Published online Feb 27, 2021. doi: 10.4254/wjh.v13.i2.261
Peer-review started: November 24, 2020
First decision: January 11, 2021
Revised: January 20, 2021
Accepted: February 12, 2021
Article in press: February 12, 2021
Published online: February 27, 2021
Two-stage hepatectomy (TSH) is a well-established surgical technique, used to treat bilateral colorectal liver metastases (CRLM) with a small future liver remnant (FLR). However, in classical TSH, drop-out is reported to be around 25%-40%, due to insufficient FLR increase or progression of disease. Trans-arterial radioembolization (TARE) has been described to control locally tumor growth of liver malignancies such as hepatocellular carcinoma, but it has been also reported to induce a certain degree of contralateral liver hypertrophy, even if at a lower rate compared to portal vein embolization or ligation.
Herein we report the case of a 75-year-old female patient, where TSH and TARE were combined to treat bilateral CRLM. According to computed tomography (CT)-scan, the patient had a hepatic lesion in segment VI-VII and two other confluent lesions in segment II-III. Therefore, one-stage posterior right sectionectomy plus left lateral sectionectomy (LLS) was planned. The liver volumetry estimated a FLR of 38% (segments I-IV-V-VIII). However, due to a more than initially planned, extended right resection, simultaneous LLS was not performed and the patient underwent selective TARE to segments II-III after the first surgery. The CT-scan performed after TARE showed a reduction of the treated lesion and a FLR increase of 55%. Carcinoembryonic antigen and CA 19.9 decreased significantly. Nearly three months later after the first surgery, LLS was performed and the patient was discharged without any postoperative complications.
According to this specific experience, TARE was used to induce liver hypertrophy and simultaneously control cancer progression in TSH settings for bilateral CRLM.
Core Tip: Two-stage hepatectomy and trans-arterial radioembolization (TARE) are usually used in advanced stage primary liver malignancies. In this case report, two-stage hepatectomy and TARE were combined, for the first time, to treat a patient with bilateral colorectal liver metastases and a small future liver remnant. In particular, TARE was performed to induce liver hypertrophy and at the same time to control tumor growth between stages, thus reducing the risk of tumor progression.
- Citation: Serenari M, Neri J, Marasco G, Larotonda C, Cappelli A, Ravaioli M, Mosconi C, Golfieri R, Cescon M. Two-stage hepatectomy with radioembolization for bilateral colorectal liver metastases: A case report. World J Hepatol 2021; 13(2): 261-269
- URL: https://www.wjgnet.com/1948-5182/full/v13/i2/261.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i2.261
Two-stage hepatectomy (TSH) has been traditionally advocated for bilateral colorectal liver metastases (CLRM) that could not be resected in a single operation[1]. In TSH, when the future liver remnant (FLR) is considered not enough, contralateral portal vein ligation (PVL) or embolization (PVE) can be performed in the first stage to increase FLR volume. However, 25%-40% of patients will not undergo the second stage due to insufficient liver hypertrophy and/or progression of disease[2]. Trans-arterial radioembolization (TARE) consists of the selective intra-arterial administration of microspheres loaded with a radioactive compound—usually yttrium90—and has been shown to control tumor growth and to induce liver hypertrophy especially in patients with primary liver cancer. More recently, it has been shown to be effective also in CLRM setting[3]. However, the combination of these two techniques has never been explored before.
A 75-year-old female patient presented herself with mild abdominal pain.
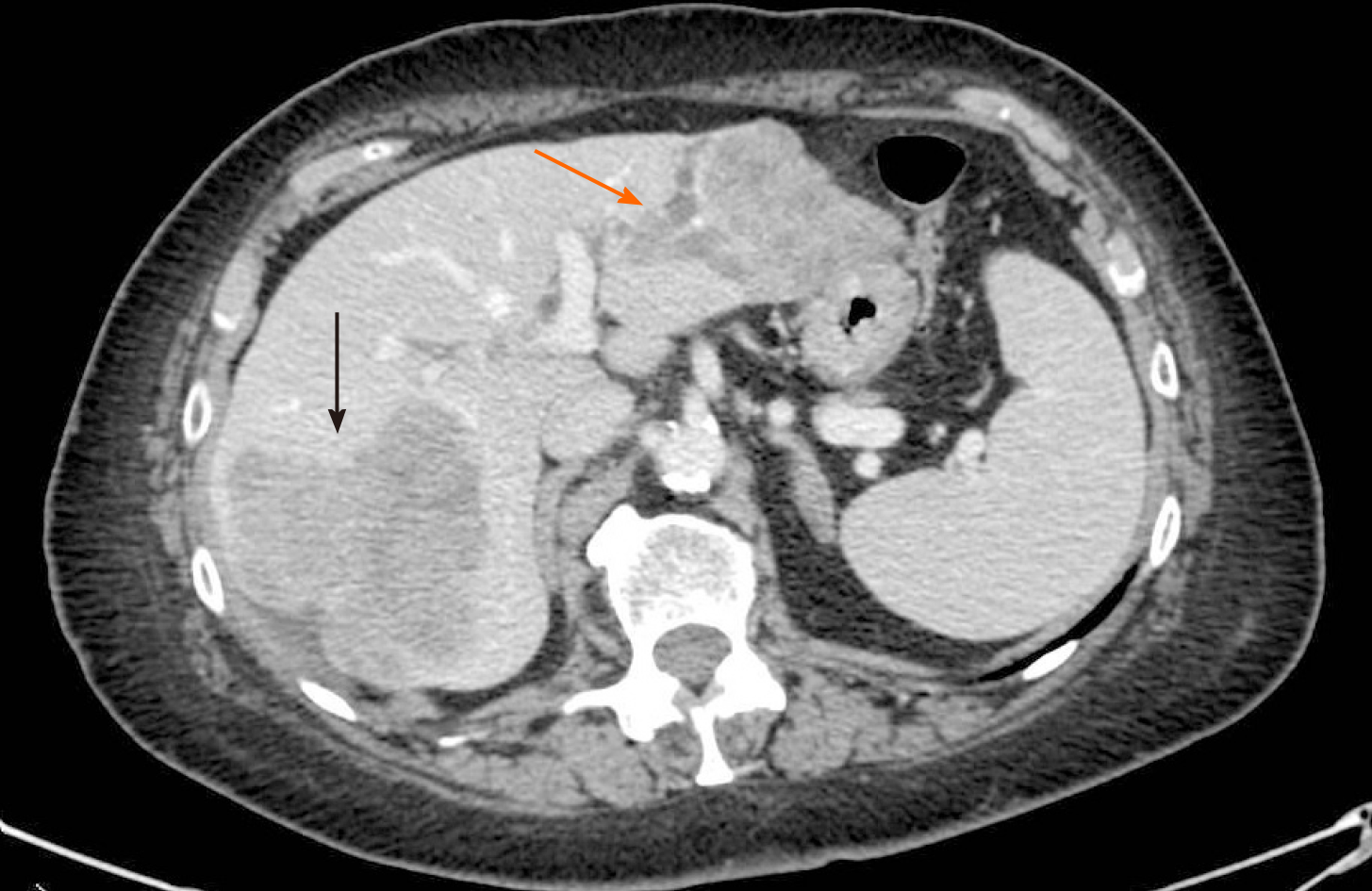
Ultrasonography and abdominal computed tomography (CT) detected three unknown hepatic lesions in segment VI-VII (n = 1) and segment II-III (n = 2), respectively. The lesion of the right lobe seemed to infiltrate the right hepatic vein whereas the other two confluent lesions in segment II-III showed a particular intrabiliary growth pattern (Figure 1).
The patient had undergone endoscopic removal of a sigmoid polyp cancer (T1NxMxR0) 3 years before.
She suffered from hypertension and hypoparathyroidism. She had no family history of cancer.
The patient had a good performance status. Physical examination was unremarkable with vital signs within the normal range of values. No jaundice was observed.
Liver function tests were normal and tumor markers were increased (carcinoembryonic antigen, CEA = 3284.9 ng/mL; CA 19-9 = 703.9 U/mL).
Esophagogastroduodenoscopy and colonoscopy were negative. According to liver volumetry, total functional liver volume (TFLV) measured 1635 mL and FLR (segments I-IV-V-VIII) 621 mL, with a resulting FLR/TFLV of 38%.
The final diagnosis of the presented case was suspected bilateral hepatic metastases from colorectal cancer.
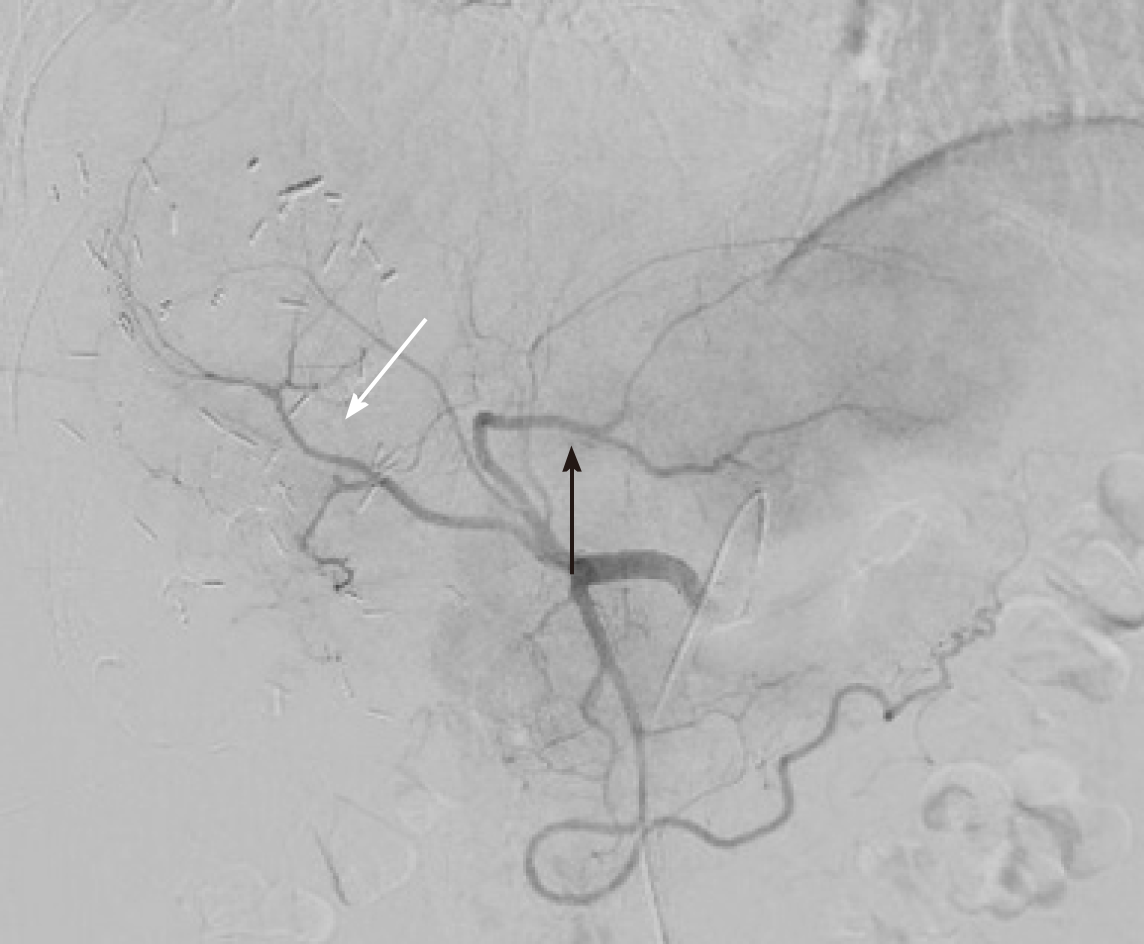
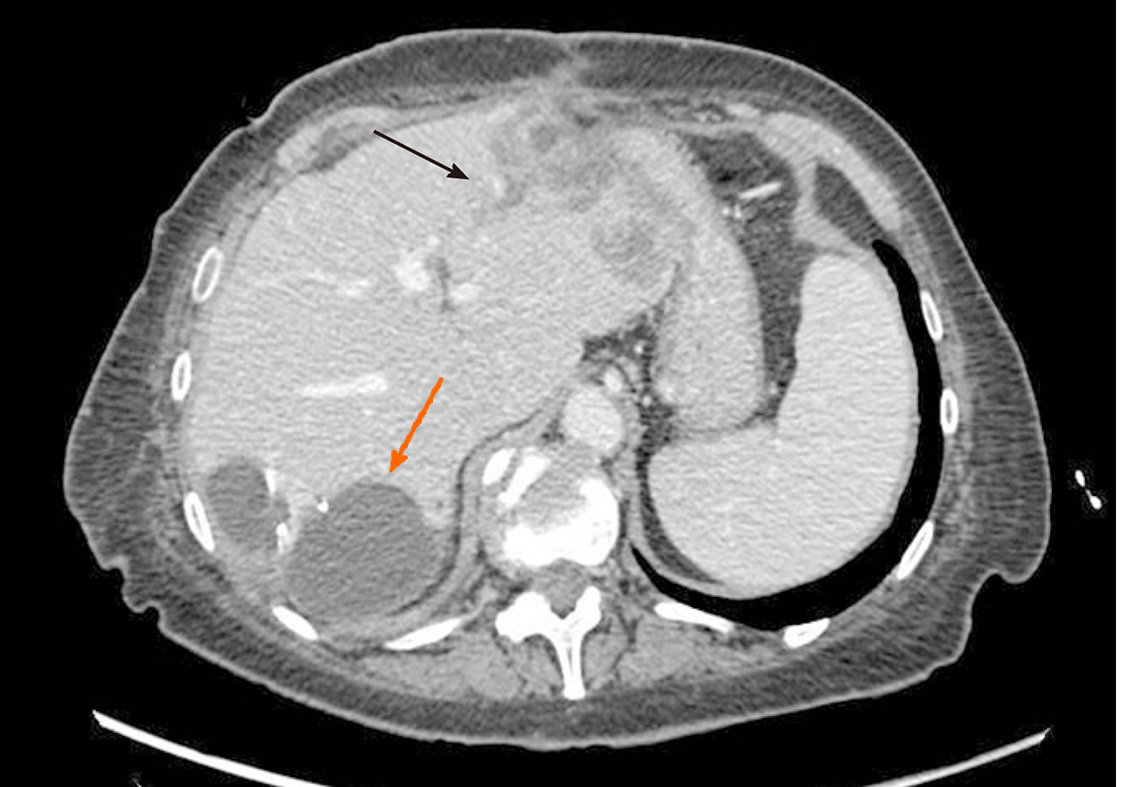
The operation started with a minimally invasive approach, but it was converted to open surgery due to diaphragm infiltration by the lesion located in the right liver. Intraoperative ultrasound showed that part of segment VIII was also involved. After detachment of the lesion from the diaphragm and its suture, a portal branch of segment V was ligated during parenchymal transection. Given the wider than initially planned hepatic surgery (segments V-VI-VII + part of segment VIII) and the difficulties encountered during the first resection, left lateral sectionectomy (LLS) was postponed. As a bridge treatment, TARE was chosen in order to control locally the disease while waiting for FLR increase. According to the CT-scan performed 10 d after surgery, FLR measured 632 mL. A small intrabdominal fluid collection was incidentally detected close to the surgical site as well as an ischemic area in segment V. The patient was discharged home on postoperative day 14, without major complications. The final diagnosis, based on histopathology of surgery specimen, was adenocarcinoma from colorectal cancer (KRAS and BRAF wild-type). TARE was carried out 11 d after discharge and realized with a single treatment (200 Gy) of Selective Internal Radiation (SIR) spheres (Sirtex Medical, Sydney, Australia) without any post-procedural complications (Figure 2). Forty-seven days after TARE, the patient underwent a new CT-scan showing a 32% reduction of the confluent lesion in segment II-III, with a surprisingly final FLR volume of 980 mL (FLR increase = 55%, FLR/TFLV = 77%) (Figure 3). CEA and CA 19.9 decreased to 93.7 ng/mL and 92.5 U/mL, respectively. After resolution of the abdominal collection by percutaneous drainage, we planned the second stage of surgery and 3 mo later after the first operation, the patient underwent LLS.
The postoperative course was uneventful. The patient did not receive any adjuvant chemotherapy and almost two years after the first surgery is still alive and free of disease.
According to this specific experience, TARE was used for the first time, combined with classical TSH, to control cancer progression between stages, waiting for adequate liver hypertrophy before the second resection.
TARE has been already shown to produce effective liver parenchyma hypertrophy in patients with primary hepatic malignancies treated with lobar 90Y radioembolization therapy[4]. After TARE, however, compared to PVE/PVL, the hypertrophy is radiation-induced rather than caused by embolization and is reached at a slower rate. According to a recent systematic review[5], the median kinetic growth rate of the controlateral lobe for patients underwent lobar TARE for CRLM was 0.8% per week compared to 6.1% of PVE. Despite a slower increase, however, a hypertrophy of 26%-47% was obtained at time intervals ranging from 44 d to 9 mo[4], of similar magnitude to that observed after PVE. In addition, Birgin et al[5] found that up to 84% of patients affected by primary and secondary hepatic malignancies had a local tumor control following TARE and about 30% of unresectable tumors underwent hepatic resection.
Review of the literature, including only studies of patients submitted to hepatectomy for CRLM after preoperative TARE (n = 18)[6-18] (Tables 1 and 2), showed that even though many of them comprised bilateral distribution of CRLM[18-20], only Pardo et al[21] reported a “two-stage resection” in 10 patients, 7 of whom underwent associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), probably from the cohort of Justinger et al[22] In this latter study, resectability of ALPPS + TARE was 85.7%. Increase of FLR in CRLM patients was reported only in few studies[22,23]. In our report, TARE to segment II-III led to a FLR increase of 55%, probably induced by the combined regenerative effect produced by the first liver resection, similar to what happens in ALPPS procedure, and by TARE itself. If a larger FLR hypertrophy was required, the role of TARE in combination also with classical portal vein occlusion techniques such as PVE/PVL or ALPPS, could have been explored. However, in this case, TARE was preferred over PVE or PVL since segment IV had to be preserved being part of the FLR. Furthermore metastasis in segment II-III could have progressed leading to the drop out of the patient. On the other side, the risk of proceeding with a second simultaneous hepatectomy or ALPPS was deemed too high. Last but not least, from the oncological point of view, this strategy may allow surgeons, without dealing with time issue, to select only patients with favorable tumor biology, according to radiological response after TARE[12].
| Ref. | Year | Type of study | Pts included, n | CRLM1, n (%) | Tumor location (n) | Bilobar n (%) | Prior resection, n (%) | Resectability, n (%) |
| Gray et al[18] | 2001 | HAI vs HAI + TARE in unresectable CRLM; RCT | 74 | 36 (48.6) | Colon (29), rectum (7) | 36/36 (100) | 0 | 1/36 (2.8) |
| Lim et al[6] | 2005 | TARE after failure of FU in unresectable CRLM; prospective | 30 | 30 (100) | NA | NA | 0 | 1/30 (3.3) |
| Sharma et al[7] | 2007 | TARE + FOLFOX4 in unresectable CRLM; prospective (phase I) | 20 | 20 (100) | Right colon (4), sigmoid (5), rectum (4), other colon sites (7) | NA | 0 | 2/20 (10) |
| Cosimelli et al[19] | 2010 | TARE in unresectable CRLM; prospective (phase II) | 50 | 50 (100) | Colon (41), rectum (9) | 35/50 (70) | 12/50 (24.0) | 2/50 (4.0) |
| Hendlisz et al[8] | 2010 | FU vs TARE + FU in unresectable CRLM; RCT | 44 | 21 (47.7) | NA | NA | NA | 1/21 (4.8) |
| Brown et al[9] | 2011 | TARE vs CHT vs no therapy before hepatectomy; case-control | 840 | 16 (1.9) | NA | NA | NA | 16/16 (100) |
| Whitney et al[10] | 2011 | TARE in unresectable liver disease; retrospective | 44 | 15 (34) | Rectum (15) | 0 | 0 | 1/15 (6.7) |
| Vouche et al[11] | 2013 | TARE in unresectable liver disease; retrospective | 83 | 8 (9.6) | NA | 0 | 0 | 1 (12.5) |
| Wang et al[20] | 2013 | TARE before liver resection for CRLM; retrospective | 24 | 24 (100) | Sigmoid (1), rectum (1), other colon sites (1), unknown (21) | 1/3 (33.3) | 0 | 3/24 (12.5) |
| Henry et al[12] | 2015 | TARE before liver resection for metastatic cancer; retrospective | 9 | 4 (44.4) | NA | NA | 0 | 4/4 (100) |
| Justinger et al[22] | 2015 | TARE in marginally resectable CRLM; retrospective | 13 | 13 (100) | Right colon (2), sigmoid (4), rectum (7) | 9/13 (69.2) | 7/13 (53.8)2 | 11/13 (84.6) |
| Moir et al[13] | 2015 | TARE in unresectable liver disease; retrospective | 44 | 22 (50) | NA | NA | NA | 4/22 (18.2) |
| Maleux et al[14] | 2016 | TARE in unresectable CRLM; NA | 88 | 71 (80.6) | NA | 58/71 (81.6) | 10/71 (14.0) | 1/71 (1.4) |
| Lewandowski et al[23] | 2016 | TARE in unresectable right-sided liver disease; retrospective | 13 | 1 (7.6) | NA | 0 | NA | 1/1 (100) |
| Wright et al[15] | 2017 | TARE in unresectable liver disease; retrospective | 465 | 6 (1.2) | NA | NA | NA | 6/6 (100) |
| van Hazel et al[16] | 2016 | FOLFOX6 vs FOLFOX6 + TARE ± Bevacizumab; RCT | 530 | 267 (50.3) | Left colon (141), right colon (72), rectum (45), other colon sites (7), unknown (2) | NA | NA | 38/267 (14.2) |
| Pardo et al[21] | 2017 | TARE before liver resection or transplantation; retrospective | 100 | 30 (30) | NA | 44/100 | 7/30 (23.3)2 | 30/30 (100) |
| Wasan et al[17] | 2017 | FOLFOX vs FOLFOX + TARE; RCT | 1103 | 554 (50.2) | Colon (421), rectum (116), unknown (17) | NA | NA | 56/554 (10.1) |
| Ref. | FLR increase % | Time TARE-surgery, median (range), mo | Type of hepatic resection (n) | Post-operative mortality % | Survival, median (range), mo | Disease free, median (range), mo | Recurrence after surgery, n (%) |
| Gray et al[18] | NA | NA | NA | 0 | 96 | NA | NA |
| Lim et al[6] | NA | NA | NA | NA | NA | 22 | 1/1 (100) |
| Sharma et al[7] | NA | NA | LLS + S6 (1), RH + S3 (1) | NA | NA | NA | NA |
| Hendlisz et al[8] | NA | NA | RH (1) | 0 | NA | 1.5 | 1/1 (100) |
| Brown et al[9] | NA | 6.5 (4-13) | NA | NA | NA | NA | NA |
| Whitney et al[10] | NA | NA | RT (1) | 0 | NA | 24 | 1/1 (100) |
| Vouche et al[11] | NA | NA | RT (1) | 0 | NA | NA | NA |
| Wang et al[20] | NA | NA (4-9) | RH (2), LH + S6 (1) | 0 | NA | NA | NA |
| Henry et al[12] | NA | 5 (2-8) | LLS (1), multiple wedge, HAI pump (1), RT + RFA (1), RT (1) | 50 | 13 (0-27) | 6.2 (1.8-10.5) | 3/4 (75) |
| Justinger et al[22] | 32.9 (ALPPS), 27.1 (no ALPPS) | 2 (1-5) | RT (4), RH (5), mesohepatectomy (1), LT (1) | 7.6 | 25 (12-38)1 | NA | NA |
| Moir et al[13] | NA | 4 (2-11) | NA | 0 | 15 (11-19)1 | NA | NA |
| Maleux et al[14] | NA | NA | S + RFA (1) | 0 | NA | NA | NA |
| Lewandowski et al[23] | 15 | 1.6 (1-7) | RT (1) | 0 | 4.8 | NA | NA |
| Wright et al[15] | NA | 9 (3-20) | RT (2), S (1), RH (3) | 16.6 | 25 (NA) | NA | NA |
| van Hazel et al[16] | NA | NA | NA | 0 | NA | NA | NA |
| Pardo et al[21] | NA | NA | NA | 10 | NA | NA | NA |
| Wasan et al[17] | NA | NA | NA | 3.6 | NA | NA | NA |
TARE in TSH setting may represent a viable option to increase resectability in patients with bilateral CLRM by stimulating liver hypertrophy and controlling locally the disease. Future larger, comparative studies may help answer the questions above.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: International Hepato-Pancreato-Biliary Association, No. ZTNR89NY2JR.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang KJ, Qiu Y S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, Aloia T. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525-536, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Baltatzis M, Siriwardena AK. Liver Resection for Colorectal Hepatic Metastases after Systemic Chemotherapy and Selective Internal Radiation Therapy with Yttrium-90 Microspheres: A Systematic Review. Dig Surg. 2019;36:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Teo JY, Allen JC Jr, Ng DC, Choo SP, Tai DW, Chang JP, Cheah FK, Chow PK, Goh BK. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford). 2016;18:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Birgin E, Rasbach E, Seyfried S, Rathmann N, Diehl SJ, Schoenberg SO, Reissfelder C, Rahbari NN. Contralateral Liver Hypertrophy and Oncological Outcome Following Radioembolization with 90Y-Microspheres: A Systematic Review. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Lim L, Gibbs P, Yip D, Shapiro JD, Dowling R, Smith D, Little A, Bailey W, Liechtenstein M. A prospective evaluation of treatment with Selective Internal Radiation Therapy (SIR-spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer. 2005;5:132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Sharma RA, Van Hazel GA, Morgan B, Berry DP, Blanshard K, Price D, Bower G, Shannon JA, Gibbs P, Steward WP. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E, Delatte P, Delaunoit T, Personeni N, Paesmans M, Van Laethem JL, Flamen P. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687-3694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Brown RE, Bower MR, Metzger TL, Scoggins CR, McMasters KM, Hahl MJ, Tatum C, Martin RC. Hepatectomy after hepatic arterial therapy with either yttrium-90 or drug-eluting bead chemotherapy: is it safe? HPB (Oxford). 2011;13:91-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Whitney R, Tatum C, Hahl M, Ellis S, Scoggins CR, McMasters K, Martin RC. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res. 2011;166:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, Gaba RC, Mulcahy MF, Baker T, Sato K, Hickey R, Ganger D, Riaz A, Fryer J, Caicedo JC, Abecassis M, Kulik L, Salem R. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | Henry LR, Hostetter RB, Ressler B, Bowser I, Yan M, Vaghefi H, Abad J, Gulec S, Schwarz RE. Liver resection for metastatic disease after y90 radioembolization: a case series with long-term follow-up. Ann Surg Oncol. 2015;22:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Moir JA, Burns J, Barnes J, Colgan F, White SA, Littler P, Manas DM, French JJ. Selective internal radiation therapy for liver malignancies. Br J Surg. 2015;102:1533-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Maleux G, Deroose C, Laenen A, Verslype C, Heye S, Haustermans K, De Hertogh G, Sagaert X, Topal B, Aerts R, Prenen H, Vanbeckevoort D, Vandecaveye V, Van Cutsem E. Yttrium-90 radioembolization for the treatment of chemorefractory colorectal liver metastases: Technical results, clinical outcome and factors potentially influencing survival. Acta Oncol. 2016;55:486-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Wright GP, Marsh JW, Varma MK, Doherty MG, Bartlett DL, Chung MH. Liver Resection After Selective Internal Radiation Therapy with Yttrium-90 is Safe and Feasible: A Bi-institutional Analysis. Ann Surg Oncol. 2017;24:906-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | van Hazel GA, Heinemann V, Sharma NK, Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland AH, Ferguson T, Rodríguez J, Kröning H, Wolf I, Ganju V, Walpole E, Boucher E, Tichler T, Shacham-Shmueli E, Powell A, Eliadis P, Isaacs R, Price D, Moeslein F, Taieb J, Bower G, Gebski V, Van Buskirk M, Cade DN, Thurston K, Gibbs P. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34:1723-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, Peeters M, Findlay M, Weaver A, Mills J, Wilson C, Adams R, Francis A, Moschandreas J, Virdee PS, Dutton P, Love S, Gebski V, Gray A; FOXFIRE trial investigators; SIRFLOX trial investigators; FOXFIRE-Global trial investigators; van Hazel G; Sharma RA. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 18. | Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, Gebski V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 438] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F; Italian Society of Locoregional Therapies in Oncology (SITILO). Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Wang LM, Jani AR, Hill EJ, Sharma RA. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J Clin Pathol. 2013;66:205-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Pardo F, Sangro B, Lee RC, Manas D, Jeyarajah R, Donckier V, Maleux G, Pinna AD, Bester L, Morris DL, Iannitti D, Chow PK, Stubbs R, Gow PJ, Masi G, Fisher KT, Lau WY, Kouladouros K, Katsanos G, Ercolani G, Rotellar F, Bilbao JI, Schoen M. The Post-SIR-Spheres Surgery Study (P4S): Retrospective Analysis of Safety Following Hepatic Resection or Transplantation in Patients Previously Treated with Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres. Ann Surg Oncol. 2017;24:2465-2473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Justinger C, Kouladouros K, Gärtner D, Tatsch K, Reimer P, Rüdiger T, Binnenhei M, Bentz M, Schön MR. Liver resection after selective internal radiotherapy (SIRT): Proof of concept, initial survival, and safety. J Surg Oncol. 2015;112:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lewandowski RJ, Donahue L, Chokechanachaisakul A, Kulik L, Mouli S, Caicedo J, Abecassis M, Fryer J, Salem R, Baker T. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol. 2016;114:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |