV infection observed in our study is in the range of prevalences (from 1% to 5%) that was reported in West Africa[9]. It is important to stress that our study concerned exclusively females living mainly in urban areas. According to the EDSM-IV report, the proportion of men and women positive for HIV-2 and whose age varies between 15 and 49 years is low (0.2%) indicating that HIV-1 predominates over HIV-2[11]. Our study confirms this observation and further indicates that there is significantly more HIV-2 in women > 50 years (5/14) than in young females (2/41), (P < 0.01). The HIV prevalence rate of 4.1%, measured in pregnant women in this study is similar to that of 7.9% (18/288) obtained in a population of pregnant women, in the neighboring country of Burkina Faso[19]. Regarding the predictive positive values measured with VIKIA HIV-1/2, our study shows that this test is more efficient in Malian young women compared to older ones (100% vs 85.7%; P < 0.05). The rate of false positive HIV results (VIKIA pos; LIA neg) in the two subpopulations, (0% in young women vs 10.5% in older ones) may explain this difference.
Our study is the first to determine molecular HCV epidemiology and to evaluate the performance of EIA HCV tests in Mali. HCV seroprevalence was 3.3% among Malian blood donors[7]. That frequency is lower than that of 6.5% measured in our study in women over 50 years, but remains well above that of 0.2% measured in young women.
The HCV prevalence rates of 0.2% and 6.5% measured respectively in young and older women in our study are in the range of prevalences (0.1%-13.8%) documented in sub-Saharan Africa[6]. Similarly, the overall prevalence of HCV (1.3%) measured in the present study in women is in the range of prevalence (1.1%-5.5%) reported in six West African countries[6]. That prevalence could be extrapolated to the general Malian population. However, the population of our study is essentially urban. It would be interesting to conduct an additional study in rural areas of Mali to confirm the quality of our data.
The prevalence of 0.2% HCV as measured by our study in pregnant women is of the same order of magnitude than the prevalences of 0.14% and 0.18% respectively reported in previous studies[16,20]. In our study, only one pregnant woman (AD3) of two HCV seropositives was found PCR positive (HCV-RNA+) and infected with genotype 2. In the other subject, OM4, the sample exhibited a discrepancy with the two tests (Innotest pos/Monolisa neg). This woman, OM4, tested negative twice by PCR on two separate samples collected at an interval of 11 mo. This suggests either a very old HCV infection with virtual disappearance of specific antibodies, or false positive results of EIA and LIA due to aspecific antibodies. In older women HCV seroprevalence is 6.5%, therefore significantly higher than in younger females (0.2%, P < 0.01). The significant variation of anti-HCV prevalence according to the age of women (> 50 years vs younger), 6.5% vs 0.2%, may suggest the hypothesis that exposure of women > 50 years to HCV infection is usually an old one. Accordingly, the prevalence of HCV-RNA was 5.6% vs 0.1 % in the two groups.
The proportion of HCV positive women was significantly higher in rural areas (6/91) compared to urban residence (11/1125, P < 0.01). That would also explain the discrepancy between the HCV seroprevalence of 0.2% in young women of whom only 2.63% came from rural areas and 6.5% observed in older women of whom 29.13% came from rural areas. The distribution of HCV genotypes 1 and 2 revealed in Mali by our study is similar to that reported in West Africa[21-23]. Our study reveals the predominance of HCV genotype 2 (11/13 or 84.6%) known to be easier to treat than genotype 1. HCV prevalence measured in our study in young women (0.2%) is close to data obtained in pregnant women in two countries of West Africa namely Niger (0%) and Benin (0.7%)[24].
As far as the performance of third-generation HCV EIA tests in the African region are concerned, there are quite controversial opinions in the literature. Indeed, some studies report poor performance of EIA tests for third and even the fourth generation[20,21,25-28]. Conversely, others have observed good performance using these tests[29-38]. In the present study, our data indicate the poor performance of EIA tests both in terms of predictive positive value (14.3% vs 7.4%) and in terms of specificity (42.9% vs 55.6) for false positive results obtained using both Monolisa (mixed test Ag-Ab) and Innotest (Ab test only), in the population of pregnant women where the prevalence of HCV is particularly low. Of note, the positive predictive values as well as the rates of false positive reactions were not significantly different using either of the two tests.
On the other hand, our observations confirm the better performance of Monolisa test used as a screening test (with 2/21 or 9.5% false positive; PPV at 88.2%) in the population of women over 50 years where the prevalence was higher.
The rates of false positive samples measured with the Monolisa test in the two populations (42.9% vs 9.5%) were significantly different (P < 0.05). Equally the positive predictive values of the Monolisa test were significantly different in the 2 populations (14.3% vs 88.2%; P < 0.01).
We also observed EIA false positive reactions which could be related to a cross-reaction by antibodies caused by poly-immunization against bacterias or parasites[39,40].
As far as the tests performance are concerned, our results suggest that for TR values > 3 (i.e., TR > 50% TRmax), the repetition of the EIA test and confirmation by the INNO-LIA HCV test are not required to anticipate a confirmed HCV infection (PPV at 93.8%; P < 0.01) and positive viraemia (PPV at 66.7%; P < 0.01).
Taking into account the 2 subpopulations separately, when TR is in the range of 1 to 3 (i.e., TR ≤ 50% TRmax), there is not a significant difference between the PPV of TR measured with LIA HCV (0% vs 33.33%) or PCR (0% vs 0%) in populations of young and older women. In contrast, when TR > 50% TRmax, the PPV of test ratio measured in older women with LIA HCV (100%) is significantly higher than that measured in younger women (50%, P < 0.01). According to PCR analysis, the PPV of TR measured in older women (92.9%) is significantly higher than 14.3% calculated in pregnant women (P < 0.01). This supports the recommendation that the patient’s age should be taken into account in the interpretation algorithm.
Our data confirm the findings of other authors who reported a high degree of accuracy between anti-HCV TR and HCV viraemia[38,41].
In all samples analyzed, no HIV/HCV co-infection was detected but in each population we observed one case of HIV/HCV co-indetermination, with the following LIA profiles: p17 (2+)/C1 (1+) and gp41 (2+)/NS3 (0.5+).
In summary, we have observed a high prevalence of HIV infection in both subpopulations of pregnant women and women of more than 50 years old. We have also reported a high prevalence of HCV 6.5% in older women. Our data indicate a good efficiency of VIKIA HIV 1/2 (BioMérieux) in pregnant women and a high performance of Monolisa HCV Ag/Ab Ultra (Biorad) in older women. In addition we have proposed an interpretation algorithm for the clinical diagnosis of HCV infection in Mali. This algorithm could be useful in the West African population. Furthermore we have reported the importance of INNO-LIA HCV test in an African population since it may detect an earlier HCV seroconversion.
Our data stress the importance of both infections in Mali, and the need to organize the management of viral hepatitis in general in Mali together with the endemic HBV infection at a prevalence of 14.7% based on HBsAg assays[42].
We are grateful to the Belgium government through the Belgian technical cooperation for sponsoring of Nouhoum Bouare’s scholarship, and the Malian government through the Professional Training and Employment Department, International Cooperation Department. We would like to warmly thank the Department of Health of Mali, Biomerieux, Biorad and Innogenetics generously supported our research project. We thank the partners and collaborators at sample collection sites (Reference Health Centers, CHU Gabriel Toure and Mother Child Hospital of Bamako) for their collaboration. We also thank Warling C, Susin F, Puglisi A, Meganck J, Olivera G, Bougoudogo F, Maiga M, Koita O, Traore S, Diarra S, Bouare M, Feu Sissoko H, Doumbia B, Dembele G, Keita M, Bagayoko D, Bagayogo M and Sanogo A, for their expert collaboration.
In Sub-Saharan Africa, the prevalence of hepatitis C virus (HCV) varies between 0.1% and 13.8 %. In Mali, HCV seroprevalence is estimated at 3.3% in the population of Malian blood donors, but is not known accurately in the population of pregnant women, and even less in the general population. Furthermore the molecular epidemiology of HCV is unknown. As for human immunodeficiency virus (HIV), the seroprevalence is 1.3% in the general population. To prevent HIV vertical transmission, the screening of infection in pregnant women is undertaken with few rapid tests in health centers providing antenatal care. The VIKIA HIV 1/2 3rd generation test (BioMerieux) is known to be highly sensitive and specific, and does not require complex instrumentation. It is therefore well suited to facilitate HIV testing, especially in poorer areas where technical facilities are unavailable.
As for the performance of the third-generation HCV enzyme immuno-assay (EIA) tests (EIA3) in the African region, there are quite controversial reports in published studies. In the present work, the authors demonstrate that the specificity is high in women over 50 years old, but much lower in the pregnant women cohort. Indeed, when test ratio (TR) > 50% TRmax, the probability for EIA3 HCV test to give a confirmed antibody reaction is 2 times higher in older women than in younger ones. The probability of a confirmed positive polymerase chain reaction (PCR) reaction (i.e., to detect HCV-RNA) is 6 times higher in older women than in younger ones.
To reduce HCV-EIA false positive results, the World Health Organization as well as other authors recommend replicating tests by using two different serological assays. In the present study, the authors propose a new interpretation algorithm which balances effectiveness and cost, based on the signal/cut-off ratio, and applicable in Mali to the general population. This algorithm may be generalized for use in other West African countries. As for the interpretation of INNO-LIA HCV assays, a single reactive NS3 band may be indicative of seroconversion. Such samples will be classified as indeterminate as per manufacturer’s instructions. In this case, it is recommended to test a sample from the same patient drawn a few weeks later. In this study, the authors demonstrated that the intensity of single NS3 bands higher than 3 may be indicative of viraemia. Indeed, in one sample of our study with an isolated NS3 band at 4+ intensity, the patient was later found positive by PCR analysis, thus indicating earlier seroconversion and active HCV infection.
The overall prevalence of HCV (1.3%) measured in our study in women is within the range of data (from 1.1% to 5.5%) collected in six West African countries. The 1.3% prevalence may be extrapolated to the Malian general population. However, since our population under study was essentially urban, an additional cohort in rural areas of Mali would be useful to confirm the estimations.
EIA3: The detection of anti-HCV antibodies in plasma or serum is based on the use of third-generation EIA, that detects mixtures of antibodies directed against various HCV epitopes. Recombinant antigens are used to capture circulating anti-HCV antibodies onto the wells of microtiter plates, microbeads, or specific holders adapted to closed automated devices. The presence of anti-HCV antibodies is revealed by anti-antibodies labeled with an enzyme that catalyzes the transformation of a substrate into a colored compound. The optical density (OD) ratio of the reaction (sample OD/internal control OD) is proportional to the amount of antibodies in the serum or plasma sample; Predictive values: The positive predictive value is the probability that when the test is reactive, the specimen does contain antibody to HCV. This may be calculated by using the simple formula: true positives/(true positives + false positives) which will give an approximate value; INNO-LIA™ HCV score: Based on the principle of an enzyme immunoassay. A sample is incubated in a compartment with a strip. The anti-HCV antibodies possibly present in the sample will bind to the HCV antigen bands fixed to the strip. Then conjugated goat anti-human immunoglobulin G (H + L) coupled to alkaline phosphatase is added and binds to the antigen/antibody complex formed previously. Incubation with enzyme substrate produces a brown color whose intensity is proportional to the amount of specific antibodies to HCV-related antigen bands. The color development is stopped by adding sulfuric acid.
The manuscript is interesting, well done and well written.
Peer reviewer: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan, Fabio, Italy
S- Editor Li JY L- Editor Hughes D E- Editor Li JY
| 1. | Trépo C, Merle P, Zoulim F. Hépatites virales B et C. Collection Pathologie, sciences, formation. Paris: Edition Jonh Libbey Eurotext 2006; 1-246. [Cited in This Article: ] |
| 2. | OMS. Hépatite C: Prévalence mondiale. Relevéépidémiologique hebdomadaire N° 49, 10 décembre 1999, OMS. WHO information Aide-mémoire N° 164 Révisé Octobre 2000. Available from: http//www.who.int. [Cited in This Article: ] |
| 3. | Delwaide J, Bourgeois N, Colle I, Robaeys G. Risk factors for hepatitis C: past, present and future. Acta Gastroenterol Belg. 2002;65:87-89. [PubMed] [Cited in This Article: ] |
| 4. | Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, Butler K. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907. [PubMed] [Cited in This Article: ] |
| 5. | Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054-1058. [PubMed] [Cited in This Article: ] |
| 6. | Madhava V, Burgess C, Drucker E. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. Lancet Infect Dis. 2002;2:293-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Diarra A, Kouriba B, Baby M, Murphy E, Lefrere JJ. HIV, HCV, HBV and syphilis rate of positive donations among blood donations in Mali: lower rates among volunteer blood donors. Transfus Clin Biol. 2009;16:444-447. [PubMed] [Cited in This Article: ] |
| 8. | AIDS epidemic update (December 2007). UNAIDS/WHO, 2007: 1-55. Available from: http://www.un-ngls.org/spip.php?page=article_s&id_article=383ref. [Cited in This Article: ] |
| 9. | Available from: http: //www.unaids.org/en/dataanalysis/knowyourepidemic/epidemiologypublications/2008reportontheglobalaidsepidemic. [Cited in This Article: ] |
| 10. | Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, Fruteau C, Noë R, Peeters M, Brookfield JF. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol. 2005;79:12515-12527. [PubMed] [Cited in This Article: ] |
| 11. | Enquête Démographique et de Santé du Mali. 2006. Available from: http//www.measuredhs.com/pubs/pdf/FR199/FR199.pdf. [Cited in This Article: ] |
| 12. | Piche J, Morgand J, Revol F, Lacoux X, Rincon M, Leportier M. CDB0075-Development of a new HIV immunochromatographic assay for the rapid detection of Human Immunodeficiency Virus antibodies. Proceedings of XVI International AIDS Conference; 2006 Aug 13-18; Toronto, Canada. . [Cited in This Article: ] |
| 13. | Plantier JC, Maniez M, Barin F, Kreplak G, Diagbouga PS, Cortes-Martins H, Menan H, Piche J. Evaluation of a new rapid test: VIKIA® HIV 1/2. Poster Presentation AIDS. 2008;THPE 0053. [Cited in This Article: ] |
| 14. | Farvacque-Vitkovic C, Casalis A, Mah D, Eghoff C. Developpement of the cities of Mali. Challenges and Priorities. World Bank Africa Region Working Paper, number serie 104/a. 2007;1-1 Available from: http//www.worldbank.org/afr/wps/wp104_english.pdf. [Cited in This Article: ] |
| 15. | Annuaire Système Local d’Information Sanitaire du Mali. 2007;1-117 Available from: http://www.sante.gov.ml/docs/pdf/slis2007.pdf. [Cited in This Article: ] |
| 16. | Faulques B, Michault A, Sevidjian B, Barau G, Pawlotsky JM, Dhumeaux D. Prevalence of hepatitis C virus infection in pregnant woman on the island of Réunion. Gastroenterol Clin Biol. 1999;23:355-358. [PubMed] [Cited in This Article: ] |
| 17. | Ndong-Atome GR, Makuwa M, Njouom R, Branger M, Brun-Vézinet F, Mahé A, Rousset D, Kazanji M. Hepatitis C virus prevalence and genetic diversity among pregnant women in Gabon, central Africa. BMC Infect Dis. 2008;8:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | INNO-LIA™ HCV Score. B30068 v4. Innogenetics. 2010;1-12. [Cited in This Article: ] |
| 19. | Ilboudo D, Sawadogo A, Simpore J. Hepatitis C and HIV co-infection in pregnant women, Ouagadougou (Burkina Faso). Editions scientifiques et médicales Elsevier SAS Médecine et maladies infectieuses. 2003;33:276-279. [DOI] [Cited in This Article: ] |
| 20. | Abid S, Fkih S, Khlass B, Cherif W, Toumi NH, Jenhani F, Boukef K. Screening and confirmation of anti-HCV antibodies in Tunisian blood donors. Transfus Clin Biol. 1997;4:221-226. [PubMed] [Cited in This Article: ] |
| 21. | Serme AK, Ilboudo PD, Samandoulgou A, Simpore J, Bougouma A, Sombie AR. Portage du virus de l’hépatite C chez les femmes enceintes et transmission mère-enfant à Ouagadougou, Burkina Faso. Santé publique: courte note n°2764, juin 2005. Bull Soc Pathol Exot. 2006;99:108-109. [Cited in This Article: ] |
| 22. | Jeannel D, Fretz C, Traore Y, Kohdjo N, Bigot A, Pê Gamy E, Jourdan G, Kourouma K, Maertens G, Fumoux F. Evidence for high genetic diversity and long-term endemicity of hepatitis C virus genotypes 1 and 2 in West Africa. J Med Virol. 1998;55:92-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 23. | Ruggieri A, Argentini C, Kouruma F, Chionne P, D'Ugo E, Spada E, Dettori S, Sabbatani S, Rapicetta M. Heterogeneity of hepatitis C virus genotype 2 variants in West Central Africa (Guinea Conakry). J Gen Virol. 1996;77:2073-2076. [PubMed] [Cited in This Article: ] |
| 24. | Nicot T, Rogez S, Denis F. Epidémiologie de l’hépatite C en Afrique. Gastroenterol Clin Biol. 1997;21:596-606. [Cited in This Article: ] |
| 25. | Hladik W, Kataaha P, Mermin J, Purdy M, Otekat G, Lackritz E, Alter MJ, Downing R. Prevalence and screening costs of hepatitis C virus among Ugandan blood donors. Trop Med Int Health. 2006;11:951-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Seremba E, Ocama P, Opio CK, Kagimu M, Thomas DL, Yuan HJ, Attar N, Lee WM. Poor performance of hepatitis C antibody tests in hospital patients in Uganda. J Med Virol. 2010;82:1371-1378. [PubMed] [Cited in This Article: ] |
| 27. | Benouda A, Boujdiya Z, Ahid S, Abouqal R, Adnaoui M. Prévalence de l’infection par le virus de l’hépatite-C au Maroc et évaluation des tests sérologiques de dépistage pour la prédiction de la virémie. Pathologie Biologie. 2009;57:368-372. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | O'Reilly JI, Ocama P, Opio CK, Alfred A, Paintsil E, Seremba E, Sofair AN. Risk Factors and Seroprevalence of Hepatitis C among Patients Hospitalized at Mulago Hospital, Uganda. J Trop Med. 2011;2011:598341. [PubMed] [Cited in This Article: ] |
| 29. | Rouet F, Chaix ML, Inwoley A, Msellati P, Viho I, Combe P, Leroy V, Dabis F, Rouzioux C. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Côte d'Ivoire: the ANRS 1236 study. J Med Virol. 2004;74:34-40. [PubMed] [Cited in This Article: ] |
| 30. | Chevaliez S, Pawlotsky JM. Hepatitis C virus serologic and virologic tests and clinical diagnosis of HCV-related liver disease. Int J Med Sci. 2006;3:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Chevaliez S, Pawlotsky JM. Hepatitis C virus: virology, diagnosis and management of antiviral therapy. World J Gastroenterol. 2007;13:2461-2466. [PubMed] [Cited in This Article: ] |
| 32. | Pawlotsky JM, Lonjon I, Hezode C, Raynard B, Darthuy F, Remire J, Soussy CJ, Dhumeaux D. What strategy should be used for diagnosis of hepatitis C virus infection in clinical laboratories? Hepatology. 1998;27:1700-1702. [PubMed] [Cited in This Article: ] |
| 33. | Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36:S65-S73. [PubMed] [Cited in This Article: ] |
| 34. | Vermeersch P, Van Ranst M, Lagrou K. Validation of a strategy for HCV antibody testing with two enzyme immunoassays in a routine clinical laboratory. J Clin Virol. 2008;42:394-398. [PubMed] [Cited in This Article: ] |
| 35. | Pirillo MF, Bassani L, Germinario EA, Mancini MG, Vyankandondera J, Okong P, Vella S, Giuliano M. Seroprevalence of hepatitis B and C viruses among HIV-infected pregnant women in Uganda and Rwanda. J Med Virol. 2007;79:1797-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Ndjomou J, Kupfer B, Kochan B, Zekeng L, Kaptue L, Matz B. Hepatitis C virus infection and genotypes among human immunodeficiency virus high-risk groups in Cameroon. J Med Virol. 2002;66:179-186. [PubMed] [Cited in This Article: ] |
| 37. | Cao J, Chen Q, Zhang H, Qi P, Liu C, Yang X, Wang N, Qian B, Wang J, Jiang S. Novel evolved immunoglobulin (Ig)-binding molecules enhance the detection of IgM against hepatitis C virus. PLoS One. 2011;6:e18477. [PubMed] [Cited in This Article: ] |
| 38. | Masson S, Volle P, Bonneau J. Virus de l’hépatite C: relations entre la sérologie, le taux d’ALAT et la présence d’ARN circulant. Transfusion Clinique et Biologique. 1998;5:S87-S88. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 39. | Aceti A, Taliani G, de Bac C, Sebastiani A. Anti-HCV false positivity in malaria. Lancet. 1990;336:1442-1443. [PubMed] [Cited in This Article: ] |
| 40. | Sonmez E, Ozerol IH, Senol M, Kizilkaya N, Sahin K, Ozbilge H. False-positive reaction between syphilis and hepatitis C infection. Isr J Med Sci. 1997;33:724-727. [PubMed] [Cited in This Article: ] |
| 41. | Seo YS, Jung ES, Kim JH, Jung YK, Kim JH, An H, Yim HJ, Yeon JE, Byun KS, Kim CD. Significance of anti-HCV signal-to-cutoff ratio in predicting hepatitis C viremia. Korean J Intern Med. 2009;24:302-308. [PubMed] [Cited in This Article: ] |
| 42. | Konaté A. Epidémiologie de l’infection par le virus de l’hépatite B en Afrique. DEVELOPPEMENT et SANTE; Information permanente des acteurs de santé. 2012;1 Available from: http://devsante.org/.../epidemiologie-de-linfection-par-le-virus-de-lhepatite. [Cited in This Article: ] |