Published online Nov 27, 2022. doi: 10.4254/wjh.v14.i11.1940
Peer-review started: September 18, 2022
First decision: November 5, 2022
Revised: November 9, 2022
Accepted: November 16, 2022
Article in press: November 16, 2022
Published online: November 27, 2022
Disparities have emerged as an important issue in many aspects of healthcare in developed countries and may be based on race, ethnicity, sex, geographical location, and socioeconomic status. For liver disease specifically, these potential disparities can affect access to care and outcome in viral hepatitis, chronic liver disease, and hepatocellular carcinoma. Shortages in hepatologists and medical providers versed in liver disease may amplify these disparities by compromising early detection of liver disease, surveillance for hepatocellular carcinoma, and prompt referral to subspecialists and transplant centers. In the United States, continued efforts have been made to address some of these disparities with better education of healthcare providers, use of telehealth to enhance access to spe
Core Tip: The liver is a uniquely complex system, the function of which can be difficult for patients to conceptualize. Given the complexity associated with managing liver disease, patients of different backgrounds can face significant disparity in care, which populations are at increased risk, and what factors cause these differences are not well understood. This review aims to summarize the disparities that exist in patients with liver disease and to bring attention to interventions that have been successful in reducing disparity among vulnerable populations.
- Citation: Sempokuya T, Warner J, Azawi M, Nogimura A, Wong LL. Current status of disparity in liver disease. World J Hepatol 2022; 14(11): 1940-1952
- URL: https://www.wjgnet.com/1948-5182/full/v14/i11/1940.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i11.1940
In 2017, an estimated 1.5 billion people worldwide had some type of chronic liver disease, and this increased by nearly 35% from the previous decade[1]. About 60% of this chronic liver disease was due to non-alcoholic fatty liver, while 38% was due to viral hepatitis and 2% due to alcohol-related liver disease. Both acute and chronic liver diseases are prevalent worldwide, but the specific etiology of liver disease has geographic variations based on underlying risk factors[2]. In general, viral hepatitis, especially hepatitis B virus (HBV) infections, is more common in Asian and African countries. Non-alcoholic fatty liver disease (NAFLD) is more common in North American and Latin American countries due to a high prevalence of obesity. In addition, underlying genetic factors, such as the Patatin-like phospholipase domain-containing protein 3 (PNPLA3) allele, may contribute to a different phenotype of liver disease[3].
However, the true prevalence of liver disease may be a function of the health care providers available to make these diagnoses. A study from the American Association for the Study of Liver Disease (AASLD)’s workforce study group reported a critical shortage of hepatology providers[4]. They identified about 8000 gastroenterologists, hepatologists, and advanced practice providers who had practices in which ≥ 50% of their time was spent in hepatology. Their modeling analysis predicted that by 2033, the United States would have 35% fewer hepatology providers than needed to care for the population. As the recognition and management of the liver disease can be complex and often require specialized care, patients with liver disease will be at risk of receiving suboptimal care based on accessibility to hepatologists. This review will discuss the studies to characterize the current status of disparities in liver disease and describe current efforts to minimize disparities.
Hepatitis A, B, and C viruses (HAV, HBV, and HCV, respectively) are the most common causes of viral hepatitis. Hepatitis Delta is less common and tends to occur as a coinfection with HBV. Hepatitis E, though less common than the others, is similar to HAV as it is transmitted via the fecal-oral route. HBV is primarily transmitted vertically, especially in Asian countries, but like HCV, this can be transmitted with direct blood contact from transfusions, intravenous drug use, other needle stick injuries, tattoos, or sexual contact. The incidence of these viruses varies based on geographic and socioeconomic factors, but there are also disparities in access to the prevention and treatment of these viruses. Outcome may also differ by race as Asians had higher HBV mortality while non-Hispanic Whites had increased HCV mortality despite the decrease in all other ethnic groups[5].
Prevention of these viruses can be accomplished with vaccination, proper screening of the blood supply for transfusion, minimizing food contamination and public education. Only HAV and HBV can be prevented with appropriate vaccines. Access to immunizations may be limited by geographic and socioeconomic factors, and populations affected by this disease prevention disparity may be at an increased risk for HBV infection. A descriptive epidemiologic study in rural China showed that HBV vaccination was more likely among those in higher income quintiles, higher education levels, and non-farm occupations[6]. A cross-sectional study from South Korea evaluated HBV vaccination rates among the homeless population, finding only 39.8% completed their HBV vaccination series[7]. In the United States (U.S.), other factors decrease the probability of vaccination, including the vaccine costs, service fees, travel costs, the time required to receive a vaccine, and older age. Overall, income was the single greatest variable affecting HBV vaccination inequality, with a relative weight of > 52%[8]. Advanced age, lower income, a lack of private insurance, and lower education levels have a marked negative influence on the completion of immunization[7]. Residing within an urban area was associated with a higher compliance rate with completing the vaccinations. Clearly, the availability of vaccinations alone is not enough to ensure universal protection by immunization.
Proper identification of patients with viral hepatitis may also depend on access to care. More than 80% of HBV in the U.S. were not diagnosed, and 40% of patients with HCV in the U.S. from 2015-2018 were unaware that they had this infection[9]. Currently, the Center for Disease Control (CDC) recommends a one-time HCV screening for all adults aged 18 or older and for all pregnant women during each pregnancy. A systematic review of 19 studies reported that screening for HCV in the general population was variable in terms of cost-effectiveness[10]. However, screening intravenous drug users, pregnant women, HIV-infected, and certain immigrant populations may be more consistently cost-effective. For HBV, the AASLD recommends screening of HBV for persons born in areas in which HBV is endemic, pregnant women, dialysis patients, or those with high-risk behavior, including intravenous drug use[11].
However, there are barriers to successful HBV screening in Asian and Pacific Islander (API) communities, including cultural beliefs of wellbeing, misinformation about HBV, and lack of access to culturally sensitive healthcare[8].
While community campaigns can promote screening for viral hepatitis, screening can only be truly effective if there is an appropriate linkage to care. Of the estimated 700000 people with HBV in the U.S., only 15% are aware of their diagnosis, and only 4.5% are receiving treatment[12]. While HCV infection is not preventable with vaccination, it is curable with direct-acting antiviral (DAA) medications. However, the high cost of these medications may have further exacerbated disparities in HCV care. In a large cohort of 29544 patients with HCV in 4 different health care systems, older age and enrollment in commercial insurance correlated with increased rates of HCV treatment[13]. Patients not receiving treatment had more comorbidities, including HIV, mental health disorders, and non-HCC cancers. Additionally, the Hispanic race and those with Medicare, Medicaid, and indigent care/no insurance were significantly less likely to receive treatment. Even after access to DAAs improved (after 2014), the treatment of chronic HCV remained low at < 20%, and patients who were Hispanic and with publicly funded or no insurance were less likely to receive treatment[13]. Jung et al[14] found socioeconomic disparities in prescribing patterns for DAA medications among Medicare patients. Schaeffer et al[15], in exploring low HCV treatment rates among underserved African Americans in San Francisco found that most of those not receiving treatment were lost to follow-up before eligibility was determined.
Other factors that affect the diagnosis and progression of viral hepatitis that may potentially be perceived as disparities include gender, genetic factors, diet, environment/geography, and other patient comorbidities. Higher rates of specific circulating microRNAs, which may increase disease progression, were found in Black patients diagnosed with HCV-mediated HCC and cirrhosis[16]. This may be a contributing factor to the high prevalence of chronic HCV in this population. An ecologic study done in New York City demonstrated a geographic disparity in HCV-related mortality[17]. Neighborhoods with higher proportions of Hispanic and Black residents, poverty, non-English speaking households, and/or lower mean education level were positively associated with HCV mortality. Women with HCV also had a lower fibrosis score at younger ages, but this disparity does not persist after 50 years of age[18]. Rates of fibrosis progression were also highest among women with low socioeconomic status and among those with HIV coinfection. This population is also less likely to achieve sustained virologic response (SVR). However, racial disparities can be eliminated once SVR is achieved in terms of liver disease progression and overall survival[19].
Vulnerable populations have been the focus of numerous efforts aiming to understand and address the disparities in the prevention, screening, and treatment of viral hepatitis. Incarcerated individuals are particularly susceptible to viral infections both chronically and acutely. Worldwide, the prevalence of HCV is 10%-30% in prisoners, with an overall prevalence of 17.7%[20]. In 2019, an HAV outbreak occurred at a county jail in Minnesota, U.S.[21]. In response, the county jail’s health service promoted HAV vaccination among inmates, increasing vaccination from 0.6 to 7.1% within three days. Homeless individuals are another vulnerable population with limited access to appropriate healthcare. HCV screening in 6767 homeless individuals in Los Angeles, California, identified that 11.4% were HCV antibody positive. More than half of these patients were viremic, but 95% of the viremic patients were treated successfully with resources that were accessible within the local community[22]. A study in New South Wales, Australia, was done to identify the hurdles to treatment in the indigenous Aboriginal people, who have a high prevalence of HCV[23]. They interviewed 39 Aboriginal Australians in-depth and identified several specific challenges, including a lack of information provided at diagnosis, the stigma associated with HCV, and concerns about treatment's side effects and efficacy. Several groups have created healthcare policies to improve HCV screening and treatment, such as the Cherokee Nation Health Services in 2012. Through their collaborative efforts with the Center for Disease Control, HCV testing and treatment were significantly increased among Cherokee individuals[24]. Similar strategies have since been implemented by the Indian Health Services, resulting in HCV screening rate increases from 7.9% to 32.5%[25]. With targeted efforts and programs, identification and treatment of viral hepatitis can be possible in these vulnerable populations.
The prevalence of NAFLD has continued to increase and now accounts for about 60% of all chronic liver disease, which surpasses all other etiologies. In fact, NASH is the leading cause of liver transplantation among Asians and Hispanics[26]. Much of the NAFLD is driven by the high prevalence of obesity and metabolic syndrome. Nearly 2 billion people in the world are currently overweight, and by 2040, one billion people are expected to be living with obesity[27]. There was a strong association between NAFLD prevalence and national economic status[28]. European countries had a higher NAFLD prevalence (28.04%) than the Middle East (12.95%) and East Asia (19.24%). A systemic review and meta-analysis published in 2019 showed that roughly 16.7% of U.S. populations and 50% of the high-risk population had NAFLD[29]. In the United States, both the NAFLD prevalence and the risk for developing non-alcoholic steatohepatitis was the highest among Hispanics, followed by Whites, and the lowest in Blacks[29]. Underlying genetic factors may affect the progression of fatty liver disease. Hepatic steatosis within Hispanic communities may be partially attributable to the high rates of obesity, metabolic syndrome, and PNPLA3 polymorphism but this disparity in hepatic steatosis may be more evident in Hispanic communities of Mexican descent[30]. Asians have more central fat deposition at a lower body mass index (BMI) and have a higher prevalence of NAFLD with BMI < 25 kg/m2, compared to Western countries[31]. It is estimated that 8-19% of people with BMI < 25 kg/m2 have NAFLD. Underlying genetic factors such as the PNPLA3 polymorphism may play a role in the development of lean NAFLD; 13%-19% of Asians have PNPLA3 rs738409 GG genotype, compared to 4% in White and 25% of Hispanics. It is also important to note that the high prevalence of HBV infections in Asian countries poses a risk for concurrent chronic HBV infection and NAFLD. Unfortunately, few studies have explored the problem of multiple contributing factors in the totality of a patient’s liver condition.
While the predisposing factors for NAFLD have not been entirely elucidated, variations in sex hormones, insulin resistance, and hyperlipidemia may contribute to gender differences apparent in NAFLD. The prevalence of NAFLD is higher in men but increases steadily with age in women, es
The shortage of hepatology providers may outpace the epidemic of patients with chronic liver disease, especially NAFLD. While primary care providers may be managing diabetes, hyperlipidemia, and obesity, there are no definitive guidelines on clinical management of NAFLD, so much of NAFLD is likely undiagnosed. A U.S.-based study showed that only 5.1% of patients with NAFLD were aware of their diagnosis[38]. Currently, universal screening for NAFLD is not recommended, but certain high-risk populations may benefit from screening for NAFLD; including metabolic syndrome, age older than 50-year-old, type 2 diabetes for more than 10 years, or obesity with BMI > 35 kg/m2[39]. Without definitive strategies for diagnosis and therapy, primary care providers may rely on their hepatology colleagues for assistance; however, referral patterns may vary by race, insurance, location, and availability of a tertiary referral center for liver disease. One study showed that only 17% of Blacks were referred to specialized care, compared to 92% of Hispanics and 97% of Whites[38]. Efforts are currently underway to develop more uniform guidelines to assist providers with NAFLD. Because patients with NAFLD have concurrent diabetes, cardiovascular disease and dyslipidemia, multidisciplinary care with various specialists is essential[40,41].
Three million deaths worldwide are due to harmful alcohol use, accounting for 5.3% of all deaths and 13.5% of deaths in people aged 20-39 years[42]. Alcohol intake and the sequelae of excessive con
Underlying genetic factors and cultural differences contribute to the behavior related to alcohol consumption and thus rates of alcohol-associated liver disease. For moderate alcohol intake, Blacks had increased mortality secondary to liver disease, but not Whites[45]. This study suggested that Black individuals were more susceptible to alcohol-associated liver injury. This observation may be due to underlying comorbidities and genetic polymorphisms. During the COVID-19 pandemic, Black patients were more likely to be treated for alcoholic hepatitis, pancreatitis, and gastritis[43]. Providers’ racial bias may also influence treatment decisions for alcoholic liver disease. A recent United Network for Organ Sharing (UNOS) based study suggested that Black patients with alcohol-associated liver disease had less access to liver transplantation, but socioeconomic factors may have confounded this conclusion[46]. A population-based study from the U.S. National Vital Statistics System from 2007 to 2016 demonstrated that non-Hispanic Whites had the highest mortality rates for alcoholic liver disease; however, increases were seen in all Asian subgroups, with the highest increase in Japanese[5].
Gender disparity for alcohol-associated liver disease can be attributed to differences in alcohol metabolism and perhaps social and cultural biases in women with heavy alcohol consumption. Men and women metabolize alcohol differently, as women typically have a higher serum alcohol level per drink[46]. Such factors may impact whether an individual seeks treatment and the severity of liver disease at presentation. For the treatment of alcohol use disorder, appropriate care provided by a mental health provider is often necessary, but only 10% of patients utilize this service. Women were less likely to seek specialist treatment for alcohol use disorder and to receive relapse prevention medications[47]. In recent years, there has been a rapid increase in the prevalence and mortality of women with alcohol-associated liver disease, especially alcoholic hepatitis[48]. This trend was more evident in Asian and Native American women. However, women with alcohol-associated liver disease were less likely to pursue liver transplant evaluation[46]. Among liver transplant recipients due to alcohol-associated liver disease, women had higher model for end-stage liver disease (MELD) scores, higher rates of renal failure requiring dialysis, and higher rates of ventilator dependence before the transplant than men[49]. Men with alcohol-associated liver disease were more likely to be listed and receive liver transplantation than their female counterparts[50]. A history of other substance abuse was also more common in men, while women more often had an underlying psychiatric illness. Women were 9% less likely than men to experience long-term graft loss, though this gender disparity was isolated to patients with additional comorbidities.
Patterns of alcohol use may differ based on geography, and this may be related to restrictions on the purchase of alcohol, laws/prosecution of drivers under the influence of alcohol, and local advertising of alcoholic beverages. Many states have policies to regulate alcohol in the United States, though specific restrictions differ by state. Hadland et al[51] showed a strong correlation between state alcohol policies and the incidence and mortality of alcohol-associated cirrhosis. Mortality of alcohol-associated cirrhosis was highest among males in Western states and states with proportionally higher American Indians/Alaska Natives. A stricter alcohol policy was associated with lower mortality in females but not males. Among non-Indigenous descendants, more robust alcohol policy environments were linked to lower alcoholic cirrhosis mortality rates in both sexes.
Autoimmune liver disease includes autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and primary biliary cholangitis (PBC). While the etiology of autoimmune liver is largely unclear, it is likely less to be related to behavioral or preventable causes. Disparities in autoimmune liver disease may be related to genetic factors, concurrent illnesses and socioeconomic issues that affect access to care. The current literature is predominantly based on White patients, and the data from racial minorities are limited[52]. Autoimmune liver disease often requires complex treatment provided by a hepatologist and often in coordination with a rheumatologist. Those with less access to specialists may experience inadequate or delayed care. In addition, racial minorities and recent immigrants may have issues with language barrier, which impacts health literacy and may result in resistance to treatment. Genetic polymorphism with the subtype of PNPLA3 in Hispanic patients with AIH predicted more severe liver disease and increased mortality after liver transplantation[53,54]. A single-center, retrospective case-control study evaluated the association between race/ethnicity and AIH[55]. After controlling age and sex, the odds of AIH were higher among Black, Latinos, or API compared to Whites. Though not quite statistically significant, Black patients trended to have higher ALT, IgG, INR, bilirubin, inflammation on biopsy, hospitalizations, and other concomitant autoimmune diseases. A small study suggested worse outcomes for Black and Asian patients with AIH[52]. A National inpatient sample-based study on PBC showed increased hospitalization rates in Hispanics and females[56]. Patients with PBC who were Black or had Medicaid have had significantly higher in-hospital mortality[57]. Black patients with PSC with inflammatory bowel disease reportedly had worse PSC-related survival outcomes and higher need for liver transplantation[52,58]. More studies are needed to clarify the impact of socioeconomic and racial disparities on each of these specific autoimmune liver diseases.
HCC is the sixth most common malignancy and the second leading cause of cancer mortality worldwide. It frequently develops in patients with underlying liver diseases, such as those with cirrhosis, HCV, or HBV infections[59]. While appropriate surveillance can facilitate early detection HCC and improve survival, most cases are found at an advanced stage. There are significant differences in the incidence of HCC by race, but this may be likely be related to differences in the underlying chronic liver disease that predisposed a patient to HCC.
HCC occurs two to four times higher in men compared to women[18,60]. Some of this is likely due to the differences in viral hepatitis between genders and behavioral risk factors which may have led to viral hepatitis. In addition, differences in comorbidities such as alcohol use, smoking, and obesity may contribute to hepatocarcinogenesis and outcome. In the United States, increased mortality rates for HCC from 2000 to 2015 were seen in less educated patients, particularly men[61]. In contrast, women had significantly lower mortality from HCC[62], possibly due to early detection and response to the first treatment10]. Studies have also suggested that estrogen may have protective role in HCC development through reduction of interleukin-6 mediated hepatic inflammatory responses[60].
While the etiology of liver disease is an essential factor affecting incidence and survival in HCC, previous U.S. studies have shown that age-adjusted HCC incidence was highest in APIs, followed by Hispanics, Blacks, and Whites[63,64]. Among Asian ethnic groups, the highest HCC incidence rates were observed among Vietnamese, Cambodians, and Laotians. Southeast Asians were twice as likely to have HCC as other Asians and an 8-fold risk compared to Whites. While Asian countries have a considerably higher burden of disease, there are subgroups within these countries that demonstrate differences in HCC. A study from China, showed differences in mortality between Mongols vs Non-Mongols, primarily attributed to the difference in underlying HBV infections[65]. For other races, Black patients had a lower survival overall from HCC than their White or Hispanic counterparts[66]. Black patients typically present with advanced stages of HCC at the time of diagnosis and are thus less likely to receive curative treatment[66,67]. Even after adjusting for stages, Blacks and Hispanics were less likely to receive curative treatment for HCC and lower healthcare utilization for HCC. Blacks had a higher risk of inpatient mortality[68]. On the other hand, Asians are more likely to have underlying HBV infection without underlying cirrhosis; therefore, they are more likely to undergo curative resection[66].
The role of socioeconomic status has been evaluated for disparities in HCC outcomes. A Surveillance, Epidemiology and End Results (SEER) database study showed that patients who reside in the area with a higher cost of living index was associated with stage I disease, smaller tumor size, and increased likelihood of surgical intervention or loco-regional therapy[69]. Earlier detection of HCC in wealthier patients may infer more effective surveillance in this population. Similar findings were found in a study based on the Korean National Health Insurance sampling cohort[70]. This study of 7325 patients showed a decreased 5-year mortality for HCC among lower-income patients and middle-aged men. HCC outcomes also vary by hospital. Safety-net hospitals in the U.S. provide much of the care for patients with lower household income, education levels, and racial minorities. HCC patients in these safety-net hospital were less likely to undergo liver resection or transplantation and had higher procedure-specific mortality[71]. Insurance and employment status may also affect specialist referral and outcome. In a study conducted in China, Wu et al[72] showed increased mortality from HCC in patients with a basic health insurance compared to those with an employment-based insurance. Unemployment may also be a factor in HCC development and care. A U.S. census and National Health Interview survey based study showed that there was that alcohol use disorder and viral hepatitis was nearly 40% higher among the unemployed, and HCC mortality was worse in the unemployed group[73].
Place of residence may also affect the incidence and survival in HCC. A U.S. based SEER database study based on 83638 patients showed that rural and suburban residents had higher HCC mortality and were less likely to receive treatment than urban residents[74]. Insurance status itself may not guarantee appropriate access to healthcare providers as patients are unable to pay for associated costs in travelling to tertiary referral centers. Lee et al[75] showed in the SEER database and CDC’s cancer registries that there was a state-level racial and ethnic disparity in the incidence of HCC. This incidence trends had some correlation with obesity rates of the states. Geographic disparities were also identified in a study from Australia, where HCC incidence was highest amongst those residing in metropolitan cities and remote areas with high Indigenous populations[76].
While primary care providers are very familiar with screening for common cancers like breast and colon, many are not aware of current surveillance guidelines for HCC. As such, access to hepatologist or gastroenterologist may improve the rate of appropriate HCC surveillance. Current AASLD guidelines for HCC surveillance recommend ultrasound every 6 mo with or without alpha feto-protein, in patients with cirrhosis, chronic HBV, and chronic HCV with greater than stage 3 fibrosis[59]. Therefore, an appropriate surveillance for HCC requires recognition of the underlying chronic liver disease, appropriate testing and then referral for contrast-enhanced imaging for suspicious lesions. Without recognition of the underlying chronic liver disease and indications, surveillance cannot occur. As mentioned previously, many patients in the U.S. have unrecognized viral hepatitis and will neither receive antiviral agents nor undergo surveillance[9,12,77]. Despite guidelines, only 17% of HCC are detected in early stages (BCLC 0 and A) in the U.S., while European countries was 10%-30%[78]. On the other hand, Japan implemented nationwide HCC surveillance in 1980’s with free testing for hepatitis B surface antigen and HCV Ab in the clinics and meticulous surveillance. This eventually led to early-stage detection of HCC in 60%-65% of patients. Factors such as race, obesity, ascites, operator’s experience may also affect the early detection rates of HCC[12,79,80]. Finally, there is likely a complex interplay between these factors, socioeconomics and access to care that all affect surveillance and early detection in HCC.
Liver transplantation is considered the definitive treatment for end stage liver disease and early-stage HCC that is not amenable to resection. Because this is a risky operation, an expensive endeavor and a limited resource, it is not unexpected that disparities would be evident. Patients must be medically suitable with no active non-HCC malignancy, no ongoing infection, and adequate cardiovascular function. In addition, patients must be medically compliant, without active substance abuse and have financial stability/medical insurance coverage to ensure adequate access to immunosuppressive medications post-transplant. Unfortunately, the COVID-19 pandemic influenced liver transplant access among the minority population. A UNOS-based study showed minority had a more significant reduction in transplant (Minority: 15% vs White: 7% reduction) and listing for transplant (14% vs 12% reduction, respectively), despite a higher median MELD score (23 vs 20, respectively) during early COVID-19 period[81]. The reduction in transplants became more prominent for patients with public insurance than private insurance. Another study from Mexico showed an unequal reduction of liver transplantation for public (88% reduction) and private (35% reduction) hospitals during the COVID-19 pandemic[82].
In the United States, the MELD-Na score is the current organ allocation method for liver transplantation. This score is predictive of mortality on the waiting list but can create disparities. Gender disparities can occur because differences in muscle mass in males vs females affects creatinine, which is a variable in MELD. The current allocation system does not account for these sex differences in creatinine and anthropometric measure. In fact, Locke et al[83] showed that women had higher waitlist mortality and less likelihood of receiving deceased donor liver transplantation. Another cohort study by Cullaro et al[84] showed that women were 10% more likely to be removed from the waitlist for being too sick for liver transplantation. One study found that women removed from the list were older, had non-HCV liver disease, and had higher rates of hepatic encephalopathy. Another cohort study on liver transplantation for NASH showed that women were less likely to be White and listed with MELD exception points[85]. In multivariable analysis, women with NASH were 19% less likely to receive liver transplantation compared to men with NASH through the follow-up period even after adjusting for multiple other factors. Overall, women were less likely to have liver transplantation while more likely to die without transplant, be removed from the waiting list due to clinical deterioration or remain on the waiting list without liver transplantation. In order to decrease gender disparities in liver transplantation, Kim et al[86] attempted to address sexual disparity by creating an updated MELD 3.0, incorporating sex difference, which may provide a better mortality prediction. Efforts are currently underway to implement this in the U.S. Another study from Toronto, Canada, highlighted that there is a broader availability of living donor liver transplants (LDLT) to women, which may provide a protective factor to minimize the gender disparity[87].
Racial disparities in transplant can be seen in wait list mortality, use of simultaneous liver-kidney transplant (SLK), LDLT and eventual outcomes. A study on the UNOS liver transplant registry showed lower waitlist mortality among Asian compared to White after correcting for the MELD score[88]. Black patients tended to have higher MELD scores at the time of listing for liver transplantation but similar rates of waitlist mortality after correcting for MELD scores. Patients with end stage liver disease often have comorbid renal dysfunction, and SLK is considered. However, Black and Hispanic patients with renal dysfunction who are listed for liver transplantation were more likely to undergo SLK than White[89]. LDLT was noticeably lower among Hispanic, Black, and Asian patients compared to White patients. Blacks in particular, were less likely to inquire about LDLT during the evaluation process[90].
Finally, there are disparities in outcome and survival after liver transplantation. Differences in outcome after transplant may be biologically driven and may also be related to medical compliance. Blacks and women with HBV had worse long-term outcomes post-transplant compared to other races[91]. Recurrence of HBV was significant for Blacks even ten years post liver transplant, affecting the survival rate. While there have been racial disparities to liver transplantation in the past, the U.S. continues to develop broader sharing policies and allocation schemes which have helped equalize access to liver transplantation for all populations[92]. Longer term coverage of immunosuppression by Medicare and other insurances will also help improve long-term outcomes after liver transplant.
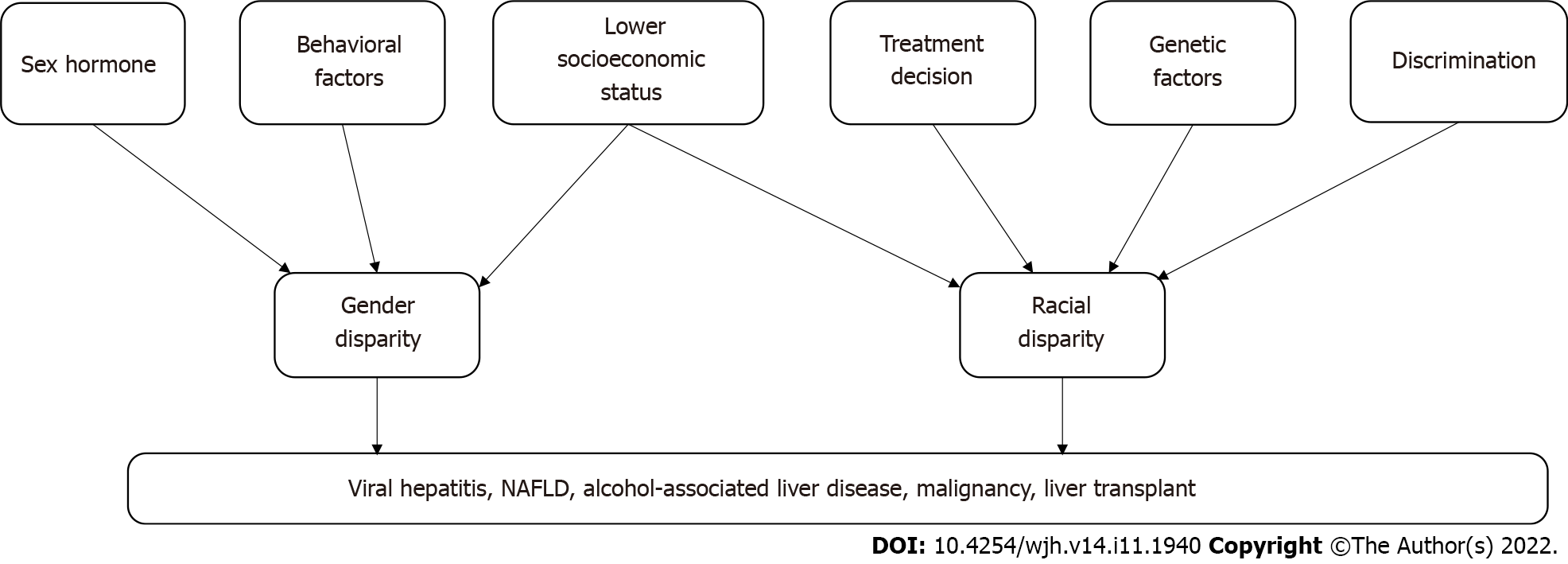
Disparities in liver disease and treatment are frequent and may be related to gender, race, geography, socioeconomic status and specific behaviors that predispose to liver disease (Figure 1). The need for complex management of these diseases, limited resources of liver transplantation and the inadequate number of specialists to care for these patients is further magnifying these disparities. Future studies should focus on evaluation of a language barrier among immigrant in regard to disparity and interventions to address these disparities.
A literature review was provided by Danielle Westmark, MLIS, IPI PMC, and Cindy Schmidt, M.D., M.L.S., from the Leon S. McGoogan Health Sciences Library at the University of Nebraska Medical Center.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bellini MI, Italy; Lee S, South Korea; Lee S, South Korea S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7855] [Cited by in F6Publishing: 7041] [Article Influence: 1173.5] [Reference Citation Analysis (1)] |
| 2. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 478] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 3. | Sliz E, Sebert S, Würtz P, Kangas AJ, Soininen P, Lehtimäki T, Kähönen M, Viikari J, Männikkö M, Ala-Korpela M, Raitakari OT, Kettunen J. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum Mol Genet. 2018;27:2214-2223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Russo MW, Fix OK, Koteish AA, Duggan K, Ditmyer M, Fuchs M, Chung RT, Reddy G. Modeling the Hepatology Workforce in the United States: A Predicted Critical Shortage. Hepatology. 2020;72:1444-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Li AA, Kim D, Kim W, Dibba P, Wong K, Cholankeril G, Jacobson IM, Younossi ZM, Ahmed A. Disparities in mortality for chronic liver disease among Asian subpopulations in the United States from 2007 to 2016. J Viral Hepat. 2018;25:1608-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Zhu D, Guo N, Wang J, Nicholas S, Wang Z, Zhang G, Shi L, Wangen KR. Socioeconomic inequality in Hepatitis B vaccination of rural adults in China. Hum Vaccin Immunother. 2018;14:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Park B, Choi KS, Lee HY, Jun JK, Park EC. Socioeconomic inequalities in completion of hepatitis B vaccine series among Korean women: results from a nationwide interview survey. Vaccine. 2012;30:5844-5848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Nguyen CT, Lin SY. Hepatitis B Screening in Asian and Pacific Islanders: New Guidelines, Old Barriers. J Immigr Minor Health. 2015;17:1585-1587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | National Center for HIV VH, STD, and TB Prevention. New estimates reveal declines in hepatitis C treatment in the U.S. between 2015 and 2020. [accessed 2022 Aug 21]. Available from: https://www.cdc.gov/nchhstp/newsroom/2021/2014-2020-hepatitis-c-treatment-estimates.html#:~:text=It's%20estimated%2040%20percent%20of,all%20people%20with%20risk%20factors. [Cited in This Article: ] |
| 10. | Geue C, Wu O, Xin Y, Heggie R, Hutchinson S, Martin NK, Fenwick E, Goldberg D; Consortium; ECDC. Cost-Effectiveness of HBV and HCV Screening Strategies--A Systematic Review of Existing Modelling Techniques. PLoS One. 2015;10:e0145022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Koh C, Zhao X, Samala N, Sakiani S, Liang TJ, Talwalkar JA. AASLD clinical practice guidelines: a critical review of scientific evidence and evolving recommendations. Hepatology. 2013;58:2142-2152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 13. | Wong RJ, Jain MK, Therapondos G, Shiffman ML, Kshirsagar O, Clark C, Thamer M. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol. 2018;113:1329-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Jung J, Du P, Feldman R, Kong L, Riley T 3rd. Racial/Ethnic and Socioeconomic Disparities in Use of Direct-Acting Antivirals Among Medicare Beneficiaries with Chronic Hepatitis C, 2014-2016. J Manag Care Spec Pharm. 2019;25:1236-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Schaeffer S, Khalili M. Reasons for HCV non-treatment in underserved African Americans: implications for treatment with new therapeutics. Ann Hepatol. 2015;14:234-242. [PubMed] [Cited in This Article: ] |
| 16. | Devhare PB, Steele R, Di Bisceglie AM, Kaplan DE, Ray RB. Differential Expression of MicroRNAs in Hepatitis C Virus-Mediated Liver Disease Between African Americans and Caucasians: Implications for Racial Health Disparities. Gene Expr. 2017;17:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Ford MM, Desai PS, Maduro G, Laraque F. Neighborhood Inequalities in Hepatitis C Mortality: Spatial and Temporal Patterns and Associated Factors. J Urban Health. 2017;94:746-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Corsi DJ, Karges W, Thavorn K, Crawley AM, Cooper CL. Influence of female sex on hepatitis C virus infection progression and treatment outcomes. Eur J Gastroenterol Hepatol. 2016;28:405-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Le AK, Zhao C, Hoang JK, Tran SA, Chang CY, Jin M, Nguyen NH, Yasukawa LA, Zhang JQ, Weber SC, Garcia G, Nguyen MH. Ethnic disparities in progression to advanced liver disease and overall survival in patients with chronic hepatitis C: impact of a sustained virological response. Aliment Pharmacol Ther. 2017;46:605-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Salari N, Darvishi N, Hemmati M, Shohaimi S, Ghyasi Y, Hossaini F, Bazrafshan MR, Akbari H, Mohammadi M. Global prevalence of hepatitis C in prisoners: a comprehensive systematic review and meta-analysis. Arch Virol. 2022;167:1025-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Zellmer L, Peters L, Silva RS. Hennepin County Adult Detention Center's Response to a 2019 Hepatitis A Outbreak in Minnesota. Am J Public Health. 2021;111:839-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Benitez TM, Fernando SM, Amini C, Saab S. Geographically Focused Collocated Hepatitis C Screening and Treatment in Los Angeles's Skid Row. Dig Dis Sci. 2020;65:3023-3031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Treloar C, Jackson C, Gray R, Newland J, Wilson H, Saunders V, Johnson P, Brener L. Care and treatment of hepatitis C among Aboriginal people in New South Wales, Australia: implications for the implementation of new treatments. Ethn Health. 2016;21:39-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mera J, Vellozzi C, Hariri S, Carabin H, Drevets DA, Miller A, Reilley B, Essex W, Gahn D, Lyons L, Leston J, Ward JW. Identification and Clinical Management of Persons with Chronic Hepatitis C Virus Infection - Cherokee Nation, 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Reilley B, Leston J, Hariri S, Neel L, Rudd M, Galope M, Ward J, Vellozzi C. Birth Cohort Testing for Hepatitis C Virus - Indian Health Service 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65:467-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649-1659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 359] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 27. | The World Obesity Federation. World Obesity Atlas 2022. [accessed 2022 Aug 24]. Available from: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022. [Cited in This Article: ] |
| 28. | Zhu JZ, Dai YN, Wang YM, Zhou QY, Yu CH, Li YM. Prevalence of Nonalcoholic Fatty Liver Disease and Economy. Dig Dis Sci. 2015;60:3194-3202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:198-210.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 30. | Shaheen M, Pan D, Schrode KM, Kermah D, Puri V, Zarrinpar A, Elisha D, Najjar SM, Friedman TC. Reassessment of the Hispanic Disparity: Hepatic Steatosis Is More Prevalent in Mexican Americans Than Other Hispanics. Hepatol Commun. 2021;5:2068-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 671] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 32. | Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019;70:1457-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 507] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 33. | Wang J, Wu AH, Stanczyk FZ, Porcel J, Noureddin M, Terrault NA, Wilkens LR, Setiawan VW. Associations Between Reproductive and Hormone-Related Factors and Risk of Nonalcoholic Fatty Liver Disease in a Multiethnic Population. Clin Gastroenterol Hepatol. 2021;19:1258-1266.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Chen XY, Wang C, Huang YZ, Zhang LL. Nonalcoholic fatty liver disease shows significant sex dimorphism. World J Clin Cases. 2022;10:1457-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Mo MQ, Huang ZC, Yang ZH, Liao YH, Xia N, Pan L. Relationship between total testosterone, sex hormone-binding globulin levels and the severity of non-alcoholic fatty liver disease in males: a meta-analysis. Ther Adv Endocrinol Metab. 2022;13:20420188221106879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Phan H, Richard A, Lazo M, Nelson WG, Denmeade SR, Groopman J, Kanarek N, Platz EA, Rohrmann S. The association of sex steroid hormone concentrations with non-alcoholic fatty liver disease and liver enzymes in US men. Liver Int. 2021;41:300-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Du T, Sun X, Yuan G, Zhou X, Lu H, Lin X, Yu X. Sex differences in the impact of nonalcoholic fatty liver disease on cardiovascular risk factors. Nutr Metab Cardiovasc Dis. 2017;27:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Le MH, Yeo YH, Cheung R, Wong VW, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011-2016. J Intern Med. 2020;287:711-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Kanwal F, Shubrook JH, Younossi Z, Natarajan Y, Bugianesi E, Rinella ME, Harrison SA, Mantzoros C, Pfotenhauer K, Klein S, Eckel RH, Kruger D, El-Serag H, Cusi K. Preparing for the NASH Epidemic: A Call to Action. Gastroenterology. 2021;161:1030-1042.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Kumar S, Wong R, Newberry C, Yeung M, Peña JM, Sharaiha RZ. Multidisciplinary Clinic Models: A Paradigm of Care for Management of NAFLD. Hepatology. 2021;74:3472-3478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Zoncapè M, Liguori A, Tsochatzis EA. Multi-disciplinary clinic models for the management of non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2022;11:586-591. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 42. | World Health Organization. Alcohol. May 9, 2022. [accessed 2022 Aug 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/alcohol. [Cited in This Article: ] |
| 43. | Damjanovska S, Karb DB, Cohen SM. Increasing Prevalence and Racial Disparity of Alcohol-Related Gastrointestinal and Liver Disease During the COVID-19 Pandemic: A Population-Based National Study. J Clin Gastroenterol. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: A modeling study. Hepatology. 2022;75:1480-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 45. | Fan L, Zhu X, Shingina A, Kabagambe EK, Shrubsole MJ, Dai Q. Racial Disparities in Associations of Alcohol Consumption With Liver Disease Mortality in a Predominantly Low-Income Population: A Report From the Southern Community Cohort Study. Am J Gastroenterol. 2022;117:1523-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Kaplan A, Wahid N, Fortune BE, Verna E, Halazun K, Samstein B, Brown RS Jr, Rosenblatt R. Black patients and women have reduced access to liver transplantation for alcohol-associated liver disease. Liver Transpl. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, Blow FC, Lok ASF. Gender Disparities in Alcohol Use Disorder Treatment Among Privately Insured Patients with Alcohol-Associated Cirrhosis. Alcohol Clin Exp Res. 2019;43:334-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Bertha M, Shedden K, Mellinger J. Trends in the inpatient burden of alcohol-related liver disease among women hospitalized in the United States. Liver Int. 2022;42:1557-1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Matsuoka L, Izzy M, Feurer ID, Rega SA, Ziogas IA, Alexopoulos SP. Sex and Gender Disparities in Pretransplant Characteristics and Relationships with Postoperative Outcomes in Liver Transplant Recipients with Alcoholic Liver Disease. Exp Clin Transplant. 2020;18:701-706. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 50. | McElroy LM, Likhitsup A, Scott Winder G, Saeed N, Hassan A, Sonnenday CJ, Fontana RJ, Mellinger J. Gender Disparities in Patients With Alcoholic Liver Disease Evaluated for Liver Transplantation. Transplantation. 2020;104:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Hadland SE, Xuan Z, Blanchette JG, Heeren TC, Swahn MH, Naimi TS. Alcohol Policies and Alcoholic Cirrhosis Mortality in the United States. Prev Chronic Dis. 2015;12:E177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Lee BT, Tana MM, Kahn JA, Dara L. We Are Not Immune: Racial and Ethnic Disparities in Autoimmune Liver Diseases. Hepatology. 2021;74:2876-2887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Wong RJ, Gish R, Frederick T, Bzowej N, Frenette C. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol. 2012;46:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Mederacke YS, Kirstein MM, Großhennig A, Marhenke S, Metzler F, Manns MP, Vogel A, Mederacke I. The PNPLA3 rs738409 GG genotype is associated with poorer prognosis in 239 patients with autoimmune hepatitis. Aliment Pharmacol Ther. 2020;51:1160-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Lee B, Holt EW, Wong RJ, Sewell JL, Somsouk M, Khalili M, Maher JJ, Tana MM. Race/ethnicity is an independent risk factor for autoimmune hepatitis among the San Francisco underserved. Autoimmunity. 2018;51:258-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Adejumo AC, Akhtar DH, Dennis BB, Cholankeril G, Alayo Q, Ogundipe OA, Kim D, Ahmed A. Gender and Racial Differences in Hospitalizations for Primary Biliary Cholangitis in the USA. Dig Dis Sci. 2021;66:1461-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Galoosian A, Hanlon C, Tana M, Cheung R, Wong RJ. Race/Ethnicity and Insurance-Specific Disparities in In-Hospital Mortality Among Adults with Primary Biliary Cholangitis: Analysis of 2007-2014 National Inpatient Sample. Dig Dis Sci. 2020;65:406-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Trivedi PJ, Crothers H, Mytton J, Bosch S, Iqbal T, Ferguson J, Hirschfield GM. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People With Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology. 2020;159:915-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 59. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2675] [Article Influence: 445.8] [Reference Citation Analysis (1)] |
| 60. | Wu EM, Wong LL, Hernandez BY, Ji JF, Jia W, Kwee SA, Kalathil S. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 61. | Ma J, Siegel RL, Islami F, Jemal A. Temporal trends in liver cancer mortality by educational attainment in the United States, 2000-2015. Cancer. 2019;125:2089-2098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Rich NE, Murphy CC, Yopp AC, Tiro J, Marrero JA, Singal AG. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;52:701-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Yang B, Liu JB, So SK, Han SS, Wang SS, Hertz A, Shariff-Marco S, Lin Gomez S, Rosenberg PS, Nguyen MH, Hsing AW. Disparities in hepatocellular carcinoma incidence by race/ethnicity and geographic area in California: Implications for prevention. Cancer. 2018;124:3551-3559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Pham C, Fong TL, Zhang J, Liu L. Striking Racial/Ethnic Disparities in Liver Cancer Incidence Rates and Temporal Trends in California, 1988-2012. J Natl Cancer Inst. 2018;110:1259-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | He WQ, Gao X, Gao L, Ma Y, Sun D, Sun J. Contrasting Trends of Primary Liver Cancer Mortality in Chinese Mongol and Non-Mongol. Asian Pac J Cancer Prev. 2021;22:2757-2763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Wagle NS, Park S, Washburn D, Ohsfeldt RL, Rich NE, Singal AG, Kum HC. Racial, Ethnic, and Socioeconomic Disparities in Curative Treatment Receipt and Survival in Hepatocellular Carcinoma. Hepatol Commun. 2022;6:1186-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, Bhuket T, Wong RJ. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016;50:423-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Kangas-Dick A, Gall V, Hilden P, Turner A, Greenbaum A, Sesti J, Paul S, Carpizo D, Kennedy T, Sadaria Grandhi M, Alexander HR, Wang S, Geffner S, August D, Langan RC. Disparities in utilization of services for racial and ethnic minorities with hepatocellular carcinoma associated with hepatitis C. Surgery. 2020;168:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Sempokuya T, Patel KP, Azawi M, Ma J, Wong LL. Increased morbidity and mortality of hepatocellular carcinoma patients in lower cost of living areas. World J Clin Cases. 2021;9:6734-6746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Kim DJ, Yoo JW, Chang JW, Yamashita T, Park EC, Han KT, Kim SJ. Does low income effects 5-year mortality of hepatocellular carcinoma patients? Int J Equity Health. 2021;20:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Hoehn RS, Hanseman DJ, Dhar VK, Go DE, Edwards MJ, Shah SA. Opportunities to Improve Care of Hepatocellular Carcinoma in Vulnerable Patient Populations. J Am Coll Surg. 2017;224:697-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Wu J, Liu C, Wang F. Disparities in Hepatocellular Carcinoma Survival by Insurance Status: A Population-Based Study in China. Front Public Health. 2021;9:742355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Singh GK, Siahpush M, Altekruse SF. Time trends in liver cancer mortality, incidence, and risk factors by unemployment level and race/ethnicity, United States, 1969-2011. J Community Health. 2013;38:926-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Zhou K, Pickering TA, Gainey CS, Cockburn M, Stern MC, Liu L, Unger JB, El-Khoueiry AB, Terrault NA. Presentation, Management, and Outcomes Across the Rural-Urban Continuum for Hepatocellular Carcinoma. JNCI Cancer Spectr. 2021;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Lee YT, Wang JJ, Luu M, Tseng HR, Rich NE, Lu SC, Nissen NN, Noureddin M, Singal AG, Yang JD. State-Level HCC Incidence and Association With Obesity and Physical Activity in the United States. Hepatology. 2021;74:1384-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Clark PJ, Stuart KA, Leggett BA, Crawford DH, Boyd P, Fawcett J, Whiteman DC, Baade PD. Remoteness, race and social disadvantage: disparities in hepatocellular carcinoma incidence and survival in Queensland, Australia. Liver Int. 2015;35:2584-2594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, Ruhl C, Unalp-Arida A. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;24:1052-1066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Kudo M. Management of Hepatocellular Carcinoma in Japan as a World-Leading Model. Liver Cancer. 2018;7:134-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Wong LL, Reyes RJ, Kwee SA, Hernandez BY, Kalathil SC, Tsai NC. Pitfalls in surveillance for hepatocellular carcinoma: How successful is it in the real world? Clin Mol Hepatol. 2017;23:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Wong LL, Hernandez B, Kwee S, Albright CL, Okimoto G, Tsai N. Healthcare disparities in Asians and Pacific Islanders with hepatocellular cancer. Am J Surg. 2012;203:726-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | MacConmara M, Wang B, Patel MS, Hwang CS, DeGregorio L, Shah J, Hanish SI, Desai D, Lynch R, Tanriover B, Zeh H 3rd, Vagefi PA. Liver Transplantation in the Time of a Pandemic: A Widening of the Racial and Socioeconomic Health Care Gap During COVID-19. Ann Surg. 2021;274:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Servin-Rojas M, Olivas-Martinez A, Ramirez Del Val F, Torres-Gomez A, Navarro-Vargas L, García-Juárez I. Transplant trends in Mexico during the COVID-19 pandemic: Disparities within healthcare sectors. Am J Transplant. 2021;21:4052-4060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Locke JE, Shelton BA, Olthoff KM, Pomfret EA, Forde KA, Sawinski D, Gray M, Ascher NL. Quantifying Sex-Based Disparities in Liver Allocation. JAMA Surg. 2020;155:e201129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 84. | Cullaro G, Sarkar M, Lai JC. Sex-based disparities in delisting for being "too sick" for liver transplantation. Am J Transplant. 2018;18:1214-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Loy VM, Joyce C, Bello S, VonRoenn N, Cotler SJ. Gender disparities in liver transplant candidates with nonalcoholic steatohepatitis. Clin Transplant. 2018;32:e13297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, Biggins SW, Wood NL, Gentry SE, Kwong AJ. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology. 2021;161:1887-1895.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 87. | Karnam RS, Chen S, Xu W, Chen C, Elangainesan P, Ghanekar A, McGilvray I, Reichman T, Sayed B, Selzner M, Sapisochin G, Galvin Z, Hirschfield G, Asrani SK, Selzner N, Cattral M, Lilly L, Bhat M. Sex Disparity in Liver Transplant and Access to Living Donation. JAMA Surg. 2021;156:1010-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Robinson A, Hirode G, Wong RJ. Ethnicity and Insurance-Specific Disparities in the Model for End-Stage Liver Disease Score at Time of Liver Transplant Waitlist Registration and its Impact on Mortality. J Clin Exp Hepatol. 2021;11:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Chang SH, Wang M, Liu X, Alhamad T, Lentine KL, Schnitzler MA, Colditz GA, Park Y, Chapman WC. Racial/Ethnic Disparities in Access and Outcomes of Simultaneous Liver-Kidney Transplant Among Liver Transplant Candidates With Renal Dysfunction in the United States. Transplantation. 2019;103:1663-1674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Nobel YR, Forde KA, Wood L, Cartiera K, Munoz-Abraham AS, Yoo PS, Abt PL, Goldberg DS. Racial and ethnic disparities in access to and utilization of living donor liver transplants. Liver Transpl. 2015;21:904-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Campsen J, Zimmerman M, Trotter J, Hong J, Freise C, Brown RS Jr, Cameron A, Ghobrial M, Kam I, Busuttil R, Saab S, Holt C, Emond JC, Stiles JB, Lukose T, Chang MS, Klintmalm G. Multicenter review of liver transplant for hepatitis B-related liver disease: disparities in gender and ethnicity. Clin Transplant. 2013;27:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Zhang Y. The Impact of the Share 35 Policy on Racial and Ethnic Disparities in Access to Liver Transplantation for Patients with End Stage Liver Disease in the United States: An Analysis from UNOS Database. Int J Equity Health. 2017;16:55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |